Influence of Natural Barriers on Small Rivers for Changes in Water Quality Parameters
Abstract
:1. Introduction
2. Materials and Methods
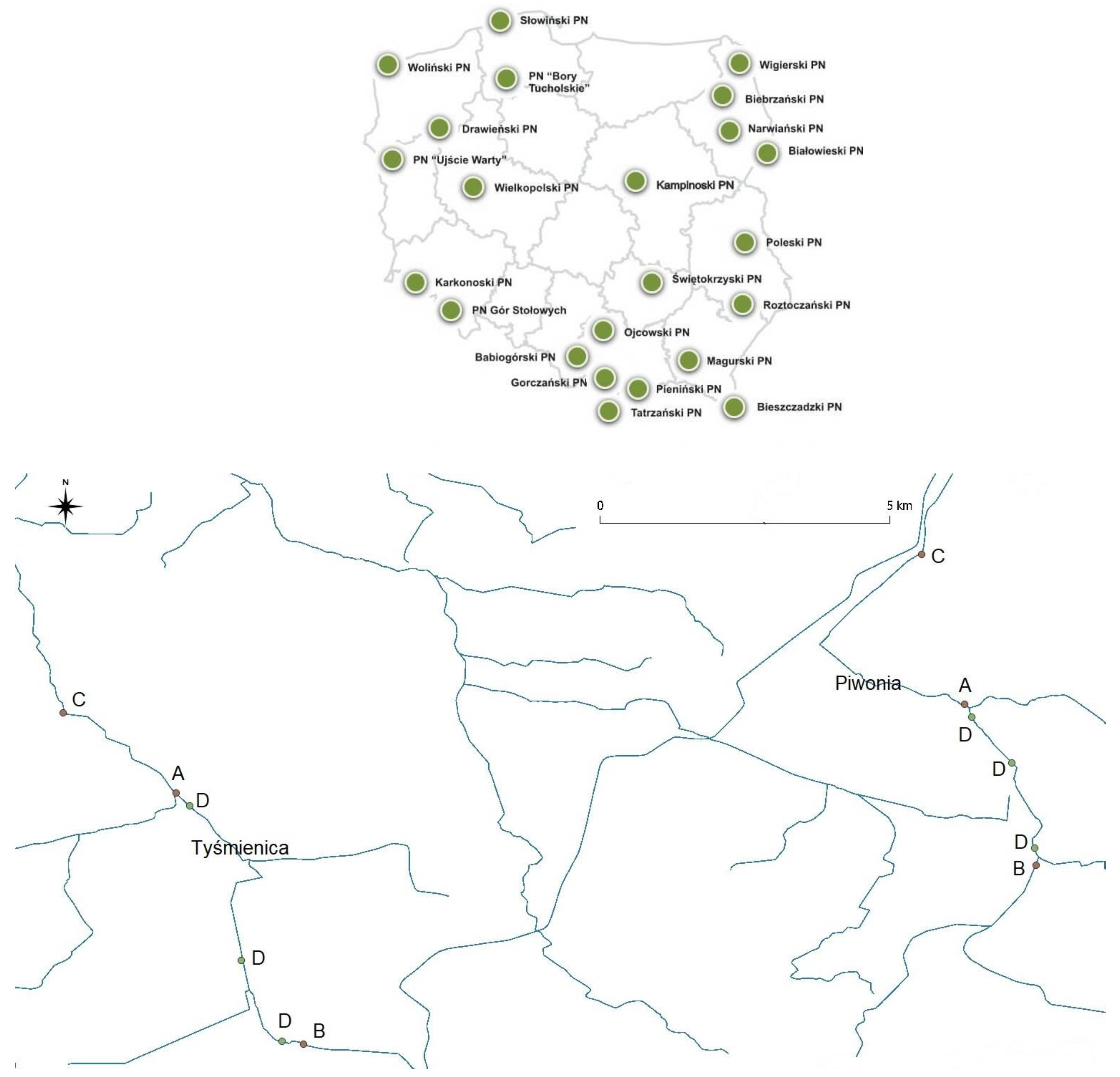
2.1. Study Area
2.2. Sample Collection
2.3. Data Analysis
3. Results and Discussion
3.1. BACI Analyses
3.2. Impact Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water pollutants: Sources and impact on the environment and human health. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Singapore, 2020; pp. 43–62. [Google Scholar]
- Oki, T.; Kanae, S. Global Hydrological Cycles and World Water Resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Szpak, D.; Boryczko, K.; Żywiec, J.; Piegoń, I.; Tchórzewska-Cieślak, B.; Rak, J.R. Risk assessment of water intakes in South-Eastern Poland in relation to the WHO requirements for water safety plans. Resources 2021, 10, 105. [Google Scholar] [CrossRef]
- Moss, B. Water pollution by agriculture. Phil. Trans. R. Soc. B 2008, 363, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Puchlik, M.; Piekutin, J.; Dyszewska, K. Analysis of the impact of climate change on surface water quality in North-Eastern Poland. Energies 2022, 15, 164. [Google Scholar] [CrossRef]
- Grzywna, A.; Bronowicka-Mielniczuk, U. Spatial and temporal variability of water quality in the Bystrzyca River Basin. Poland. Water 2020, 12, 190. [Google Scholar] [CrossRef]
- Wróbel, M.; Krysztofiak-Kaniewska, A. Long-term dynamics of and potential management strategies for the beaver (Castor fiber) population in Poland. Eur. Zool. J. 2020, 87, 116–121. [Google Scholar] [CrossRef]
- Połeć, K.; Grzywna, A.; Tarkowska-Kukuryk, M.; Bronowicka-Mielniczuk, U. Changes in the ecological status of rivers caused by the functioning of natural barriers. Water 2022, 14, 1522. [Google Scholar] [CrossRef]
- Florek, M.; Domaradzki, P.; Skałecki, P.; Ryszkowska-Siwko, M.; Ziomek, M.; Tajchman, K.; Gondek, M.; Pyz-Łukasik, R. Content and solubility of collagen and their relation to proximate composition and shear force of meat from different anatomical location in carcass of European Beaver (Castor fiber). Foods 2022, 11, 1288. [Google Scholar] [CrossRef]
- Nica, A.; Petrea, M.S.; Simionov, I.A.; Antache, A.; Cristea, V. Ecological impact of European Beaver, Castor fiber. Sci. Pap. Ser. D Anim. Sci. 2022, 65, 640–647. [Google Scholar]
