Praseodymium(III) Removal from Aqueous Solutions Using Living and Non-Living Arthrospira platensis Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiments with Living A. platensis Biomass
2.2. Experiments with Non-Living A. platensis Biomass
2.3. Biomass Productivity and Biochemical Tests
2.4. Statistical Analysis
2.5. Data Analysis
3. Results and Discussion
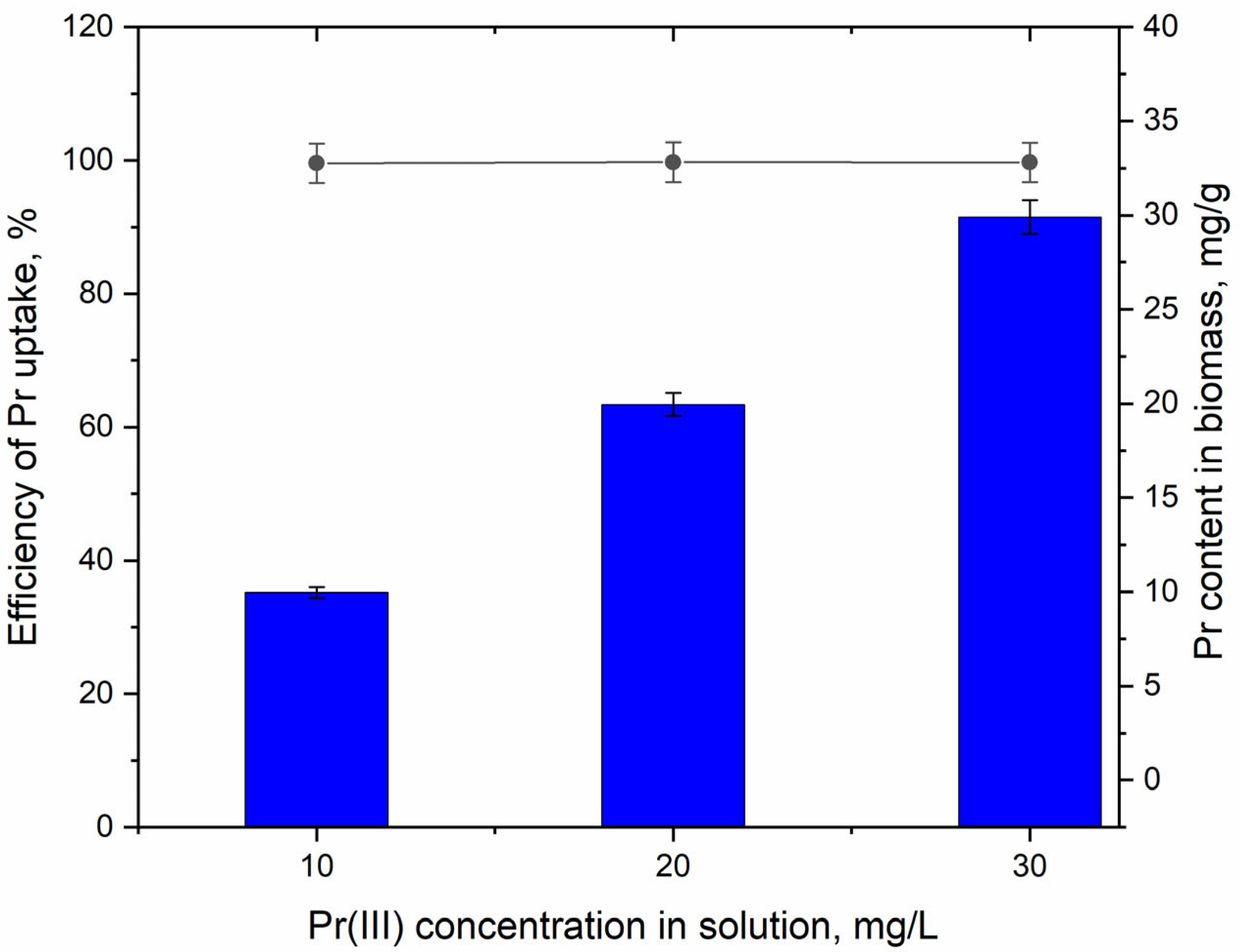
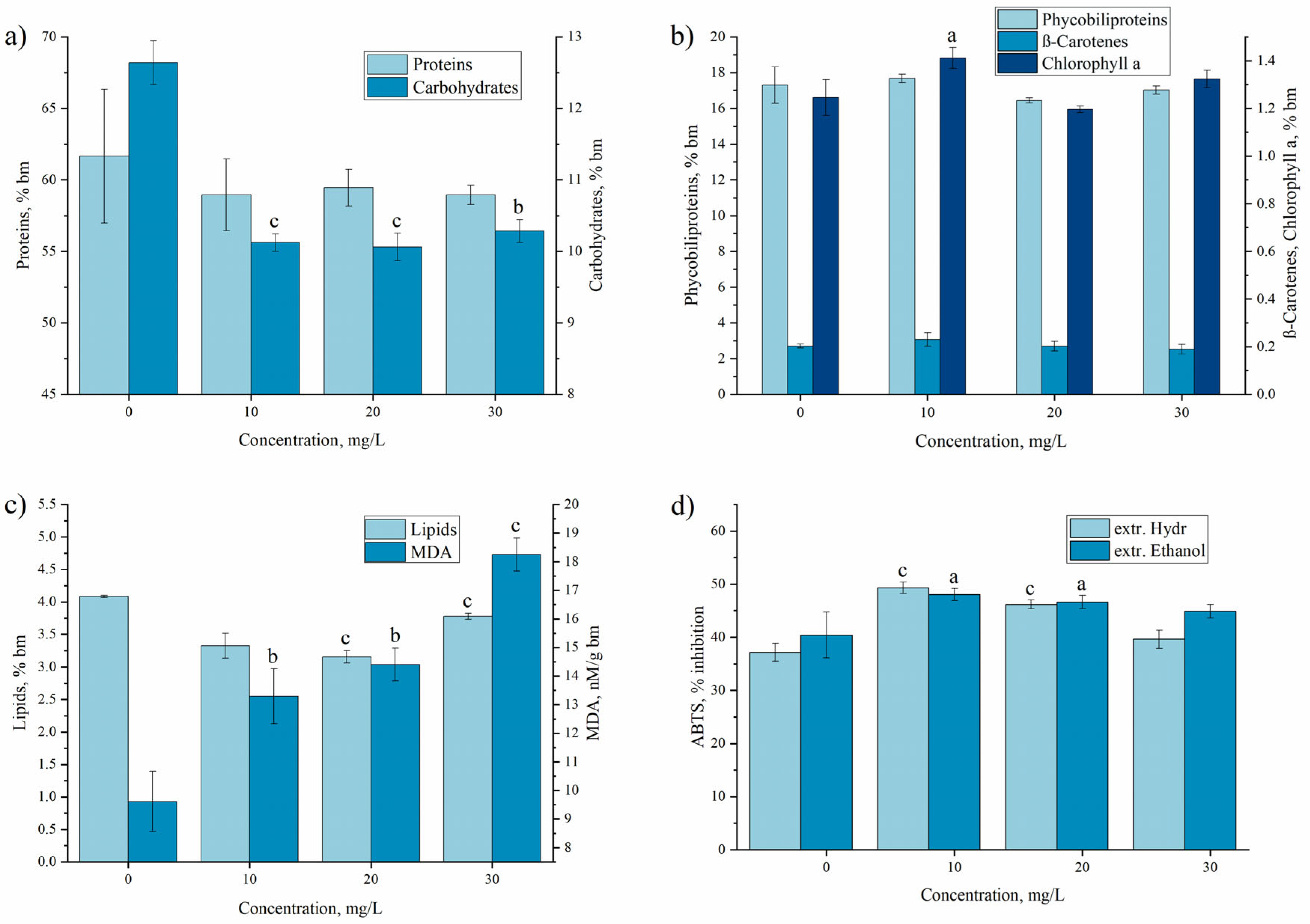
3.1. Praseodium Uptake by Living A. platensis and Its Effect on Biomass
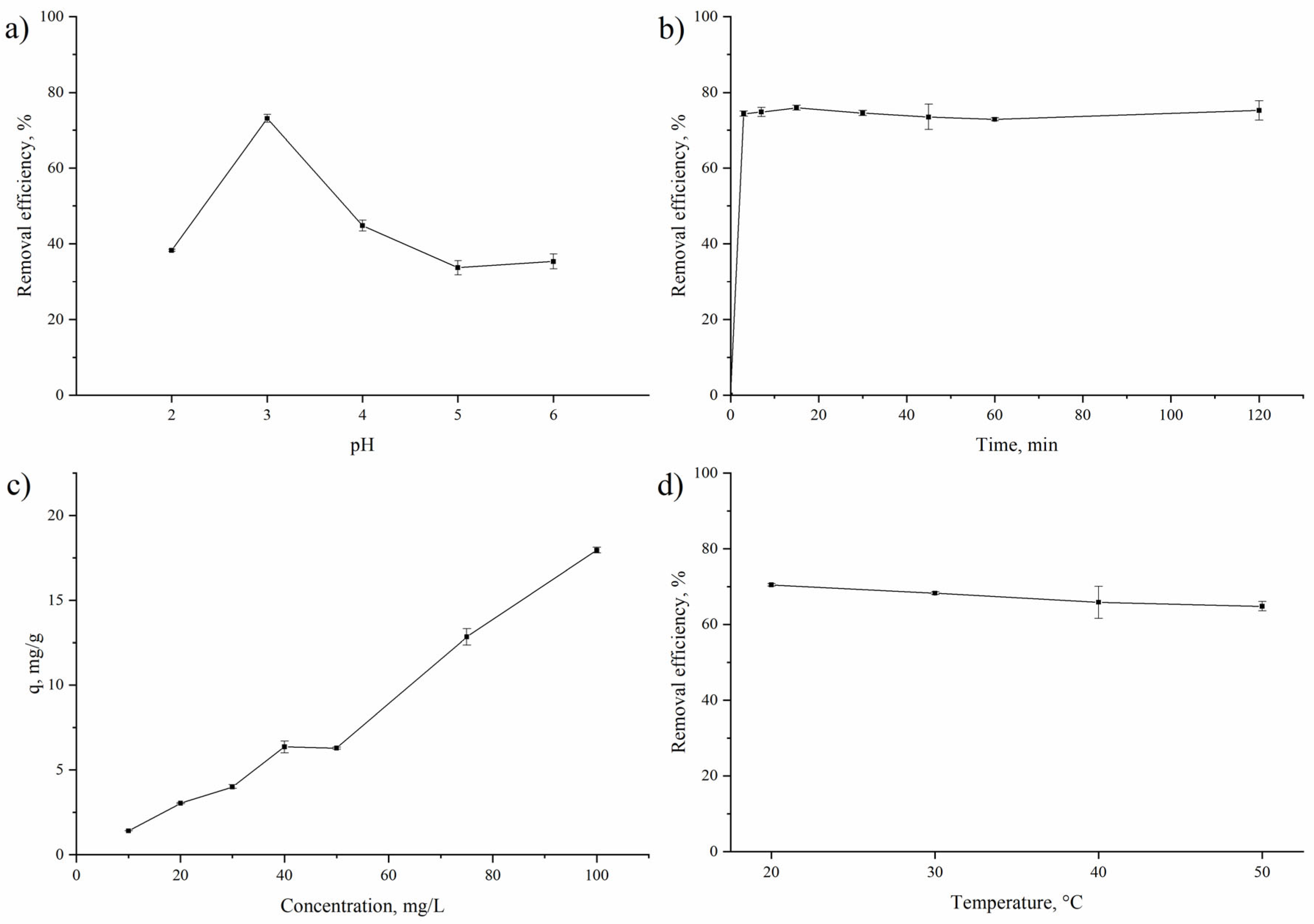
3.2. Praseodium Removal by Non-Living A. platensis Biomass under Different Experimental Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef]
- Elbashier, E.; Mussa, A.; Hafiz, M.A.; Hawari, A.H. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.Y. Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Negrea, A.; Gabor, A.; Davidescu, C.M.; Ciopec, M.; Negrea, P.; Duteanu, N.; Barbulescu, A. Rare Earth Elements Removal from Water Using Natural Polymers. Sci. Rep. 2018, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Hsu, H.S.; Liang, S.T.; Roldan, M.J.M.; Lee, J.S.; Ger, T.R.; Hsiao, C. Der An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 2022, 10, 1617. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Diep, P.; Mahadevan, R.; Yakunin, A.F. Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 2018, 6, 157. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations 2018, 5, 54. [Google Scholar] [CrossRef]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable application of biosorption and bioaccumulation of persistent pollutants in wastewater treatment: Current practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef]
