Assessment of Current Blue Energy Technologies and Their Potential Applications on Romanian Water Resources
Abstract
1. Introduction
2. Technologies for Obtaining Energy from Salinity Gradient
2.1. Pressure Retarded Osmosis
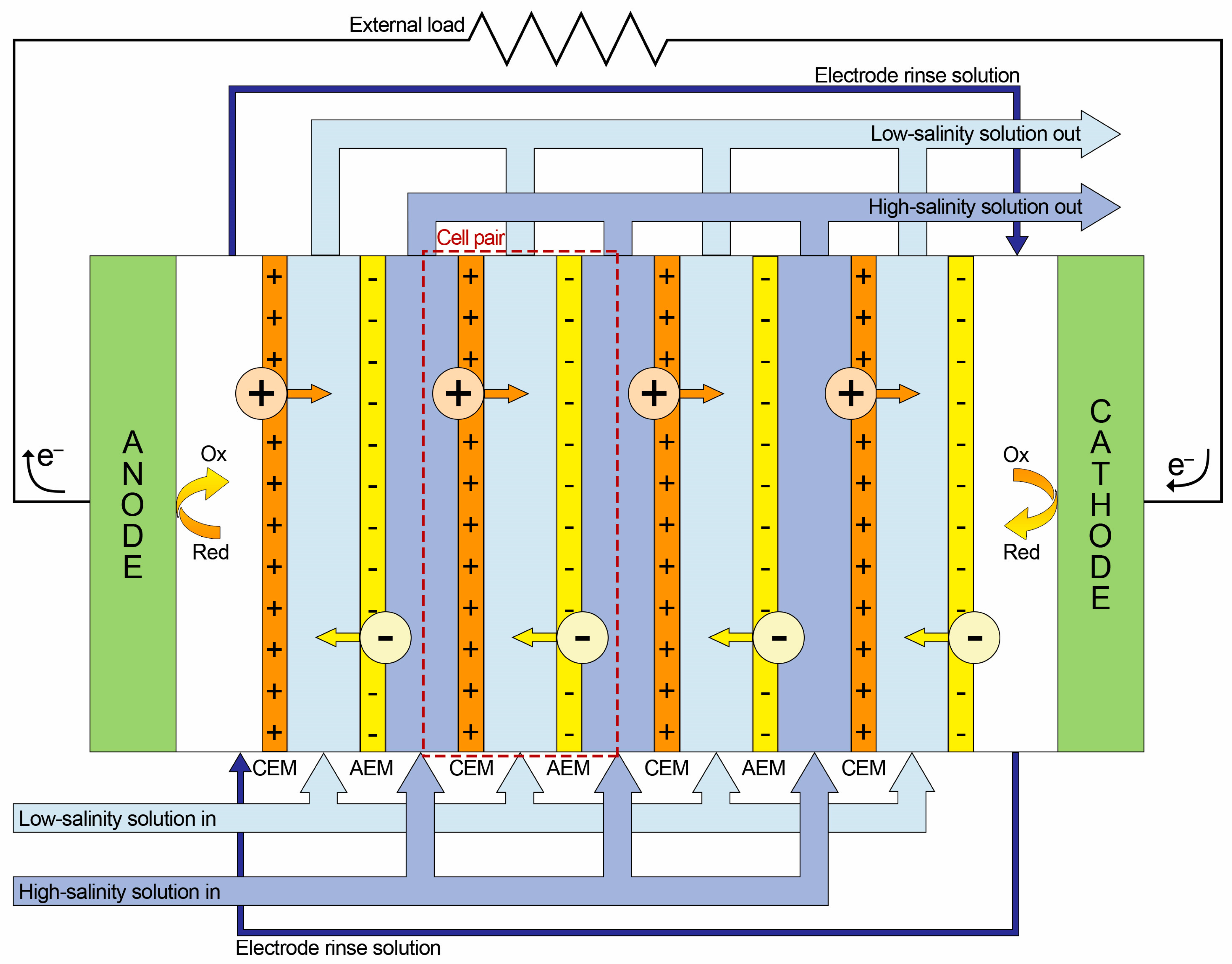
2.2. Reverse Electrodialysis (RED)
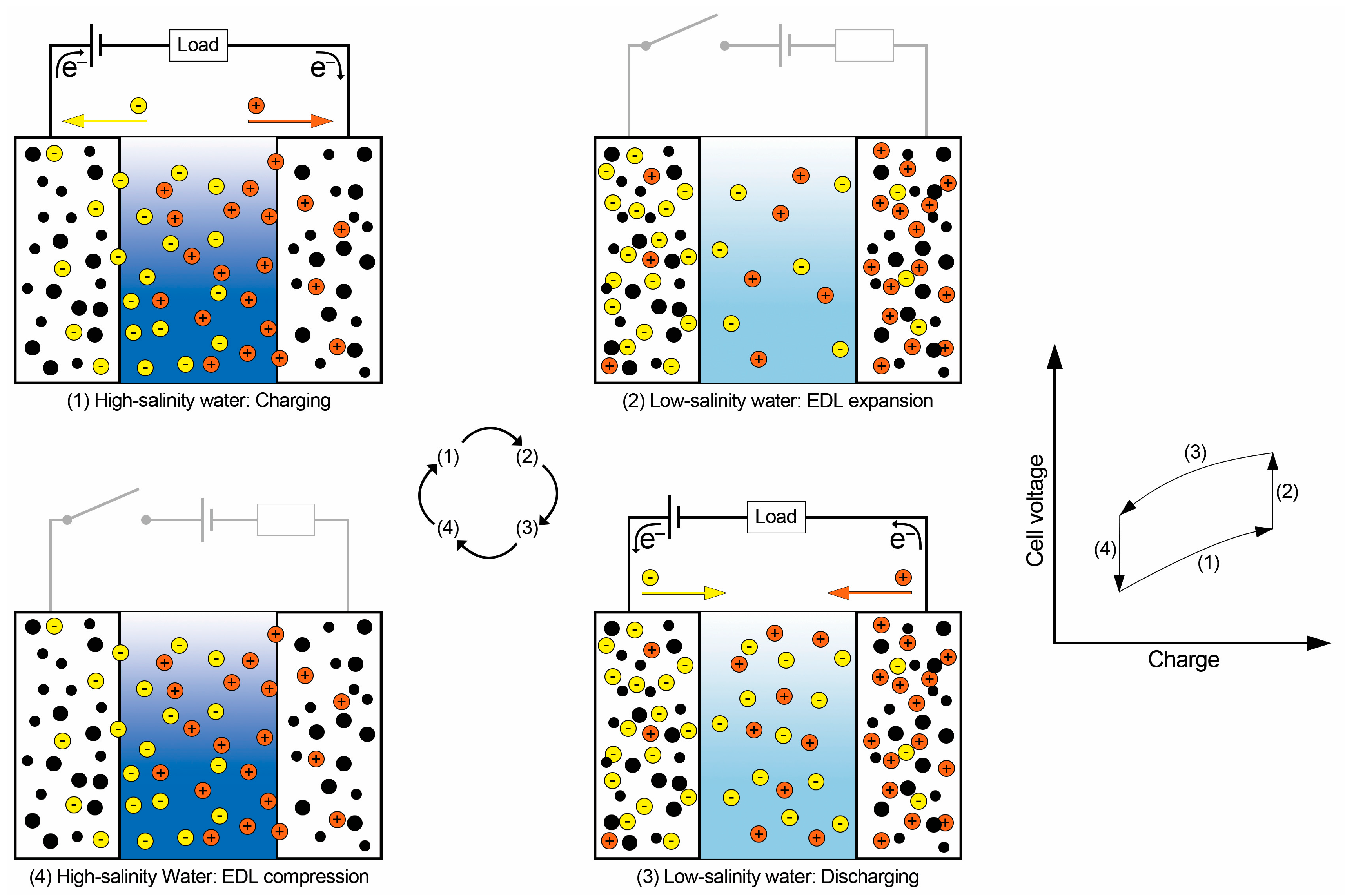
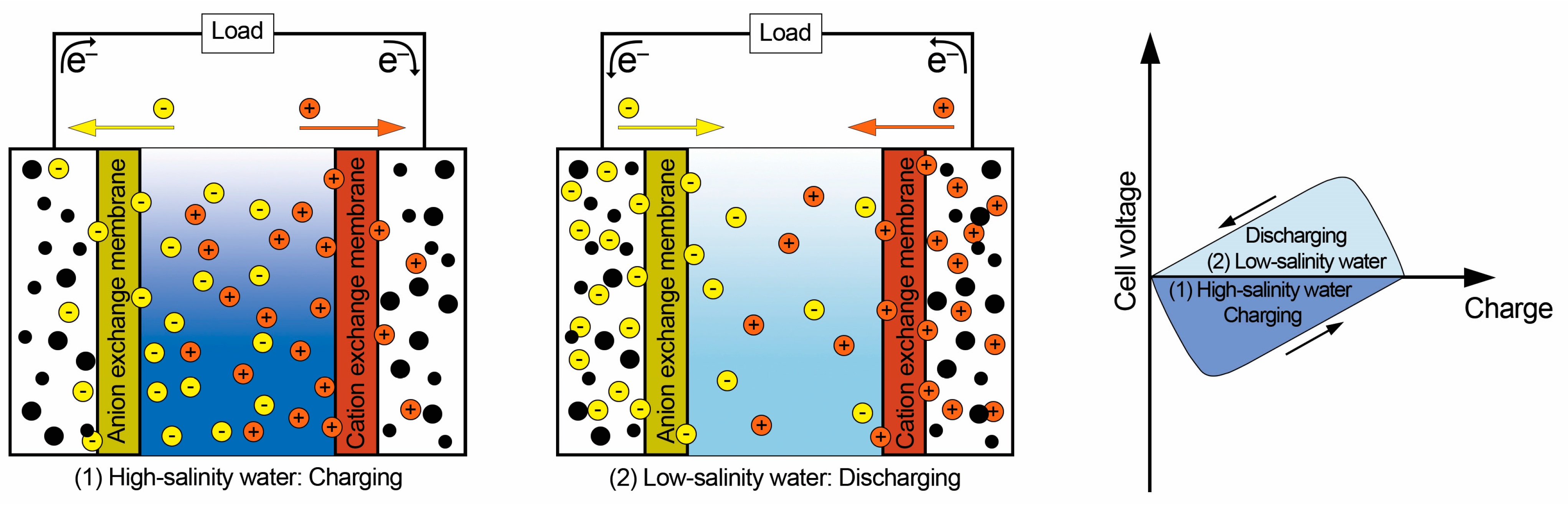
2.3. Capacitive Mixing (CapMix)
3. Pilot Centers for the Generation of Blue Energy
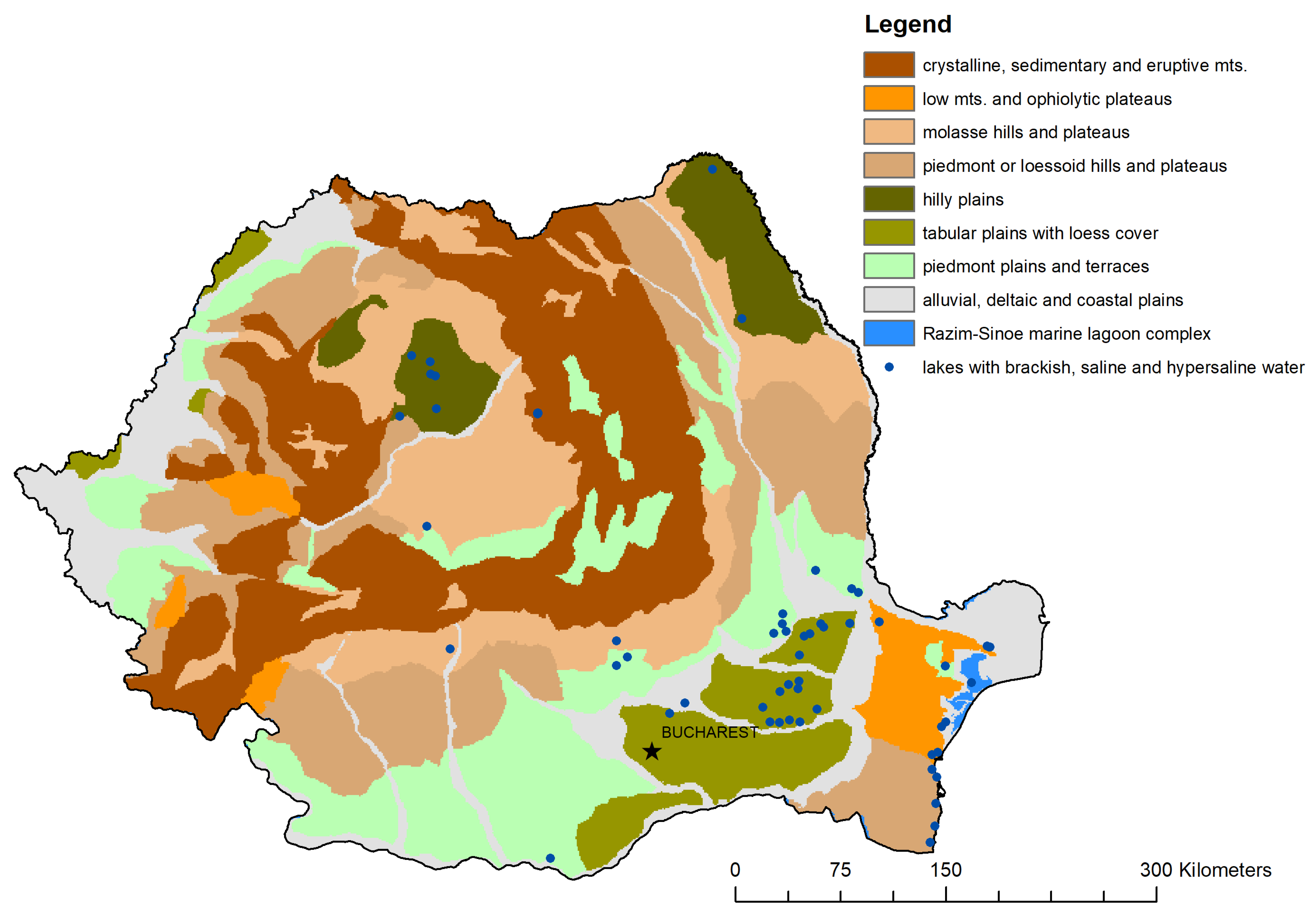
4. The Potential Applicability of Salinity Gradient in Romania
- -
- Danube River floodplain, near Zimnicea (Balta Suhaia);
- -
- Prahova River catchment (Balta Filipești-Târg);
- -
- Ialomița River catchment (Căldărușani, Balta Maia, Fundata, Reviga, Șchiauca, Amara—Slobozia, Iezer, Stachina);
- -
- Buzău River catchment (Balta Amara—Buzău, Balta Albă, Jirlău);
- -