- Brazier, R.E.; Puttock, A.; Graham, H.A.; Auster, R.E.; Davies, K.H.; Brown, C.M.L. Beaver: Nature’s ecosystem engineers. Wire’s Water 2021, 8, e1494. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, B.P.; Fritz, K.; Golden, H.E.; Newcomer-Johnson, T.A.; Rech, J.A.; Levy, J.; Fain, J.; McCarty, J.L.; Johnson, B.; Keng Vang, T.; et al. A global review of beaver dam impacts: Stream conservation implications across biomes. Glob. Ecol. Conserv. 2022, 37, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Mantel, S.K. Estimating the uncertainty in simulating the impacts of small farm dams on streamflow regimes in South Africa. Hydrol. Sci. J.–J. Sci. Hydrol. 2010, 55, 578–592. [Google Scholar] [CrossRef]
- Puttock, A.; Graham, H.A.; Cunliffe, A.M.; Elliott, M.; Brazier, R.E. Eurasian beaver activity increases water storage. attenuates flow and mitigates diffuse pollution from intensively-managed grasslands. Sci. Total Environ. 2017, 576, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Tetzlaff, D.; Gelbrecht, J.; Kleine, L.; Soulsby, C. Riparian wetland rehabilitation and beaver re-colonization impacts on hydrological processes and water quality in a lowland agricultural catchment. Sci. Total Environ. 2020, 699, 134302. [Google Scholar] [CrossRef]
- Shepherd, N.L.; Nairn, R.W. Induced mobilization of stored metal precipitates from beaver (Castor canadensis) created wetlands on a mine drainage impacted stream. Wetl. Ecol. Manag. 2022, 30, 127–137. [Google Scholar] [CrossRef]
- Kalvīte, Z.; Lībiete, Z.; Kļaviņš, I.; Bārdule, A.; Bičkovskis, K. The impact of beaver dam removal on the chemical properties of water in drainage ditches in peatland forests. Scand. J. For. Res. 2021, 36, 1–14. [Google Scholar] [CrossRef]
- Thompson, J.; Pelc, C.E.; Brogan, W.R., III; Jordan, T.E. The multiscale effects of stream restoration on water quality. Ecol. Eng. 2018, 124, 7–18. [Google Scholar] [CrossRef]
- Thompson, J.; Pelc, C.E.; Jordan, T.E. Water quality sampling methods may bias evaluations of watershed management practices. Sci. Total Environ. 2021, 765, 142739. [Google Scholar] [CrossRef]
- West Polesie Transboundary Biosphere Reserve. Belarus/Poland/Ukraine. UNESCO 2012. Available online: https://en.unesco.org/biosphere/eu-na/west-polesie (accessed on 2 April 2023).
