Preparation of Cationic Cellulose as a Natural Flocculant/Sorbent and Its Application in Three Water Treatment Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
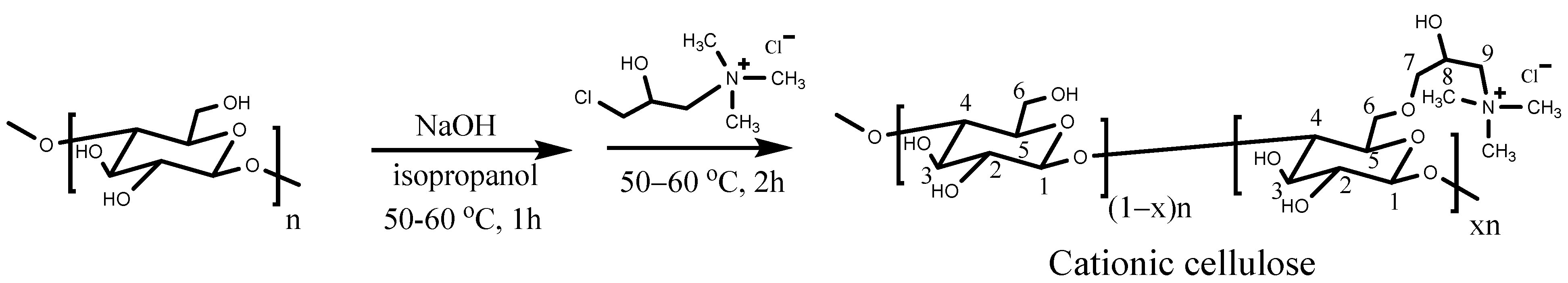
2.2. Preparation of Cationic Cellulose
2.3. Characterization of Cationic Cellulose
2.4. Degree of Substitution (DS)
2.5. Harvesting of Microalgae by Flocculation
2.6. Flocculation Removal of TSS from Dairy Wastewater
2.7. Complexation Removal of Methyl Orange (MO)
3. Results
3.1. Structural Analysis
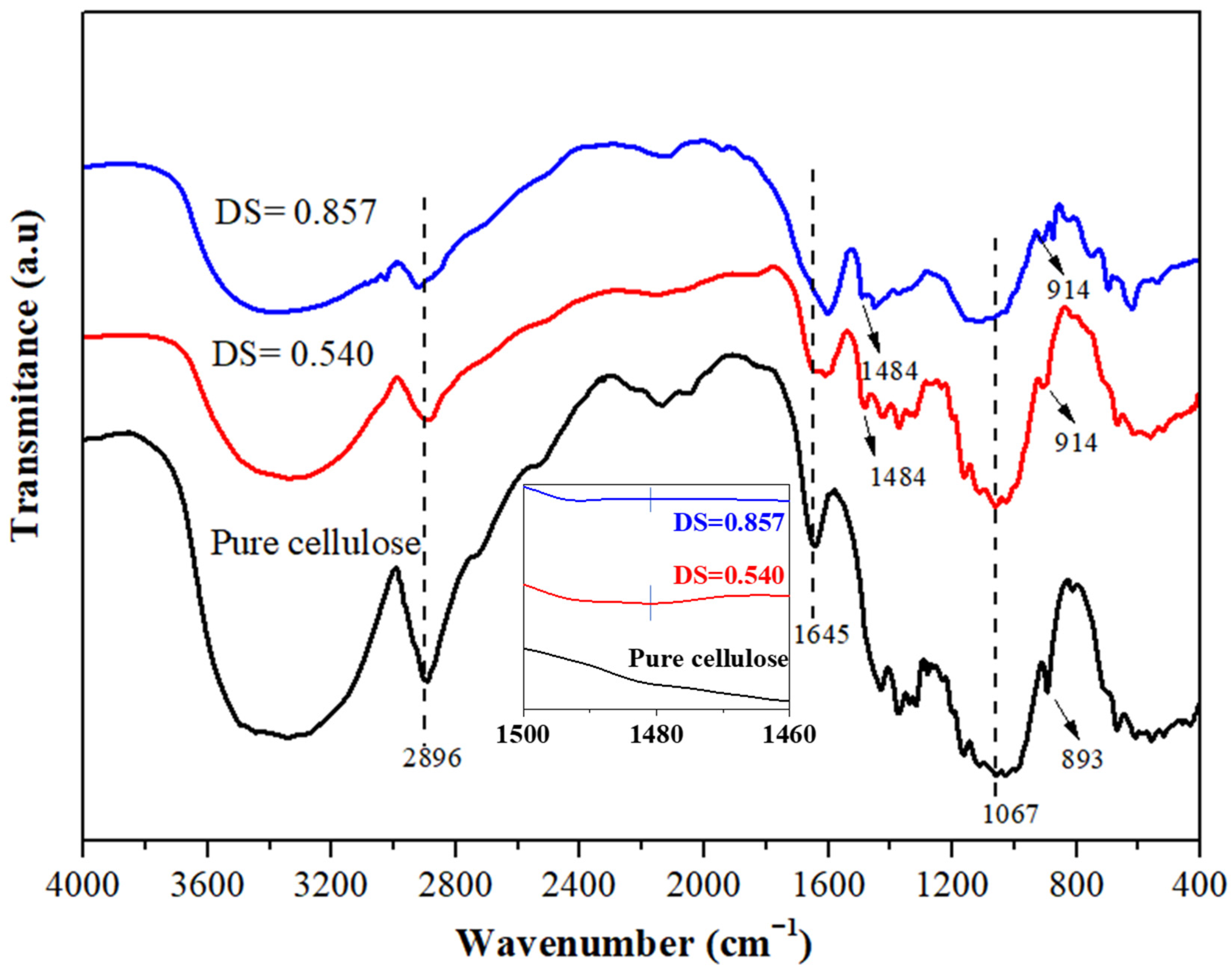
3.1.1. FTIR Spectrum
3.1.2. DSC Thermogram
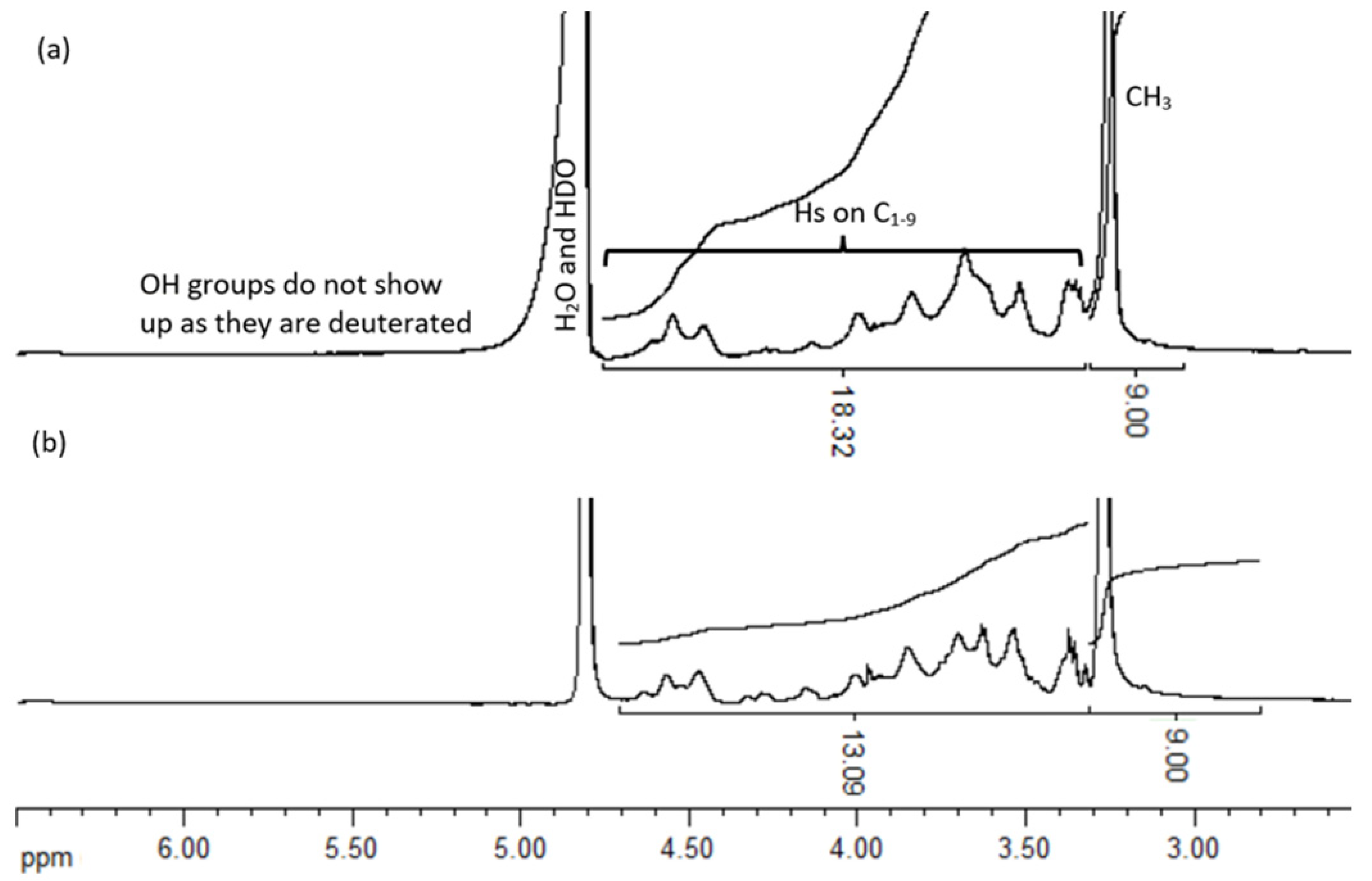
3.1.3. NMR Spectrum
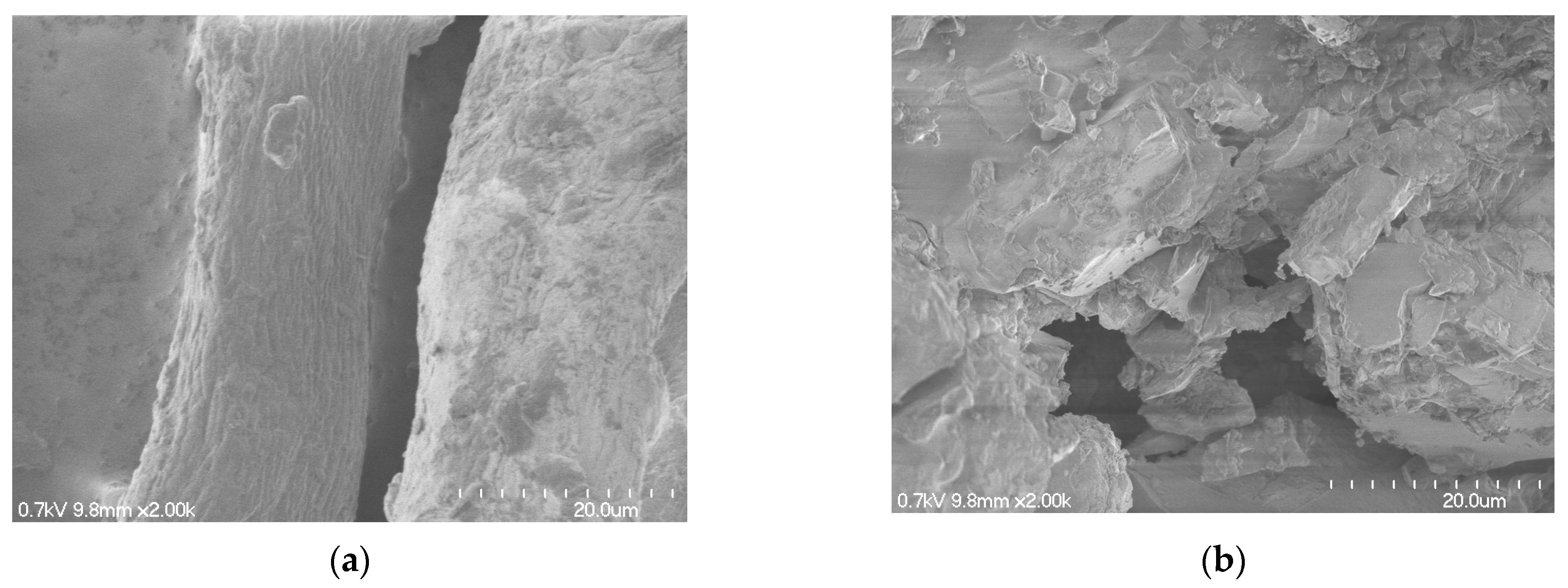
3.1.4. Scanning Electron Microscopy
3.2. Flocculation Experiments
3.2.1. Algal Harvesting
3.2.2. Solid–Liquid Separation of Dairy Wastewater
3.2.3. Methyl Orange
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sirviö, J.; Honka, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Synthesis of highly cationic water-soluble cellulose derivative and its potential as novel biopolymeric flocculation agent. Carbohydr. Polym. 2011, 86, 266–270. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhu, H.; Cheng, J.-H. Structure modification and property improvement of plant cellulose: Based on emerging and sustainable nonthermal processing technologies. Food Res. Int. 2022, 156, 111300. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Si, C.; Li, B. Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind. Crops Prod. 2016, 94, 736–745. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L. New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system. Food Res. Int. 2013, 52, 387–400. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Zhang, X.; You, T.-T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustainable Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z. Recent advances in the application of cellulose derivatives for removal of contaminants from aquatic environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Jin, H.; Zha, C.; Gu, L. Direct dissolution of cellulose in NaOH/thiourea/urea aqueous solution. Carbohydr. