Adsorption of Heavy Metals and Biocides from Building Runoff onto Granular Activated Carbon—The Influence of Different Fractions of Dissolved Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. DOM Fractionation via Centrifugation
2.2. Granular Activated Carbon Preparation
2.3. Adsorption of Fractionated DOM and Pollutants onto GAC
2.4. Pollutants Co-Presence during the Adsorption Process
2.5. Liquid Chromatography–Mass Spectrometry Analysis
2.6. Adsorption Modelling using Freundlich and Langmuir Equations
3. Results and Discussion
3.1. DOM and Granular Activated Carbon Prepared
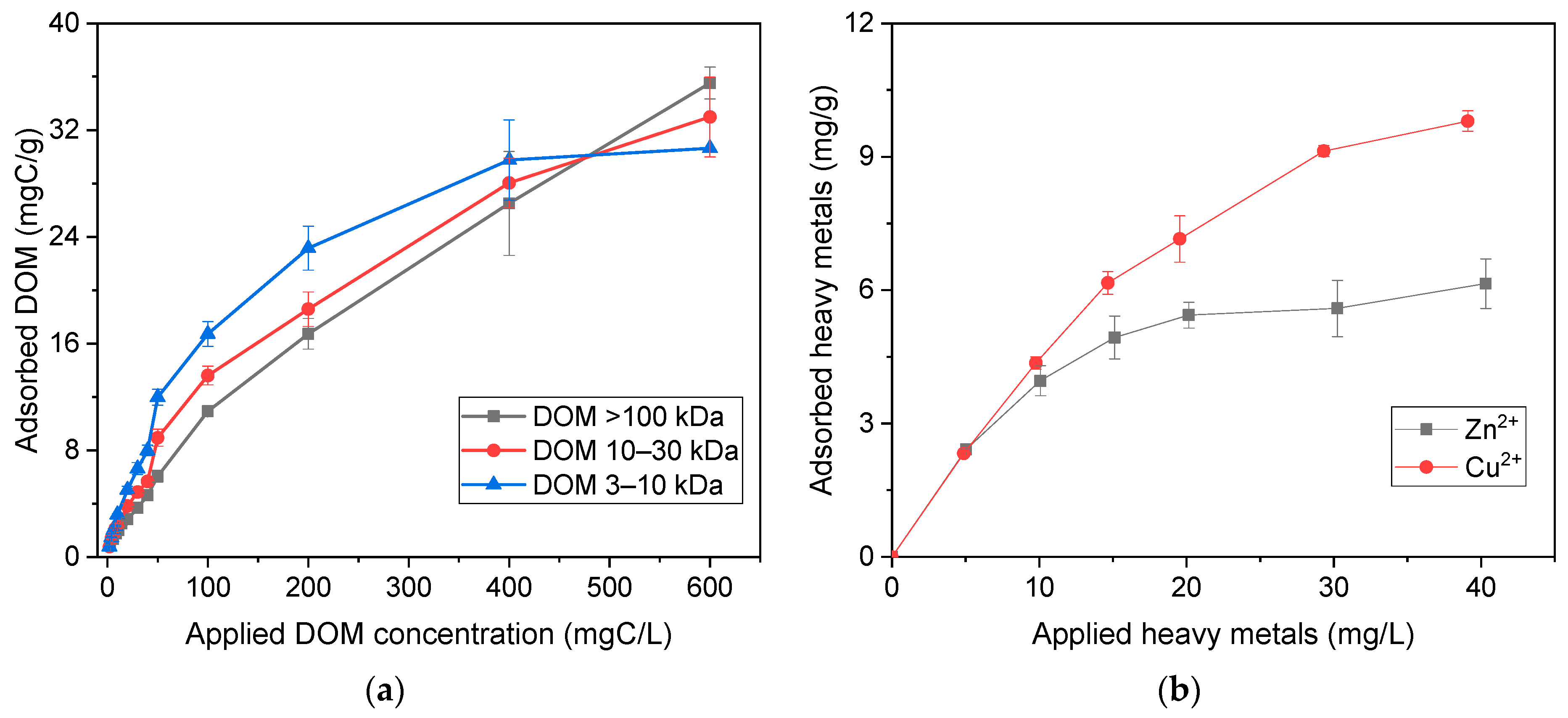
3.2. The Adsorption of DOM onto GAC
3.3. The Adsorption of Heavy Metals onto GAC
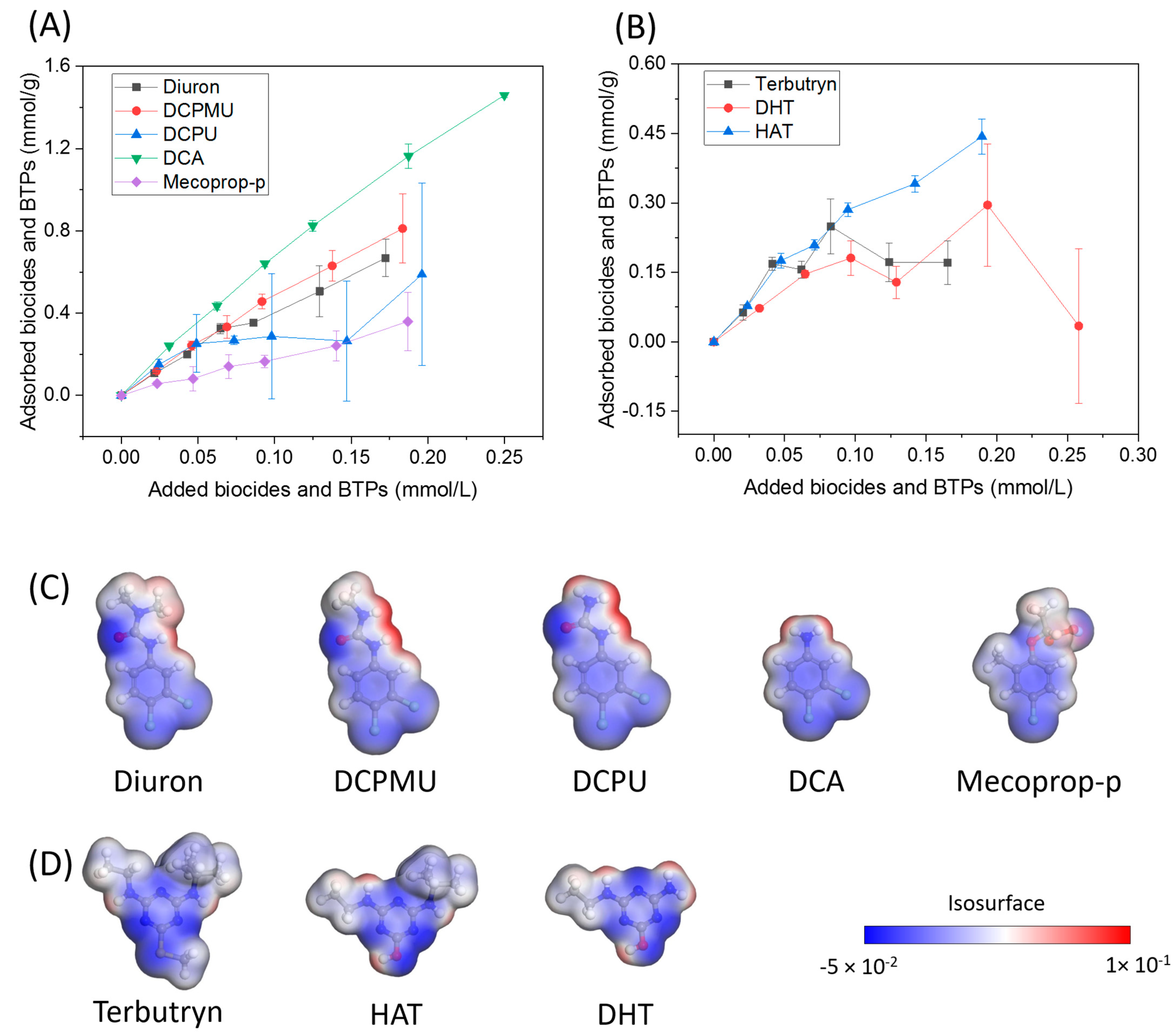
3.4. The Adsorption of Biocides and Biocide Transformation Products onto GAC
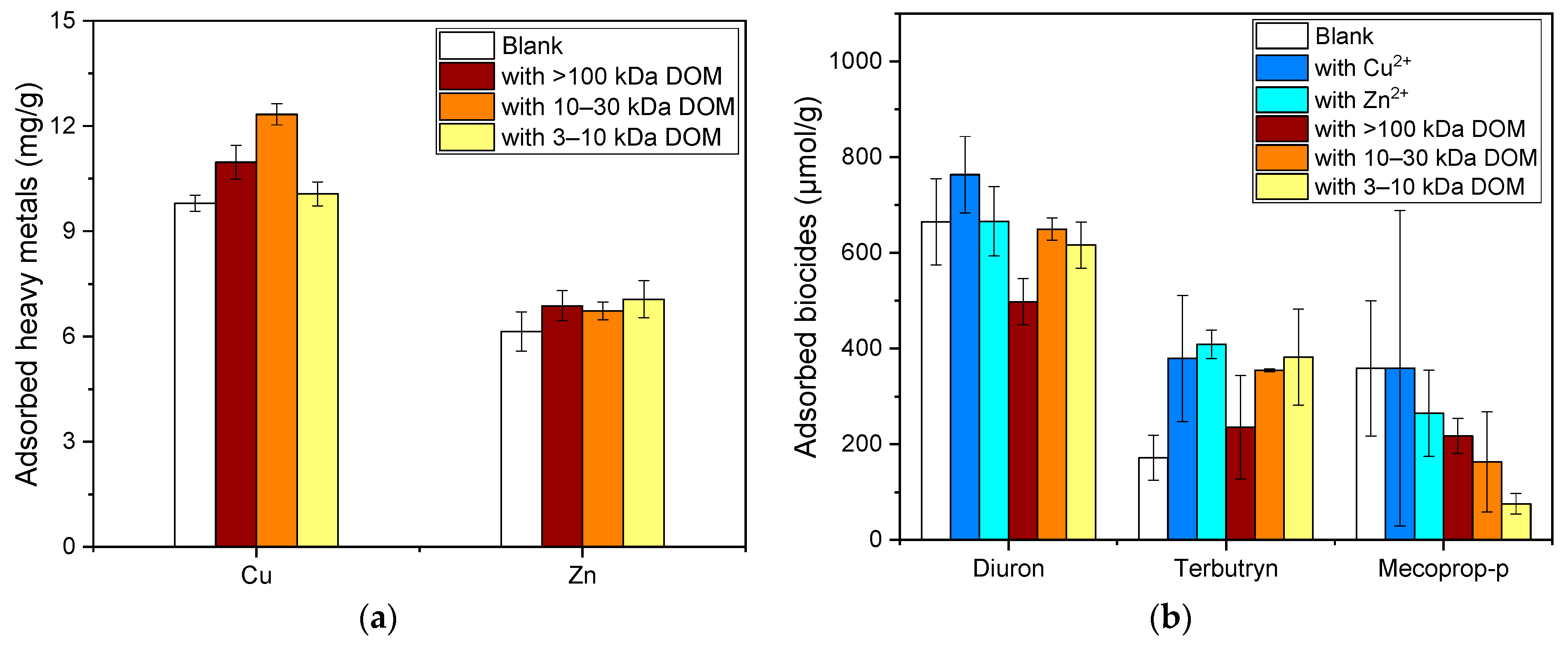
3.5. The Influence of Co-Presence on the Adsorption Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luthy, R.G.; Sharvelle, S.; Dillon, P. Urban Stormwater to Enhance Water Supply. Environ. Sci. Technol. 2019, 53, 5534–5542. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Conventional and Decentralized Urban Stormwater Management: A Comparison through Case Studies of Singapore and Berlin, Germany. Urban Water J. 2017, 14, 113–124. [Google Scholar] [CrossRef]
- Galster, S.; Helmreich, B. Copper and Zinc as Roofing Materials—A Review on the Occurrence and Mitigation Measures of Runoff Pollution. Water 2022, 14, 291. [Google Scholar] [CrossRef]
- Athanasiadis, K.; Horn, H.; Helmreich, B. A Field Study on the First Flush Effect of Copper Roof Runoff. Corros. Sci. 2010, 52, 21–29. [Google Scholar] [CrossRef]
- Charters, F.J.; Cochrane, T.A.; O’Sullivan, A.D. The Influence of Urban Surface Type and Characteristics on Runoff Water Quality. Sci. Total Environ. 2021, 755, 142470. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Nordqvist, K.; Marsalek, J.; Viklander, M. Building Surface Materials as Sources of Micropollutants in Building Runoff: A Pilot Study. Sci. Total Environ. 2019, 680, 190–197. [Google Scholar] [CrossRef]
- Styszko, K.; Bollmann, U.E.; Bester, K. Leaching of Biocides from Polymer Renders under Wet/Dry Cycles—Rates and Mechanisms. Chemosphere 2015, 138, 609–615. [Google Scholar] [CrossRef]