- Rozporządzenie Rady Ministrów z dnia 10 kwietnia 1990 r. w sprawie utworzenia Poleskiego Parku Narodowego. Dz. Ustaw 1990, 27, 155.
- ISO 5667-6:2016-12; Water Quality—Sampling—Part 6: Guidance on Sampling of Rivers and Streams. PKN: Warszawa, Poland, 2016.
- ISO 15705:2002; Water Quality: Determination of the Chemical Oxygen Demand Index (ST-COD), Small-Scale Sealed-Tube Method. PKN: Warszawa, Poland, 2002.
- ISO 5815-1:2019; Water Quality—Determination of Biochemical Oxygen Demand after n Days (BODn), Dilution and Seeding Method with Allylthiourea Addition. ISO: Geneva, Switzerland, 2019.
- ISO 6878:2004; Water Quality-Determination of Phosphorus-Ammonium Molybdate Spectrometric Method. ISO: Geneva, Switzerland, 2004.
- Stewart-Oaten, A.; Bence, J.R. Temporal and spatial variation in environmental impact assessment. Ecol. Monogr. 2001, 71, 305–339. [Google Scholar] [CrossRef]
- Weber, N.; Bouwes, N.; Pollock, M.M.; Volk, C.; Wheaton, J.M.; Wathen, G. Alteration of stream temperature by natural and artificial beaver dams. PLoS ONE 2017, 12, e0176313. [Google Scholar] [CrossRef]
- Dou, M.; Li, G.; Li, C. Quantitative relations between chemical oxygen demand concentration and its influence factors in the sluice-controlled river reaches of Shaying River, China. Environ. Monit. Assess. 2015, 187, 4139. [Google Scholar] [CrossRef]
- Rozhkova-Timina, I.O.; Popkov, V.K.; Mitchell, P.J.; Kirpotin, S.N. Beavers as ecosystem engineers–a review of their positive and negative effects. IOP Conf. Ser. Earth Environ. Sci. 2018, 201, e012015. [Google Scholar] [CrossRef]
- Tomczyk, P.; Wiatkowski, M. Impact of a small hydropower plant on water quality dynamics in a diversion and natural river channel. Energie 2021, 50, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Prambudy, H.; Supriyatin, T.; Setiawan, F. The testing of chemical oxygen demand (COD) and biological oxygen demand (BOD) of river water in Cipager Cirebon. J. Phys. Conf. Ser. 2019, 1360, 012010. [Google Scholar] [CrossRef]
- Gibbs, D. The effect of beaver ponds on the nutrient composition in the water column and the sediments of Cart Creek. Ecosystems 2014, 3, 1–8. [Google Scholar]
- Čiuldienė, D.; Vigricas, E.; Belova, O.; Aleinikovas, M.; Armolaitis, K. The effect of beaver dams on organic carbon. nutrients and methyl mercury distribution in impounded waterbodies. Wildl. Biol. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Błȩdzki, L.A.; Bubier, J.L.; Moulton, L.A.; Kyker-Snowman, T.D. Downstream effects of beaver ponds on the water quality of New England first-and second-order streams. Ecohydrology 2011, 4, 698–707. [Google Scholar] [CrossRef]
- Strzelec, M.; Białek, K.; Spyra, A. Activity of beavers as an ecological factor that affects the benthos of small rivers-a case study in the Żylica River (Poland). Biologia 2018, 73, 577–588. [Google Scholar] [CrossRef]
