Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Adsorption Kinetic Experiments
2.3. Adsorption Isotherm Experiments
2.4. Analytical Methods
3. Results and Discussion
3.1. Adsorption Kinetics and Modeling
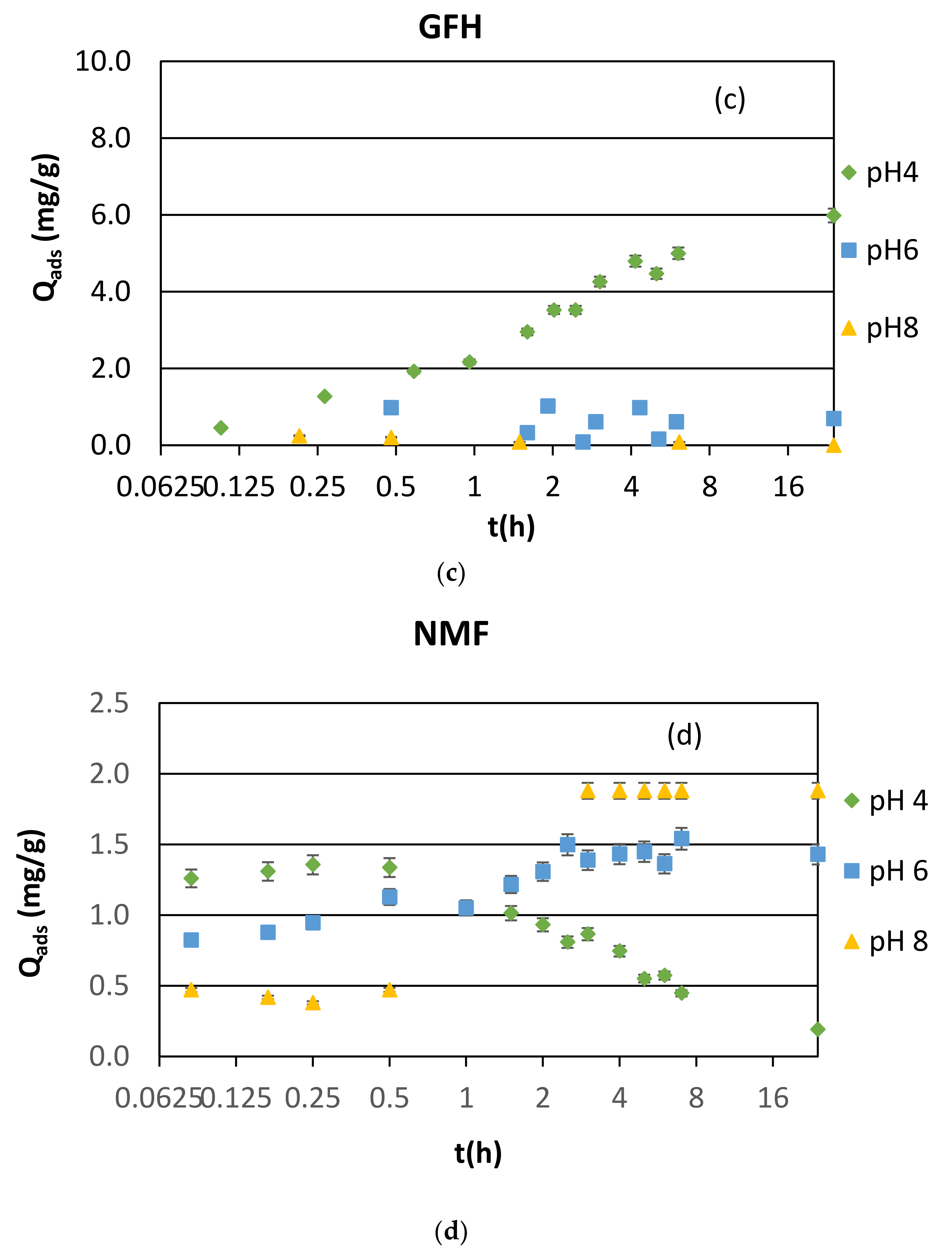
3.1.1. Synthetic Samples and Initial pH
3.1.2. Real Samples
3.1.3. Synthetic Samples and Equilibrium pH
3.2. Adsorption Isotherms and Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharti, V. Fluoride Sources, Toxicity and Its Amelioration: A Review. Peertechz J. Environ. Sci. Toxicol. 2017, 2, 021–032. [Google Scholar] [CrossRef]
- Scientific Committee on Health and Environmental Risks (SCHER). Critical Review of Any New Evidence on the Hazard Profile, Health Effects, and Human Exposure to Fluoride and the Fluoridating Agents of Drinking Water; European Union: Brussels, Belgium, 2010; ISSN 1831-4775. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality, 4th ed; World Health Organization: Geneva, Switzerland, 2011; Volume 1, ISBN 978-92-4-154815-1. [Google Scholar]
- Biswas, G.; Kumari, M.; Adhikari, K.; Dutta, S. A Critical Review on Occurrence of Fluoride and Its Removal through Adsorption with an Emphasis on Natural Minerals. Curr. Pollut. Rep. 2017, 3, 104–119. [Google Scholar] [CrossRef]
- European Commission EU. Directive 98/83/EC Council Directive of 3 November 1998 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 1998, L330, 32–54. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive (Eu) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020, 435, 1–62. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 3 April 2022).