- Paijens, C.; Bressy, A.; Frère, B.; Moilleron, R. Biocide Emissions from Building Materials during Wet Weather: Identification of Substances, Mechanism of Release and Transfer to the Aquatic Environment. Environ. Sci. Pollut. Res. 2020, 27, 3768–3791. [Google Scholar] [CrossRef]
- Uhlig, S.; Colson, B.; Schoknecht, U. A Mathematical Approach for the Analysis of Data Obtained from the Monitoring of Biocides Leached from Treated Materials Exposed to Outdoor Conditions. Chemosphere 2019, 228, 271–277. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Schwerd, R.; Scherer, C.; Schwitalla, C.; Johann, S.; Rommel, S.H.; Helmreich, B. Influence of Façade Orientation on the Leaching of Biocides from Building Façades Covered with Mortars and Plasters. Sci. Total Environ. 2020, 734, 139465. [Google Scholar] [CrossRef]
- Linke, F.; Olsson, O.; Preusser, F.; Kümmerer, K.; Schnarr, L.; Bork, M.; Lange, J. Sources and Pathways of Biocides and Their Transformation Products in Urban Storm Water Infrastructure of a 2 Ha Urban District. Hydrol. Earth Syst. Sci. 2021, 25, 4495–4512. [Google Scholar] [CrossRef]
- Wicke, D.; Tatis-Muvdi, R.; Rouault, P.; Zerball-van Baar, P.; Dünnbier, U.; Rohr, M.; Burkhardt, M. Emissions from Building Materials—A Threat to the Environment? Water 2022, 14, 303. [Google Scholar] [CrossRef]
- Burkhardt, M.; Zuleeg, S.; Vonbank, R.; Bester, K.; Carmeliet, J.; Boller, M.; Wangler, T. Leaching of Biocides from Façades under Natural Weather Conditions. Environ. Sci. Technol. 2012, 46, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, T.D.; Müller, S.R.; Voegelin, A.; Schwarzenbach, R.P. Bituminous Roof Sealing Membranes as Major Sources of the Herbicide (R,S)-Mecoprop in Roof Runoff Waters: Potential Contamination of Groundwater and Surface Waters. Environ. Sci. Technol. 1998, 32, 3465–3471. [Google Scholar] [CrossRef]
- Bukhardt, M.; Kupper, T.; Hean, S.; Haag, R.; Schmid, P.; Haag, R.; Rossi, L.; Boller, M. Release of Biocides from Urban Areas into Aquatic Systems; IWA Newslatter: London, UK, 2007; pp. 15–17. [Google Scholar]
- Schwerd, R.; Hübner, S.; Schwitalla, C.; Scherer, C. Freisetzung von Mecoprop aus Polymerbitumendachbahnen. In Aqua Urbanica Congerence Proceedings 2018; Technical University of Kaiserslautern: Kaiserslautern, Germany, 2018; pp. 317–320. [Google Scholar]