- Padina Plain, near Făurei (Choibășești or Chichinețu, Plașcu, Tătaru);
- -
- Brăila Plain (Plopu, Lutu Alb);
- -
- Siret River Catchment (Balta Tălăbasca, Balta Mălina, Balta Cătușa);
- -
- Prut River catchment (Viișoara);
- -
- Dobrogea region (Babadag, Razelm complex, Tașaul, Gargalîc, Mangalia, Sărat-Greci, Tătlăgeac).
Estimation of the Theoretical Amount of Energy Released When Mixing Water Streams with Different Salinity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bilardi, S.; Calabrò, P.S.; Moraci, N. A Review of the Hydraulic Performance of Permeable Reactive Barriers Based on Granular Zero Valent Iron. Water 2023, 15, 200. [Google Scholar] [CrossRef]
- Shadman, M.; Roldan-Carvajal, M.; Pierart, F.G.; Haim, P.A.; Alonso, R.; Silva, C.; Osorio, A.F.; Almonacid, N.; Carreras, G.; Maali Amiri, M. A Review of Offshore Renewable Energy in South America: Current Status and Future Perspectives. Sustainability 2023, 15, 1740. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Elimelech, M. Viability of Harvesting Salinity Gradient (Blue) Energy by Nanopore-Based Osmotic Power Generation. Engineering 2022, 9, 51–60. [Google Scholar] [CrossRef]
- Wu, Q.; Fang, Y.; Wang, X. Improvement in Operation Efficiency of Shallow Geothermal Energy System—A Case Study in Shandong Province, China. Water 2023, 15, 1409. [Google Scholar] [CrossRef]
- European Commission (2020). Communication from the Commission to the European Parliament, the European Council, the Council, the European economic and Social Committee and the Committee of the Regions: An EU Strategy to Harness the Potential of Offshore Renewable Energy for a Climate Neutral Future. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0741&from=EN (accessed on 24 March 2023).
