Abstract
Ni-ethylenediaminetetraacetic acid (Ni-EDTA) poses serious threats to the ecological environment and human health, due to its acute toxicity and low biodegradability. The decomplexation efficiency of Ni-EDTA through the conventional Fenton process has been constrained to pH; thus, other appropriate approaches are required to destroy the stable chelate structure at a neutral pH. In this study, the effect of operating parameters such as the pH, Fe2+ concentration, particle electrode dosage, current density, and coexisting ions was studied. The results revealed that the 3D-EF system owned advantages for the removal of Ni-EDTA in the broadening of the pH application window. The Ni-EDTA removal efficiency in the 3D-EF system reached 84.89% after 120 min at a pH of 7. In addition, the presence of coexisting ions slightly affected the decomplexation efficiency of Ni-EDTA.
1. Introduction
Ethylenediaminetetraacetic acid (EDTA) is widely used as a stabilizer of some metal ions in the process of plating and metallurgy, as it can react with Ni, Cu, and Fe to form stable chelates with different structures [1,2]. Ni as a typical plating metal is easy with EDTA to form a stable complex Ni-ethylenediaminetetraacetic acid (Ni-EDTA), and its high stability and acute toxicity to environment can be detrimental to life. Ni-EDTA usually has high stability and low biodegradability, resulting in lower removal efficiency of Ni-EDTA in conventional treatments [3]. Therefore, how to effectively remove metal–EDTA complexes remains a challenge, and numerous studies have been conducted to explore the treatment methods of metal–EDTA complexes from wastewater. Some promising treatments, such as non-thermal plasma oxidation/alkaline [4,5], microwave-assisted Fenton reaction [6], zero-valent iron [7], and ozone-based oxidation process [8], have been demonstrated to be effective in removing metal–EDTA complexes, but these methods can achieve a better removal performance of metal–EDTA at acidic conditions, and the high capital costs of these methods limits their widespread application.
Electrochemical advanced oxidation processes (EAOPs) are considered to be the most promising processes for the treatment of the metal–EDTA complex, with benefits including high versatility, mild conditions, and environmental compatibility [9,10]. Previous studies have shown that the hydroxyl radical (·OH) generated based on the electro-Fenton (EF) systems has the characteristics of strong oxidation, high reactivity, and harmless, and it has exhibited satisfactory efficiency in decomposing [11,12]. Basturk et al. (2021) studied the removal efficiency of antibiotics in the EF process and found that the removal efficiency of antibiotics exceeded 98% at a pH of 2.99 [13]. The Stupar team reported that the mineralization efficiency of acid blue 111 in the EF system achieved up to 76% at a pH of 2.5 [14]. Interestingly, outstanding results were achieved in the EF process with a Ti/Ru0.3Ti0.7O2 as a cathode in the study of Gamarra-Güere et al. (2019), who completed methylparaben degradation in both synthetic and real water at a pH of 3 [15]. However, the EF system can achieve a high removal efficiency only in a lower pH range. The problem of strong dependence on pH restricts its practical application. To overcome this drawback, a three-dimensional electro-Fenton (3D-EF) system based on an EF system filled with some particle electrodes has received extensive attention.
Particles are polarized to form a tiny electrolyzer in the 3D-EF system that not only can provide a shorter mass transfer distance but also improve the processing efficiency and enlarge the pH application window [16]. Lu et al. (2022) found a synergistic effect of the 3D-EF system in the presence of CuO-doped red mud particle electrodes and found that the system exhibited a higher degradation efficiency of ciprofloxacin at an initial pH of 7.0 [17]. It has also been reported that used Fe3O4/N-doped reduced graphene oxide as particle electrodes by Zhang et al. (2020) for bisphenol A (BPA) removal in a 3D-EF system. As a result, BPA exhibited excellent degradation efficiency within 90 min [18]. It can be noted that Mohammadi et al. (2018) demonstrated highly efficient degradation of ibuprofen and naproxen at near-neutral pH in a 3D-EF system with Fe-coated nickel foam particle electrodes [19].
The particle electrodes and polar plates play an important role in the efficiency of electrochemical treatment in the 3D-EF system. Granular activated carbon (GAC) is recognized as one of the most outstanding particle electrode materials due to its abundance and relatively low cost [20]. Graphite is a perfect electrode material, as it has excellent electrical conductivity and nontoxicity [21]. Previous studies have suggested that graphite cathode [22,23], graphite anode [24], and graphite bipolar plates [25] are promising polar plate materials for removing organic contaminants.
In this study, a 3D-EF system was constructed with cheap and easily available graphite as polar plates and GAC introduced as particle electrodes. The widening effect of the pH application window in the 3D-EF system was evaluated by the decomplexation performance of Ni-EDTA. The effect of process parameters (including Fe2+, particle electrode, current density, and coexisting ions) on the 3D-EF system was further studied. In addition, the reaction kinetics of each factor in the 3D-EF system were also fitted. This study provides a view of practical applications for the decomplexation of Ni-EDTA in different pH water environments.
2. Materials and Methods
2.1. Materials
Analytical purity ethylenediaminetetraacetic acid disodium salt (Na2EDTA∙2H2O) was bought from Macklin Biochemical Co., Ltd. (Shanghai, China). The reagents of nickel sulfate (NiSO4∙6H2O), sodium chloride (NaCl), sodium nitrate (Na2NO3), disodium hydrogen phosphate (Na2HPO4·12H2O), and sodium sulfate (Na2SO4) were of analytical grade and obtained from Damao Chemical Reagent in China Factory (Tianjin, China). Sulfuric acid (H2SO4) was bought from Kaixin Chemical Reagent Co., Ltd. (Hengyang, China). Ferrous sulfate (FeSO4∙7H2O) and sodium hydroxide (NaOH) were from Xilong Scientific Co., Ltd. (Shantou, China). GAC (particle size, 0.6–2 mm) was received as a particle from Aladdin Reagents Co., Ltd. (Shanghai, China). The Ni-EDTA (1000 mg/L) stock solution was prepared by dissolving NiSO4 and Na2EDTA∙2H2O in the molar ratio of 1:1 with ultrapure water with stewing for 24 h.
2.2. Experimental Setup
A glass reactor device (0.5 L) was used for Ni-EDTA decomplexation. Graphite bipolar plates (10 cm × 5 cm, 7 cm plate spacing) were used as the cathode and anode in the reactor devices, respectively. The 3D-EF system was realized by filling a certain dose of GAC (0.6–2 mm) particle electrodes in the reactor. Desired 50 mg/L Ni-EDTA reserve solution was added to the reactor, and then the solution pH was adjusted by 0.2 mol/L NaOH and H2SO4 immediately. Reactions were initiated when 0.047 mol/L Na2SO4 was added to the solution. Aliquots were sampled at predetermined time intervals, and samples’ pH was adjusted to 11 by NaOH and H2SO4 to form hydroxide precipitates, which were then removed.
2.3. Experimental Procedure
2.3.1. Degradation of Ni-EDTA by 3D-EF
An initial pH of 3, Fe2+ dosage of 1 mmol/L, GAC of 2 g/L, and current density of 4 mA/cm2 were used to study the superiority of the 3D-EF system. The EF system was performed by using the same reactor devices without GAC. GAC was used as an absorbent to remove Ni-EDTA in the GAC system.