- Fernández-Calviño, D.; Rousk, J.; Bååth, E.; Bollmann, U.E.; Bester, K.; Brandt, K.K. Isothiazolinone Inhibition of Soil Microbial Activity Persists despite Biocide Dissipation. Soil Biol. Biochem. 2023, 178, 108957. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Lok, C.S.C.; Marhoon, A.; Schwerd, R.; Johann, S.; Helmreich, B. Modelling the Environmental Fate and Behavior of Biocides Used in Façades Covered with Mortars and Plasters and Their Transformation Products. Build. Environ. 2022, 216, 108991. [Google Scholar] [CrossRef]
- Bork, M.; Lange, J.; Graf-Rosenfellner, M.; Hensen, B.; Olsson, O.; Hartung, T.; Fernández-Pascual, E.; Lang, F. Urban Storm Water Infiltration Systems Are Not Reliable Sinks for Biocides: Evidence from Column Experiments. Sci. Rep. 2021, 11, 7242. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, D.; Loganathan, P.; Johir, M.A.H.; Kandasamy, J.; Vigneswaran, S. Enhanced Removal of Nutrients, Heavy Metals, and PAH from Synthetic Stormwater by Incorporating Different Adsorbents into a Filter Media. Water Air Soil Pollut. 2021, 232, 96. [Google Scholar] [CrossRef]
- Ulrich, B.A.; Im, E.A.; Werner, D.; Higgins, C.P. Biochar and Activated Carbon for Enhanced Trace Organic Contaminant Retention in Stormwater Infiltration Systems. Environ. Sci. Technol. 2015, 49, 6222–6230. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S. Simultaneous Adsorption of Copper Ions and Humic Acid onto an Activated Carbon. J. Colloid Interface Sci. 2004, 280, 334–342. [Google Scholar] [CrossRef]
- Ko, D.; Mines, P.D.; Jakobsen, M.H.; Yavuz, C.T.; Hansen, H. Chr.B.; Andersen, H.R. Disulfide Polymer Grafted Porous Carbon Composites for Heavy Metal Removal from Stormwater Runoff. Chem. Eng. J. 2018, 348, 685–692. [Google Scholar] [CrossRef]
- Vatankhah, H.; Riley, S.M.; Murray, C.; Quiñones, O.; Steirer, K.X.; Dickenson, E.R.V.; Bellona, C. Simultaneous Ozone and Granular Activated Carbon for Advanced Treatment of Micropollutants in Municipal Wastewater Effluent. Chemosphere 2019, 234, 845–854. [Google Scholar] [CrossRef]
- Tang, L.; Ma, X.Y.; Wang, Y.; Zhang, S.; Zheng, K.; Wang, X.C.; Lin, Y. Removal of Trace Organic Pollutants (Pharmaceuticals and Pesticides) and Reduction of Biological Effects from Secondary Effluent by Typical Granular Activated Carbon. Sci. Total Environ. 2020, 749, 141611. [Google Scholar] [CrossRef]
- Golea, D.M.; Jarvis, P.; Jefferson, B.; Moore, G.; Sutherland, S.; Parsons, S.A.; Judd, S.J. Influence of Granular Activated Carbon Media Properties on Natural Organic Matter and Disinfection By-Product Precursor Removal from Drinking Water. Water Res. 2020, 174, 115613. [Google Scholar] [CrossRef]