- Ecke, F.; Levanoni, O.; Audet, J.; Carlson, P.; Eklöf, K.; Hartman, G.; McKie, B.; Ledesma, J.; Segersten, J.; Truchy, A.; et al. Meta-analysis of environmental effects of beaver in relation to artificial dams. Environ. Res. Lett. 2017, 12, 113002. [Google Scholar] [CrossRef]
- Bason, C.W.; Kroes, D.E.; Brinson, M.M. The effect of beaver ponds on water quality in rural coastal plain streams. Southeast. Nat. 2017, 16, 584–602. [Google Scholar] [CrossRef]
- Correll, D.L.; Jordan, T.E.; Weller, D.E. Beaver Pond biogeochemical effects in the Maryland Coastal Plain. Biogeochemistry 2000, 49, 217–239. [Google Scholar] [CrossRef]
- Lazar, J.G.; Addy, K.; Gold, A.J.; Groffman, P.M.; McKinney, R.A.; Kellogg, D.Q. Beaver ponds: Resurgent nitrogen sinks for rural watersheds in the Northeastern United States. J. Environ. Qual. 2015, 44, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.; Neilson, B.T.; Brahney, J. Source or sink? Quantifying beaver pond influence on non-point source pollutant transport in the Intermountain West. J. Environ. Manag. 2021, 285, 112127. [Google Scholar] [CrossRef] [PubMed]
- Gugała, M.; Sikorska, A.; Kapela, K.; Zarzecka, K. Seasonal changes in selected indicators of water quality of the Łydynia river. J. Ecol. Eng. 2016, 17, 149–153. [Google Scholar] [CrossRef]
- Pal, M.; Samal, N.R.; Roy, P.K.; Roy, M.B. Electrical conductivity of lake water as environmental monitoring—A case study of Rudra agar Lake. J. Environ. Sci. 2015, 9, 66–71. [Google Scholar]
- Margolis, B.E.; Castro, M.S.; Raesly, R.L. The impact of beaver impoundments on the water chemistry of two Appalachian streams. Can. J. Fish. Aquat. 2001, 58, 2271–2283. [Google Scholar] [CrossRef]
- Koschorreck, M.; Herzsprung, P.; Brands, E.; Kirch, P.M.; Dalbeck, L. Minor effect of beaver dams on stream dissolved organic carbon in the catchment of a German drinking water reservoir. Limnologica 2016, 61, 36–43. [Google Scholar] [CrossRef]
- Shaw, M.; Yazbek, L.; Singer, D.; Herndon, E. Seasonal mixing from intermittent flow drives concentration—Discharge behavior in a stream affected by coal mine drainage. Hydrol. Process. 2020, 34, 3669–3682. [Google Scholar] [CrossRef]
- Kangur, M.; Puusepp, L.; Buhvestova, O.; Haldna, M.; Kangur, K. Spatio-temporal variability of surface sediment phosphorus fractions and water phosphorus concentration in Lake Peipsi (Estonia/Russia). Est. J. Earth Sci. 2013, 62, 171–180. [Google Scholar] [CrossRef]
- Ignatius, A.R.; Rasmussen, T.C. Small reservoir effects on headwater water quality in the rural-urban fringe. Georgia Piedmont. USA. J. Hydrol. Reg. Stud. 2016, 8, 145–161. [Google Scholar] [CrossRef]
- Janiszewski, P.; Wozniak, A.; Janiszewska, L. Wpływ tam bobrowych na zmiany w przegrodzonym cieku wodnym. Zesz. Probl. Postępów Nauk. Rol. 2005, 506, 197–204. [Google Scholar]
- Barnes, D.M.; Mallik, A.U. Use of woody plants in construction of beaver dams in northern Ontario. Can. J. Zool. 1996, 74, 1781–1786. [Google Scholar] [CrossRef]
- St-Pierre, M.L.; Labbé, J.; Darveau, M.; Imbeau, L.; Mazerolle, M.J. Factors affecting abundance of beaver dams in forested landscapes. Wetlands 2017, 37, 941–949. [Google Scholar] [CrossRef]