- Altıok, E.; Kaya, T.Z.; Güler, E.; Kabay, N.; Bryjak, M. Performance of Reverse Electrodialysis System for Salinity Gradient Energy Generation by Using a Commercial Ion Exchange Membrane Pair with Homogeneous Bulk Structure. Water 2021, 13, 814. [Google Scholar] [CrossRef]
- Cîrstea, Ş.D.; Martiş, C.S.; Cîrstea, A.; Constantinescu-Dobra, A.; Fülöp, M.T. Current Situation and Future Perspectives of the Romanian Renewable Energy. Energies 2018, 11, 3289. [Google Scholar] [CrossRef]
- Turkenburg, W.C. World Energy Assessment: Energy and the Challenge of Sustainability; UNDP: New York, NY, USA, 2000; Chapter 7; p. 221. [Google Scholar]
- Alvarez-Silva, O.A.; Osorio, A.F.; Winter, C. Practical global salinity gradient energy potential. Renew. Sustain. Energy Rev. 2016, 60, 1387–1395. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Jenita Rani, G.; Ng, F.L.; Periasamy, V.; Pappathi, M.; Rajan, M.J.; Al-Sehemi, A.G.; Pannipara, M.; Phang, S.-M.; Aziz, M.A.; et al. 3D Flower–Like FeWO4/CeO2 Hierarchical Architectures on rGO for Durable and High-Performance Microalgae Biophotovoltaic Fuel Cells. Appl. Biochem. Biotechnol. 2020, 192, 751–769. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Sheet, S.; Sathishkumar, Y.; Lee, Y.S.; Phang, S.-M.; Periasamy, V.; Gnana Kumar, G. Titania/reduced graphene oxide composite nanofibers for the direct extraction of photosynthetic electrons from microalgae for biophotovoltaic cell applications. Appl. Phys. A 2018, 124, 769. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Raj Kumar, T.; Pannipara, M.; Al-Sehemi, A.G.; Senthilkumar, N.; Angelaalincy, M.J.; Varalakshmi, P.; Phang, S.M.; Periasamy, V.; Gnana Kumar, G. Ruthenium oxide/tungsten oxide composite nanofibers as anode catalysts for the green energy generation of Chlorella vulgaris mediated biophotovoltaic cells. Environ. Prog. 2019, 38, e13262. [Google Scholar] [CrossRef]
- Micale, G.; Cipollina, A.; Tamburini, A. Chapter 1: Salinity gradient energy. In Sustainable Energy from Salinity Gradients; Elsevier B.V.: Amsterdam, The Netherlands, 2016; pp. 1–18. [Google Scholar]
- Zou, Z.; Liu, L.; Meng, S.; Bian, X. Comparative study on the performance of capacitive mixing under different operational modes. Energy Rep. 2022, 8, 7325–7335. [Google Scholar] [CrossRef]
- Simoes, C.; Vital, B.; Saakes, T.M.; Brilman, W. Scaled-up multistage reverse electrodialysis pilot study with natural waters. Chem. Eng. J. 2022, 450, 138412. [Google Scholar] [CrossRef]
- Jin, D.; Xi, R.; Xu, S.; Wang, P.; Wu, X. Numerical simulation of salinity gradient power generation using reverse electrodialysis. Desalination 2021, 512, 13. [Google Scholar] [CrossRef]
- Rahman, M.M. Membranes for Osmotic Power Generation by Reverse Electrodialysis. Membranes 2023, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Choi, Y.; Lee, S.; Park, C.-g.; Hwang, T.; Jung, N. Comparison of Pretreatment Methods for Salinity Gradient Power Generation Using Reverse Electrodialysis (RED) Systems. Membranes 2022, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Mora, D.; de Rijck, A. Blue Energy: Salinity Gradient Power in Practice; Report No. GSDR 2015 Brief; Wageningen University and Research Centre: Wageningen, The Netherlands, 2009. [Google Scholar]
- Rai Khare, V.; Dubey, R. Blue Energy: Power from the Sea—A Review. In Proceedings of the Green Technology Jaipur Conference: National Conference of Green Technology by DST and ISTE, Jaipur, India, 28–29 March 2012. [Google Scholar]
- Matsuyama, K.; Makabe, R.; Ueyama, T.; Sakai, H.; Saito, K.; Okumura, T.; Hayashi, H.; Tanioka, A. Power generation system based on pressure retarded osmosis with a commercially-available hollow fiber PRO membrane module using seawater and freshwater. Desalination 2021, 499, 114805. [Google Scholar] [CrossRef]
- Obode, E.I.; Badreldin, A.; Adham, S.; Castier, M.; Abdel-Wahab, A. Techno-Economic Analysis towards Full-Scale Pressure Retarded Osmosis Plants. Energies 2023, 16, 325. [Google Scholar] [CrossRef]
- Straub, A.P.; Deshmukh, A.; Elimelech, M. Pressure-retarded osmosis for power generation from salinity gradients: Is it viable? Energy Environ. Sci. 2016, 9, 31–48. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Abdel-Wahab, A.; Adham, S.; Suk Han, D.; Phuntsho, S.; Suwaileh, W.; Hilal, N.; Shon, H.K. Salinity gradient energy generation by pressure retarded osmosis: A review. Desalination 2021, 500, 114841. [Google Scholar] [CrossRef]
- Radu, V.M.; Boaja, I.P.; Ivanov, A.A.; Dinca, G.; Szabo, R. Forthcoming Opportunities for Obtaining Energy from New Renewable Sources in Romania. In Renewable Energy Systems in Smart Grid. Lecture Notes in Electrical Engineering; Kolhe, M.L., Ed.; Springer: Singapore, 2022; p. 938. [Google Scholar] [CrossRef]