- Belkouteb, N.; Franke, V.; McCleaf, P.; Köhler, S.; Ahrens, L. Removal of Per- and Polyfluoroalkyl Substances (PFASs) in a Full-Scale Drinking Water Treatment Plant: Long-Term Performance of Granular Activated Carbon (GAC) and Influence of Flow-Rate. Water Res. 2020, 182, 115913. [Google Scholar] [CrossRef]
- Zhu, P.; Knoop, O.; Helmreich, B. Interaction of Heavy Metals and Biocide/Herbicide from Stormwater Runoff of Buildings with Dissolved Organic Matter. Sci. Total Environ. 2022, 814, 152599. [Google Scholar] [CrossRef] [PubMed]
- Rommel, S.H.; Ebert, V.; Huber, M.; Drewes, J.E.; Helmreich, B. Spatial Distribution of Zinc in the Topsoil of Four Vegetated Infiltration Swales Treating Zinc Roof Runoff. Sci. Total Environ. 2019, 672, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Khamis, K.; Croghan, D.; Hernandez Gonzalez, L.M.; Rivera, V.A.; Phillips, C.B.; Packman, A.I.; Miller, W.M.; Hawke, R.G.; Hannah, D.M.; et al. Green Roof Vegetation Management Alters Potential for Water Quality and Temperature Mitigation. Ecohydrology 2021, 14, e2321. [Google Scholar] [CrossRef]
- Urbanczyk, M.M.; Bester, K.; Borho, N.; Schoknecht, U.; Bollmann, U.E. Influence of Pigments on Phototransformation of Biocides in Paints. J. Hazard. Mater. 2019, 364, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Paijens, C.; Bressy, A.; Frère, B.; Tedoldi, D.; Mailler, R.; Rocher, V.; Neveu, P.; Moilleron, R. Urban Pathways of Biocides towards Surface Waters during Dry and Wet Weathers: Assessment at the Paris Conurbation Scale. J. Hazard. Mater. 2021, 402, 123765. [Google Scholar] [CrossRef]
- Schoknecht, U.; Mathies, H.; Lisec, J. Leaching and Transformation of Film Preservatives in Paints Induced by Combined Exposure to Ultraviolet Radiation and Water Contact under Controlled Laboratory Conditions. Water 2021, 13, 2390. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Kalaruban, M.; Loganathan, P.; Nguyen, T.V.; Nur, T.; Hasan Johir, M.A.; Nguyen, T.H.; Trinh, M.V.; Vigneswaran, S. Iron-Impregnated Granular Activated Carbon for Arsenic Removal: Application to Practical Column Filters. J. Environ. Manag. 