- Lgs. D. 31/2001 Decreto Legislativo 2 Febbraio 2001. n. 31. Attuazione Della Direttiva 98/83/CE Relativa Alla Qualità Delle Acque Destinate Al Consumo Umano. Gazzetta Ufficiale n. 52 del 3 marzo 2001—Supplemento Ordinario n. 41. Available online: https://web.camera.it/parlam/leggi/deleghe/01031dl.htm (accessed on 3 April 2022).
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Fewtrell, L.; Magara, Y. Fluoride in Drinking Water; WHO Drinking; IWA Publishing: London, UK; Alliance House: London, UK, 2006; ISBN 1900222965. [Google Scholar]
- Solanki, Y.S.; Agarwal, M.; Maheshwari, K.; Gupta, S.; Shukla, P.; Gupta, A.B. Removal of Fluoride from Water by Using a Coagulant (Inorganic Polymeric Coagulant). Environ. Sci. Pollut. Res. 2021, 28, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.K.; Ohanian, E.; Giddings, M.; Toft, P.; Magara, Y.; Jackson, P. Fluoride in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Habuda-Stanić, M.; Ravančić, M.; Flanagan, A. A Review on Adsorption of Fluoride from Aqueous Solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Pandian, S.; Panchal, H. Overview of Fluoride Removal from Water Using Separation Techniques. Environ. Technol. Innov. 2021, 21, 101246. [Google Scholar] [CrossRef]
- Ghosh, D.; Medhi, C.R.; Purkait, M.K. Treatment of Fluoride Containing Drinking Water by Electrocoagulation Using Monopolar and Bipolar Electrode Connections. Chemosphere 2008, 73, 1393–1400. [Google Scholar] [CrossRef]
- Das, D.; Nandi, B.K. Simultaneous Removal of Fluoride and Fe (II) Ions from Drinking Water by Electrocoagulation. J. Environ. Chem. Eng. 2020, 8, 103643. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of Fluoride Removal from Water Environment by Adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Ramdani, A.; Taleb, S.; Benghalem, A.; Ghaffour, N. Removal of Excess Fluoride Ions from Saharan Brackish Water by Adsorption on Natural Materials. Desalination 2010, 250, 408–413. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dutta, S.; Ray, S.; Halder, G. A Comparative Study on Defluoridation Capabilities of Biosorbents: Isotherm, Kinetics, Thermodynamics, Cost Estimation, and Eco-Toxicological Study. Environ. Sci. Pollut. Res. 2018, 25, 17473–17489. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A Review of Emerging Adsorbents and Current Demand for Defluoridation of Water: Bright Future in Water Sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and Trace Elements in Tap Water from Italy. J. Geochem. Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- Ergul, S.; Ferranti, F.; Sappa, G. Arsenic in the Aquifer Systems of Viterbo Region. Central Italy: Distribution and Geochemistry. Rend. Online Della Soc. Geol. Ital. 2013, 24, 116–118. [Google Scholar]
- Chiavola, A.; D’Amato, E.; Boni, M.R. Comparison of different iron oxide adsorbents for combined arsenic, vanadium and fluoride removal from drinking water. Int. J. Environ. Sci. Technol. 2019, 16, 6053–6064. [Google Scholar] [CrossRef]
- López-Guzmán, M.; Alarcón-Herrera, M.T.; Irigoyen-Campuzano, J.R.; Torres-Castañón, L.A.; Reynoso-Cuevas, L. Simultaneous Removal of Fluoride and Arsenic from Well Water by Electrocoagulation. Sci. Total Environ. 2019, 678, 181–187. [Google Scholar] [CrossRef]