- Abbasi-Garravand, E.; Mulligan, C.N. Feasibility of Pressure-Retarded Osmosis for Electricity Generation at Low Temperatures. Membranes 2021, 11, 556. [Google Scholar] [CrossRef]
- Han, G.; Feng, Y. Recent Development of Pressure Retarded Osmosis (PRO) Hollow Fiber Membranes; Chapter 22—Hollow Fiber Membranes; Chung, T.-S., Feng, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 473–493. [Google Scholar] [CrossRef]
- Nur Hidayati, O.; Nalan, K.; Enver, G. Principles of reverse electrodialysis and development of integrated-based system for power generation and water treatment: A review. Rev. Chem. Eng. 2022, 38, 921–958. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Veerman, J.; Vermaas, D.A. Sustainable Energy from Salinity Gradients. In Chapter 4—Reverse Electrodialysis: Fundamentals; Woodhead Publishing: Sawston, UK, 2016; pp. 77–133. [Google Scholar]
- Iglesias, G.R.; Fernández, M.M.; Ahualli, S.; Jiménez, M.L.; Kozynchenko, O.P.; Delgado, Á.V. Materials selection for optimum energy production by double layer expansion methods. J. Power Sources 2014, 261, 371–377. [Google Scholar] [CrossRef]
- Rica, R.A.; Brogioli, D.; Ziano, R.; Salerno, D.; Mantegazza, F. Ions Transport and Adsorption Mechanisms in Porous Electrodes During Capacitive-Mixing Double Layer Expansion (CDLE). J. Phys. Chem. C 2012, 116, 16934–16938. [Google Scholar] [CrossRef]
- Brogioli, D.; Zhao, R.; Biesheuvel, P.M. A prototype cell for extracting energy from a water salinity difference by means of double layer expansion in nanoporous carbon electrodes. Energy Environ. Sci. 2011, 4, 772–777. [Google Scholar] [CrossRef]
- Sales, B.B.; Burheim, O.S.; Liu, F.; Schaetzle, O.; Buisman, C.J.N.; Hamelers, H.V.M. Impact of Wire Geometry in Energy Extraction from Salinity Differences Using Capacitive Technology. Environ. Sci. Technol. 2012, 46, 12203–12208. [Google Scholar] [CrossRef]
- La Mantia, F.; Pasta, M.; Deshazer, H.D.; Logan, B.E.; Cui, Y. Batteries for efficient energy extraction from a water salinity difference. Nano Lett. 2011, 11, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, H.M.H.; Seyedsadjadi, S.A.; Ghaffarinejad, A. Application of electrodeposited cobalt hexacyanoferrate film to extract energy from water salinity gradients. RSC Adv. 2015, 5, 30032–30037. [Google Scholar] [CrossRef]
- Ye, M.; Pasta, M.; Xie, X.; Dubrawski, K.L.; Xu, J.; Liu, C.; Cui, Y.; Criddle, C.S. Charge-Free Mixing Entropy Battery Enabled by Low-Cost Electrode Materials. ACS Omega 2019, 4, 11785–11790. [Google Scholar] [CrossRef]
- Statkraft Osmotic Power Plant. Available online: https://www.power-technology.com/projects/statkraft-osmotic/ (accessed on 6 April 2023).
- Kurihara, M.; Takeuchi, H. SWRO-PRO System in “Mega-ton Water System” for Energy Reduction and Low Environmental Impact. Water 2018, 10, 48. [Google Scholar] [CrossRef]
- Fujifilm Starts Production Line for Blue Energy Membranes in Tilburg, the Netherlands. Available online: https://www.dutchwatersector.com/news/fujifilm-starts-production-line-for-blue-energy-membranes-in-tilburg-the-netherlands/ (accessed on 6 April 2023).
- Abad Castelos, M. The Black Sea and Blue Energy: Challenges, Opportunities and the Role of the European Union. In The Future of the Law of the Sea; Andreone, G., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Gâștescu, P. Lacurile din Republica Populară Romînă. Geneză și Regim Hidrologic; Editura Academiei Republicii Populare Romîne: București, Romania, 1963. [Google Scholar]
- Gâștescu, P. Lacurile din România. Limnologie Regională; Editura Academiei Republicii Socialiste România: București, Romania, 1971. [Google Scholar]
- Pricăjan, A. Apele Minerale și Termale din România; Editura Tehnică: București, Romania, 1972. [Google Scholar]
- Bulgăreanu, V.-A.; Godeanu, D.; Izvoranu, V. New Data regarding the lake Sărătura 3 in Independeța (Murighiol), Tulcea county, Romania. Rev. Roum. Biol.-Biol. Végét. 1989, 34, 51–56. [Google Scholar]
- Bulgăreanu, V.-A.; Călinescu, E.; Cehlarov, A.; Cușmirenco, G.; Enache, G.; Opriș, E.; Prodănescu, I.; Rădan, S.; Urcan, T. Limnology and peloidogenesis evolution of “chot”—Type lake Sărătura 1—Murighiol (Independența village, Tulcea county, Romania). Rev. Roum. Géol., Géophys. Géogr. Geologie 1990, 34, 83–94. [Google Scholar]
- Momeu, L.; Cîmpean, M.; Battes, K. Hidrobiologie; Presa Universitară Clujeană: Cluj, Romania, 2018. [Google Scholar]
- Map View. Available online: https://earth.google.com/web/search/Lakes+of+Ocna+Sibiului (accessed on 3 April 2023).
