Monitoring of Microbial Contamination of Groundwater in the Upper Choluteca River Basin, Honduras
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Sample Collection
2.2. Hydrogeological Conditions
2.3. Determination of Fecal Contamination Indicators
2.4. Phenotypic Identification of Escherichia coli
2.5. DNA Extraction and Identification of Phylogenetic Groups
2.6. PCR Product Sequencing
3. Results and Discussion
3.1. Microbiological Analysis
3.2. Risk Analysis
3.3. Phylogenetic Analysis of E. coli Strains
3.4. Analysis of the Groundwater Quality Situation in the Choluteca River Basin of Honduras
3.5. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaplin, M. Do we underestimate the importance of water in cell biology? Nat. Rev. Mol. Cell Biol. 2006, 7, 861–866. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Drinking Water Quality: Training Pack; WHO: Geneva, Switzerland, 2000.
- Barrantes, K.; Chacón, L.; Morales, E.; Rivera-Montero, L.; Pino, M.; Jiménez, A.G.; Mora, D.C.; Jiménez, P.S.; Silva, B.; Romero-Esquivel, L.G. Occurrence of pathogenic microorganisms in small drinking-water systems in Costa Rica. J. Water Health 2022, 20, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Nwabor, O.F.; Nnamonu, E.; Martins, P.; Ani, O. Water and waterborne diseases: A review. Int. J. Trop. Dis. Health 2016, 12, 1–14. [Google Scholar]
- Robertine, L.-F.; Payne, V.K.; Honorine, N.T.; Mounchili, S.; Saturine, M.M.; Manjuh, B.R.; Aboubakar, N.N.; Roland, B. Trends of potential waterborne diseases at different health facilities in Bamboutos Division, West Region, Cameroon: A retrospective appraisal of routine data from 2013 to 2017. J. Water Health 2021, 19, 616–628. [Google Scholar] [CrossRef]
- Cabral, J.P. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Bennett, S.D.; Lowther, S.A.; Chingoli, F.; Chilima, B.; Kabuluzi, S.; Ayers, T.L.; Warne, T.A.; Mintz, E. Assessment of water, sanitation and hygiene interventions in response to an outbreak of typhoid fever in Neno District, Malawi. PLoS ONE 2018, 13, e0193348. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, Z.H.; Islam, M.S.; Imran, K.M.; Hakim, S.A.I.; Worth, M.; Ahmed, A.; Hossan, S.; Haider, M.; Islam, M.R.; Hossain, F. Occurrence of Escherichia coli and faecal coliforms in drinking water at source and household point-of-use in Rohingya camps, Bangladesh. Gut Pathog. 2019, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Nyangwencha, J.; Kaluli, J.; Home, P.; Murage, H. Access to Safe Drinking Water and Water-Borne Diseases in Masaba North District, Kenya. JKUAT Annual Scientific Conference, Nairobi, Kenya; pp. 688–694. Available online: http://41.204.187.99/index.php/jscp/article/view/750 (accessed on 13 February 2023).
- Kumar, P.; Srivastava, S.; Banerjee, A.; Banerjee, S. Prevalence and predictors of water-borne diseases among elderly people in India: Evidence from Longitudinal Ageing Study in India, 2017–2018. BMC Public Health 2022, 22, 993. [Google Scholar] [CrossRef]
- Saravanan, V.; Ayessa Idenal, M.; Saiyed, S.; Saxena, D.; Gerke, S. Urbanization and human health in urban India: Institutional analysis of water-borne diseases in Ahmedabad. Health Policy Plan. 2016, 31, 1089–1099. [Google Scholar] [CrossRef] [Green Version]
- Aram, S.A.; Saalidong, B.M.; Osei Lartey, P. Comparative assessment of the relationship between coliform bacteria and water geochemistry in surface and ground water systems. PLoS ONE 2021, 16, e0257715. [Google Scholar] [CrossRef]
- World Health Organization. Water for Health: Taking Charge; World Health Organization: Geneva, Switzerland, 2001.
- Centers for Disease Control and Prevention. Waterborne Disease. Available online: https://www.cdc.gov/ncezid/what-we-do/2021-highlights/waterborne-disease.html (accessed on 13 February 2023).