2019, 239, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Q.; Wu, M.Y.; Hu, L.M.; Lee, B.C.Y.; Ong, S.L.; Wang, P.; Hu, J.Y. Organics Removal and In-Situ Granule Activated Carbon Regeneration in FBR-Fenton/GAC Process for Reverse Osmosis Concentrate Treatment. Water Res. 2020, 183, 116119. [Google Scholar] [CrossRef] [PubMed]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Mosteo, R.; Ormad, M.P. Comparative Adsorption of Ciprofloxacin on Sugarcane Bagasse from Ecuador and on Commercial Powdered Activated Carbon. Sci. Total Environ. 2021, 750, 141498. [Google Scholar] [CrossRef]
- Solanki, A.; Boyer, T.H. Physical-Chemical Interactions between Pharmaceuticals and Biochar in Synthetic and Real Urine. Chemosphere 2019, 218, 818–826. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Zhang, Z. 2.29 Desulfurization Materials. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Oxford, UK, 2018; pp. 944–979. ISBN 978-0-12-814925-6. [Google Scholar]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on Potential Applications of Biomass for the Separation of Heavy Metals from Water and Wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Schreiber, B.; Brinkmann, T.; Schmalz, V.; Worch, E. Adsorption of Dissolved Organic Matter onto Activated Carbon—The Influence of Temperature, Absorption Wavelength, and Molecular Size. Water Res. 2005, 39, 3449–3456. [Google Scholar] [CrossRef]
- Shimabuku, K.K.; Kennedy, A.M.; Mulhern, R.E.; Summers, R.S. Evaluating Activated Carbon Adsorption of Dissolved Organic Matter and Micropollutants Using Fluorescence Spectroscopy. Environ. Sci. Technol. 2017, 51, 2676–2684. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, W.; Ray, M.B. Adsorption Isotherms and Kinetics for the Removal of Algal Organic Matter by Granular Activated Carbon. Sci. Total Environ. 2022, 806, 150885. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475–10489. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, L.; Nguyen, T.A.H.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D. Copper and Zinc Adsorption by Softwood and Hardwood Biochars under Elevated Sulphate-Induced Salinity and Acidic pH Conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, M.; Shao, S. Optimization of Target Biochar for the Adsorption of Target Heavy Metal Ion. Sci. Rep. 2022, 12, 13662. [Google Scholar] [CrossRef] [PubMed]
- Cibati, A.; Foereid, B.; Bissessur, A.; Hapca, S. Assessment of Miscanthus × Giganteus Derived Biochar as Copper and Zinc Adsorbent: Study of the Effect of Pyrolysis Temperature, pH and Hydrogen Peroxide Modification. J. Clean. Prod. 2017, 162, 1285–1296. [Google Scholar] [CrossRef]