- Grosjean, N.; Le Jean, M.; Berthelot, C.; Chalot, M.; Gross, E.M.; Blaudez, D. Accumulation and fractionation of rare earth elements are conserved traits in the Phytolacca genus. Sci. Rep. 2019, 9, 18458. [Google Scholar] [CrossRef]
- Čížková, M.; Mezricky, P.; Mezricky, D.; Rucki, M.; Zachleder, V.; Vítová, M. Bioaccumulation of Rare Earth Elements from Waste Luminophores in the Red Algae, Galdieria phlegrea. Waste Biomass Valorization 2021, 12, 3137–3146. [Google Scholar] [CrossRef]
- Castillo, J.; Maleke, M.; Unuofin, J.; Cebekhulu, S.; Gómez-Arias, A. Microbial recovery of rare earth elements. In Environmental Technologies to Treat Rare Earth Element Pollution: Principles and Engineering; IWA Publishing: London, UK, 2022; pp. 179–205. ISBN 9781789062236. [Google Scholar]
- Andrès, Y.; Le Cloirec, P.; Texier, A.C. Rare earth elements removal by microbial biosorption: A review. Environ. Technol. 2003, 24, 1367–1375. [Google Scholar] [CrossRef]
- Al-Amin, A.; Parvin, F.; Chakraborty, J.; Kim, Y.I. Cyanobacteria mediated heavy metal removal: A review on mechanism, biosynthesis, and removal capability. Environ. Technol. Rev. 2021, 10, 44–57. [Google Scholar] [CrossRef]
- Sarup, R.; Behl, K.; Joshi, M.; Nigam, S. Heavy metal removal by cyanobacteria. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Elsevier: Amsterdam, The Netherlands, 2021; pp. 441–466. ISBN 9780128229651. [Google Scholar]
- Ghorbani, E.; Nowruzi, B.; Nezhadali, M.; Hekmat, A. Metal removal capability of two cyanobacterial species in autotrophic and mixotrophic mode of nutrition. BMC Microbiol. 