Appraisal of the Temporospatial Migration and Potential Ecotoxicity of Phthalic Acid Esters in Municipal Effluents, Rivers and Dam—A Catchment-Wide Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Target PAE Selection
2.2. Chemicals
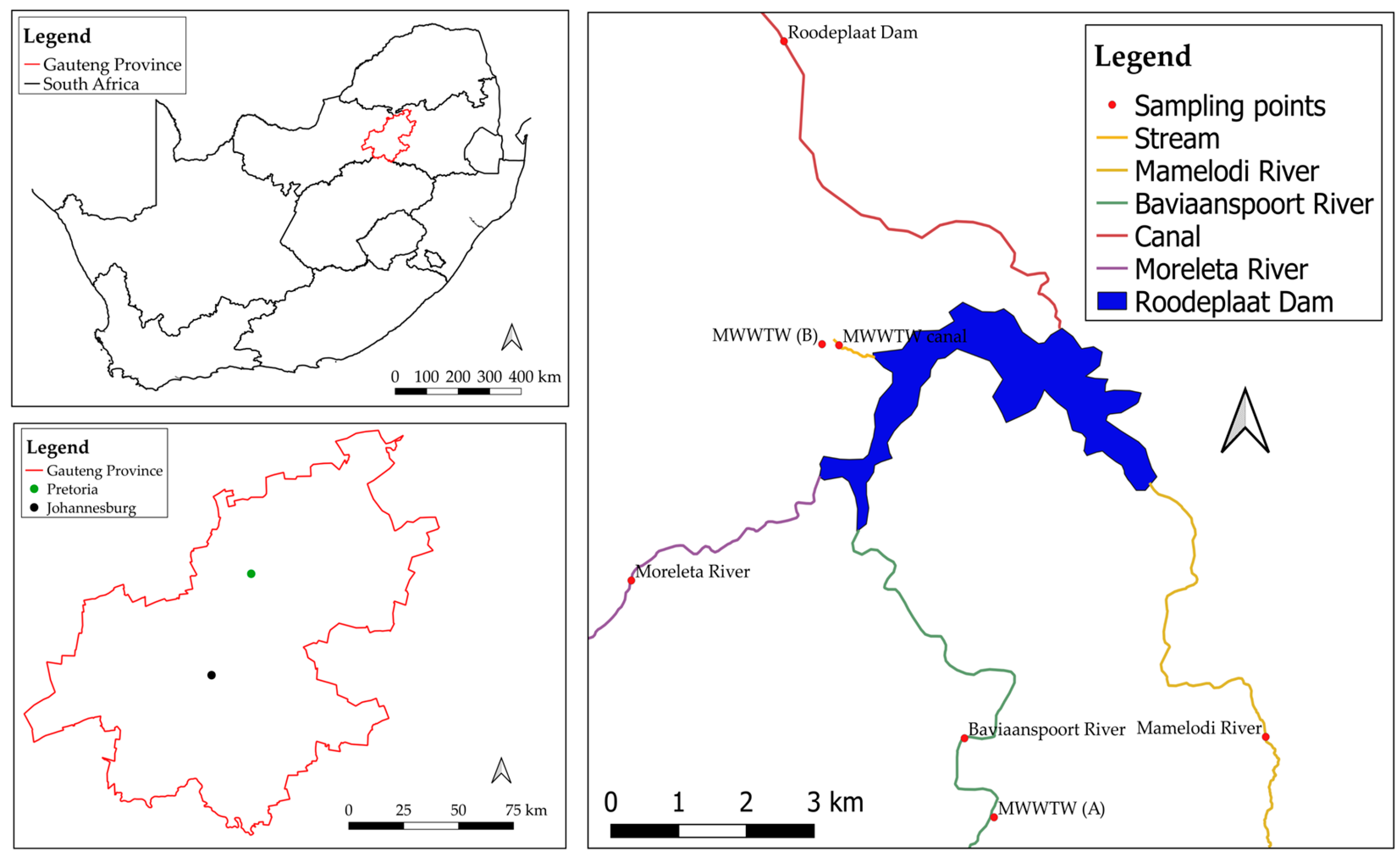
2.3. Study Area and Sampling
2.4. Liquid–Liquid Extraction Process and Extracts Clean-Up
2.5. GC–FID Analysis
2.6. Quantification, Detection Limit and Recoveries
2.7. Data Analysis, Quality Control and Quality Assurance
2.8. Potential Ecotoxicological Risk Assessment
3. Results and Discussion
3.1. Concentrations of PAEs in Studied MWWTW Effluents, Rivers and the Dam Catchment
3.1.1. Concentrations of PAEs in Studied MWWTW Effluents
3.1.2. Concentrations of PAEs in Water Samples within the Studied Rivers
3.1.3. Concentrations of PAEs within the Roodeplaat Dam Catchment
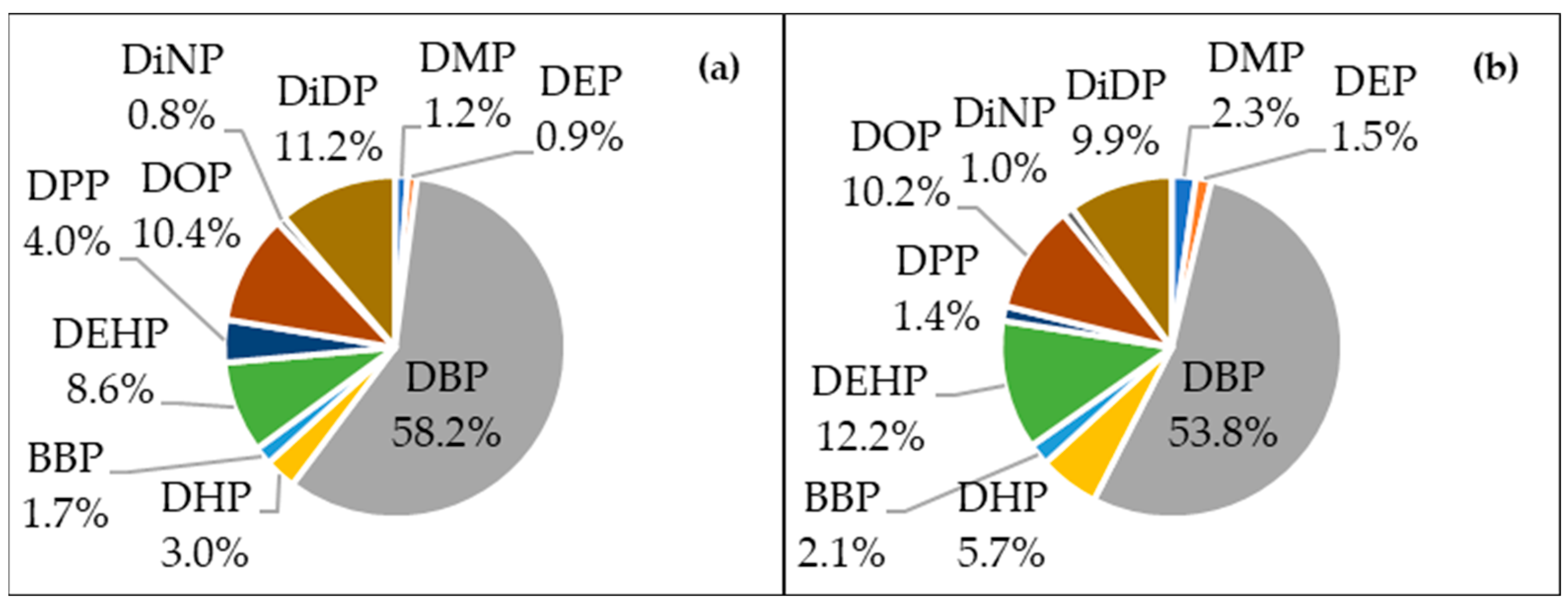
3.2. Temporospatial Distributions of PAEs in MWWTWs, Rivers and the Dam Catchment
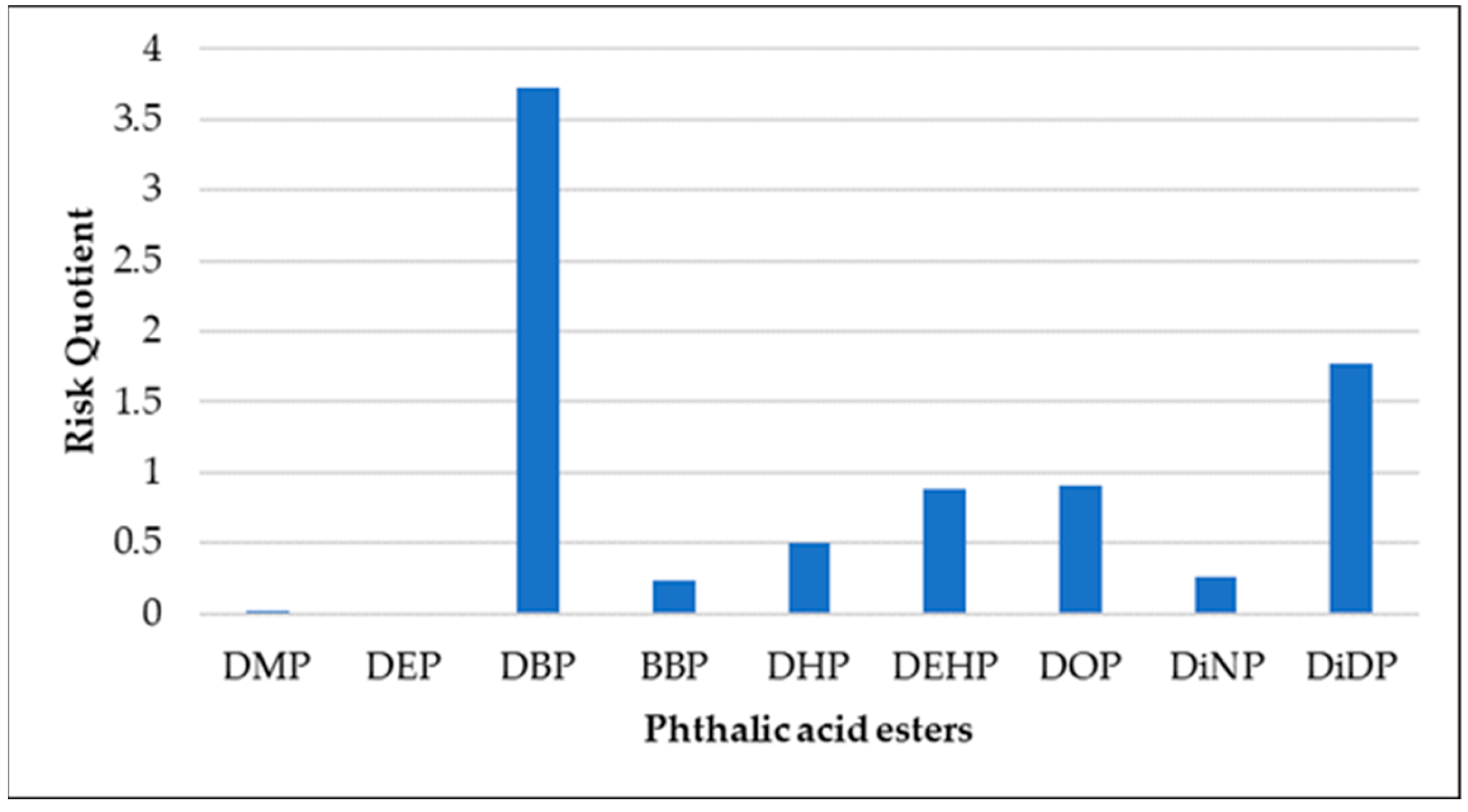
3.3. Ecotoxicological Risk Assessment
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farré, M.; Kantiani, L.; Petrovic, M.; Pérez, S.; Barceló, D. Achievements and future trends in the analysis of emerging organic contaminants in environmental samples by mass spectrometry and bioanalytical techniques. J. Chromatogr. A 2012, 1259, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Baloyi, N.D.; Tekere, M.; Maphangwa, K.W.; Masindi, V. Insights into the prevalence and impacts of phthalate esters in aquatic ecosystems. Front. Environ. Sci. 2021, 9, 684190. [Google Scholar] [CrossRef]
- Nantaba, F.; Ulrich-Palm, W.; Wasswa, J.; Bouwman, H.; Kylin, H.; Kümmerer, K. Temporal dynamics and ecotoxicological risk assessment of personal care products, phthalate ester plasticizers, and organophosphorus flame retardants in water from Lake Victoria. Chemosphere 2021, 262, 127716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.; Choi, M.J.; Park, J.; Nam, T.; Cho, J. Anthropogenic occurrence of phthalate esters in beach seawater in the Southeast Coast Region, South Korea. Water J. 2020, 12, 122. [Google Scholar] [CrossRef]
- Chiellini, F.; Ferri, M.; Morelli, A.; Dipaola, L.; Latini, G. Perspective on alternatives to phthalate plasticized poly (vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013, 38, 1067–1088. [Google Scholar] [CrossRef]
- Malarvannan, G.; Onghena, M.; Verstraete, S.; Van Puffelen, E.; Jacobs, A.; Vanhorebeek, I.; Verbruggen, S.; Joosten, K.; Van den Berghe, G.; Jorens, P.; et al. Phthalate and alternative plasticizers in indwelling medical devices in pediatric intensive care units. J. Hazard. Mater. 2019, 363, 64–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef]