- Chiavola, A.; Stoller, M.; Di Palma, L.; Boni, M.R. Magnetic Core Nanoparticles Coated by Titania and Alumina for Water and Wastewater Remediation from Metal Contaminants. Chem. Eng. Trans. 2017, 60, 205–210. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Stoller, M.; Chianese, A.; Boni, M.R. Application of Iron Based Nanoparticles as Adsorbents for Arsenic Removal from Water. Chem. Eng. Trans. 2016, 47, 325–330. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Sirini, P.; Caretti, C.; Gori, R. Arsenic Removal from a Highly Contaminated Groundwater by a Combined Coagulation-Filtration-Adsorption Process. Water Air Soil Pollut. 2019, 230, 87. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Li, Y.; Arai, Y. Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions. Soil Syst. 2020, 4, 36. [Google Scholar] [CrossRef]
- USEPA. Technologies and Costs for Removal of Arsenic from Drinking Water EPA 815-R-00-028; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Streat, M.; Hellgardt, K.; Newton, N.L.R. Hydrous Ferric Oxide as an Adsorbent in Water Treatment. Process Saf. Environ. Prot. 2008, 86, 21–30. [Google Scholar] [CrossRef]
- Pirilä, M.; Martikainen, M.; Ainassaari, K.; Kuokkanen, T.; Keiski, R.L. Removal of Aqueous As(III) and As(V) by Hydrous Titanium Dioxide. J. Colloid Interface Sci. 2011, 353, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance Evaluation of Small Sized Powdered Ferric Hydroxide as Arsenic Adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Belkasmi, A.I.; Kastoyiannis, I.A.; Ernst, M. Pre-Deposited Dynamic Membrane Adsorber Formed of Microscale Conventional Iron Oxide-Based Adsorbents to Remove Arsenic from Water: Application Study and Mathematical Modeling. J. Chem. Technol. Biotechnol. 2021, 96, 1504–1514. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Ji, M.; Jung, W.; Lee, S.-H.; Kim, S.-J.; Lee, G.; Song, H.; Choi, J.-Y.; Yang, J.-S.; et al. Defluoridation from Aqueous Solutions by Granular Ferric Hydroxide (GFH). Water Res. 2009, 43, 490–498. [Google Scholar] [CrossRef]
- Purolite. Engineering Bulletin-Arsenic Removal for Commercial and Residential Applications Using Purolite FerrIXTM A33E. 2018. Available online: www.purolite.com (accessed on 3 April 2022).
- Naser, J.A.; Ahmed, Z.W.; Ali, E.H. Nanomaterials Usage as Adsorbents for the Pollutants Removal from Wastewater; A Review. Mater. Today Proc. 2021, 42, 2590–2595. [Google Scholar] [CrossRef]
- Vuppala, S.; Stoller, M.; Chiavola, A.; Kanaev, A.; Cheng, K. Synthesis of Core-Shell Nanoparticles for the Removal of Toxic Pollutants in Aqueous Medium. Chem. Eng. Trans. 2018, 70, 1819–1824. [Google Scholar] [CrossRef]
- Wajima, T.; Umeta, Y.; Narita, S.; Sugawara, K. Adsorption Behavior of Fluoride Ions Using a Titanium Hydroxide-Derived Adsorbent. Desalination 2009, 249, 323–330. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Zhang, X.; Zhou, W.; Huang, J.; Yu, G. Removal of Fluoride from Water Using Titanium-Based Adsorbents. Front. Environ. Sci. Eng. China 2010, 4, 414–420. [Google Scholar] [CrossRef]