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Wang, B.; Shi, S.; Xiong, L.; Chen, X. Synthesis and characterization of quaternized bacterial cellulose prepared in homogeneous aqueous solution. Carbohydr. Polym. 2016, 136, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Tao, H.; Bangal, P.R. Synthesis and flocculation behavior of cationic cellulose prepared in a NaOH/urea aqueous solution. Clean: Soil, Air, Water 2009, 37, 39–44. [Google Scholar] [CrossRef]
- Kono, H. Cationic flocculants derived from native cellulose: Preparation, biodegradability, and removal of dyes in aqueous solution. Resour.-Effic. Technol. 2017, 3, 55–63. [Google Scholar] [CrossRef]
- Sievänen, K.; Kavakka, J.; Hirsilä, P.; Vainio, P.; Karisalmi, K.; Fiskari, J.; Kilpeläinen, I. Cationic cellulose betainate for wastewater treatment. Cellulose 2015, 22, 1861–1872. [Google Scholar] [CrossRef]
- Glibert, P.M. From hogs to HABs: Impacts of industrial farming in the US on nitrogen and phosphorus and greenhouse gas pollution. Biogeochemistry 2020, 150, 139–180. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, R.; Code, K.R.; Leonard, J. Advanced treatment of liquid swine manure using physico-chemical treatment. J. Hazard. Mater. 2011, 186, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Westerman, P. Solid-liquid separation of annual manure for odor control and nutrient management. Appl. Eng. Agric. 1997, 13, 385–393. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-based livestock wastewater treatment (MbWT) as a circular bioeconomy approach: Enhancement of biomass productivity, pollutant removal and high-value compound production. J. Environ. Manag. 2022, 308, 114612. [Google Scholar] [CrossRef]
- Osabutey, A.; Haleem, N.; Uguz, S.; Min, K.; Samuel, R.; Albert, K.; Anderson, G.; Yang, X. Growth of Scenedesmus dimorphus in swine wastewater with versus without solid–liquid separation pretreatment. Bioresour. Technol. 2023, 369, 128434. [Google Scholar] [CrossRef]