2.3.2. The pH Window Was Widened in 3D-EF System
The widening of the pH application window of the 3D-EF system was evaluated at different initial pH values of 3–9, Fe2+ dosage of 1 mmol/L, GAC of 2 g/L, and current density of 4 mA/cm2.
2.3.3. Parameter Optimization of Ni-EDTA Degradation by 3D-EF
Fe2+ dosage, GAC dosage, and current density were used to evaluate the parameters of Ni-EDTA degradation in 3D-EF system. Under the optimal pH condition, an Fe2+ dosage of 0.5–2.0 mmol/L, GAC of 1–6 g/L, and current density of 2–12 mA/cm2 were discussed in the decomplexation of Ni-EDTA in the system.
2.3.4. The Effect of Coexisting Ions in 3D-EF System
The most suitable conditions, namely the pH, Fe2+ dosage, particle electrode, and current density, were prepared. The effect of coexisting ions (Cl−, NO3−, and HPO42−) in the 3D-EF system on Ni-EDTA decomplexation was investigated.
2.4. Analytical and Statistical Methods
The pH value of the solution was monitored by a potentiometric titrator meter (PB-10, Sartorius). Experimental samples were taken at a certain time (10, 20, 30, 60, 90, and 120 min) for the determination of Ni2+. Samples were diluted 500 times and passed through 0.22 μm membranes for detection. Each experiment was performed in three replicates to ensure the repeatability of the experiment. Inductively Coupled Plasma–Mass Spectrometry (ICP–MS, NexlON 2000, PerkinElmer, Waltham, MA, USA) was used to analyze the Ni2+ level of the filtrate. Origin 2018 software was used to analyze data.
The Ni-EDTA removal efficiency (R) was calculated by using Equation (1).
R = (1 − Ct/C0) × 100%
The kinetics of Ni-EDTA degradation were characterized by the first-order equation, and the reaction rate constants (Kobs) were determined according to Equation (2).
where C0 (mg/L) and Ct (mg/L) are the Ni2+ initial concentration and the concentration at time, t, respectively.
Ln (C0/Ct) = Kobst
3. Results and Discussion
3.1. The Advantages of Ni-EDTA Decomplexation in 3D-EF System
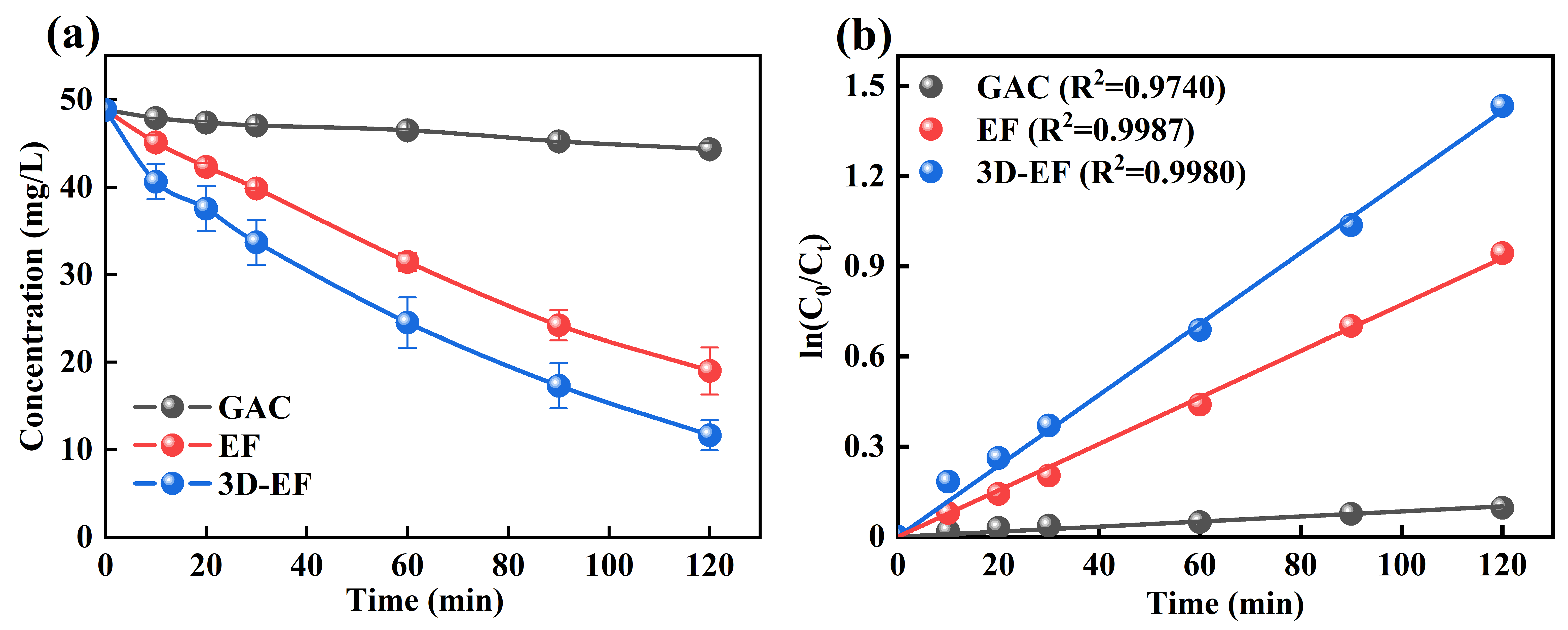
The concentration of Ni-EDTA decreased gradually with the extension of reaction time (Figure 1a). The results showed that the performance of the 3D-EF system was higher than that of the EF and GAC systems, respectively. After 120 min, the residual concentrations of Ni-EDTA in each system were 44.33, 18.99, and 11.64 mg/L, respectively, and the removal rates were calculated to be 9.17%, 61.08%, and 76.14%. From Figure 1b, we can see that the Kobs of 3D-EF system was higher than that of other systems, creating a sharp contrast. This illustration was consistent with Zhang et al. (2020) [18].
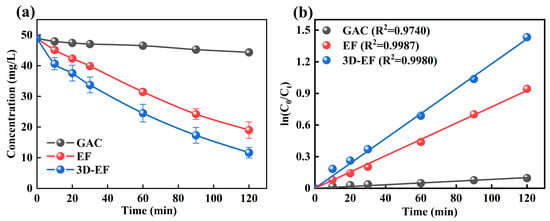
Figure 1.
Decomplexation of Ni-EDTA in different systems (a) and kinetics fitting lines (b).
The 3D-EF system based on GAC particle electrodes gives an excellent Ni-EDTA decomplexation performance. In the 3D-EF system, GAC particle electrodes become independent electrodes through electrostatic induction, which could not only expand the electrode specific surface area but also increase space utilization [26]. Moreover, the mass transfer effect could be improved due to the shorter distance between the GAC particle electrode in the 3D-EF system. Hence, the 3D-EF system could provide a higher removal efficiency of Ni-EDTA compared with the conventional EF system, thus greatly enhancing the current efficiency, the rate of chemical reaction, and the treatment effect [27,28].