- Papapetrou, M.; Kumpavat, K. 10—Environmental Aspects and Economics of Salinity Gradient Power (SGP) Processes. In Sustainable Energy from Salinity Gradients; Cipollina, A., Micale, M., Eds.; Woodhead Publishing Series in Energy: Sawston, UK, 2016; pp. 315–335. [Google Scholar] [CrossRef]
- Romanian Waters National Administration. Hydrographic Basin Management Plan 2023—Annex 6.1.2.E. The Updated National Management Plan 2021 Related to the Portion of the International River Basin of the Danube River that is Included in the Territory of Romania. Available online: https://rowater.ro/despre-noi/descrierea-activitatii/managementul-european-integrat-resurse-de-apa/planurile-de-management-ale-bazinelor-hidrografice/planuri-de-management-nationale/ (accessed on 11 May 2023).
- Map View. Available online: https://earth.google.com/web/@44.63230635,28.61319476,128.55605331a,336879.05068047d,35y,0h,0t,0r (accessed on 11 May 2023).



| Energy Type | Source | Technology | Energy Product | Application |
|---|---|---|---|---|
| Bioenergy | waste and biomass | Combustion | Heat (cooking, space heating) Process heat, steam, electricity | Widely applied |
| Gasification/power production | Electricity, heat (CHP). | Demonstration phase | ||
| Gasification/fuel production | Hydrocarbons, methanol, H2 | Development phase | ||
| Hydrolysis and fermentation | Ethanol | Commercially applied; under development | ||
| Pyrolysis/production of liquid fuels | Bio-oils | Pilot phase; some technical barriers | ||
| Pyrolysis/production of solid fuels | Charcoal | Widely applied | ||
| Extraction | Biodiesel | Applied, expensive | ||
| Digestion | Biogas | Commercially applied | ||
| Solar | sun | Photovoltaic solar energy conversion | Electricity | Widely applied; rather expensive; |
| Solar thermal electricity | Heat, steam, electricity | Demonstrated; | ||
| Low-temperature solar energy use | Heat (water and space heating, cooking, drying) and cold | Commercially applied and demonstrated | ||
| Passive solar energy use | Heat, cold, light, ventilation | Demonstrations and applications; | ||
| Artificial photosynthesis | H2 or hydrogen rich fuels | Fundamental and applied research | ||
| Wind | wind | Water pumping and battery charging | Movement, power | Small wind machines, widely applied |
| Onshore wind turbines | Electricity | Widely applied commercially | ||
| Offshore wind turbines | Electricity | Development and demonstration phase | ||
| Hydropower | water | Hydro plants and dams | Power, electricity | Commercially applied |
| Geothermal | earth | Geothermal and head pumps | Heat, steam, electricity | Commercially applied |
| Marine | waves, tides, salinity, currents | Tidal energy | Electricity | Applied, expensive |
| Wave energy | Electricity | Research, development, and demonstration phase | ||
| Current energy | Electricity | Research and development phase | ||
| Ocean thermal energy conversion | Heat, electricity | Research, development, and demonstration phase | ||
| Salinity gradient/osmotic energy | Electricity | Theoretical option | ||
| Marine biomass production | Fuels | Research and development phase |