- Nanda, M.; Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Gautam, P.; Bahuguna, V.; Chauhan, P.K. Micro-pollutant Pb(II) mitigation and lipid induction in oleaginous microalgae Chlorella sorokiniana UUIND6. Environ. Technol. Innov. 2021, 23, 101613. [Google Scholar] [CrossRef]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; Castro, J.d.S.; Couto, E.d.A.d.C.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci. Total Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater treatment by seven species of microalgae and an algal bloom: Biomass production, N and P removal kinetics and harvestability. Water Res. 2015, 83, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Garcia, D.F.; Cuellar-Bermudez, S.P.; del Rio-Hinojosa, E.; Betancourt, F.; Aleman-Nava, G.S.; Parra-Saldivar, R. Potential land microalgae cultivation in Mexico: From food production to biofuels. Algal Res. 2019, 39, 101459. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Gururani, P.; Vlaskin, M.S.; Parveen, A.; Nanda, M.; Kurbatova, A.; Gautam, P.; Grigorenko, A.V. Bio-flocculation of oleaginous microalgae integrated with municipal wastewater treatment and its hydrothermal liquefaction for biofuel production. Environ. Technol. Innov. 2022, 26, 102340. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants—A review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Yang, X.; Ni, L. Synthesis of hybrid hydrogel of poly(AM co DADMAC)/silica sol and removal of methyl orange from aqueous solutions. Chem. Eng. J. 2012, 209, 194–200. [Google Scholar] [CrossRef]
- Lau, Y.-Y.; Wong, Y.-S.; Teng, T.-T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv. 2015, 5, 34206–34215. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Lafi, R.; Abdellaoui, L.; Montasser, I.; Hafiane, A. Removal of methyl orange from aqueous solution onto modified extracted cellulose from Stipa tenacissima L. Int. J. Environ. Anal. Chem. 2022, 102, 8124–8140. [Google Scholar] [CrossRef]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Synthesis and Characterization of Starch-Based Cationic Flocculants for Harvesting Microalgae. 2012. Available online: https://conservancy.umn.edu/bitstream/handle/11299/140051/1/Li_umn_0130M_13143.pdf (accessed on 17 May 2023).