- Ronnquist, A.L.; Westbrook, C.J. Beaver dams: How structure flow state, and landscape setting regulate water storage and release. Sci. Total Environ. 2021, 785, 147333. [Google Scholar] [CrossRef]
- Woo, M.K.; Waddington, J.M. Effects of beaver dams on subarctic wetland hydrology. Arctic 1990, 2, 223–230. [Google Scholar] [CrossRef]
- Camara, M.; Jamil, N.R.; Abdullah, A.F.B. Impact of land uses on water quality in Malaysia: A review. Ecol. Process. 2019, 8, 10. [Google Scholar] [CrossRef]
- Meneses, B.M.; Reis, R.; Vale, M.J.; Saraiva, R. Land use and land cover changes in Zêzere watershed (Portugal)—Water quality implications. Sci. Total Environ. 2015, 527, 439–447. [Google Scholar] [CrossRef]
- Mazur, A. Quantity and quality of surface and subsurface runoff from an eroded loess slope used for agricultural purposes. Water 2018, 10, 1132. [Google Scholar] [CrossRef]
- Mazur, A. Surface and subsurface water runoff and selected matter components from the forested loess slope. J. Ecol. Eng. 2018, 19, 259–266. [Google Scholar] [CrossRef]
- Mazur, A. Losses of chemical nutrients of plants and soil as a result of the outflow of water from the sodded loess slope. Przemysł Chem. 2018, 12, 2154–2157. [Google Scholar]
- Tsegaye, T.; Sheppard, D.; Islam, K.R.; Tadesse, W.; Atalay, A.; Marzen, L. Development of chemical index as a measure of in-stream water quality in response to land-use and land cover changes. Water Air Soil Pollut. 2006, 174, 161–179. [Google Scholar] [CrossRef]
- Grzywna, A.; Bronowicka-Mielniczuk, U.; Połeć, K. Spatio-temporal changes of water pollution, and its sources and consequences in the Bug River, Poland. Desalination Water Treat. 2021, 243, 18–36. [Google Scholar] [CrossRef]
- Koskinen, M.; Tahvanainen, T.; Sarkkola, S.; Menberu, M.W.; Laurén, A.; Sallantaus, T.; Nieminen, M. Restoration of nutrient-rich forestry-drained peatlands poses a risk for high exports of dissolved organic carbon, nitrogen, and phosphorus. Sci. Total Environ. 2017, 586, 858–869. [Google Scholar] [CrossRef]
- Laine, M.P.; Strömmer, R.; Arvola, L. Nitrogen release in pristine and drained peat profiles in response to water table fluctuations: A mesocosm experiment. Appl. Environ. Soil Sci. 2013, 2013, 694368. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Infrastruktury z dnia 25 czerwca 2021 r. w sprawie klasyfikacji stanu ekologicznego, potencjału ekologicznego i stanu chemicznego oraz sposobu klasyfikacji stanu jednolitych części wód powierzchniowych, a także środowiskowych norm jakości dla substancji priorytetowych. Dz. Ustaw 2021, 1475. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 2 April 2023).


| Season | Habitat | EC | BOD5 | COD | TN | N-NH4 | N-NO2 | N-NO3 | TP | P-PO4 | SO42− | Cl− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| spring | B | min | −17.7 | −0.37 | −0.4 | −0.63 * | −0.039 | 0.004 | −0.03 | −0.116 | −0.040 | −0.65 | −9.76 |