- Alemu, S.; Mulugeta, E.; Zewge, F.; Chandravanshi, B.S. Water Defluoridation by Aluminium Oxide–Manganese Oxide Composite Material. Environ. Technol. 2014, 35, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical Modeling of Arsenic(V) Adsorption onto Iron Oxyhydroxides in an Adsorption-Submerged Membrane Hybrid System. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef] [PubMed]
- Ohs, B.; Krödel, M.; Wessling, M. Adsorption of Carbon Dioxide on Solid Amine-Functionalized Sorbents: A Dual Kinetic Model. Sep. Purif. Technol. 2018, 204, 13–20. [Google Scholar] [CrossRef]
- Wang, M.; Yu, X.; Yang, C.; Yang, X.; Lin, M.; Guan, L.; Ge, M. Removal of Fluoride from Aqueous Solution by Mg-Al-Zr Triple-Metal Composite. Chem. Eng. J. 2017, 322, 246–253. [Google Scholar] [CrossRef]
- Wang, J.; Chen, N.; Feng, C.; Li, M. Performance and Mechanism of Fluoride Adsorption from Groundwater by Lanthanum-Modified Pomelo Peel Biochar. Environ. Sci. Pollut. Res. 2018, 25, 15326–15335. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride Removal from Water by Adsorption-A Review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Eskandarpour, A.; Onyango, M.S.; Ochieng, A.; Asai, S. Removal of Fluoride Ions from Aqueous Solution at Low PH Using Schwertmannite. J. Hazard. Mater. 2008, 152, 571–579. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mondal, M.; Banerjee, S.; Halder, G. Elucidation of the Sorptive Uptake of Fluoride by Ca 2+ -Treated and Untreated Algal Biomass of Nostoc Sp. (BTA394): Isotherm, Kinetics, Thermodynamics and Safe Disposal. Process Saf. Environ. Prot. 2017, 107, 334–345. [Google Scholar] [CrossRef]









| Name | Composition and Production | Average Size (mm) | Bulk Density (g/cm3) | Specific Surface (m2/g) |
|---|---|---|---|---|
| GFH, Granular Ferric Hydroxide | Made by 50–60% Fe2O3 | 0.2–2 | 1.6 | 250–300 |
| GAC, Granular Activated Carbon | Produced by steam activation of coal | 0.6–0.7 | 0.47 | 500–1100 |
| TiO2, Titanium dioxide | Titanium oxide-based granulation | 0.25–2 | 0.705 | 200–300 |
| A33E, Hybrid Ion Exchange Adsorber | Polystyrene crosslinked with divinylbenzene hybrid ion exchange resin (Cl− form) infused with iron oxide nanoparticles [25] | 0.3–1.2 | 0.79–0.98 | 810.4 9 ± 38.2 [26] |
| NMF, Fine Magnetic Nanomaterial | Ferromagnetic nanoparticles with a SiO2 core shell and titanium dioxide coating (37.5% by weight of TiO2) | 11.8 × 10−3 | ND | 110 |
| NMG, Granular Magnetic Material | Ferromagnetic nanoparticles with a SiO2 core shell and titanium dioxide coating (37.5% by weight of TiO2) | 1–2 | ND | 110 |
| Test | C0 (mg/L F−) | M/V (g/L) | pH (Unit) | Adsorbent Type |
|---|---|---|---|---|
| 1a | 2.5 | 1 | initial | GAC, GFH, A33E, TiO2 |
| 1b | 2.5 | 5 | ||
| 1c | 5 | 1 | ||
| 1d | 5 | 5 | ||
| 1e | 10 | 1 | ||
| 1f | 10 | 5 | ||
| 1g | 10 | 10 | ||
| 1h | 2.5 | 1 | NMF, NMG | |
| 2a | 2.4 (real samples) | 1 | initial | GHF, A33E, TiO2, |
| 2b | 2.4 (real samples) | 5 | ||
| 3a | 5 | 1 | 4–6–8 | GAC, GFH, A33E, TiO2 |
| 3b | 2.5 | GFH, A33E, TiO2 | ||