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Deepa, D.; Keerthana, R.; Pratheep Kumar, R.; Suryaprakash, R. Primary treatment of dairy wastewater using bio based natural coagualnts. Mater. Today Proc. 2022, 60, 616–621. [Google Scholar] [CrossRef]
- Marinho, Y.F.; Oliveira, C.Y.B.; Malafaia, C.B.; Cahú, T.B.; Oliveira, A.P.S.; Napoleão, T.H.; Bezerra, R.S.; Paiva, P.G.; Gálvez, A.O. A circular approach for the efficient recovery of astaxanthin from Haematococcus pluvialis biomass harvested by flocculation and water reusability. Sci. Total Environ. 2022, 841, 156795. [Google Scholar] [CrossRef]
- Haleem, N.; Osabutey, A.; Albert, K.; Zhang, C.; Min, K.; Anderson, G.; Yang, X. Flocculation of livestock wastewater using cationic starch prepared from potato peels. Environ. Sci. Water Res. Technol. 2023. [Google Scholar] [CrossRef]
- Sang, Y.; Xiao, H. Preparation and application of cationic cellulose fibers modified by in situ grafting of cationic PVA. Colloids Surf. A 2009, 335, 121–127. [Google Scholar] [CrossRef]
- Jahan, M.S.; Saeed, A.; He, Z.; Ni, Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2011, 18, 451–459. [Google Scholar] [CrossRef]
- Loubaki, E.; Ourevitch, M.; Sicsic, S. Chemical modification of chitosan by glycidyl trimethylammonium chloride. Characterization of modified chitosan by 13C-and 1H-NMR spectroscopy. Eur. Polym. J. 1991, 27, 311–317. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Anttila, A.-K.; Pirttilä, A.M.; Liimatainen, H.; Kilpeläinen, I.; Niinimäki, J.; Hormi, O. Cationic wood cellulose films with high strength and bacterial anti-adhesive properties. Cellulose 2014, 21, 3573–3583. [Google Scholar] [CrossRef]
- Zhang, F.; Pang, Z.; Dong, C.; Liu, Z. Preparing cationic cotton linter cellulose with high substitution degree by ultrasonic treatment. Carbohydr. Polym. 2015, 132, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, Y.; Shao, Z.; Zhang, F.; Qin, M. Preparing cationic cellulose derivative in NaOH/urea aqueous solution and its performance as filler modifier. BioResources 2015, 10, 7782–7794. [Google Scholar] [CrossRef]