- Ren, L.; Lin, Z.; Liu, H.; Hu, H. Bacteria-mediated phthalic acid esters degradation and related molecular mechanisms. Appl. Microbiol. Biotechnol. 2018, 102, 1085–1096. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Ventrice, P.; Ventrice, D.; Russo, E.; Sarro, G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ. Toxicol. Phar. 2013, 36, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, J.E.; Choe, W.; Kim, T.; Lee, J.Y.; Kho, Y.; Choi, K.; Zoh, K.D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, C.; Gye, M.C.; Lee, Y. Assessment of endocrine-disrupting activities of alternative chemicals for bis(2-ethylhexyl) phthalate. Environ. Res. 2019, 172, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; He, W.; Liu, W.X.; Yang, B.; He, Q.S.; Yang, C.; Xu, F.L. Impacts of anthropogenic activities on spatial variations of phthalate esters in water and suspended particulate matter from China’s lakes. Sci. Total Environ. 2020, 724, 138281. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Gao, X.; Wang, X.; Li, J.; Fan, B.; Zhao, S.; Liu, Z. Exposure and tiered ecological risk assessment of phthalate esters in the surface water of Poyang Lake, China. Chemosphere 2021, 262, 127864. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Wang, Q.; Guo, L.; Wang, C.; Wang, Z.; Zhang, S.; Wu, Q. Construction of hypercrosslinked polymers for high-performance solid phase microextraction of phthalate esters from water samples. J. Chromatogr. A 2021, 1641, 461972. [Google Scholar] [CrossRef]
- Chaudhary, G.; Jasrotia, A.; Raj, P.; Kaur, R.; Kumari, A.; Rajput, V.D.; Minkina, T.; Mandzhieva, S.; Kaur, R. Contamination of water and sediments of Harike Wetland with phthalate esters and associated risk assessment. Water 2023, 15, 1009. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; Van der Ploeg, M.; Van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–67. [Google Scholar] [CrossRef]
- Selvaraj, K.K.; Sundaramoorthy, G.; Ravichandran, P.K.; Girijan, G.K.; Sampath, S.; Ramaswamy, B.R. Phthalate esters in water and sediments of the Kaveri River, India: Environmental levels and ecotoxicological evaluations. Environ. Geochem. Health 2015, 37, 83–96. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Staples, C.A.; Adams, W.J.; Parkerton, T.F.; Gorsuch, J.W.; Biggingers, G.R.; Reiner, K.H. Environmental toxicology review—Aquatic toxicity of eighteen phthalate esters. Environ. Toxicol. Chem. 1997, 16, 875–891. [Google Scholar] [CrossRef]
- Das, M.T.; Kumar, S.S.; Ghosh, P.; Sha, G.; Malyan, S.K.; Bajar, S.; Thakur, I.S.; Singh, L. Remediation strategies for mitigation of phthalate pollution: Challenges and future perspectives. J. Hazard. Mater. 2021, 409, 124496. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Qin, N.; Kong, X.; Liu, W.; He, Q.; Ouyang, H.; Yang, C.; Jiang, Y.; Wang, Q.; Yang, B.; et al. Spatio-temporal distributions and the ecological and health risks of phthalate esters (PAEs) in the surface water of a large, shallow Chinese lake. Sci. Total Environ. 2013, 461–462, 672–680. [Google Scholar] [CrossRef]
- Salaudeen, T.; Okoh, O.; Agunbiade, F.; Okoh, A. Fate and impact of phthalates in activated sludge treated municipal wastewater on the water bodies in the Eastern Cape, South Africa. Chemosphere 2018, 203, 336–344. [Google Scholar] [CrossRef]
- Henkel, C.; Hüffer, T.; Hofmann, T. The leaching of phthalates from PVC can be determined with an infinite sink approach. MethodsX 2019, 6, 2729–2734. [Google Scholar] [CrossRef]
