Degradation of Polystyrene Nanoplastics in UV/NaClO and UV/PMS Systems: Insights into Degradation Efficiency, Mechanism, and Toxicity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Procedure
2.3. Analytical Methods
2.4. Theoretical Computation Methods
3. Results and Discussion
3.1. Turbidity Removal of PS-NP
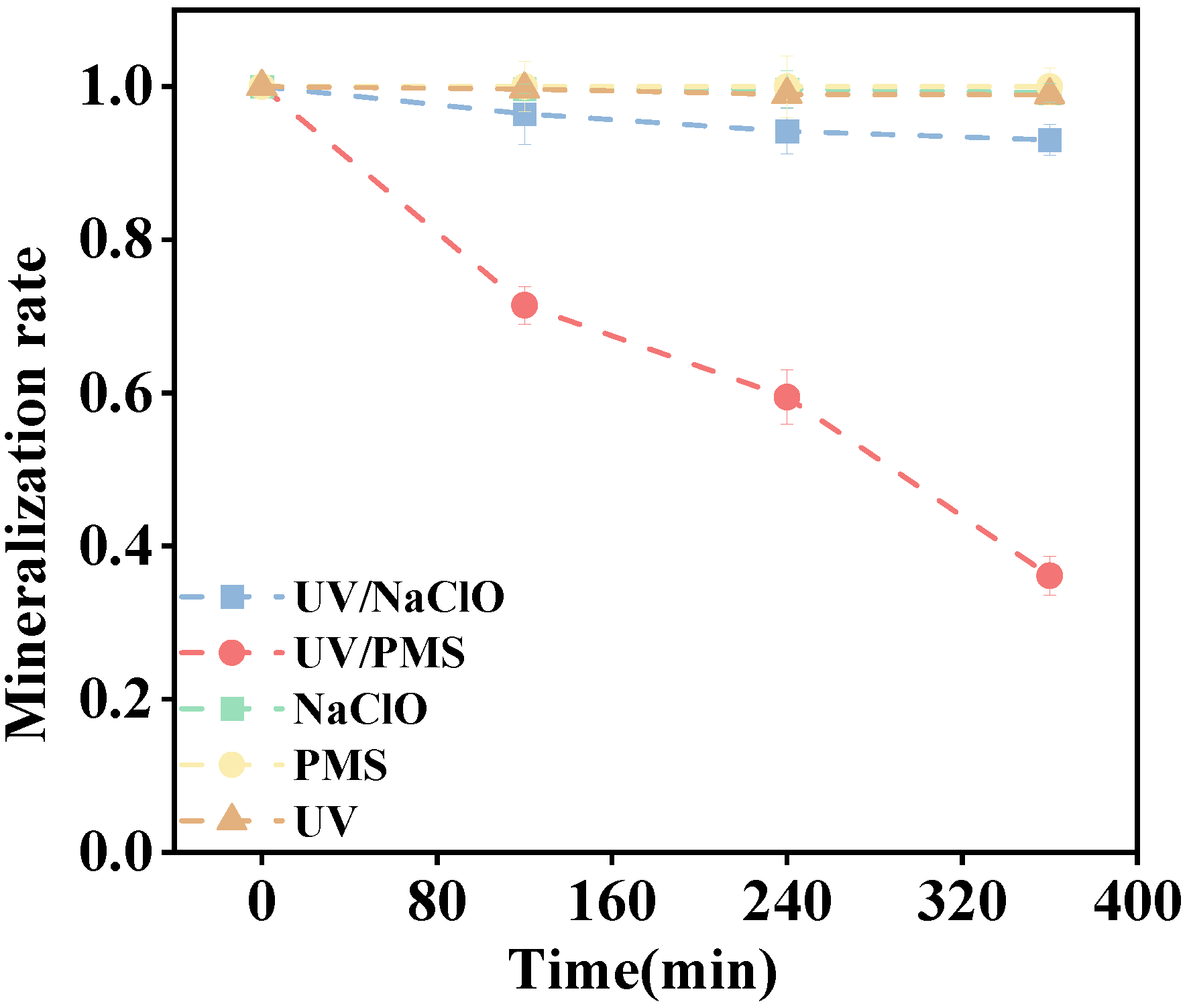
3.2. Mineralization of PS-NP in Different Oxidation Systems
3.3. Degradation Mechanisms and Pathway of PS-NP by UV/PMS and UV/NaClO
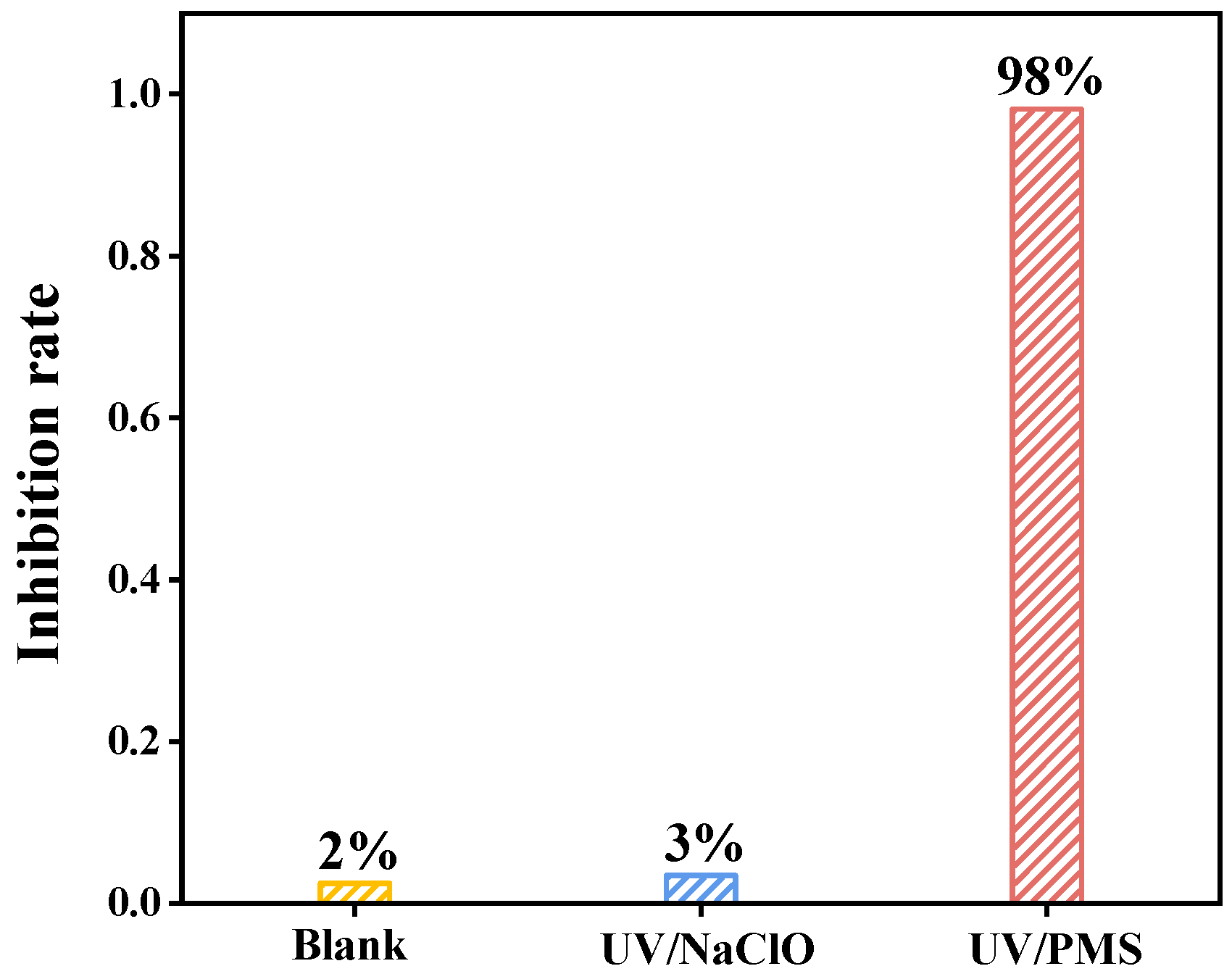
3.4. Toxicity Assessment of Degradation Intermediates
4. Conclusions
- (1)
- The UV/NaClO and UV/PMS systems achieved 78.20% and 94.30% turbidity removal in 360 min of reaction time. However, the corresponding mineralization rates were 7.00% and 63.90%, indicating that UV/NaClO could not completely mineralize nanoplastics into inorganic substances, but only decompose it into smaller organic molecules.
- (2)
- DFT calculation coupled with HPLC analysis proposed the degradation pathways of PS-NP in two oxidative systems. The different reaction sites and energy barriers of SO4•− and •Cl on PS-NP resulted in the differences between the mineralization rate and degradation intermediates.