| Lake Name | County | Area (km2) | Depth (m) | Conc. (g/L) | Year | Water Class Type | Cation Group | Origin |
|---|---|---|---|---|---|---|---|---|
| Căldărușani | Ilfov | 2.24 | 4.0 | 0.52 | 1956 | bicarbonatic | Na | fluviatile liman |
| Balta Maia | Ialomița | 0.2 | - | 0.70 | 1954 | bicarbonatic | Ca | fluviatile liman |
| Fundata | Ialomița | 5 | - | 12.64 | 1956 | sulfatic | Na | fluviatile liman |
| Reviga | Ialomița | - | - | 5.73 | 1956 | bicarbonatic | Na | fluviatile liman |
| Schiauca | Ialomița | 1.0 | - | 5.73 | 1956 | chloridic | Na | fluviatile liman |
| Amara-Slobozia | Ialomița | 1.32 | 3.0 | 20.06 | 1956 | chloridic | Na | fluviatile liman |
| Iezer-Ialomița | Ialomița | 1.42 | - | 2.07 | 1956 | chloridic | Na | fluviatile liman |
| Strachina | Ialomița | 5.64 | - | 9.15 | 1957 | chloridic | Na | fluviatile liman |
| Balta Amara | Buzău | 6.98 | - | 7.93 | 1957 | Na | fluviatile liman | |
| Balta Albă | Buzău | 10.68 | - | 1.07 | 1957 | bicarbonatic | Na | fluviatile liman |
| Balta Jirlău | Brăila | 10.68 | - | 3.11 | 1956 | bicarbonatic | Na | fluviatile liman |
| Cîineni (Sărat) | Brăila | 0.56 | - | 29.25 | 1957 | chloridic | Na | fluviatile liman |
| Batogu | Brăila | 1.31 * | - | 59.13 | 1957 | chloridic | Na | karsto-klastic |
| Chioibășești | Brăila | 1.18 * | - | 9.04 | sulfatic | Na | karsto-klastic | |
| Plașcu | Brăila | 1.88 | - | 8.58 | sulfatic | Na | karsto-klastic | |
| Tătaru | Brăila | 3.28 | - | 12.50 | sulfatic | Na | karsto-klastic | |
| Ianca | Brăila | 3.32 | - | 34.46 | chloridic | Na | karsto-klastic | |
| Plopul | Brăila | 1.80 | - | 18.89 | chloridic | Na | karsto-klastic | |
| Movila Miresii | Brăila | 1.80 | - | 137.97 | chloridic | Na | karsto-klastic | |
| Lutu Alb | Brăila | 3.1 3.45 * | - | 21.58 | chloridic | Na | karsto-klastic | |
| Lacul Sărat-Brăila | Brăila | 1.72 | - | 83.96 | 1956 | sulfatic | Na | karsto-klastic |
| Balta Filipești-Târg | Prahova | 0.02 * | - | 0.72 | 1955 | bicarbonatic | Ca | floodplain lake |
| Balta Tălăbasca | Galați | 1.44 | - | 0.82 | 1953 | bicarbonatic | Ca | floodplain lake |
| Balta Mălina | Galați | 0.8 * | - | 0.72 | 1953 | bicarbonatic | Ca | floodplain lake |
| Balta Cătușa | Galați | 0.42 * | - | 0.61 | 1953 | bicarbonatic | Ca | floodplain lake |
| Viișoara | Botoșani | 0.55 * | - | 17.60 | 1956 | chloridic | Na | pond |
| Balta Suhaia | Teleorman | 31.20 | - | 0.92 | bicarbonatic | Na | floodplain lake | |
| Babadag | Tulcea | 22.52 | - | 1.76 | 1957 | chloridic | Na | fluviatile-maritime liman |
| Razelm (at Sarinasuf) | Tulcea | 393.30 | 3.0 | 1.03 | 1956 | chloridic | Na | sea lagoon |
| Razelm (at Jurilovca) | Tulcea | 2.52 | 1957 | chloridic | Na | |||
| Duingi (Histria) | Tulcea | 3.76 * | - | 36.99 | 1954 | chloridic | Na | sea lagoon |
| Tăbăcăriei | Constanța | 1.10 | - | 0.80 | 1957 | bicarbonatic | Na | sea lagoon |
| Siutghiol | Constanța | 21.05 | 18.0 | 0.75 | 1957 | bicarbonatic | Na | sea lagoon |
| Tașaul | Constanța | 18.30 | 5.6 | 1.85 | 1957 | chloridic | Na | fluviatile-maritime liman |
| Gargalîc (Corbu) | Constanța | 3.10 | - | 2.97 | 1957 | chloridic | Na | fluviatile-maritime liman |