- Chirila, L.; Popescu, A.; Cutrubinis, M.; Stanculescu, I.; Moise, V.I. The influence of gamma irradiation on natural dyeing properties of cotton and flax fabrics. Radiat. Phys. Chem. 2018, 145, 97–103. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Jiang, Z.; Cai, T.; Li, H.; Li, H.; Li, A.; Cheng, R. Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J. Hazard. Mater. 2013, 254–255, 36–45. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Van Haver, L.; Nayar, S. Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res. 2017, 24, 167–180. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Bakonyi, P.; Nemestóthy, N.; Xia, A.; Banu J, R.; Kumar, G. A review on chemical mechanism of microalgae flocculation via polymers. Biotechnol. Rep. 2019, 21, e00302. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Exposito, P.; Campano, C.; van de Ven, T.G.; Negro, C.; Blanco, A. Microalgae harvesting with the novel flocculant hairy cationic nanocrystalline cellulose. Colloids Surf. B 2019, 178, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Hansel, P.A.; Riefler, R.G.; Stuart, B.J. Efficient flocculation of microalgae for biomass production using cationic starch. Algal Res. 2014, 5, 133–139. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Polymer based flocculants: Review of water purification applications. J. Water Process. Eng. 2022, 48, 102938. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L. Harvesting freshwater microalgae with natural polymer flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Mohseni, F.; Moosavi Zenooz, A. Flocculation of Chlorella vulgaris with alum and pH adjustment. Biotechnol. Appl. Biochem. 2022, 69, 1112–1120. [Google Scholar] [CrossRef]
- Devi, M.G.; Dumaran, J.J.; Feroz, S. Dairy Wastewater Treatment Using Low Molecular Weight Crab Shell Chitosan. J. Inst. Eng. Ser. E 2012, 93, 9–14. [Google Scholar] [CrossRef]
- Rolf, J.; Weide, T.; Brügging, E.; Wetter, C. The Application of Biodegradable Flocculants Derived From Potato Starch for Nutrient Recovery in Pig Manure. Global J. Agric. Res. 2021, 9, 1–15. Available online: https://ssrn.com/abstract=3905166 (accessed on 17 May 2023).