- Chi, J.; Gao, J. Effects of Potamogeton Crispus L.-bacteria interactions on the removal of phthalate acid esters from surface water. Chemosphere 2015, 119, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, X.; Zhang, X.; Li, X.; Li, D.; Dang, Y. C8-modified magnetic graphene oxide based solid-phase extraction coupled with dispersive liquid-liquid microextraction for detection of trace phthalate acid esters in water samples. Ecotoxicol. Environ. Saf. 2019, 170, 789–795. [Google Scholar] [CrossRef]
- Gao, L.; Tang, Y.; Wang, C.; Yao, L.; Zhang, J.; Gao, R.; Tang, X.; Chong, T.; Zhang, H. Highly efficient amphiphilic magnetic nanocomposites based on a simple sol-gel modification for adsorption of phthalate esters. J. Colloid Interf. Sci. 2019, 552, 142–152. [Google Scholar] [CrossRef]
- Aarab, N.; Lemaire-Gony, S.; Unruh, E.; Hansen, P.D.; Larsen, B.K.; Andersen, O.K.; Narbonne, J.F. Preliminary study of responses in mussel (Mytilus Edilus) exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether. Aquat. Toxicol. 2006, 78, S86–S92. [Google Scholar] [CrossRef]
- Arambourou, H.; Planelló, R.; Llorente, L.; Fuertes, I.; Barata, C.; Delorme, N.; Noury, P.; Herrero, O.; Villeneuve, A.; Bonnineau, C. Chironomus Riparius exposure to field-collected contaminated sediments: From subcellular effect to whole-organism response. Sci. Total Environ. 2019, 671, 874–882. [Google Scholar] [CrossRef]
- Gao, D.W.; Li, Z.; Wang, H.; Liang, H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.; Wollenberger, L.; Santos, E.; Paull, G.; Van Look, K.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, J.; Li, B.; Geng, J.; Ma, L.; Qi, H.; Zhang, A.; Zhao, Z. The spatiotemporal distribution and potential risk assessment of 19 phthalate acid esters in wastewater treatment plants in China. Environ. Sci. Pollut. Res. 2021, 28, 6780–6791. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Elkhatib, R.; Al-Rajoudi, T.; Al-Qudaihi, G. Assessing the concentration of phthalate esters (PAEs) and bisphenol A (BPA) and the genotoxic potential of treated wastewater (final effluent) in Saudi Arabia. Sci. Total Environ. 2017, 578, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, T.; Zhou, Z.; Yu, N.; He, Z.; Li, B.; Shi, Y.; Ma, D. Occurrence and fate of phthalate esters in wastewater treatment plants in Qingdao, China. Hum. Ecol. Risk Assess. 2019, 25, 1547–1563. [Google Scholar] [CrossRef]
- Olujimi, O.O.; Fatoki, O.S.; Odendaal, J.P.; Daso, A.P. Chemical monitoring and temporal variation in levels of endocrine disrupting chemicals (priority phenols and phthalate esters) from selected wastewater treatment plant and freshwater ecosystems in Republic of South Africa. Microchem. J. 2012, 101, 11–23. [Google Scholar] [CrossRef]
- He, M.J.; Lu, J.F.; Wang, J.; Wei, S.Q.; Hageman, K.J. Phthalate esters in biota, air and water in an agricultural area of Western China, with emphasis on bioaccumulation and human exposure. Sci. Total Environ. 2020, 698, 134264. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Bornman, M.; Ravandhalala, L.; Chimuka, L.; Genthe, B.; Adeniyi, A. Phthalate ester plasticizers in freshwater ecosystems of Venda, South Africa and potential health effects. Water SA 2010, 36, 117–126. [Google Scholar] [CrossRef]