3.2. pH Application Window of 3D-EF for Ni-EDTA Complex Breaking
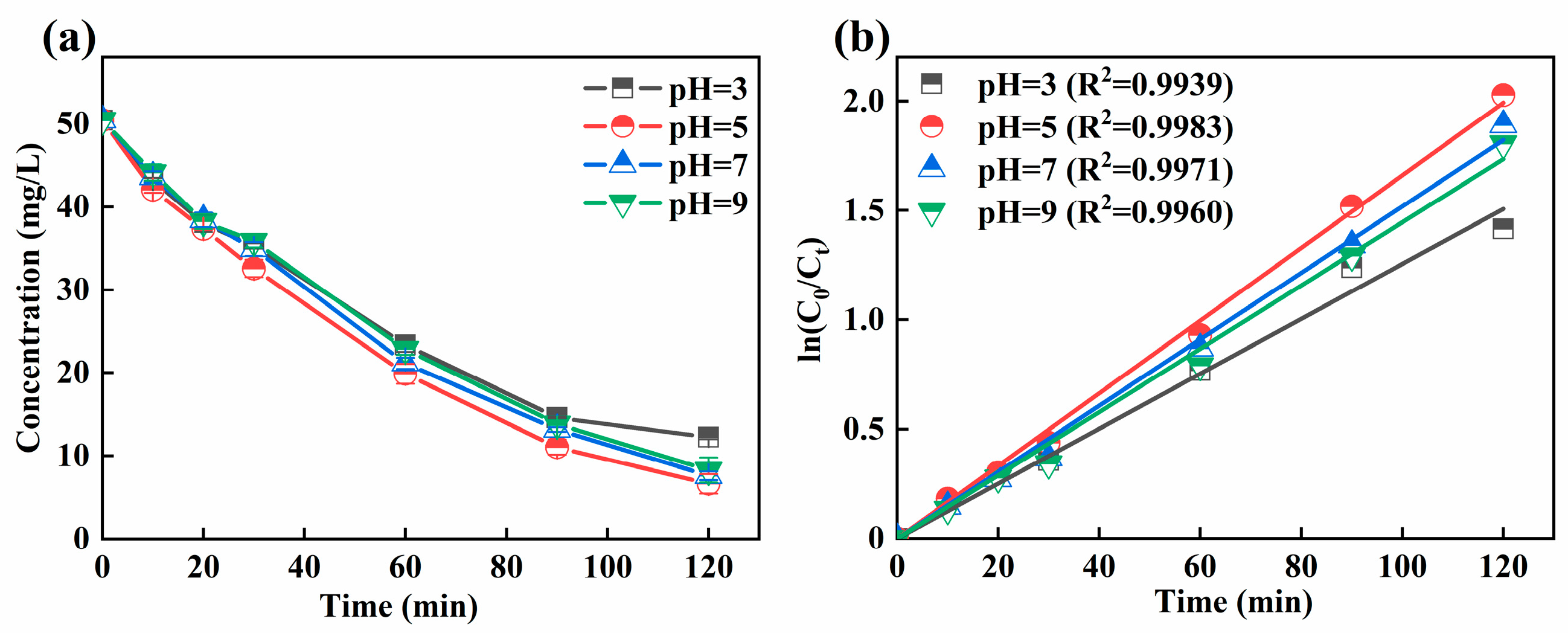
The pH is related to the generation of H2O2 in the cathode and to the present form of iron ions, which further can affect the treatment performance of 3D-EF system. A too-high or too-low pH would result in a change in treatment performance. It could be seen from Figure 2a that the residual concentration of Ni-EDTA at different pH values was 12.25, 6.64, 7.62, and 8.29 mg/L in 3D-EF system, respectively, which can also be effectively removed at a near-neutral pH. The Kobs within 120 min was the highest at a pH of 5 (Figure 2b). Kobs increased first and then decreased with the increase of the initial pH, which was consistent with the removal trend of Ni-EDTA.
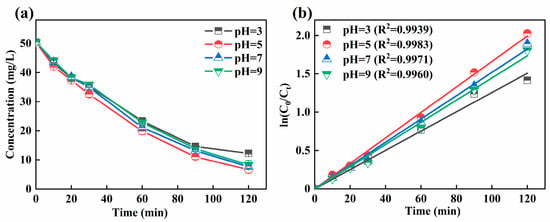
Figure 2.
Decomplexation of Ni-EDTA with different pH values (a) and kinetics fitting lines (b).
As shown in Table 1, in many 3D-EF studies, lower pH values tended to have a higher contaminant removal rate. Liu et al. (2019) found that the mineralization efficiency of m-cresol at a pH of 2.0 was about average, at 70% [29]. Dargahi et al. (2021) determined that the three-dimensional sono-electro-Fenton achieved 96.2% removal efficiency for 2,4-dichlorophenoxyacetic acid within 60 min, at a pH of 3.0 [30]. In addition, Wan et al. (2021) found that the 3D-EF reactor with AC@Ti-Cu-Ni-Zn-Sb-Mn particles serving as particle electrodes could achieve the degradation of p-aminophenol at a pH of 7.0 [31]. Compared with other research reports, this study used GAC particle electrodes without additional preparation; inexpensive and easily available graphite was used as the polar plate to construct a 3D-EF system which can achieve higher Ni-EDTA removal efficiency and break through the application window of pH.
In this study, the 3D-EF system with the GAC particle electrode could break through the limitation of pH in the traditional EF process and broaden the application window of pH; thus, better removal efficiency of Ni-EDTA was achieved at a near-neutral pH. The 3D-EF system could produce a hydrogen evolution reaction at a lower pH, which reduced the generation of H2O2 and ·OH. The 3D-EF system inhibited the formation of ·OH at a higher pH, produced Fe(OH)3 precipitation, and significantly reduced the decomposition of H2O2 into H2O and O2 [32]. However, the EF system needs to be carried out under acidic conditions. Fe(OH)+ would be generated at a lower pH, and it could compete with Fe2+ to react with H2O2. The reaction rate of Fe(OH)+ with H2O2 was slower, resulting in the lower generation of ·OH. On the other hand, Fe(OH)3 precipitation was easy to form and cover the particle electrodes at a higher pH, which resulted in current loss and increased the processing costs [33]. The 3D-EF system has excellent removal of Ni-EDTA in the near-neutral pH range, and the pH was not an adjusted pH (pH = 5) in the following experiments.

Table 1.
Some typical 3D-EF works at different pH values.
Table 1.