- Pan, Y.; Wang, D.; Mei, K.; Tang, T. Optimization modeling and mechanism discussion on specific industrial coal-washing wastewater treatment. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Zhai, S.; Li, Y.; Dong, W.; Zhao, H.; Ma, K.; Zhang, H.; Wang, H.; Zhao, Y.; Li, X.; Cai, Z. Cationic cotton modified by 3-chloro-2-hydroxypropyl trimethyl ammonium chloride for salt-free dyeing with high levelling performance. Cellulose 2022, 29, 633–646. [Google Scholar] [CrossRef]
- Muniz, G.L.; Borges, A.C.; Silva, T.C.F.d. Performance of natural coagulants obtained from agro-industrial wastes in dairy wastewater treatment using dissolved air flotation. J. Water Process. Eng. 2020, 37, 101453. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, A.; Wu, J.; Zhao, J.; Yan, H. Adsorption Behaviors and Mechanisms of Methyl Orange on Heat-Treated Palygorskite Clays. Ind. Eng. Chem. Res. 2012, 51, 14026–14036. [Google Scholar] [CrossRef]
- Duan, S.; Tang, R.; Xue, Z.; Zhang, X.; Zhao, Y.; Zhang, W.; Zhang, J.; Wang, B.; Zeng, S.; Sun, D. Effective removal of Pb(II) using magnetic Co0.6Fe2.4O4 micro-particles as the adsorbent: Synthesis and study on the kinetic and thermodynamic behaviors for its adsorption. Colloids Surf. A 2015, 469, 211–223. [Google Scholar] [CrossRef]
- Lafi, R.; Hafiane, A. Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J. Taiwan Inst. Chem. Eng. 2016, 58, 424–433. [Google Scholar] [CrossRef]

| CC Dose Level (mg/L) | Initial Algal Biomass (mg/L) | Final Algal Biomass (mg/L) | Removal of Dry Algal Biomass (%) |
|---|---|---|---|
| 80 | 1950 ± 550 | 1016 ± 103 | 47.9 |
| 125 | 1950 ± 550 | 766 ± 103 | 60.7 |
| 160 | 1950 ± 550 | 450 ± 141 | 76.9 |
| 200 | 1950 ± 550 | 267 ± 24 | 86.3 |
| 250 | 1950 ± 550 | 183 ± 47 | 90.6 |
| 300 | 1950 ± 550 | 133 ± 24 | 93.0 |
| pH | Microalgae | Dairy Wastewater | Methyl Orange | |||
|---|---|---|---|---|---|---|
| Final DAB (mg/L) | RE (%) | Final TSS (mg/L) | RE (%) | Final MO (mg/L) | RE (%) | |