| max | −55.2 * | −0.56 * | −2.5 | 0.19 | 0.041 | −0.030 * | −0.26 | −0.163 * | −0.063 * | −6.65 | −0.76 | ||
| mean | −36.4 | −0.46 * | −1.0 | −0.22 | 0.001 | −0.013 * | −0.16 | −0.139 | −0.051 * | −3.65 | −5.26 | ||
| A | min | −11.5 | −0.57 * | −1.8 | −1.07 * | −0.051 | −0.003 | −0.17 | −0.103 | −0.100 * | −3.60 | −7.76 | |
| max | −94.5 * | −0.91 * | −12.4 * | −0.27 | 0.089 * | 0.026 * | −1.29 * | −0.122 | −0.063 * | 3.65 | −0.76 | ||
| mean | −53.0 * | −0.74 * | −9.6 | −0.65 * | 0.019 | −0.011 * | −0.56 | −0.109 | −0.081 * | 0.01 | −4.26 | ||
| summer | B | min | −15.5 | −0.11 | −3.8 | −0.61 * | 0.078 * | −0.006 | −0.17 * | −0.149 * | −0.043 | −14.0 * | −2.01 |
| max | −50.6 * | −0.21 | −3.1 | −1.24 | 0.065 * | −0.017 * | −1.13 * | −0.163 * | −0.062 * | −4.33 | 0.99 | ||
| mean | −33.1 | −0.15 | −3.4 | −0.92 * | 0.072 * | −0.011 * | −0.52 | −0.156 * | −0.053 * | −9.18 | −0.51 | ||
| A | min | −10.4 | −0.70 * | −7.2 | −0.53 * | 0.059 | −0.013 * | −0.33 | −0.155 * | −0.043 | −12.3 * | −2.01 | |
| max | −69.0 * | −0.85 * | −11.6 * | −1.61 * | 0.079 * | −0.016 * | −1.25 * | −0.221 * | −0.081 * | −6.33 | −1.01 | ||
| mean | −39.7 | −0.77 * | −9.4 | −1.07 * | 0.069 * | −0.014 * | −0.64 * | −0.188 * | −0.062 * | −9.33 | −1.51 | ||
| autumn | B | min | −18.9 | −0.48 * | −1.5 | −0.13 | 0.027 | −0.020 * | −0.54 | −0.132 | −0.032 | −1.32 | 0.51 |
| max | −24.2 | −0.18 | −12.2 * | 0.00 | 0.062 * | −0.022 * | 0.54 | −0.232 * | −0.077 * | −10.3 * | −2.19 | ||
| mean | −21.5 | −0.33 | −6.8 | −0.06 | 0.044 | −0.021 * | 0.00 | −0.182 * | −0.050 * | −5.82 | −0.84 | ||
| A | min | −63.7 * | −0.47 * | −4.0 | −0.05 | 0.025 | −0.022 * | −0.07 | −0.134 | −0.042 | −10.3 * | 1.39 | |
| max | −13.6 | −0.41 * | −13.9 * | −1.39 * | 0.061 * | −0.020 * | −1.04 * | −0.247 * | −0.083 * | −2.32 | −1.51 | ||
| mean | −38.6 | −0.44 * | −8.9 | −0.67 * | 0.043 | −0.021 * | −0.52 | −0.191 * | −0.063 * | −6.32 | −0.06 | ||
| winter | B | min | −4.4 | −0.20 | 2.0 | −0.24 | 0.011 | −0.001 | −0.24 | −0.027 | −0.002 | −4.67 | −0.03 |
| max | −25.1 | −0.34 | −2.8 | −0.51 * | 0.031 | 0.009 | −0.49 | −0.131 | −0.038 | −14.6 * | −12.0 * | ||
| mean | −14.7 | −0.27 | −0.4 | −0.37 | 0.016 | 0.004 | −0.36 | −0.079 | −0.021 | −9.67 | −6.03 | ||
| A | min | 7.2 | −0.52 * | −1.9 | −0.01 | 0.031 | −0.001 | −0.02 | −0.113 | −0.052 * | −3.67 | −0.03 | |
| max | −23.5 | −0.20 | −9.0 | −0.91 * | 0.092 * | 0.014 * | −0.89 * | −0.181 * | −0.012 | −8.67 | −11.0 * | ||
| mean | −8.2 | −0.36 | −5.5 | −0.50 | 0.061 * | 0.006 | −0.53 | −0.147 * | −0.032 | −6.17 | −5.53 |
| Season | Habitat | EC | BOD5 | COD | TN | N-NH4 | N-NO2 | N-NO3 | TP | P-PO4 | SO42− | Cl− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| spring | B | min | −96.1 * | −0.74 * | −17.2 * | −1.04 * | 0.167 * | 0.004 | −1.44 * | −0.006 | 0.017 | −10.2 | −10.49 * |
| max | 16.9 | 0.14 | −11.6 * | −0.07 | 0.097 * | −0.031 * | 0.30 | −0.100 | −0.050 * | −6.2 | −1.49 | ||