- Centers for Disease Control and Prevention. Global Wash Fast Facts. Available online: https://www.cdc.gov/healthywater/global/wash_statistics.html (accessed on 13 February 2023).
- United Nations World Water Assessment Programme. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; UNESCO: Paris, France, 2018.
- Megdal, S.B. Invisible water: The importance of good groundwater governance and management. NPJ Clean Water 2018, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Velis, M.; Conti, K.I.; Biermann, F. Groundwater and human development: Synergies and trade-offs within the context of the sustainable development goals. Sustain. Sci. 2017, 12, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, B.L.; Lawrence, A.R.; Chilton, P.; Adams, B.; Calow, R.C.; Klinck, B.A. Groundwater and Its Susceptibility to Degradation: A Global Assessment of the Problem and Options for Management; United Nations Environment Programme: Nairobi, Kenya, 2003.
- Siebert, S.; Burke, J.; Faures, J.-M.; Frenken, K.; Hoogeveen, J.; Döll, P.; Portmann, F.T. Groundwater use for irrigation—A global inventory. Hydrol. Earth Syst. Sci. 2010, 14, 1863–1880. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, B.R.; Fakhreddine, S.; Rateb, A.; de Graaf, I.; Famiglietti, J.; Gleeson, T.; Grafton, R.Q.; Jobbagy, E.; Kebede, S.; Kolusu, S.R. Global water resources and the role of groundwater in a resilient water future. Nat. Rev. Earth Environ. 2023, 4, 87–101. [Google Scholar] [CrossRef]
- Kurwadkar, S. Occurrence and distribution of organic and inorganic pollutants in groundwater. Water Environ. Res. 2019, 91, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, T.; Cuthbert, M.; Ferguson, G.; Perrone, D. Global Groundwater Sustainability, Resources, and Systems in the Anthropocene. Annu. Rev. Earth Planet. Sci. 2020, 48, 431–463. [Google Scholar] [CrossRef] [Green Version]
- Beyene, G.; Aberra, D.; Fufa, F. Evaluation of the suitability of groundwater for drinking and irrigation purposes in Jimma Zone of Oromia, Ethiopia. Groundw. Sustain. Dev. 2019, 9, 100216. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality. Vol. 3, Surveillance and Control of Community Supplies. Vol. 3, Vigilancia y Control de los Abastecimientos de Agua a la Comunidad; World Health Organization: Geneva, Switzerland, 1997.
- Devane, M.L.; Moriarty, E.; Weaver, L.; Cookson, A.; Gilpin, B. Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 2020, 185, 116204. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Ramos-Ramírez, L.d.C.; Romero-Bañuelos, C.A.; Jiménez-Ruíz, E.I.; Palomino-Hermosillo, Y.A.; Saldaña-Ahuactzi, Z.; Martínez-Laguna, Y.; Handal-Silva, A.; Castañeda-Roldán, E.I. Coliform bacteria in san Pedro lake, western Mexico. Water Environ. Res. 2021, 93, 384–392. [Google Scholar] [CrossRef]
- Castro Fernández, M.F.; Cárdenas Manosalva, I.R.; Colmenares Quintero, R.F.; Montenegro Marín, C.E.; Diaz Cuesta, Y.E.; Escobar Mahecha, D.; Pérez Vásquez, P.A. Multitemporal Total Coliforms and Escherichia coli Analysis in the Middle Bogotá River Basin, 2007–2019. Sustainability 2022, 14, 1769. [Google Scholar] [CrossRef]
- Ercumen, A.; Pickering, A.J.; Kwong, L.H.; Arnold, B.F.; Parvez, S.M.; Alam, M.; Sen, D.; Islam, S.; Kullmann, C.; Chase, C. Animal feces contribute to domestic fecal contamination: Evidence from E. coli measured in water, hands, food, flies, and soil in Bangladesh. Environ. Sci. Technol. 2017, 51, 8725–8734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Dixit, O.V.A.; Vangchhia, B.; Condamine, B.; Dion, S.; Bridier-Nahmias, A.; Denamur, E.; Gordon, D. Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environ. Microbiol. 2019, 21, 3107–3117. [Google Scholar] [CrossRef]
- Naciones Unidas. Agenda 2030 y los Objetivos de Desarrollo Sostenible: Una Oportunidad para América Latina y el Caribe; United Nations: Santiago, Chile, 2018; ISBN 978-92-1-058643-6.