- (3)
- Toxicity assessment of degradation intermediates showed that the inhibition rate of luminescent bacteria originated by the UV/NaClO and UV/PMS systems were 2.97% and 98.19%. This suggested that the acute toxicity of PS-NP degradation intermediates using UV/PMS should be given attention.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Fennell, T.R.; Johnson, L.M. Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources. NanoImpact 2021, 21, 100302. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef][Green Version]
- Remucal, C.K.; Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 565–579. [Google Scholar] [CrossRef]
- Zhou, P.; Di Giovanni, G.D.; Meschke, J.S.; Dodd, M.C. Enhanced Inactivation of Cryptosporidium parvum Oocysts during Solar Photolysis of Free Available Chlorine. Environ. Sci. Technol. Lett. 2014, 1, 453–458. [Google Scholar] [CrossRef][Green Version]
- Oliver, B.G.; Carey, J.H. Photochemical production of chlorinated organics in aqueous solutions containing chlorine. Environ. Sci. Technol. 1977, 11, 893–895. [Google Scholar] [CrossRef]
- Tian, F.-X.; Ye, W.-K.; Xu, B.; Hu, X.-J.; Ma, S.-X.; Lai, F.; Gao, Y.-Q.; Xing, H.-B.; Xia, W.-H.; Wang, B. Comparison of UV-induced AOPs (UV/Cl2, UV/NH2Cl, UV/ClO2 and UV/H2O2) in the degradation of iopamidol: Kinetics, energy requirements and DBPs-related toxicity in sequential disinfection processes. Chem. Eng. J. 2020, 398, 125570. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wu, W.; Liu, C.; Dionysiou, D.D.; Deng, Y.; Zhou, D. Activation of persulfate with vanadium species for PCBs degradation: A mechanistic study. Appl. Catal. B Environ. 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, T.; Xu, Z.; Li, Y.; Li, B.; Tian, S. UV facilitated synergistic effects of polymetals in ore catalyst on peroxymonosulfate activation: Implication for the degradation of bisphenol S. Chem. Eng. J. 2022, 431, 133989. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Li, T.; Chen, Z.; Jiang, Q.; Zhao, Z.; Liang, X.; Hu, C. Unraveling the High-Activity Origin of Single-Atom Iron Catalysts for Organic Pollutant Oxidation via Peroxymonosulfate Activation. Environ. Sci. Technol. 2021, 55, 8318–8328. [Google Scholar] [CrossRef]
- Keerthana Devi, M.; Karmegam, N.; Manikandan, S.; Subbaiya, R.; Song, H.; Kwon, E.E.; Sarkar, B.; Bolan, N.; Kim, W.; Rinklebe, J.; et al. Removal of nanoplastics in water treatment processes: A review. Sci. Total Environ. 2022, 845, 157168. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.-A. Chapter 13—Nanoplastics in the Aquatic Environment. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–399. [Google Scholar]
- Geetha, N.; Bhavya, G.; Abhijith, P.; Shekhar, R.; Dayananda, K.; Jogaiah, S. Insights into nanomycoremediation: Secretomics and mycogenic biopolymer nanocomposites for heavy metal detoxification. J. Hazard. Mater. 2021, 409, 124541. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Removal Effectiveness of Nanoplastics (<400 nm) with Separation Processes Used for Water and Wastewater Treatment. Water 2020, 12, 635. [Google Scholar]
- Schwaferts, C.; Sogne, V.; Welz, R.; Meier, F.; Klein, T.; Niessner, R.; Elsner, M.; Ivleva, N.P. Nanoplastic Analysis by Online Coupling of Raman Microscopy and Field-Flow Fractionation Enabled by Optical Tweezers. Anal. Chem. 2020, 92, 5813–5820. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, H.; Chen, B.; Pan, H.; Qiu, Z. Insights into chloroacetaldehydes degradation by 254 nm ultraviolet: Kinetics, products, and influencing factors. J. Environ. Chem. Eng. 2021, 9, 104571. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Saha, A.; Si, M.K.; Ganguly, B. A new strategy to generate super and hyper acids with simple organic molecules exploiting σ-hole interaction. Phys. Chem. Chem. Phys. 2019, 21, 17772–17778. [Google Scholar] [CrossRef]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The Roles of Reactive Species in Micropollutant Degradation in the UV/Free Chlorine System. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef][Green Version]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