Some typical 3D-EF works at different pH values.
| Anode | Cathode | Particle Electrode | Process Parameters | Efficiency | Reference |
|---|---|---|---|---|---|
| Ti/RuO2 | Ti/RuO2 | Activated carbon fiber loaded with MnOx | pH = 2 | Total organic carbon: 70% | [29] |
| SS316/β-PbO2 | Stainless-steel plate | Powder activated carbon/Fe3O4 | pH = 3 | 2,4-dichlorophenoxyacetic acid: 96.2% COD: 92.3% Total organic carbon: 86.5% | [30] |
| RuO2-IrO2-Ti | Activated carbon fiber | Cu-Fe/Sodium alginate Carbon | pH = 5.4 | Fulvic acid: 81.1% | [34] |
| Ti/RuO2 | Ti/RuO2 | GAC | pH = 5.56 | Amoxicillin: 98.98% Total organic carbon: 47.6% | [35] |
| Ti/RuO2-IrO2-Ta2O5 | Stainless-steel plate | AC@Ti-Cu-Ni-Zn-Sb-Mn | pH = 7 | P-aminophenol: 99.87% | [31] |
3.3. Influence Factors on Decomplexation of Ni-EDTA
3.3.1. Effect of Fe2+ Dosage
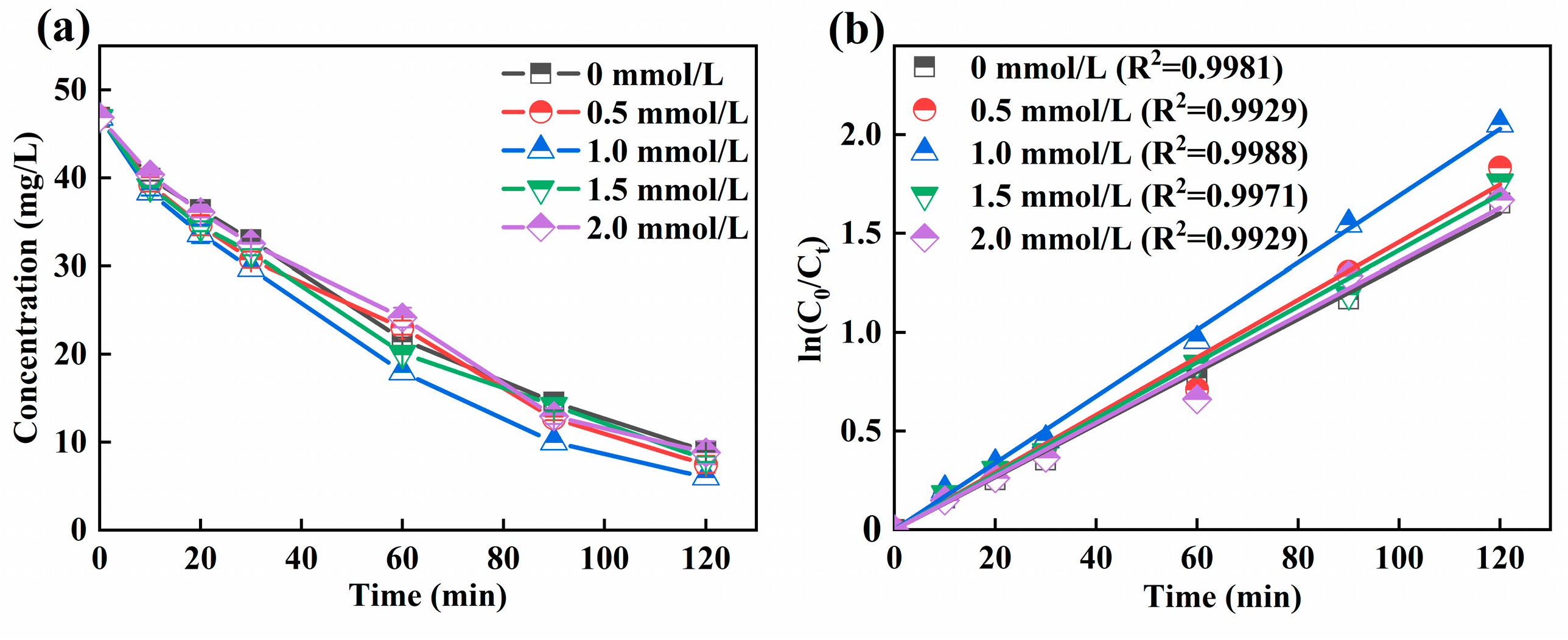
Figure 3a illustrated that the effect of Fe2+ dosage on the Ni-EDTA. With the increase of Fe2+ dosage, the removal efficiency of Ni-EDTA in the 3D-EF system first increased and then decreased. At an Fe2+ dosage of 1 mmol/L, the removal efficiency of Ni-EDTA in the 3D-EF system was the highest. Figure 3b showed that Kobs reached a maximum at an Fe2+ dosage of 1 mmol/L. An Fe2+ dosage was a key factor in the 3D-EF system. In the absence of Fe2+, the decomplexation of Ni-EDTA mainly depends on the oxidation of plates and particle electrodes.
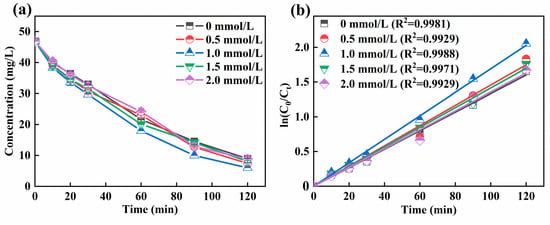
Figure 3.
Decomplexation of Ni-EDTA in different Fe2+ dosages (a) and kinetics fitting lines (b).
The activity of micro-electrolysis was weak when the Fe2+ dosage was smaller, but the Ni-EDTA removal efficiency also increased gradually with the increasing Fe2+ dosage to generate more ·OH [36]. When the dosage of Fe2+ was too high, the contact probability between Fe2+ and H2O2 increased, and the amount of ·OH produced increased. However, excessive Fe2+ would react with ·OH to generate Fe3+, and a large amount of ·OH was consumed, further reducing the oxidation capacity and Ni-EDTA removal efficiency in the 3D-EF system [18,37].