| Techirghiol | Constanța | 10.68 | 9.0 | 95.52 | 1953 | chloridic | Na | fluviatile-maritime liman |
| Mangalia | Constanța | 2.6 | - | 1.29 | 1949 | chloridic | Na | fluviatile-maritime liman |
| Lacu Sărat Ocnele Mari | Vâlcea | 0.02 * | - | 262.13 | 1954 | chloridic | Na | karsto-saline |
| Stelei-Țintea | Prahova | 0.005 * | - | 79.11 | 1955 | chloridic | Na | karsto-saline |
| Sărat-Telega | Prahova | 0.004 * for all | - | 117.73 | 1955 | chloridic | Na | karsto-saline |
| Ursu-Sovata | Mureș | 0.038 | 18.9 | 256.70 | 1953 | chloridic | Na | karsto-saline |
| Aluniș-Sovata | Mureș | 0.003 | 7.4 | 77.76 | 1954 | chloridic | Na | karsto-saline |
| Șerpilor-Sovata | Mureș | - | - | 232.65 | 1954 | chloridic | Na | karsto-saline |
| Roșu-Sovata | Mureș | 0.001 | 2.5 | 233.82 | 1954 | chloridic | Na | karsto-saline |
| Ocnița-Ocna Sibiului | Sibiu | - | - | 230.19 | 1953 | chloridic | Na | karsto-saline |
| Roman-Turda | Cluj | - | 7 | 77.78 | 1951 | chloridic | Na | karsto-saline |
| Geaca | Cluj | 0.55 Cătina 1.63 Țaga | 2.89 Cătina 3.00 Țaga | 0.72 Geaca | 1953 | bicarbonatic | Ca | pond |
| Zaul-de-Cîmpie | Mureș | 1.17 | 1.95 | 1.78 | 1955 | bicarbonatic | Ca | pond |
| Iazul Paharnicul | Iași | Presently dried | - | 0.91 | 1959 | bicarbonatic | Ca | pond |
| Știucilor | Cluj | 0.80 | 12.0 | 1.02 | 1958 | bicarbonatic | Ca | pond |
| Sărăturile I (Murighiol) | Tulcea | 0.2 | 2.60 | 36.11 | 1960 | chloridic | Na | mixed ** |
| Sărăturile II (Murighiol) | Tulcea | - | 28.41 | 1960 | chloridic | Na | mixed ** | |
| Sărat-Greci | Tulcea | Presently dried | - | 3.03 | 1960 | chloridic | Na | fluviatile liman |
| Tătlăgeac | Constanța | 0.84 | - | 0.79 | 1960 | chloridic | Mg | fluviatile-maritime liman |
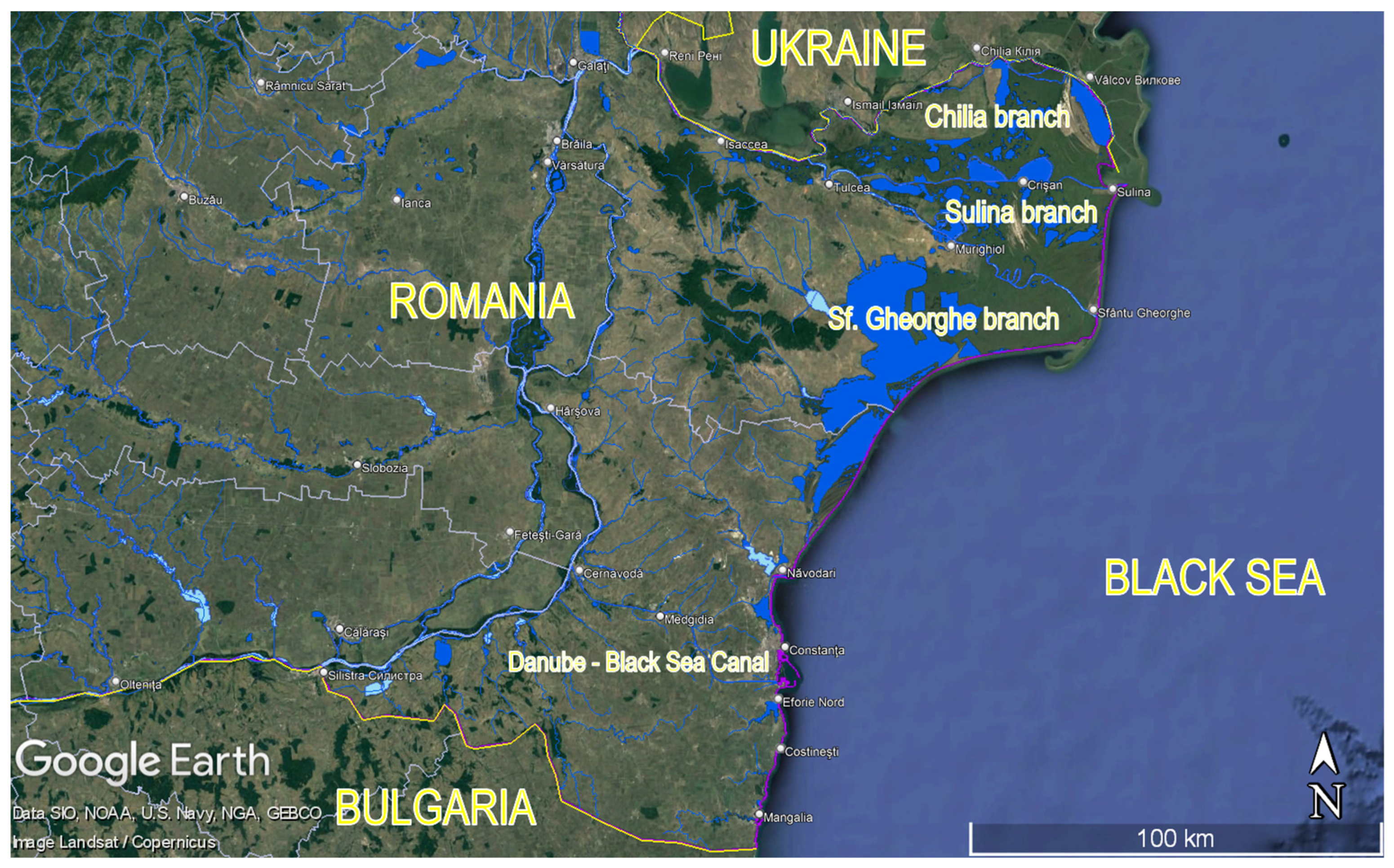
| River Pathway | Average Flow (m3·s−1) | TP (MW) | AP (MW) |
|---|---|---|---|
| Danube—upstream branching into its Delta | 5900 | 3100 | 100 |
| Chilia Branch | 3000 | 1600 | 50 |
| Sf. Gheorghe Branch | 1800 | 950 | 30 |
| Sulina Branch | 1200 | 650 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, V.-M.; Ivanov, A.-A.; Vîjdea, A.-M.; Alexe, V.-E.; Dincă, G.; Filiuță, A.-E.; Cetean, V.-M. Assessment of Current Blue Energy Technologies and Their Potential Applications on Romanian Water Resources. Water 2023, 15, 2063. https://doi.org/10.3390/w15112063
Radu V-M, Ivanov A-A, Vîjdea A-M, Alexe V-E, Dincă G, Filiuță A-E, Cetean V-M. Assessment of Current Blue Energy Technologies and Their Potential Applications on Romanian Water Resources. Water. 2023; 15(11):2063. https://doi.org/10.3390/w15112063
Chicago/Turabian StyleRadu, Violeta-Monica, Alexandru-Anton Ivanov, Anca-Marina Vîjdea, Veronica-Elena Alexe, George Dincă, Andra-Elena Filiuță, and Valentina-Maria Cetean. 2023. "Assessment of Current Blue Energy Technologies and Their Potential Applications on Romanian Water Resources" Water 15, no. 11: 2063. https://doi.org/10.3390/w15112063
APA StyleRadu, V.-M., Ivanov, A.-A., Vîjdea, A.-M., Alexe, V.-E., Dincă, G., Filiuță, A.-E., & Cetean, V.-M. (2023). Assessment of Current Blue Energy Technologies and Their Potential Applications on Romanian Water Resources. Water, 15(11), 2063. https://doi.org/10.3390/w15112063