| 4 | 495 ± 212 | 74.6 ± 5.3 | 24,303 ± 100 | 53.4 ± 1.9 | 27.7 ± 1.8 | 44.5 ± 2.1 |
| 5 | 313 ±110 | 84.0 ± 5.3 | 23,216 ±126 | 54.8 ± 1.4 | 20.7 ± 4.1 | 58.7 ± 5.0 |
| 6 | 250 ± 126 | 87.2 ± 7.2 | 23,516 ± 122 | 54.3 ± 1.2 | 17.3 ± 0.5 | 65.3 ± 0.7 |
| 7 | 181 ± 174 | 90.5 ± 1.2 | 23,545 ± 358 | 54.2 ± 2.1 | 17.9 ± 1.3 | 64.3 ± 1.6 |
| 8 | 178 ± 420 | 90.9 ± 1.2 | 24,860± 178 | 51.8 ± 0.3 | 24.7 ± 2.2 | 50.7 ± 2.6 |
| 9 | 228 ± 112 | 86.3 ± 2.4 | 29,100 ± 187 | 43.5 ± 0.6 | 28.0 ± 2.7 | 44.0 ± 1.7 |
| 10 | 816 ± 532 | 58.2 ± 1.2 | 32,850 ± 161 | 36.2 ± 0.8 | 42.0 ± 4.1 | 16.0 ± 3.0 |
| CC Dose Level (mg/L) | Initial TSS (mg/L) | Final TSS (mg/L) | TSS Removal (%) |
|---|---|---|---|
| 80 | 51,466 ± 675 | 26,685 ± 900 | 47.1 |
| 125 | 51,466 ± 675 | 25,735 ± 369 | 49.9 |
| 160 | 51,466 ± 675 | 25,350 ± 444 | 50.5 |
| 200 | 51,466 ± 675 | 24,270 ± 101 | 53.3 |
| 250 | 51,466 ± 675 | 24,700 ± 180 | 52.3 |
| CC Dose Level (mg/L) | Initial MO (mg/L) | Final MO (mg/L) | MO Removal (%) | qe (mg/g) |
|---|---|---|---|---|
| 50 | 50 | 35.7 ± 2.2 | 24.3 | 243 |
| 100 | 50 | 31.0 ± 5.0 | 27.9 | 140 |
| 150 | 50 | 22.8 ± 0.7 | 53.0 | 177 |
| 200 | 50 | 22.5 ± 1.6 | 51.8 | 130 |
| 250 | 50 | 22.6 ± 2.6 | 49.4 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haleem, N.; Zhang, C.; Jamal, Y.; Albert, K.; Fan, D.; Yao, B.; Hussain, F.; Yang, X. Preparation of Cationic Cellulose as a Natural Flocculant/Sorbent and Its Application in Three Water Treatment Scenarios. Water 2023, 15, 2021. https://doi.org/10.3390/w15112021
Haleem N, Zhang C, Jamal Y, Albert K, Fan D, Yao B, Hussain F, Yang X. Preparation of Cationic Cellulose as a Natural Flocculant/Sorbent and Its Application in Three Water Treatment Scenarios. Water. 2023; 15(11):2021. https://doi.org/10.3390/w15112021
Chicago/Turabian StyleHaleem, Noor, Cheng Zhang, Yousuf Jamal, Karlee Albert, Dongdong Fan, Bin Yao, Fida Hussain, and Xufei Yang. 2023. "Preparation of Cationic Cellulose as a Natural Flocculant/Sorbent and Its Application in Three Water Treatment Scenarios" Water 15, no. 11: 2021. https://doi.org/10.3390/w15112021