3.3.2. Effect of Particle Electrode Dosage
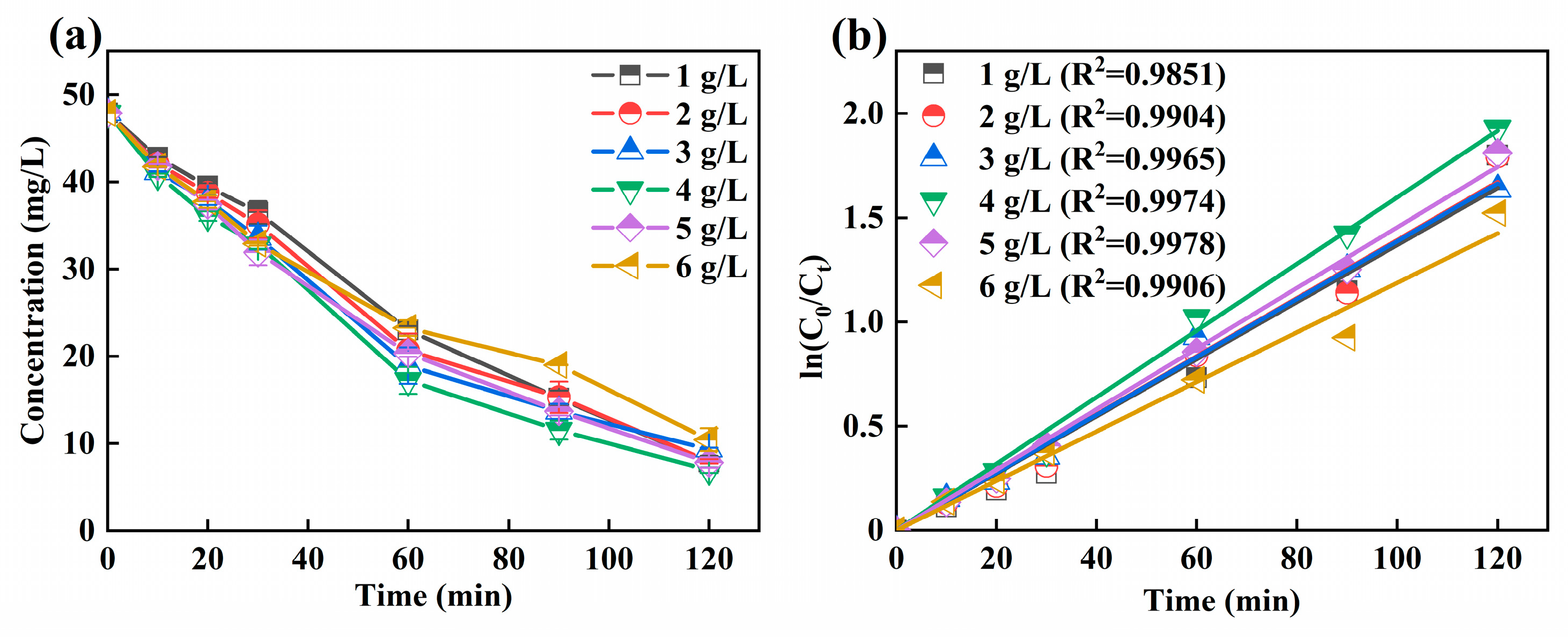
It could be observed from Figure 4a that the residual concentration of Ni-EDTA decreases first and then increases with the increase of particle electrode dosage. When the particle electrode dosage was 4 g/L, the removal efficiency of Ni-EDTA was the highest, and the concentration of Ni-EDTA decreased from 47.89 to 6.96 mg/L. As shown in Figure 4b, the Kobs reached the maximum when the particle electrode dosage was 4 g/L, which has a faster reaction rate than other particle electrode doses.
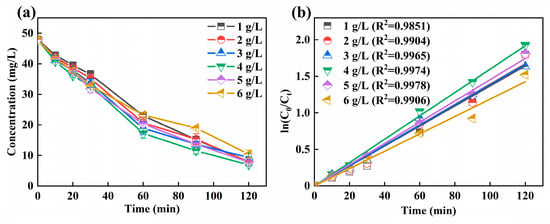
Figure 4.
Decomplexation of Ni-EDTA in different particle electrode dosages (a) and kinetics fitting lines (b).
The particle electrode was evenly distributed in the electrolyzer, resulting in the increase of electrode specific surface area and shortened electrode distance. Thus, it was a crucial factor affecting the treatment efficiency of the 3D-EF system and greatly improved the treatment efficiency for Ni-EDTA. The appropriate particle electrode could increase the electrode surface area and current efficiency, and the Ni-EDTA removal efficiency was further improved [38]. When the particle electrodes continued to be added, the current efficiency and mass transfer efficiency would not continue to improve after the polarized particle electrodes reached saturation in a limited reaction space. The short-circuit current formed between the mutually bonded particle electrodes further restricts the removal of pollutants, resulting in the decline of the decomplexation performance of Ni-EDTA [27].
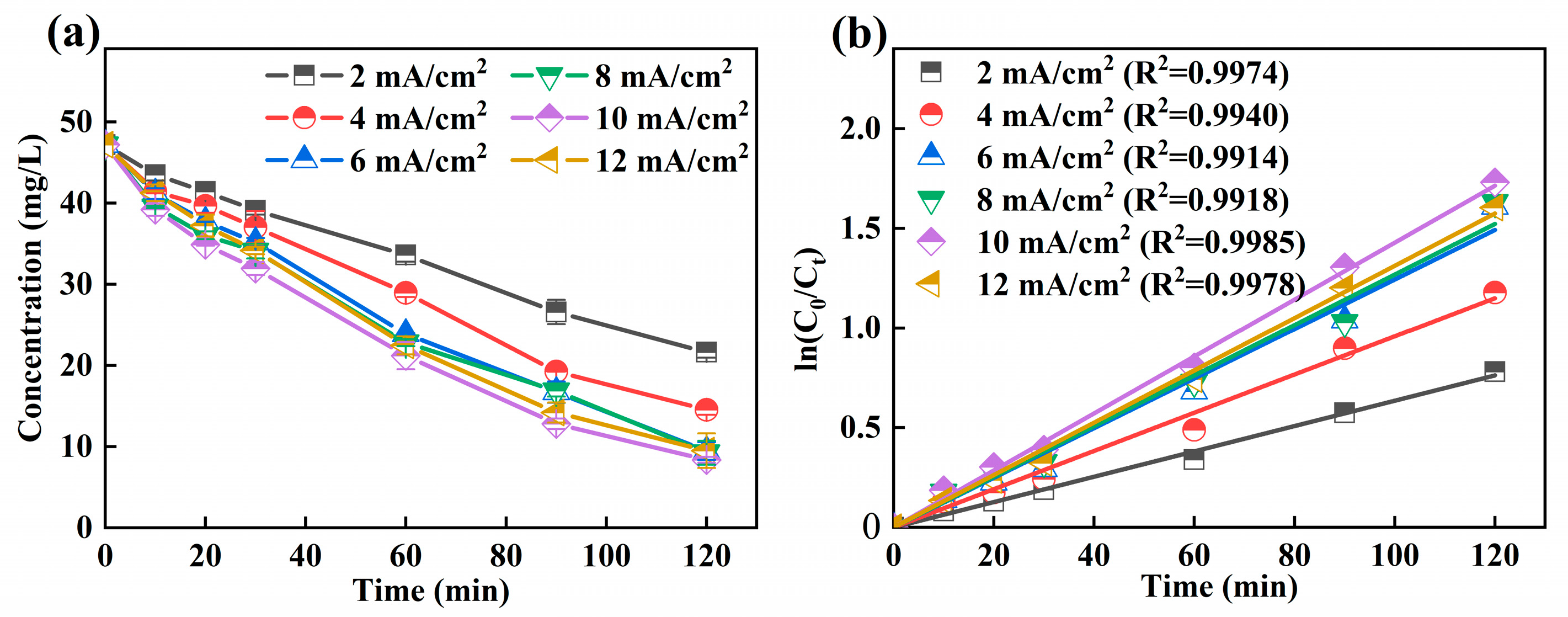
3.3.3. Effect of Current Density
The effect of different current density on the decomplexation of Ni-EDTA was studied. The results are shown in Figure 5a; when the current density was 2 mA/cm2, the residual concentration of Ni-EDTA was the highest after 120 min, and the Ni-EDTA concentration was removed from 47.19 to 21.63 mg/L. At a current density of 10 mA/cm2, the residual concentration of Ni-EDTA was 8.36 mg/L after 120 min, and it achieved the best removal efficiency of Ni-EDTA, at 82.28%. It was found that, by continuing to increase the current density, the removal efficiency of Ni-EDTA decreased and was only 79.87% at a current density of 12 mA/cm2. In Figure 5b, the Kobs is much larger than that of the other current density experimental groups at a current density of 10 mA/cm2.