- Sibali, L.L.; Okonkwo, J.O.; McCrindle, R.I. Determination of selected phthalate esters compounds in water and sediments by capillary gas chromatography and flame ionization detector. J. Environ. Sci. Health A 2013, 48, 1365–1377. [Google Scholar] [CrossRef]
- Paluselli, A.; Aminot, Y.; Galgani, F.; Net, S.; Sempéré, R. Occurrence of phthalate acid esters (PAEs) in the Northwestern Mediterranean Sea and the Rhone River. Prog. Oceanogr. 2018, 163, 221–231. [Google Scholar] [CrossRef]
- Okpara, K.E.; Phoungthong, K.; Agbozu, I.; Edwin-Isotu, E.; Techato, K. Phthalate esters in tap water, Southern Thailand: Daily exposure and cumulative health risk in infants, lactating mothers, pregnant and nonpregnant women. Int. J. Environ. Res. Public Health 2022, 19, 2187. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, P.; Qiu, C.; Li, J.; Hu, S.; Sun, L.; Bai, Y.; Gao, F.; Li, C.; Liu, N.; et al. Occurrence, migration and health risk of phthalates in tap water, barrelled water and bottled water in Tianjin, China. J. Hazard. Mater. 2021, 408, 124891. [Google Scholar] [CrossRef] [PubMed]
- Abdolahnejad, A.; Gheisari, L.; Karimi, M.; Norastehfar, N.; Ebrahimpour, K.; Mohammadi, A.; Ghanbari, R.; Ebrahimi, A.; Jafari, N. Monitoring and health risk assessment of phthalate esters in household’s drinking water of Isfahan, Iran. Int. J. Environ. Sci. Technol. 2019, 16, 7409–7416. [Google Scholar] [CrossRef]
- Dada, E.O.; Ikeh, R.K. Phthalates and metal concentrations in drinking water in Lagos, Nigeria. J. Health Pollut. 2018, 8, 180603. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Morueco, N.; Gonzalez-Alonso, S.; Valcarcel, C. Phthalate occurrence in rivers and tap water from central Spain. Sci. Total Environ. 2014, 500–501, 139–146. [Google Scholar] [CrossRef]
- Sohn, J.; Kim, S.; Koschorreck, J.; Kho, Y.; Choi, K. Alteration of sex hormone levels and steroidogenic pathway by several low molecular weight phthalates and their metabolites in male zebrafish (Danio rerio) and/or human adrenal cell (H295R) line. J. Hazard. Mater. 2016, 320, 45–54. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Choi, K.; Kim, K.T. Comparative analysis of endocrine disrupting effects of major phthalates in employed two cell lines (MVLN and H295R) and embryonic zebrafish assay. Environ. Res. 2019, 172, 319–325. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A. Effect of phthalates on development, reproduction, fat metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2019, 654, 969–977. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Noma, A. Solid phase extraction method for selective determination of phthalate esters in the aquatic environment. Water Air Soil Pollut. 2002, 140, 85–98. [Google Scholar] [CrossRef]
- Baloyi, N.D. Prevalence of Endocrine Disrupting Phthalate Esters in Selected Foods and Food Wrappers from Some Supermarkets Around Pretoria, South Africa. Master’s Thesis, University of South Africa, Pretoria, South Africa, 2012. [Google Scholar]