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.B.; Körsten, S.; Rösken, L.M.; Cappel, F.; Beresko, C.; Ankerhold, G.; Schönleber, A.; Geimer, S.; Ecker, D.; Wehner, S. Cyanobacterial promoted enrichment of rare earth elements europium, samarium and neodymium and intracellular europium particle formation. RSC Adv. 2019, 9, 32581–32593. [Google Scholar] [CrossRef] [PubMed]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Biosorption and Bioaccumulation Capacity of Arthospira platensis toward Europium Ions. Water 2022, 14, 2128. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Application of Cyanobacteria Arthospira platensis for Bioremediation of Erbium-Contaminated Wastewater. Materials 2022, 15, 6101. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Biosorption and Bioaccumulation Capacity of Arthrospira platensis toward Yttrium Ions. Metals 2022, 12, 1465. [Google Scholar] [CrossRef]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; Op den Camp, H.J.M. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ. Microbiol. 2014, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Zachleder, V.; Vítová, M. Rare earth elements and algae: Physiological effects, biorefi nery and recycling. In Algal Biorefineries: Volume 2: Products and Refinery Design; Springer International Publishing: Cham, Switzerland, 2015; pp. 339–363. ISBN 9783319202006. [Google Scholar]
- Valcheva-Traykova, M.; Saso, L.; Kostova, I. Involvement of Lanthanides in the Free Radicals Homeostasis. Curr. Top. Med. Chem. 2014, 14, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Safonov, A.; Tregubova, V.; Ilin, V.; Cepoi, L.; Chiriac, T.; Rudi, L.; Frontasyeva, M.V. Uptake of metals from single and multi-component systems by spirulina platensis biomass. Ecol. Chem. Eng. S 2016, 23, 401–412. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Grozdov, D.; Pavlov, S.; Djur, S. Accumulation of dysprosium, samarium, terbium, lanthanum, neodymium and ytterbium by Arthrospira platensis and their effects on biomass biochemical composition. J. Rare Earths 2021, 39, 1133–1143. [Google Scholar] [CrossRef]