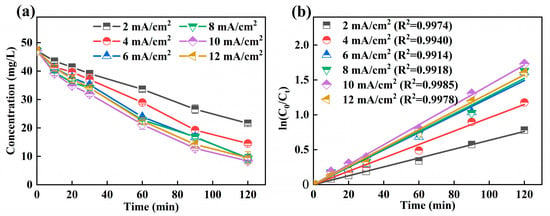
Figure 5.
Decomplexation of Ni-EDTA in different current density (a) and kinetics fitting lines (b).
The increase of current density further increases the electron density on the electrode, and the H2O2 generating ability was also enhanced. Fe2+ reacted with H2O2 by Equation (3) to generate ·OH, and the amount of ·OH increased accordingly. Moreover, the increasing current density would also increase the electron transfer between electrodes, accelerate its direct oxidation reaction at the electrode surface, and increase the Ni-EDTA removal efficiency [39,40]. A high current density will accelerate the decomposition of H2O2 and increase the probability of competitive side reactions of hydrogen evolution (Equation (4)) and oxygen evolution (Equation (5)). Further ·OH was scavenged, and the removal efficiency of Ni-EDTA was reduced [18,41].
Fe2+ + H2O2 → Fe3+ + OH− + ∙OH
2H+ + 2e− → H2
2H2O → O2 + 4H+ + 4e−
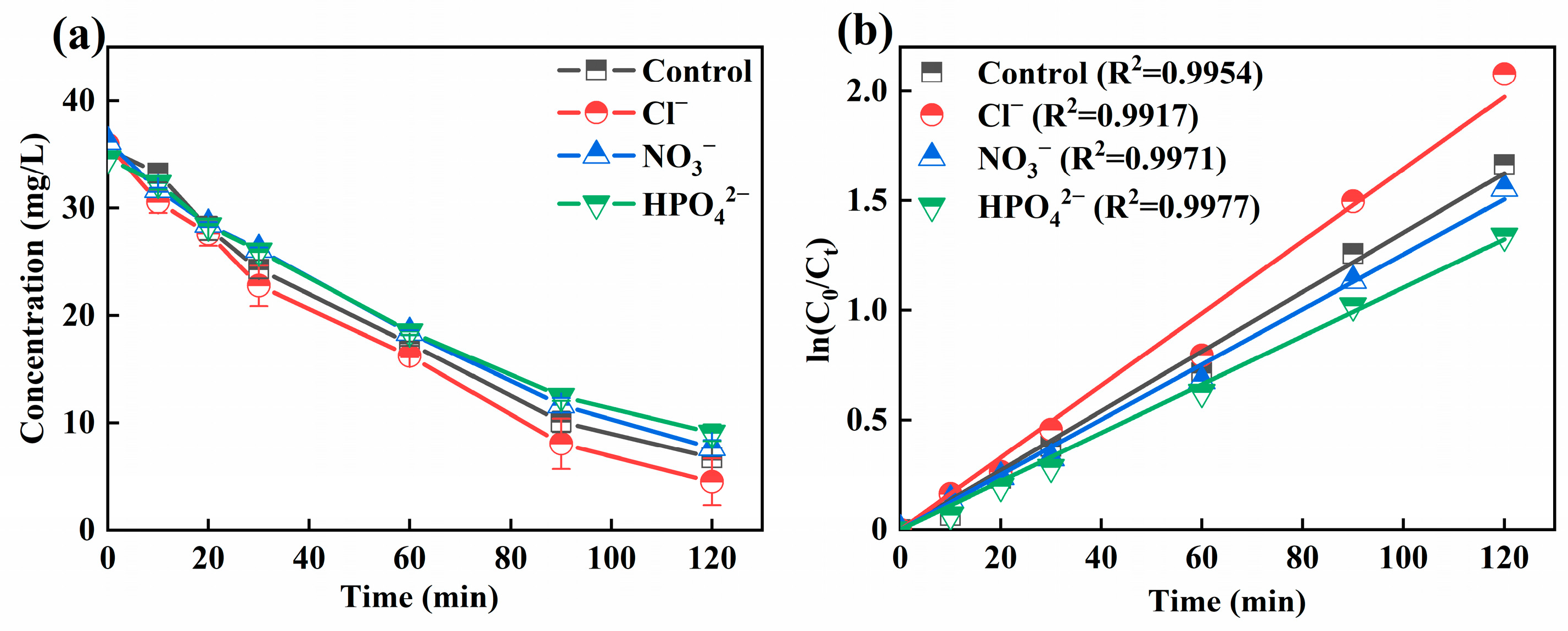
3.4. Effect of Coexisting Ions on Ni-EDTA Removal
Actual wastewater contains specific pollutants and a variety of ions. Coexisting ions would have competitive adsorption with H2O2, which would have negatively affected the treatment effect of the 3D-EF system. Figure 6a displayed the effect of Cl−, NO3−, and HPO42− on the removal efficiency of Ni-EDTA. In the control group, the concentration of Ni-EDTA reached 81.04% after 120 min. The removal efficiency of Ni-EDTA reached 87.47% in the presence of Cl−; it can be seen that Cl− promotes the removal of Ni-EDTA. After adding NO3− and HPO42−, the removal effect of Ni-EDTA was reduced: 78.87% and 73.79%, respectively. Therefore, NO3− and HPO42− could inhibit the removal effect of Ni-EDTA, and the inhibition effect followed as HPO42− > NO3−. Figure 6b reflected that the Kobs of Cl− was higher than that of other groups, indicating that Cl− accelerated the decomplexation of Ni-EDTA.
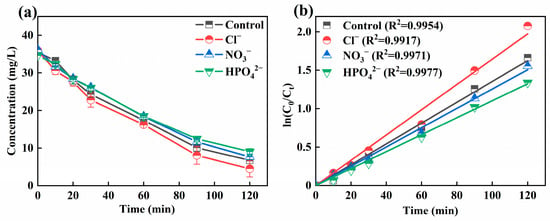
Figure 6.
Decomplexation of Ni-EDTA in coexisting ions (a) and kinetics fitting lines (b).
Cl− could accelerate the decomplexation of Ni-EDTA in the 3D-EF system. This could be explained by the fact that Cl− generated Cl2 through hydrogen evolution at anode, which further hydrolyzed to form strongly oxidized ClO− and then promoted the removal of Ni-EDTA (Equations (6)–(8)) [42,43]. However, NO3− and HPO42− inhibited the removal effect of Ni-EDTA, because the standard electrode potential of NO3−/NO2− (0.01 V) was higher than that of Fe2+/Fe (−0.44 V). When the NO3− existed within the 3D-EF system, NO3− would compete with Ni-EDTA for electrons. Meanwhile, NO3− could scavenge with ·OH to further generate NO3· with a lower reaction activity, which led to a decrease in the Ni-EDTA removal effect (Equation (9)) [44,45]. In the 3D-EF system, HPO42− could quench a certain amount of ·OH to form less reactive H2PO4· (Equation (10)), resulting in a lower removal efficiency of Ni-EDTA [46].