- Kim, D.-W.; Wee, J.-H.; Yang, C.-M.; Yang, K.S. Efficient Removals of Hg and Cd in Aqueous Solution through NaOH-Modified Activated Carbon Fiber. Chem. Eng. J. 2020, 392, 123768. [Google Scholar] [CrossRef]
- Baup, S.; Wolbert, D.; Laplanche, A. Importance of Surface Diffusivities in Pesticide Adsorption Kinetics onto Granular Versus Powdered Activated Carbon: Experimental Determination and Modeling. Environ. Technol. 2002, 23, 1107–1117. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of Antibiotics on Graphene and Biochar in Aqueous Solutions Induced by π-π Interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef]
- Apul, O.G.; Wang, Q.; Zhou, Y.; Karanfil, T. Adsorption of Aromatic Organic Contaminants by Graphene Nanosheets: Comparison with Carbon Nanotubes and Activated Carbon. Water Res. 2013, 47, 1648–1654. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of Diclofenac Sodium from Water Using Oxidized Activated Carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Xiaozhen, F.; Xing, L.; Zhenglin, H.; Kaiyuan, Z.; Guosheng, S. DFT Study of Common Anions Adsorption at Graphene Surface Due to Anion-π Interaction. J. Mol. Model. 2022, 28, 225. [Google Scholar] [CrossRef]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Diuron Multilayer Adsorption on Activated Carbon from CO2 Activation of Grape Seeds. Chem. Eng. Commun. 2016, 203, 103–113. [Google Scholar] [CrossRef]
- de Souza, F.M.; dos Santos, O.A.A. Adsorption of Diuron from Aqueous Solution onto Commercial Organophilic Clay: Kinetic, Equilibrium and Thermodynamic Study. Environ. Technol. 2020, 41, 603–616. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.; Healy, M.G.; Ryan, P.C.; Mellander, P.-E.; Morrison, L.; O’Driscoll, J.H.; Siggins, A. Batch Adsorption of Herbicides from Aqueous Solution onto Diverse Reusable Materials and Granulated Activated Carbon. J. Environ. Manag. 2022, 323, 116102. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating Binding Characteristics of Cadmium and Copper to DOM Derived from Compost and Rice Straw Using EEM-PARAFAC Combined with Two-Dimensional FTIR Correlation Analyses. J. Hazard. Mater. 2018, 344, 539–548. [Google Scholar] [CrossRef]