- Clara, M.; Windhofer, G.; Hartl, W.; Braun, K.; Simon, M.; Gans, O.; Scheffknecht, C.; Chovanec, A. Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 2010, 78, 1078–1084. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Bo, T.; Li, H.; Crittenden, J.C. Occurrence and risk assessment of selected phthalates in drinking water from waterworks in China. Environ. Sci. Pollut. Res. 2015, 22, 10690–10698. [Google Scholar] [CrossRef]
- Hadjmohammadi, M.R.; Ranbari, E. Utilization of homogeneous liquid–liquid extraction followed by HPLC-UV as a sensitive method for the extraction and determination of phthalate esters in environmental water samples. Int. J. Environ. Anal. Chem. 2011, 2, 1312–1324. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Afonso, M.d.M.; Palenzuela-López, J.A.; Rodríguez-Delgado, M.Á. Reduced graphene oxide-coated magnetic-nanoparticles as sorbent for the determination of phthalates in environmental samples by micro-dispersive solid-phase extraction followed by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2018, 1565, 36–47. [Google Scholar] [CrossRef]
- Weizhen, Z.; Xiaowei, Z.; Peng, G.; Ning, W.; Zini, L.; Jian, H.; Zheng, Z. Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environ. Sci. Pollut. Res. 2020, 27, 12550–12565. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfi, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeedi, R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in Tehran, Iran. Environ. Res. 2019, 173, 469–479. [Google Scholar] [CrossRef]
- European Commission. Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances, Part II; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Adeniyi, A.A.; Okodeyi, O.O.; Yusuf, K.A. Flame ionization gas chromatographic determination of phthalate esters in water, surface sediments and fish species in Ogun River catchments, Ketu, Lagos, Nigeria. Environ. Monit. Assess. 2011, 172, 561–569. [Google Scholar] [CrossRef]
- Santhi, V.A.; Mustafa, A.M. Assessment of organochlorine pesticides and plasticisers in the Selangor River Basin and possible pollution sources. Environ. Monit. Assess. 2013, 185, 1541–1554. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Zhang, H.H.; Li, J.L.; Yang, G.P. Determination of phthalic acid esters in seawater and sediment by solid-phase microextraction and gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2017, 45, 348–356. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, J.B.; Gao, M.; Shi, G.Z.; Fu, X.J.; Cai, P.; Lv, Y.F.; Guo, Z.B.; Shan, C.Q.; Yang, Z.B.; et al. Occurrence and distribution of phthalate esters in freshwater aquaculture fishponds in Pearl River Delta, China. Environ. Pollut. 2019, 245, 883–888. [Google Scholar] [CrossRef]
- Chen, H.; Mao, W.; Shen, Y.; Feng, W.; Mao, G.; Zhao, T.; Yang, L.; Yang, L.; Meng, C.; Li, Y.; et al. Distribution, source, and environmental risk assessment of phthalate esters (PAEs) in water, suspended particulate matter, and sediment of a typical Yangtze River Delta City. China. Environ. Sci. Pollut. Res. 2019, 26, 24609–24619. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Water Quality Standards Handbook, 2nd ed.; USEPA: Washington, DC, USA, 1994. [Google Scholar]
- Koo, H.H.; Lee, B.M. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health Part A 2004, 67, 1901–1914. [Google Scholar] [CrossRef]