- Goecke, F.; Vítová, M.; Lukavský, J.; Nedbalová, L.; Řezanka, T.; Zachleder, V. Effects of rare earth elements on growth rate, lipids, fatty acids and pigments in microalgae. Phycol. Res. 2017, 65, 226–234. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Gao, S.; Shan, C. Praseodymium enhanced the tolerance of maize seedlings subjected to cadmium stress by up-regulating the enzymes in the regeneration and biosynthetic pathways of ascorbate and glutathione. Plant Soil Environ. 2021, 67, 633–642. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Shan, C. Effect of praseodymium on the postharvest quality of Lilium longiflorum cut flowers. New Zeal. J. Crop. Hortic. Sci. 2022. [Google Scholar] [CrossRef]
- Ziekhrü, M.; Imchen, P.; Phucho, T.; Devi, M.I. Synthesis, characterization, antioxidant and antibacterial studies of praseodymium complex with glutathione. Curr. Sci. 2023, 124, 554–561. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Sathishkumar, M.; Balasubramanian, R. Interaction of rare earth elements with a brown marine alga in multi-component solutions. Desalination 2011, 265, 54–59. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H.A. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci. Rep. 2022, 12, 3256. [Google Scholar] [CrossRef]
- Kusaka, E.; Kamata, Y.; Fukunaka, Y.; Nakahiro, Y. Effect of hydrolysed metal cations on the liquid-liquid extraction of silica fines with cetyltrimethylammonium chloride. Colloids Surf. A Physicochem. Eng. Asp. 1998, 139, 155–162. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J. Entrapment of brown seaweeds (Turbinaria conoides and Sargassum wightii) in polysulfone matrices for the removal of praseodymium ions from aqueous solutions. J. Rare Earths 2015, 33, 1196–1203. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. Biosorption of lanthanide (praseodymium) using Ulva lactuca: Mechanistic study and application of two, three, four and five parameter isotherm models. J. Environ. Biotechnol. Res. 2015, 1, 10–17. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, J.; Shen, C.; Chen, Q. Adsorption and desorption of praseodymium (III) from aqueous solution using D72 resin. Chinese J. Chem. Eng. 2012, 20, 823–830. [Google Scholar] [CrossRef]
- El-Dessouky, S.I.; El-Sofany, E.A.; Daoud, J.A. Studies on the sorption of praseodymium (III), holmium (III) and cobalt (II) from nitrate medium using TVEX-PHOR resin. J. Hazard. Mater. 2007, 143, 17–23. [Google Scholar] [CrossRef]
- Boulaiche, W.; Belhamdi, B.; Hamdi, B.; Trari, M. Kinetic and equilibrium studies of biosorption of M(II) (M = Cu, Pb, Ni, Zn and Cd) onto seaweed Posidonia oceanica fibers. Appl. Water Sci. 2019, 9, 173. [Google Scholar] [CrossRef]
- Aguiar, A.B.S.; Costa, J.M.; Santos, G.E.; Sancinetti, G.P.; Rodriguez, R.P. Removal of Metals by Biomass Derived Adsorbent in Its Granular and Powdered Forms: Adsorption Capacity and Kinetics Analysis. Sustain. Chem. 2022, 3, 535–550. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Use of algal biorefinery waste and waste office paper in the development of xerogels: A low cost and eco-friendly biosorbent for the effective removal of congo red and Fe (II) from aqueous solutions. J. Environ. Manag. 2020, 262, 110380. [Google Scholar] [CrossRef] [PubMed]