2Cl− → Cl2 + 2e−
Cl2 + H2O ↔ HOCl + Cl− + H+
HOCl ↔ H+ + OCl−
NO3− + ∙OH → NO3· + OH−
HPO42− + ∙OH → OH− + H2PO4·
4. Conclusions
In summary, the 3D-EF system is a novel and effective method for the decomplexation of Ni-EDTA. The decomplexation performance of Ni-EDTA in the 3D-EF system was superior to its performance in the GAC and EF system. The removal efficiency of Ni-EDTA reached 84.89% at a near-neutral pH in the 3D-EF system. The 3D-EF system with GAC particle electrode could break through the limitation of pH and broaden the application window of pH. Influencing factors analysis by selecting Ni-EDTA as a target contaminant suggested that Fe2+ of 1 mmol/L, GAC of 4 g/L, and current density of 10 mA/cm2 accelerated the Ni-EDTA decomplexation. In addition, the presence of coexisting ions can affect the decomplexation of Ni-EDTA.
Author Contributions
Methodology, software, investigation, data curation, and writing—original draft preparation, J.P. and Y.M.; methodology, validation, investigation, resources, writing—review and editing, and funding acquisition, X.H.; writing—review and editing and supervision, J.Y.; investigation and data curation, F.Y.; validation, resources, and supervision, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of Shenzhen Science and Technology Plan, grant number KJYY20180206180737010; the university-level supporting projects by Shenzhen polytechnic, grant number 6020320003K; the startup foundation for introducing talent of NUIST, National key R&D program of China, grant number 2018YFD0900805; and the startup foundation for Natural Science Fund Project of Colleges in Jiangsu Province, grant number 20KJB610010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are included in the article and available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.; Zhou, W.; Yang, L.; Chen, Y.; Hu, Y. Co-N-doped MoO2 modified carbon felt cathode for removal of EDTA-Ni in electro-Fenton process. Environ. Sci. Pollut. Res. 2018, 25, 22754–22765. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, F.; Bi, X.; Chen, D.; Huang, Z.; Li, Y.; Sun, S.; Liu, J. Decomposition of Nickel (Ⅱ)-ethylenediaminetetraacetic acid by Fenton-like reaction over oxygen vacancies-based Cu-Doped Fe3O4@ γ-Al2O3 catalyst: A synergy of oxidation and adsorption. Chemosphere 2019, 221, 563–572. [Google Scholar] [CrossRef]
- Liang, S.; Hu, X.; Xu, H.; Lei, Z.; Wei, C.; Feng, C. Mechanistic insight into the reaction pathway of peroxomonosulfate-initiated decomplexation of EDTA-NiII under alkaline conditions: Formation of high-valent Ni intermediate. Appl. Catal. B Environ. 2021, 296, 120375. [Google Scholar] [CrossRef]
- Cao, Y.; Qian, X.; Zhang, Y.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Wang, T. Decomplexation of EDTA-chelated copper and removal of copper ions by non-thermal plasma oxidation/alkaline precipitation. Chem. Eng. J. 2019, 362, 487–496. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Xue, M.; Song, R.; Zhang, Y.; Qu, G.; Wang, T. High-efficient decomplexation of Cu-EDTA and Cu removal by high-frequency non-thermal plasma oxidation/alkaline precipitation. Sep. Purif. Technol. 2021, 257, 117885. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Zhang, X.; Dong, W.; Cao, Z.; He, L.; Wang, X. Rapid decomplexation of Ni-EDTA by microwave-assisted Fenton reaction. Chem. Eng. J. 2020, 381, 122703. [Google Scholar] [CrossRef]
- Fei, L.; Ren, S.; Xijun, M.; Ali, N.; Jing, Z.; Yi, J.; Bilal, M. Efficient removal of EDTA-chelated Cu (II) by zero-valent iron and peroxydisulfate: Mutual activation process. Sep. Purif. Technol. 2021, 279, 119721. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Pham, T.T.; Pham, H.G.; Hoang, B.L.; Nguyen, T.H.; Nguyen, T.H.; Tran, T.H.; Ngo, H.H. Fenton/ozone-based oxidation and coagulation processes for removing metals (Cu, Ni)-EDTA from plating wastewater. J. Water Process Eng. 2021, 39, 101836. [Google Scholar] [CrossRef]
- Nidheesh, P.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, W.; Xu, L.; Zhou, C.; Sun, Y.; Niu, J. Treatment of Ni-EDTA containing wastewater by electrochemical degradation using Ti3+ self-doped TiO2 nanotube arrays anode. Chemosphere 2021, 278, 130465. [Google Scholar] [CrossRef]
- Sirés, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E. Degradation of clofibric acid in acidic aqueous medium by electro-Fenton and photoelectro-Fenton. Chemosphere 2007, 66, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Luo, Z.; Yang, J.; Zhi, D.; Zhou, Y. FeII FeIII layered double hydroxide modified carbon felt cathode for removal of ciprofloxacin in electro-Fenton process. Environ. Res. 2021, 197, 111144. [Google Scholar] [CrossRef] [PubMed]
- Basturk, I.; Varank, G.; Murat-Hocaoglu, S.; Yazici-Guvenc, S.; Can-Güven, E.; Oktem-Olgun, E.E.; Canli, O. Simultaneous degradation of cephalexin, ciprofloxacin, and clarithromycin from medical laboratory wastewater by electro-Fenton process. J. Environ. Chem. Eng. 2021, 9, 104666. [Google Scholar] [CrossRef]
- Stupar, S.L.; Grgur, B.N.; Radišić, M.M.; Onjia, A.E.; Ivanković, N.D.; Tomašević, A.V.; Mijin, D.Ž. Oxidative degradation of Acid Blue 111 by electro-assisted Fenton process. J. Water Process Eng. 2020, 36, 101394. [Google Scholar] [CrossRef]
- Gamarra-Güere, C.D.; Dionisio, D.; Santos, G.O.S.; Lanza, M.R.V.; de Jesus Motheo, A. Application of Fenton, photo-Fenton and electro-Fenton processes for the methylparaben degradation: A comparative study. J. Environ. Chem. Eng. 2022, 10, 106992. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Cheng, J.; Hu, C.; Yang, Z.; Wu, C. Three-dimensional particle electrode system treatment of organic wastewater: A general review based on patents. J. Clean. Prod. 2021, 308, 127324. [Google Scholar] [CrossRef]
- Lu, C.; Wei, G.; Ba, J.; Li, Q.; Zhang, L.; Li, Z.; Cen, L.; Ma, J.; Song, Y. Three-dimensional electro-Fenton degradation of ciprofloxacin catalyzed by CuO doped red mud particle electrodes: Electrodes preparation, kinetics and mechanism. J. Environ. Chem. Eng. 2022, 10, 107151. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Wu, P.; Duan, Y.; Zhou, L.; Lai, Y.; Wang, F.; Li, S. Three-dimensional heterogeneous Electro-Fenton system with a novel catalytic particle electrode for Bisphenol A removal. J. Hazard. Mater. 2020, 393, 120448. [Google Scholar] [CrossRef]