- Genç-Fuhrman, H.; Mikkelsen, P.S.; Ledin, A. Simultaneous Removal of As, Cd, Cr, Cu, Ni and Zn from Stormwater Using High-Efficiency Industrial Sorbents: Effect of PH, Contact Time and Humic Acid. Sci. Total Environ. 2016, 566, 76–85. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive Sorption of Cd, Cr, Cu, Ni, Pb and Zn from Stormwater Runoff by Five Low-Cost Sorbents; Effects of Co-Contaminants, Humic Acid, Salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, N.; Wang, X.; Wang, Y.; Wang, L.; Li, X.; Hu, X. Adsorption Properties of Granular Activated Carbon-Supported Titanium Dioxide Particles for Dyes and Copper Ions. Sci. Rep. 2018, 8, 6463. [Google Scholar] [CrossRef]
- Eeshwarasinghe, D.; Loganathan, P.; Vigneswaran, S. Simultaneous Removal of Polycyclic Aromatic Hydrocarbons and Heavy Metals from Water Using Granular Activated Carbon. Chemosphere 2019, 223, 616–627. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Z.; Wu, Z.; Ye, B.-C. Adsorption Behaviors of Atrazine and Cr(III) onto Different Activated Carbons in Single and Co-Solute Systems. Powder Technol. 2018, 329, 207–216. [Google Scholar] [CrossRef]
- Radian, A.; Mishael, Y. Effect of Humic Acid on Pyrene Removal from Water by Polycation-Clay Mineral Composites and Activated Carbon. Environ. Sci. Technol. 2012, 46, 6228–6235. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, P. Comparative Study on Pharmaceuticals Adsorption in Reclaimed Water Desalination Concentrate Using Biochar: Impact of Salts and Organic Matter. Sci. Total Environ. 2017, 601, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Feng, T.; Gao, R.; Ma, Y.; Wang, W.; Zhou, Q.; Li, A. Ultrahigh Selective Adsorption of Zwitterionic PPCPs Both in the Absence and Presence of Humic Acid: Performance and Mechanism. J. Hazard. Mater. 2018, 348, 117–124. [Google Scholar] [CrossRef] [PubMed]
| Chemicals | Molecular Structure b | Molecular Formula a | Molecular Weight a | Water Solubility (mg/L) a | LogD (pH = 7) b |
|---|---|---|---|---|---|
| Diuron | 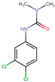 | C9H10Cl2N2O | 233.09 | 37.4–42 (−3.11) c | 2.53 |
| Mecoprop-p | 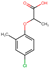 | C10H11ClO3 | 214.64 | 880 (0.0) c | −0.25 |
| Terbutryn |  | C10H19N5S | 241.36 | 35.9 (−3.65) c | 2.7 |
| 1-(3,4-Dichlorophenyl)-3-methylurea (DCPMU) | 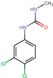 | C8H8Cl2N2O | 219.06 | (−3.08) c | 2.31 |
| 1-(3,4-Dichlorophenyl)urea (DCPU) |  | C7H6Cl2N2O | 205.04 | (−3.08) c | 2.09 |
| 3,4-Dichloroaniline (DCA) | 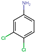 | C6H5Cl2N | 162.01 | (−2.75) c | 2.35 |