- Wang, W.-L.; Zhang, X.; Wu, Q.-Y.; Du, Y.; Hu, H.-Y. Degradation of natural organic matter by UV/chlorine oxidation: Molecular decomposition, formation of oxidation byproducts and cytotoxicity. Water Res. 2017, 124, 251–258. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ding, J.; Song, Z.; Yang, B.; Zhang, C.; Guan, B. Degradation of nano-sized polystyrene plastics by ozonation or chlorination in drinking water disinfection processes. Chem. Eng. J. 2022, 427, 131690. [Google Scholar] [CrossRef]
- Xie, R.; Ji, J.; Guo, K.; Lei, D.; Fan, Q.; Leung, D.Y.C.; Huang, H. Wet scrubber coupled with UV/PMS process for efficient removal of gaseous VOCs: Roles of sulfate and hydroxyl radicals. Chem. Eng. J. 2019, 356, 632–640. [Google Scholar] [CrossRef]
- Zrinyi, N.; Pham, A.L.-T. Oxidation of benzoic acid by heat-activated persulfate: Effect of temperature on transformation pathway and product distribution. Water Res. 2017, 120, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.; Aeschbacher, M.; Salhi, E.; Canonica, S.; von Gunten, U.; Sander, M. Chemical Oxidation of Dissolved Organic Matter by Chlorine Dioxide, Chlorine, And Ozone: Effects on Its Optical and Antioxidant Properties. Environ. Sci. Technol. 2013, 47, 11147–11156. [Google Scholar] [CrossRef][Green Version]






| Removal Strategies | Type of Material | Time | Removal Efficiency (%) | Limitations | Reference |
|---|---|---|---|---|---|
| Filtration | 0.22 μm syringe filter 3 μm Whatman filter | 10 min | 32 ± 12 92 ± 3 | Larger nanoplastics can retain in the fractions and are not suitable for larger particles. | [17] |
| Ultrafiltration | 0.45 μm Express PLUS PES filters | 27 min | 74.0 | The particle may escape from the treatment and the time duration is also poor. | [18] |
| Flocculation | 150 rpm impeller rotational speed | 50 min | 77 ± 15 | Design parameters have to improve with studies and the order of reaction should be high. | [19] |
| Centrifugation | 10,000 rpm speed with various steps | 15 min | 98.41 | This process takes a longer duration to remove the plastics. | [20] |
| Photocatalytic reaction | UV rays | 27 min | 17.1 ± 0.55 | Phototransformation of nanoplastics could be different and photoreactive activity can be high in the water. | [21] |
| Compound List | Molecular Formula | Chemical Structure | ESI(+)MS m/z |
|---|---|---|---|
| P1 | C16OH16 |  | 224 |
| P2 | C15O2H12 |  | 224 |
| P3 | C7OH6 | 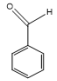 | 106 |
| P4 | C10OH12 |  | 148 |
| P5 | C6OH6 | 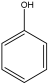 | 94 |
| P6 | C6ClH5 |  | 112 |
| P7 | C6H6 | 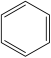 | 78 |
| P8 | C8OH8 |  | 120 |
| P9 | C8H10 |  | 106 |
| P10 | C7H8 | 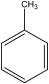 | 92 |
| P11 | C7O2H6 |  | 122 |
| P12 | C2O2H | CH3COOH | 57 |
| P13 | C3OH6 | CH3COCH3 | 58 |
| P14 | CO2H2 | HCOOH | 46 |
| Common Product | Fish (LC50, 96 h) | Daphnia (LC50, 48 h) | Green Algae (EC50, 96 h) |
|---|---|---|---|
| Butyrophenone | 31.37 | 18.92 | 18.11 |
| Benzene | 5.3 | 10.3 | 29 |
| Toluene | 31.7 | 3.8 | 9.4 |
| Phenol | 8.9 | 3.1 | 150 |
| Benzoic acid | 1300.78 | 730.08 | 518.37 |
| Acetophenone | 162 | 106.80 | 70.24 |
| Toxicity Range (mg/L) | Class |
|---|---|
| LC50/EC50 ≤ 1 | Very toxic |
| 1 < LC50/EC50 ≤ 10 | Toxic |
| 10 < LC50/EC50 ≤ 100 | Harmful |
| LC50/EC50 > 100 | Not harmful |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Chen, F.; Yang, L.; Deng, L.; Shi, Z. Degradation of Polystyrene Nanoplastics in UV/NaClO and UV/PMS Systems: Insights into Degradation Efficiency, Mechanism, and Toxicity Evaluation. Water 2023, 15, 1920. https://doi.org/10.3390/w15101920
Cai Y, Chen F, Yang L, Deng L, Shi Z. Degradation of Polystyrene Nanoplastics in UV/NaClO and UV/PMS Systems: Insights into Degradation Efficiency, Mechanism, and Toxicity Evaluation. Water. 2023; 15(10):1920. https://doi.org/10.3390/w15101920
Chicago/Turabian StyleCai, Yishu, Fan Chen, Lingfang Yang, Lin Deng, and Zhou Shi. 2023. "Degradation of Polystyrene Nanoplastics in UV/NaClO and UV/PMS Systems: Insights into Degradation Efficiency, Mechanism, and Toxicity Evaluation" Water 15, no. 10: 1920. https://doi.org/10.3390/w15101920
APA StyleCai, Y., Chen, F., Yang, L., Deng, L., & Shi, Z. (2023). Degradation of Polystyrene Nanoplastics in UV/NaClO and UV/PMS Systems: Insights into Degradation Efficiency, Mechanism, and Toxicity Evaluation. Water, 15(10), 1920. https://doi.org/10.3390/w15101920