- Sung, H.H.; Kao, W.Y.; Su, Y.J. Effects and toxicity of phthalate esters to hemocytes of giant freshwater prawn, Macrobrachium rosenbergii. Aquat. Toxicol. 2003, 64, 25–37. [Google Scholar] [CrossRef]
- Xu, H.; Shao, X.; Zhang, Z.; Zou, Y.; Wu, X.; Yang, L. Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicol. Environ. Saf. 2013, 93, 39–44. [Google Scholar] [CrossRef]
- Golshan, M.; Hatef, A.; Socha, M.; Milla, S.; Butts, I.A.E.; Carnevali, O.; Rodina, M.; Sokołowska-Mikołajczyk, M.; Fontaine, P.; Linhart, O.; et al. Di-(2-ethylhexyl)-phthalate disrupts pituitary and testicular hormonal functions to reduce sperm quality in mature goldfish. Aquat. Toxicol. 2015, 163, 16–26. [Google Scholar] [CrossRef]



| Phthalates | Acronyms | Common Applications | Molecular Weight (g/mol) | Chemical Formula | CAS Number |
|---|---|---|---|---|---|
| Dimethyl phthalate | DMP | Cosmetics and insecticides | 194.2 | C16H22O4 | 131-11-3 |
| Diethyl phthalate | DEP | Cosmetics, pharmaceuticals and insecticides | 222.2 | C12H14O4 | 84-66-2 |
| Di-n-butyl phthalate | DBP | Cosmetics and pharmaceuticals | 278.4 | C16H22O4 | 84-74-2 |
| Benzyl butyl phthalate | BBP | Adhesives, sealants, food packaging and upholstery | 312.4 | C19H20O4 | 85-68-7 |
| Diphenyl phthalate | DPP | Automotive, construction and medical devices | 318.2 | C20H14O4 | 84-62-8 |
| Dihexyl phthalate | DHP | Cosmetic, plastic additives and rubber products | 334.5 | C20H30O4 | 84-75-3 |
| Bis(2-ethylhexyl) phthalate | DEHP | Medical devices, PVC, packaging products and plastic additives | 390.6 | C24H38O4 | 117-81-7 |
| Di-n-octyl phthalate | DOP | Cosmetics, medical devices and pesticides | 390.6 | C24H38O4 | 117-84-0 |
| Diisononyl phthalate | DiNP | Construction, electrical and automotive products | 418.6 | C26H42O4 | 28553-12-0 |
| Diisodecyl phthalate | DiDP | Construction, PVC and packaging products | 446.7 | C28H46O4 | 26761-40-0 |
| Sampling Point | Location | Description of the Sampling Point |
|---|---|---|
| MWWTW A | S 25°41.369′ E 028°21.704′ | Located about 7 km from the dam, with effluents being discharged into Baviaanspoort River, which connects to the Roodeplaat Dam downstream. |
| MWWTW B | S 25°37.394′ E 028°20.185′ | Located to the north-east of Pretoria and upstream of the Roodeplaat Dam, which is less than a kilometre away. Effluents are discharged into an adjacent water-holding catchment, which then flows into the dam downstream. |
| MWWTW canal | S 25°37.403′ E 028°20.335′ | This canal serves as a stream or channel for effluents from MWWTW B through the water holding catchment between MWWTW B and the Roodeplaat Dam, which is less than a kilometre away. |
| Mamelodi River | S 25°40.695′ E 028°24.100′ | Part of Edendalspruit, located north-west of Leeuwfontein and about 6 km south-east of the Roodeplaat Dam. The river is a tributary of various streams running from, and through, Mamelodi townships and recreational areas, including schools and shopping complexes. |