| mean | −35.2 | −0.20 | −13.0 * | −0.61 * | 0.127 * | −0.012 * | −0.55 | −0.052 | −0.015 | −8.03 | −6.32 | ||
| A | min | −96.8 * | −0.97 * | −23.9 * | −1.77 * | 0.147 * | 0.004 | −1.47 * | −0.030 | 0.024 | −9.2 | −10.49 * | |
| max | 10.2 | −0.12 | −10.0 * | −0.09 | 0.047 | −0.031 * | 0.14 | −0.158 * | −0.097 * | −6.2 | −0.49 | ||
| mean | −41.3 | −0.41 | −17.2 * | −1.00 * | 0.107 * | −0.012 * | −0.74 | −0.109 | −0.027 | −8.2 | −6.16 | ||
| summer | B | min | −27.9 | −2.24 * | −19.3 * | 0.29 | 0.197 * | 0.004 | 1.14 * | 0.059 | 0.068 * | −6.76 | −5.21 |
| max | 121.1 * | −0.71 | −9.3 | 1.30 * | 0.347 * | 0.036 * | 0.25 | 0.213 * | 0.014 | 11.24 * | 10.79 * | ||
| mean | 62.4 * | −1.33 * | −13.4 * | 0.89 * | 0.229 * | 0.024 * | 0.43 | 0.121 | 0.039 | −1.43 | 1.12 | ||
| A | min | 20.3 | −2.02 * | −18.5 * | 0.59 | 0.160 * | 0.007 | 1.16 * | −0.081 | 0.050 * | −8.76 | −4.21 | |
| max | 172.1 * | −1.49 * | −19.3 * | 1.54 * | 0.390 * | 0.078 * | −0.36 | 0.141 * | 0.001 | 18.24 * | 4.79 | ||
| mean | 82.5 * | −1.76 * | −18.9 * | 0.93 * | 0.210 * | 0.035 * | 0.32 | 0.053 | 0.026 | 0.07 | 0.12 | ||
| autumn | B | min | −81.5 * | −0.76 * | −12.3 * | 0.35 | 0.191 * | −0.009 | −0.28 | −0.071 | −0.029 | −16.9 * | −2.8 |
| max | 4.6 | 0.35 | −2.6 | −0.59 | −0.109 * | 0.041 * | 0.16 | −0.022 | −0.013 | −3.9 | 2.6 | ||
| mean | −28.6 | −0.23 | −6.1 | −0.093 | 0.013 * | 0.009 | −0.092 | −0.053 | −0.020 | −10.9 * | 0 | ||
| A | min | −59 * | −1.21 * | −12.3 * | 0.64 * | 0.211 * | −0.009 | −0.34 | −0.054 | −0.025 | −16.9 * | −2 | |
| max | −1.4 | 0.80 * | −0.4 | −0.41 | −0.109 * | 0.031 * | 0.16 | 0.005 | −0.009 | −3.9 | 4.4 | ||
| mean | −24.9 | −0.04 | −5.9 | −0.089 | 0.121 * | 0.005 | −0.155 | −0.034 | −0.016 | −10.9 * | 0.97 | ||
| winter | B | min | −38.8 | −1.0 * | −6.9 | −1.30 * | −0.180 * | −0.020 * | −0.60 | −0.045 | −0.011 | −24 * | 0 |
| max | 40.7 * | −0.1 | −4.2 | −0.21 * | 0.050 | 0.010 * | −0.02 | −0.212 * | −0.084 * | −10 * | −3.15 | ||
| mean | 6.7 | −0.63 * | −5.7 | −0.84 * | −0.110 * | −0.012 * | −0.38 | −0.125 | −0.040 | −18.7 * | −1.82 | ||
| A | min | −36.1 | −1.3 * | −9.7 | −1.42 * | −0.190 * | −0.020 * | −0.60 | −0.030 | −0.011 | −24 * | −3.15 | |
| max | 21.8 | −0.76 * | −4.5 | −0.57 * | 0.060 | 0.025 * | −0.16 | −0.220 * | −0.074 * | −11 * | −0.15 | ||
| mean | −6.1 | −1.03 * | −6.5 | −0.93 * | −0.108 * | 0.001 | −0.31 | −0.112 | −0.040 | −18.5 * | −1.65 |
| River | Habitats | EC | BOD5 | COD | TN | N-NH4 | N-NO2 | N-NO3 | TP | P-PO4 | SO42− | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyśmienica | B | −26.43 | −0.30 | −2.90 | −0.392 | 0.0333 | −0.0103 * | −0.1100 | −0.1390 | −0.0433 | −7.08 | −3.16 |
| A | −34.88 | −0.58 | −8.73 | −0.737 * | 0.0480 | −0.0100 * | −0.5525 | −0.1588 * | −0.0598 * | −5.45 | −2.84 | |
| Piwonia | B | −0.47 | −0.60 * | −9.56 | −0.163 | 0.0900 | 0.0023 | −0.1480 | −0.0148 | −0.0090 | −9.77 | −2.71 |
| A | 2.53 | −0.81 * | −12.16 * | −0.273 | 0.1033 * | 0.0073 | −0.2243 | −0.0707 | −0.0143 | −9.38 | −1.68 |