- Varshini, C.J.S.; Das, D.; Das, N. Optimization of parameters for praseodymium(III) biosorption onto biowaste materials using response surface methodology: Equilibrium, kinetic and regeneration studies. Ecol. Eng. 2015, 81, 321–327. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Al-Zaban, M.I.; Albarakaty, F.M.; Abdelwahab, S.F.; Hassan, S.H.A.; Fawzy, M.A. Kinetic, Isotherm and Thermodynamic Aspects of Zn2+ Biosorption by Spirulina platensis: Optimization of Process Variables by Response Surface Methodology. Life 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Morales-Barrera, L.; Flores-Ortiz, C.M.; Cristiani-Urbina, E. Single and binary equilibrium studies for Ni2+ and Zn2+ biosorption onto lemna gibba from aqueous solutions. Processes 2020, 8, 1089. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Rodlovskaya, E.; Khiem, L.H. Yeast—As Bioremediator of Silver-Containing Synthetic Effluents. Bioengineering 2023, 10, 398. [Google Scholar] [CrossRef]
- Masry, B.A.; Abu Elgoud, E.M.; Rizk, S.E. Modeling and equilibrium studies on the recovery of praseodymium (III), dysprosium (III) and yttrium (III) using acidic cation exchange resin. BMC Chem. 2022, 16, 37. [Google Scholar] [CrossRef]
- Wang, S.; Hamza, M.F.; Vincent, T.; Faur, C.; Guibal, E. Praseodymium sorption on Laminaria digitata algal beads and foams. J. Colloid Interface Sci. 2017, 504, 780–789. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Shvetsova, M.; Frontasyeva, M. Zinc removal from model solution and wastewater by Arthrospira (Spirulina) Platensis biomass. Int. J. Phytoremediation 2018, 20, 901–908. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Gundorina, S.; Demčák, Š.; Frontasyeva, M.; Kamanina, I. Biosorption of nickel from model solutions and electroplating industrial effluenusing cyanobacterium arthrospira platensis. Desalin. Water Treat. 2018, 120, 158–165. [Google Scholar] [CrossRef]