| Baviaanspoort River | S 25°40.705′ E 028°21.443′ | Part of the Pienaars River, which is a tributary of the Crocodile River, originating in the east of Pretoria and flowing northwards into the Roodeplaat Dam, which is located about 6 km downstream. |
| Moreleta River | S 25°39.379′ E 028°18.501′ | A short section (which runs for approximately 20 km between Haakdoornbult and the Klipvoor Dam) of the Pienaars River, which is a tributary of the Crocodile River. Tributaries of the Pienaars River include the Moreletaspruit, which originates in the east of Pretoria, within Tshwane Municipality, and flows northwards into the Roodeplaat Dam. |
| Roodeplaat Dam | S 25°34.849′ E 028°19.849′ | Located about 22 km north-east of Pretoria and north of Mamelodi townships. The dam was constructed in 1956 to provide a continuous water supply to the surrounding landowners. It was later developed into a key water source for the northern areas of the City of Tshwane, including the Montana, Wonderboom and Magaliesberg reservoirs, which are used as a direct water supply for Doornpoort and the surrounding areas. |
| PAEs | % Recovery | Response Factor | Slope (Calibration Curve) | IDL (ng/L) |
|---|---|---|---|---|
| DMP | 76.6 ± 1.94 | 0.456 ± 0.0073 | 0.741 | 32.6 |
| DEP | 51.6 ± 2.02 | 0.515 ± 0.0064 | 0.820 | 25.7 |
| DBP | 71.9 ± 3.03 | 0.625 ± 0.0049 | 0.976 | 16.8 |
| BBP | 63 ± 4.22 | 0.666 ± 0.0057 | 1.0 | 21.8 |
| DPP | 50.7 ± 0.84 | 1.83 ± 0.0048 | 0.890 | 17.7 |
| DHP | 56.5 ± 2.24 | 1.05 ± 0.0057 | 3.57 | 5.29 |
| DEHP | 63.4 ± 3.12 | 0.735 ± 0.0038 | 1.12 | 11.1 |
| DOP | 61.2 ± 3.25 | 0.731 ± 0.0025 | 1.09 | 7.4 |
| DiNP | 71.6 ± 4.98 | 0.821 ± 0.0045 | 1.12 | 13.3 |
| DiDP | 80.6 ± 3.29 | 0.874 ± 0.0054 | 0.733 | 24.3 |
| PAEs | (NOEC) (mg/L) | Number of Trophic Levels | Assessment Factors | PNEC (mg/L) |
|---|---|---|---|---|
| DMP | 1, 9.6 | 2 | 50 | 0.02 |
| DEP | 1.65, 3.65, 3.8, 10, 13 | 3 | 10 | 0.165 |
| DBP | 0.1, 0.21, 0.28, 0.42, 1.3 | 3 | 10 | 0.01 |
| BBP | 0.1, 0.20, 0.26, 0.35, 0.36, 0.82 | 3 | 10 | 0.01 |
| DHP | 0.03, 0.08, 0.11, 0.18, 0.20, 0.22 | 3 | 10 | 0.003 |
| DEHP | 0.052, 0.054, 0.077, 0.1, 0.16 | 3 | 10 | 0.0052 |
| DOP | 0.32, 3.2 | 2 | 50 | 0.006 |
| DiNP | 0.034, 0.06, 0.14, 0.16, 1.0, 1.8 | 3 | 10 | 0.003 |
| DiDP | 0.03, 0.07, 0.1, 0.14, 0.37,0.8 | 3 | 10 | 0.003 |
| PAEs | Detection Frequency (%) | Conc. (μg/L) | ||
|---|---|---|---|---|
| Minimum | Mean (STDev) | Maximum (MEC) | ||
| DMP | 89.7 | nd | 0.237 ± 0.149 | 0.470 |
| DEP | 84.1 | nd | 0.159 ± 0.142 | 1.89 |
| DBP | 100 | 0.472 | 7.45 ± 4.93 | 37.9 |
| BBP | 95.2 | nd | 1.41 ± 1.02 | 2.25 |
| DPP | 99.2 | nd | 0.255 ± 0.368 | 3.25 |
| DHP | 91.3 | nd | 0.594 ± 0.425 | 1.49 |
| DEHP | 98.4 | nd | 0.348 ± 0.507 | 4.64 |
| DOP | 100 | 0.02 | 1.38 ± 1.05 | 5.46 |
| DiNP | 92.1 | nd | 0.121 ± 0.129 | 0.80 |
| DiDP | 99.2 | nd | 1.40 ± 1.10 | 5.30 |
| Σ10PAEs | n/a | 0.492 | 13.6 ± 9.82 | 63.2 |
| PAEs | Mean (STDev) (μg/L) | ||
|---|---|---|---|
| MWWTW A | MWWTW B | MWWTW Canal | |
| DMP | 0.232 ± 0.138 | 0.216 ± 0.151 | 0.234 ± 0.162 |
| DEP | 0.139 ± 0.087 | 0.138 ± 0.085 | 0.15 ± 0.093 |
| DBP | 8.04 ± 3.64 | 6.72 ± 2.54 | 6.76 ± 3.02 |
| BBP | 0.263 ± 0.266 | 0.175 ± 0.091 | 0.186 ± 0.124 |
| DPP | 0.266 ± 0.208 | 0.332 ± 0.365 | 0.416 ± 0.575 |