| Parameter | Habitats | Season | Land use | Habitats: Season | Habitats: Land Use | Season: Land Use | Habitats: Season: Land Use |
|---|---|---|---|---|---|---|---|
| EC | 0.197 | 0.0442 * | 4.59 × 10−7 *** | 0.399 | 0.394 | 0.377 | 0.599 |
| BOD5 | 0.139 | 4.23 × 10−4 ** | 0.0016 ** | 0.399 | 0.399 | 0.0229 * | 0.506 |
| COD | 0.196 | 0.272 | 0.0271 * | 0.398 | 0.399 | 0.428 | 0.599 |
| TN | 0.151 | 0.242 | 3.33 × 10−5 *** | 0.396 | 0.390 | 0.660 | 0.604 |
| N-NH4 | 0.174 | 4.09 × 10−6 *** | 1.36 × 10−5 *** | 0.399 | 0.389 | 0.0079 ** | 0.599 |
| N-NO2 | 0.191 | 0.0513 * | 5.11 × 10−5 *** | 0.388 | 0.386 | 0.738 | 0.546 |
| N-NO3 | 0.181 | 0.0611 * | 5.82 × 10−4 *** | 0.388 | 0.396 | 0.198 | 0.598 |
| TP | 0.193 | 1.36 × 10−5 *** | 9.40 × 10−4 *** | 0.396 | 0.397 | 0.194 | 0.449 |
| P-PO4 | 0.185 | 5.87 × 10−6 *** | 1.13 × 10−5 *** | 0.398 | 0.396 | 0.135 | 0.557 |
| SO42− | 0.138 | 3.66 × 10−6 *** | 0.462 | 0.372 | 0.392 | 0.486 | 0.600 |
| Cl− | 0.196 | 0.104 | 2.01 × 10−4 *** | 0.398 | 0.394 | 0.181 | 0.598 |
| Parameters | Meadows | Scrubs | Forest | TGES |
|---|---|---|---|---|
| EC | 423.3 a | 400.5 a | 305.25 b | 576 |
| BOD5 | 2.96 a | 3.09 a | 2.4 b | 4.1 |
| COD | 48.95 a | 49.70 a | 45.55 b | 79 |
| TN | 3.63 a | 3.84 a | 2.55 b | 4.5 |
| N-NH4 | 0.39 a | 0.22 b | 0.17 b | 0.68 |
| N-NO2 | 0.019 a | 0.020 a | 0.011 b | 0.03 |
| N-NO3 | 0.88 b | 1.47 a | 1.01 a | 2.5 |
| TP | 0.388 a | 0.229 a | 0.153 b | 0.40 |
| P-PO4 | 0.1063 a | 0.0893 a | 0.0725 b | 0.102 |
| SO42− | 27 | 27 | 28 | 64.8 |
| Cl− | 12.5 a | 9.5 a | 4.5 b | 29.4 |
| Parameters | Spring | Summer | Autumn | Winter | TGES |
|---|---|---|---|---|---|
| EC | 351.9 b | 359.6 ab | 400.25 a | 402.05 a | 576 |
| BOD5 | 2.89 b | 2.65 b | 3.28 a | 2.47 c | 4.1 |
| COD | 49.00 | 47.45 | 47.15 | 48.55 | 79 |
| TN | 3.38 | 3.34 | 3.31 | 3.34 | 4.5 |
| N-NH4 | 0.16 c | 0.39 a | 0.18 c | 0.27 b | 0.68 |
| N-NO2 | 0.016 ab | 0.023 a | 0.012 b | 0.012 b | 0.03 |
| N-NO3 | 1.20 a | 1.06 c | 1.02 c | 1.09 b | 2.5 |
| TP | 0.191 c | 0.334 a | 0.229 b | 0.195 bc | 0.40 |
| P-PO4 | 0.0695 b | 0.1045 a | 0.0845 ab | 0.0955 ab | 0.102 |
| SO42− | 26 b | 31 a | 27 ab | 25 b | 64.8 |
| Cl− | 9.0 | 7.4 | 7.9 | 10.9 | 29.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Połeć, K.; Grzywna, A. Influence of Natural Barriers on Small Rivers for Changes in Water Quality Parameters. Water 2023, 15, 2065. https://doi.org/10.3390/w15112065
Połeć K, Grzywna A. Influence of Natural Barriers on Small Rivers for Changes in Water Quality Parameters. Water. 2023; 15(11):2065. https://doi.org/10.3390/w15112065
Chicago/Turabian StylePołeć, Katarzyna, and Antoni Grzywna. 2023. "Influence of Natural Barriers on Small Rivers for Changes in Water Quality Parameters" Water 15, no. 11: 2065. https://doi.org/10.3390/w15112065
APA StylePołeć, K., & Grzywna, A. (2023). Influence of Natural Barriers on Small Rivers for Changes in Water Quality Parameters. Water, 15(11), 2065. https://doi.org/10.3390/w15112065