| 3c | 10 | GFH, A33E, TiO2 | ||
| 3d | 2.5 | NMF |
| Kinetic Model | Nonlinear Form | Linear Form | Linear Plot |
|---|---|---|---|
| Zeroth | |||
| First | |||
| Second | |||
| Saturation | |||
| Pseudo-first | |||
| Pseudo-second | |||
| Weber and Morris | |||
| Bangham |
| C0 (mg/L) | M/V (g/L) | pH (Unit) | Adsorbent |
|---|---|---|---|
| 2.5–5–10 | 1–5 | initial | GAC, GFH, A33E, TiO2 |
| 2.5–5–10 | 1 | 4–6–8 | GFH, A33E, TiO2 |
| Isotherm Model | Nonlinear Form | Linear Form | Linear Plot |
|---|---|---|---|
| Langmuir | |||
| Freundlich | |||
| BET | |||
| Temkin |
| Qmax (mg/g) | |||
|---|---|---|---|
| Dose (g/L)/ | 1 | 5 | 10 |
| Adsorbent | |||
| A33E | 2.0741 | 0.8030 | 0.4167 |
| TiO2 | 5.1151 | 2.0218 | 1.0373 |
| GFH | 0.1071 | 0.1564 | 0.1379 |
| GAC | 0.5446 | 0.5553 | 0.2942 |
| A33E | R2 | b (L/mg) | Kf (mg/g) | n | Kb | Qmax (mg/g) | B | A (L/mg) |
|---|---|---|---|---|---|---|---|---|
| Langmuir | 0.8089 | −0.0071 | −27.9330 | |||||
| Freundlich | 0.7756 | 0.2237 | 1.0676 | |||||
| BET | 0.0314 | 26.0800 | 7.6687 | |||||
| Temkin | 0.6597 | 0.7475 | 0.9750 | |||||
| TiO2 | R2 | b (L/mg) | Kf (mg/g) | n | Kb | Qmax (mg/g) | B | A (L/mg) |
| Langmuir | 0.9826 | 2.0400 | 4.1900 | |||||
| Freundlich | 0.9798 | 2.4900 | 2.2400 | |||||
| BET | 0.9900 | 874.5000 | 5.7200 | |||||
| Temkin | 0.9747 | 2.8600 | 13.6600 |
| A33E | R2 | b (L/mg) | Kf (mg/g) | n | Kb | Qmax (mg/g) | B | A (L/mg) |
|---|---|---|---|---|---|---|---|---|
| pH = 4 | ||||||||
| Langmuir | 0.9825 | 32.9761 | 4.0000 | |||||
| Freundlich | 0.8571 | 2.4300 | 2.6500 | |||||
| BET | 0.8206 | 506,334.3000 | 6.5800 | |||||
| Temkin | 0.7908 | 1.2084 | 8.5844 | |||||
| pH = 6 | ||||||||
| Langmuir | 0.9825 | −1.5738 | −5.9400 | |||||
| Freundlich | 0.9880 | 0.2800 | 0.9100 | |||||
| BET | 0.2663 | −15,324.0000 | −16.3100 | |||||
| Temkin | 0.9883 | 1.4595 | 0.7438 | |||||
| pH = 8 | ||||||||
| Langmuir | 0.0029 | 5.4603 | 7.3584 | |||||
| Freundlich | 0.0236 | 0.8638 | −11.7600 | |||||
| BET | 0.9453 | −943,598.9999 | 0.5299 | |||||
| Temkin | 0.0197 | 0.000 | −0.0696 | |||||
| TiO2 | R2 | b (L/mg) | Kf (mg/g) | n | Kb | Qmax (mg/g) | B | A (L/mg) |
| pH = 4 | ||||||||
| Langmuir | 0.7952 | −92.5523 | −19.4175 | |||||
| Freundlich | 0.8785 | 5.9910 | 0.9553 | |||||
| BET | 0.0165 | −48,999 | −102.04 | |||||
| Temkin | 0.9771 | 3.7664 | 5.1332 | |||||
| pH = 6 | ||||||||
| Langmuir | 0.9228 | 7.8827 | 8.3612 | |||||
| Freundlich | 0.8730 | 1.0035 | 1.6474 | |||||
| BET | 0.9103 | 223,778.777 | 4.9700 | |||||
| Temkin | 0.9565 | 2.1353 | 1.1572 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavola, A.; D’Amato, E.; Di Marcantonio, C. Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions. Water 2022, 14, 1423. https://doi.org/10.3390/w14091423
Chiavola A, D’Amato E, Di Marcantonio C. Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions. Water. 2022; 14(9):1423. https://doi.org/10.3390/w14091423
Chicago/Turabian StyleChiavola, Agostina, Emilio D’Amato, and Camilla Di Marcantonio. 2022. "Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions" Water 14, no. 9: 1423. https://doi.org/10.3390/w14091423