| Atrazine-desisopropyl-2-hydroxy (DHT) | 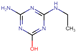 | C5H9N5O | 155.16 | (−1.8) c | 0.59 |
| Terbutylazine-2-hydroxy (HAT) | 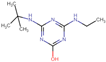 | C9H17N5O | 211.26 | (−2.67) c | 1.94 |
| Compounds | Langmuir Modelling | Freundlich Modelling | ||||
|---|---|---|---|---|---|---|
| q0 (mg/g, μmol/g) | KL | R2 | b | n | R2 | |
| DOM > 100 K | 59.4 ± 5.2 a | (2.58 ± 0.42) × 10−3 | 0.991 | 0.55 ± 0.04 | 1.50 ± 0.03 | 0.998 |
| DOM 10–30 K | 41.4 ± 2.4 a | (6.33 ± 0.92) × 10−3 | 0.986 | 1.23 ± 0.15 | 1.89 ± 0.08 | 0.990 |
| DOM 3–10 K | 34.3 ± 1.7 a | (15.6 ± 2.02) × 10−3 | 0.985 | 2.09 ± 0.31 | 2.15 ± 0.14 | 0.975 |
| Cu2+ | 157.4 ± 0.5 b | 0.72 ± 0.16 | 0.974 | 4.42 ± 0.25 | 3.52 ± 0.31 | 0.984 |
| Zn2+ | 85.7 ± 4.6 b | 2.56 ± 1.15 | 0.823 | 3.49 ± 0.17 | 5.72 ± 0.61 | 0.961 |
| Diuron | 1621.7 ± 437.6 b | 0.026 ± 0.01 | 0.978 | 1.41 ± 0.25 | 1.35 ± 0.11 | 0.983 |
| Mecoprop | N.A. | N.A. | N.A. | 0.222 ± 0.06 | 0.992 ± 0.09 | 0.981 |
| Terbutryn | 219.2 ± 46.0 b | 0.29 ± 0.27 | 0.707 | 23.7 ± 11.0 | 4.96 ± 4.01 | 0.623 |
| DCPMU | 2506.2 ± 310.4 b | 0.021 ± 0.004 | 0.997 | 14.8 ± 1.2 | 1.25 ± 0.05 | 0.997 |
| DHT | 495.6 ± 362.9 b | 0.04 ± 0.05 | 0.763 | 5.0 ± 3.6 | 1.56 ± 0.61 | 0.785 |
| DCPU | 304.3 ± 17.9 b | 0.64 ± 0.20 | 0.970 | 34.2 ± 6.0 | 5.65 ± 2.26 | 0.919 |
| DCA | 2888.8 ± 191.3 b | 0.059 ± 0.01 | 0.997 | 35.4 ± 1.5 | 1.48 ± 0.04 | 0.998 |
| HAT | N.A. | N.A. | N.A. | 9.76 ± 1.7 | 1.52 ± 0.13 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Sottorff, I.; Zhang, T.; Helmreich, B. Adsorption of Heavy Metals and Biocides from Building Runoff onto Granular Activated Carbon—The Influence of Different Fractions of Dissolved Organic Matter. Water 2023, 15, 2099. https://doi.org/10.3390/w15112099
Zhu P, Sottorff I, Zhang T, Helmreich B. Adsorption of Heavy Metals and Biocides from Building Runoff onto Granular Activated Carbon—The Influence of Different Fractions of Dissolved Organic Matter. Water. 2023; 15(11):2099. https://doi.org/10.3390/w15112099
Chicago/Turabian StyleZhu, Panfeng, Ignacio Sottorff, Tong Zhang, and Brigitte Helmreich. 2023. "Adsorption of Heavy Metals and Biocides from Building Runoff onto Granular Activated Carbon—The Influence of Different Fractions of Dissolved Organic Matter" Water 15, no. 11: 2099. https://doi.org/10.3390/w15112099