| DHP | 0.554 ± 0.378 | 0.615 ± 0.508 | 0.629 ± 0.454 |
| DEHP | 1.21 ± 0.721 | 1.38 ± 0.959 | 1.54 ± 0.889 |
| DOP | 1.08 ± 0.675 | 1.32 ± 0.792 | 1.72 ± 1.04 |
| DiNP | 0.099 ± 0.054 | 0.079 ± 0.059 | 0.08 ± 0.072 |
| DiDP | 1.20 ± 0.973 | 1.68 ± 1.55 | 1.48 ± 0.783 |
| Σ10PAEs | 13.1 ± 7.13 | 12.7 ± 7.11 | 13.2 ± 7.17 |
| PAEs | Mean (STDev) (μg/L) | ||
|---|---|---|---|
| Mamelodi River | Baviaanspoort River | Moreleta River | |
| DMP | 0.224 ± 0.149 | 0.282 ± 0.143 | 0.238 ± 0.17 |
| DEP | 0.173 ± 0093 | 0.156 ± 0.095 | 0.157 ± 0.097 |
| DBP | 9.76 ± 9.75 | 7.08 ± 3.03 | 6.71 ± 3.84 |
| BBP | 0.391 ± 0.192 | 0.146 ± 0.062 | 0.387 ± 0.684 |
| DPP | 0.635 ± 0.983 | 0.205 ± 0.145 | 0.237 ± 0.144 |
| DHP | 0.584 ± 0.442 | 0.589 ± 0.345 | 0.596 ± 0.454 |
| DEHP | 1.6 ± 1.23 | 1.16 ± 0.731 | 1.19 ± 1.04 |
| DOP | 1.42 ± 1.08 | 1.51 ± 1.37 | 0.983 ± 0.742 |
| DiNP | 0.171 ± 0.102 | 0.186 ± 0.228 | 0.091 ± 0.054 |
| DiDP | 1.28 ± 0.944 | 1.29 ± 0.834 | 1.32 ± 0.937 |
| Σ10PAEs | 16.2 ± 14.9 | 12.6 ± 6.98 | 11.9 ± 8.16 |
| PAEs | Dry Season (μg/L) | Wet Season (μg/L) | ||||
|---|---|---|---|---|---|---|
| Minimum | Mean (STDev) | Maximum | Minimum | Mean (STDev) | Maximum | |
| DMP | nd | 0.146 ± 0.114 | 0.382 | 0.129 | 0.331 ± 0.067 | 0.403 |
| DEP | nd | 0.108 ± 0.097 | 0.29 | 0.049 | 0.211 ± 0.079 | 0.512 |
| DBP | 0.66 | 7.15 ± 6.16 | 27.4 | 1.27 | 7.76 ± 1.88 | 10.9 |
| BBP | nd | 0.368 ± 0.41 | 1.12 | 0.111 | 0.82 ± 0.231 | 1.15 |
| DPP | nd | 0.211 ± 0.207 | 0.79 | 0.099 | 0.298 ± 0.363 | 1.78 |
| DHP | 0.011 | 1.06 ± 1.0 | 3.05 | 0.359 | 1.76 ± 0.669 | 2.95 |
| DEHP | 0.041 | 0.496 ± 0.644 | 2.62 | 0.115 | 0.2 ± 0.085 | 0.41 |
| DOP | 0.051 | 1.28 ± 1.17 | 4.08 | 0.569 | 1.48 ± 0.603 | 2.76 |
| DiNP | nd | 0.096 ± 0.131 | 0.63 | 0.029 | 0.145 ± 0.076 | 0.332 |
| DiDP | 0.081 | 1.38 ± 1.27 | 4.74 | 0.662 | 1.42 ± 0.493 | 2.5 |
| Σ10PAEs | 0.844 | 12.3 ± 11.2 | 45.1 | 3.39 | 14.4 ± 4.55 | 23.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloyi, N.D.; Tekere, M.; Maphangwa, K.W.; Masindi, V. Appraisal of the Temporospatial Migration and Potential Ecotoxicity of Phthalic Acid Esters in Municipal Effluents, Rivers and Dam—A Catchment-Wide Assessment. Water 2023, 15, 2061. https://doi.org/10.3390/w15112061
Baloyi ND, Tekere M, Maphangwa KW, Masindi V. Appraisal of the Temporospatial Migration and Potential Ecotoxicity of Phthalic Acid Esters in Municipal Effluents, Rivers and Dam—A Catchment-Wide Assessment. Water. 2023; 15(11):2061. https://doi.org/10.3390/w15112061
Chicago/Turabian StyleBaloyi, Ntsako Dellas, Memory Tekere, Khumbudzo Walter Maphangwa, and Vhahangwele Masindi. 2023. "Appraisal of the Temporospatial Migration and Potential Ecotoxicity of Phthalic Acid Esters in Municipal Effluents, Rivers and Dam—A Catchment-Wide Assessment" Water 15, no. 11: 2061. https://doi.org/10.3390/w15112061
APA StyleBaloyi, N. D., Tekere, M., Maphangwa, K. W., & Masindi, V. (2023). Appraisal of the Temporospatial Migration and Potential Ecotoxicity of Phthalic Acid Esters in Municipal Effluents, Rivers and Dam—A Catchment-Wide Assessment. Water, 15(11), 2061. https://doi.org/10.3390/w15112061