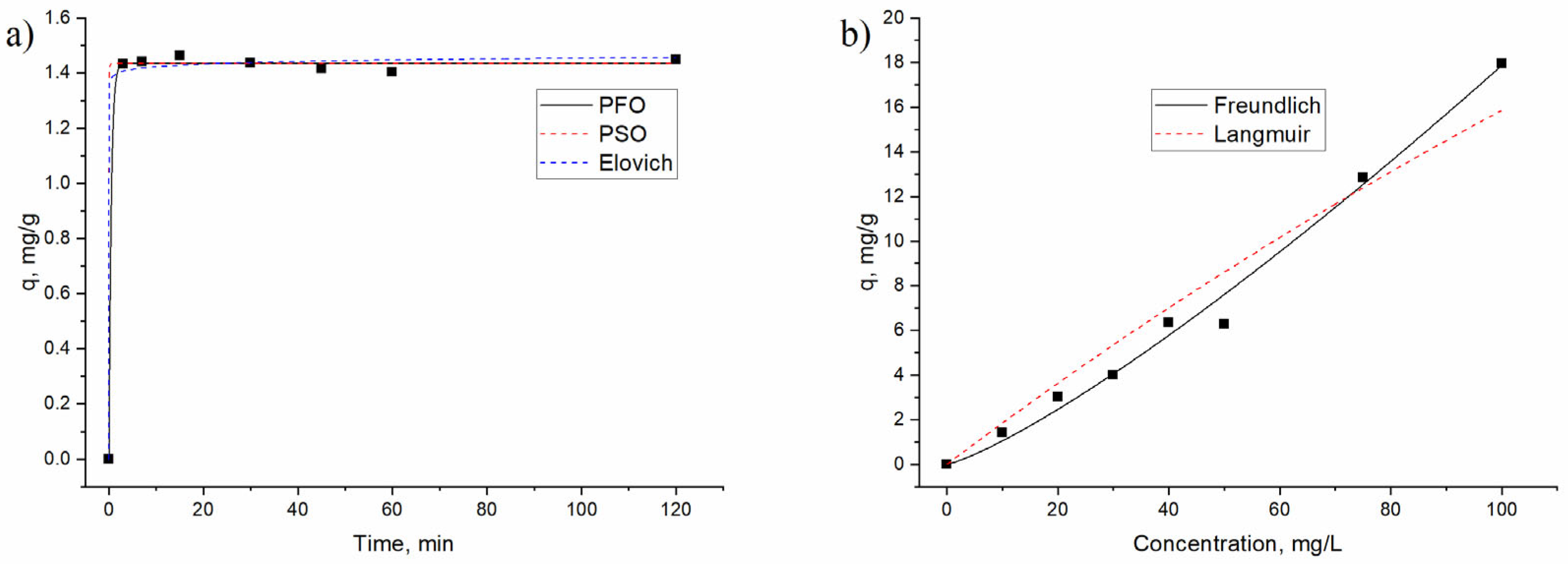
| Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | α | β | R2 |
| 1.43 | 2.13 | 0.999 | 1.43 | 26.5 | 0.998 | 3.96 × 10−43 | 75.2 | 0.997 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qm | b | R2 | Kf | n | R2 |
| 99.3 | 0.0019 | 0.952 | 0.062 | 1.2 | 0.99 |
| Temperature K | ΔG° kJ/mol | ΔH° kJ/mol | ΔS° J/mol |
|---|---|---|---|
| 293 | −15.0 | −7.0 | 27.4 |
| 303 | −15.3 | ||
| 313 | −15.6 | ||
| 323 | −15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Praseodymium(III) Removal from Aqueous Solutions Using Living and Non-Living Arthrospira platensis Biomass. Water 2023, 15, 2064. https://doi.org/10.3390/w15112064
Yushin N, Zinicovscaia I, Cepoi L, Chiriac T, Rudi L, Grozdov D. Praseodymium(III) Removal from Aqueous Solutions Using Living and Non-Living Arthrospira platensis Biomass. Water. 2023; 15(11):2064. https://doi.org/10.3390/w15112064
Chicago/Turabian StyleYushin, Nikita, Inga Zinicovscaia, Liliana Cepoi, Tatiana Chiriac, Ludmila Rudi, and Dmitrii Grozdov. 2023. "Praseodymium(III) Removal from Aqueous Solutions Using Living and Non-Living Arthrospira platensis Biomass" Water 15, no. 11: 2064. https://doi.org/10.3390/w15112064
APA StyleYushin, N., Zinicovscaia, I., Cepoi, L., Chiriac, T., Rudi, L., & Grozdov, D. (2023). Praseodymium(III) Removal from Aqueous Solutions Using Living and Non-Living Arthrospira platensis Biomass. Water, 15(11), 2064. https://doi.org/10.3390/w15112064