- Mohammadi, H.; Bina, B.; Ebrahimi, A. A novel three-dimensional electro-Fenton system and its application for degradation of anti-inflammatory pharmaceuticals: Modeling and degradation pathways. Process Saf. Environ. Prot. 2018, 117, 200–213. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, M.; Zhang, C.; Jiang, Y.; Bi, Z.; Yang, J. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts. Chem. Eng. J. 2013, 233, 185–192. [Google Scholar] [CrossRef]
- Chu, Y.; Su, H.; Lv, R.; Zhang, X. Enhanced electro-reduction of Fe3+ to Fe2+ by acidified carbon nanotube-modified graphite cathode and its application in a novel Fenton process for p-nitrophenol degradation. J. Water Process Eng. 2021, 40, 101912. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Porous graphite felt electrode with catalytic defects for enhanced degradation of pollutants by electro-Fenton process. Chem. Eng. J. 2021, 403, 126270. [Google Scholar] [CrossRef]
- Song, D.; Li, J.; Wang, Z.; Zhao, C. Performance of graphite felt as anodes in the electro-Fenton oxidation systems: Changes in catalysis, conductivity and adsorption properties. Appl. Surf. Sci. 2020, 532, 147450. [Google Scholar] [CrossRef]
- Kuleyin, A.; Gök, A.; Akbal, F. Treatment of textile industry wastewater by electro-Fenton process using graphite electrodes in batch and continuous mode. J. Environ. Chem. Eng. 2021, 9, 104782. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Y.; Xu, L.-N.; Ni, J.-R. Removal of Acid Orange 7 in simulated wastewater using a three-dimensional electrode reactor: Removal mechanisms and dye degradation pathway. Chemosphere 2010, 78, 46–51. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Li, P.; Zhang, Y.; Yan, Q.; Zhao, Y. Electrochemical treatment of industrial wastewater using a novel layer-upon-layer bipolar electrode system (nLBPEs). Chem. Eng. J. 2013, 215, 157–161. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, J.; Zhao, C.; He, X.; Yang, G. Fabrication of slag particle three-dimensional electrode system for methylene blue degradation: Characterization, performance and mechanism study. Chemosphere 2018, 213, 377–383. [Google Scholar] [CrossRef]
- Liu, W.; Duan, P.; Hu, X.; Gao, J.; Zhou, F. Fabrication of Efficient Nano-MnOx/ACF Particle Electrodes and their Application in the Electrooxidation of m-Cresol in the 3-D Electrode System. Ind. Eng. Chem. Res. 2019, 58, 22114–22123. [Google Scholar] [CrossRef]
- Dargahi, A.; Hasani, K.; Mokhtari, S.A.; Vosoughi, M.; Moradi, M.; Vaziri, Y. Highly effective degradation of in a three-dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J. Environ. Chem. Eng. 2021, 9, 105889. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, F.; Meng, Y.; Guo, M.; Tang, C.; Shi, Y.; Ke, Y.; Hu, R. Three-dimensional electrochemical degradation of p-aminophenol with efficient honeycomb block AC@Ti-Cu-Ni-Zn-Sb-Mn particle electrodes. Sep. Purif. Technol. 2021, 267, 118662. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiu, S.; Deng, F.; Zhu, Y.; Li, G.; Ma, F. Three-dimensional electro-Fenton system with iron foam as particle electrode for folic acid wastewater pretreatment. Sep. Purif. Technol. 2019, 224, 463–474. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, B.; Tian, S.; Zhao, X. The synergism between electro-Fenton and electrocoagulation process to remove Cu-EDTA. Appl. Catal. B Environ. 2018, 227, 252–257. [Google Scholar] [CrossRef]
- Li, M.; Qin, X.; Cui, J.; Guo, R.; Guo, C.; Wang, Z.; Li, T. Three-dimensional Electro-Fenton degradation for fulvic acids with Cu-Fe bimetallic aerogel-like carbon as particle electrode and catalyst: Electrode preparation, operation parameter optimization and mechanism. J. Environ. Chem. Eng. 2021, 9, 105573. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Ni, J.; Xu, Y.; Song, N.; Gao, M. Highly efficient removal of amoxicillin from water by three-dimensional electrode system within granular activated carbon as particle electrode. J. Water Process Eng. 2020, 38, 101656. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Wang, H.; Dong, W.; Wang, W. Sequential application of Fenton and ozone-based oxidation process for the abatement of Ni-EDTA containing nickel plating effluents. Chemosphere 2018, 202, 238–245. [Google Scholar] [CrossRef]
- Li, M.; Zeng, Z.; Li, Y.; Arowo, M.; Chen, J.; Meng, H.; Shao, L. Treatment of amoxicillin by O3/Fenton process in a rotating packed bed. J. Environ. Manag. 2015, 150, 404–411. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Lai, L.; Lai, B. Electrolysis assisted persulfate with annular iron sheet as anode for the enhanced degradation of 2,4-dinitrophenol in aqueous solution. J. Hazard. Mater. 2018, 344, 778–787. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, N.; Olvera-Vargas, H.; Brillas, E.; Gadri, A.; Ammar, S.; Oturan, M.A. Pyrite as a sustainable catalyst in electro-Fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment. Water Res. 2016, 94, 52–61. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Yu, Z.; Liu, Y.; Huang, J.; Qian, L.; Xiong, J. Three-dimensional electro-Fenton degradation of Rhodamine B with efficient Fe-Cu/kaolin particle electrodes: Electrodes optimization, kinetics, influencing factors and mechanism. Sep. Purif. Technol. 2019, 210, 60–68. [Google Scholar] [CrossRef]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B Environ. 2013, 140, 92–97. [Google Scholar] [CrossRef]
- Lan, Y.; Coetsier, C.; Causserand, C.; Serrano, K.G. On the role of salts for the treatment of wastewaters containing pharmaceuticals by electrochemical oxidation using a boron doped diamond anode. Electrochim. Acta 2017, 231, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, D.; Yilmaz, A.E. Treatment of slaughterhouse wastewater with the electrochemical oxidation process: Role of operating parameters on treatment efficiency and energy consumption. J. Water Process Eng. 2019, 31, 100834. [Google Scholar] [CrossRef]
- Jiang, Z.; Lv, L.; Zhang, W.; Du, Q.; Pan, B.; Yang, L.; Zhang, Q. Nitrate reduction using nanosized zero-valent iron supported by polystyrene resins: Role of surface functional groups. Water Res. 2011, 45, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zou, X.; Mao, J.; Wu, X. Decomposition of sulfadiazine in a sonochemical Fe0-catalyzed persulfate system: Parameters optimizing and interferences of wastewater matrix. Appl. Catal. B Environ. 2016, 185, 31–41. [Google Scholar] [CrossRef]
- Teng, X.; Li, J.; Wang, J.; Liu, J.; Ge, X.; Gu, T. Effective degradation of atrazine in wastewater by three-dimensional electrochemical system using fly ash-red mud particle electrode: Mechanism and pathway. Sep. Purif. Technol. 2021, 267, 118661. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).