- FA-PNUD-MiAmbiente+/Secretaría de Recursos Naturales y Ambiente. Adaptación Basada en Ecosistemas en el Corredor Boscoso Central de Tegucigalpa; FA-PNUD-MiAmbiente+/Secretaría de Recursos Naturales y Ambiente: Tegucigalpa, Honduras, 2021. [Google Scholar]
- Akpataku, K.V.; Gnazou, M.D.; Nomesi, T.Y.A.; Nambo, P.; Doni, K.; Bawa, L.M.; Djaneye-Boundjou, G. Physicochemical and Microbiological Quality of Shallow Groundwater in Lomé, Togo. J. Geosci. Environ. Prot. 2020, 8, 162–179. [Google Scholar]
- Valenzuela, M.; Lagos, B.; Claret, M.; Mondaca, M.A.; Pérez, C.; Parra, O. Fecal contamination of groundwater in a small rural dryland watershed in central Chile. Chil. J. Agric. Res. 2009, 69, 235–243. [Google Scholar] [CrossRef]
- Bartram, J.; Ballance, R. Water Quality Monitoring: A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Khan, F.M.; Gupta, R. Escherichia coli (E. coli) as an Indicator of Fecal Contamination in Groundwater: A Review. In Sustainable Development of Water and Environment: Proceedings of the ICSDWE2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 225–235. [Google Scholar]
- Ministerio de Salud de Honduras. Norma Técnica Para la Calidad del Agua Potable Honduras; Ministerio de Salud de Honduras: Tegucigalpa, Honduras, 1995.
- Borrego, A.; Romero, P. Study of the microbiological pollution of a Malaga littoral area II. In Relationschip between Fecal Coliforms and Fecal Streptococci; VIèJournée Étude Pollutions: Cannes, France, 1982; pp. 561–569. [Google Scholar]
- Geldreich, E.E.; Kenner, B.A. Concepts of fecal streptococci in stream pollution. J. Water Pollut. Control Fed. 1969, 41, R336–R352. [Google Scholar]
- Bisimwa, A.M.; Kisuya, B.; Kazadi, Z.M.; Muhaya, B.B.; Kankonda, A.B. Monitoring faecal contamination and relationship of physicochemical variables with faecal indicator bacteria numbers in Bukavu surface waters, tributaries of Lake Kivu in Democratic Republic of Congo. Hyg. Environ. Health Adv. 2022, 3, 100012. [Google Scholar] [CrossRef]
- Coyne, M.S.; Howell, J. The fecal coliform/fecal streptococci ratio (FC/FS) and water quality in the bluegrass region of Kentucky. Soil Sci. News Views 1994, 15, 9. [Google Scholar]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [Green Version]
- Odonkor, S.T.; Mahami, T. Escherichia coli as a tool for disease risk assessment of drinking water sources. Int. J. Microbiol. 2020, 2020, 2534130. [Google Scholar] [CrossRef]
- Moshi, H.A.; Shilla, D.A.; Kimirei, I.A.; O’Reilly, C.; Clymans, W.; Bishop, I.; Loiselle, S.A. Community monitoring of coliform pollution in Lake Tanganyika. PLoS ONE 2022, 17, e0262881. [Google Scholar] [CrossRef] [PubMed]
- Lescat, M.; Clermont, O.; Woerther, P.L.; Glodt, J.; Dion, S.; Skurnik, D.; Djossou, F.; Dupont, C.; Perroz, G.; Picard, B.; et al. Commensal Escherichia coli strains in Guiana reveal a high genetic diversity with host-dependant population structure. Environ. Microbiol. Rep. 2013, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Lescat, M.; O’Brien, C.L.; Gordon, D.M.; Tenaillon, O.; Denamur, E. Evidence for a human-specific Escherichia coli clone. Environ. Microbiol. 2008, 10, 1000–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]

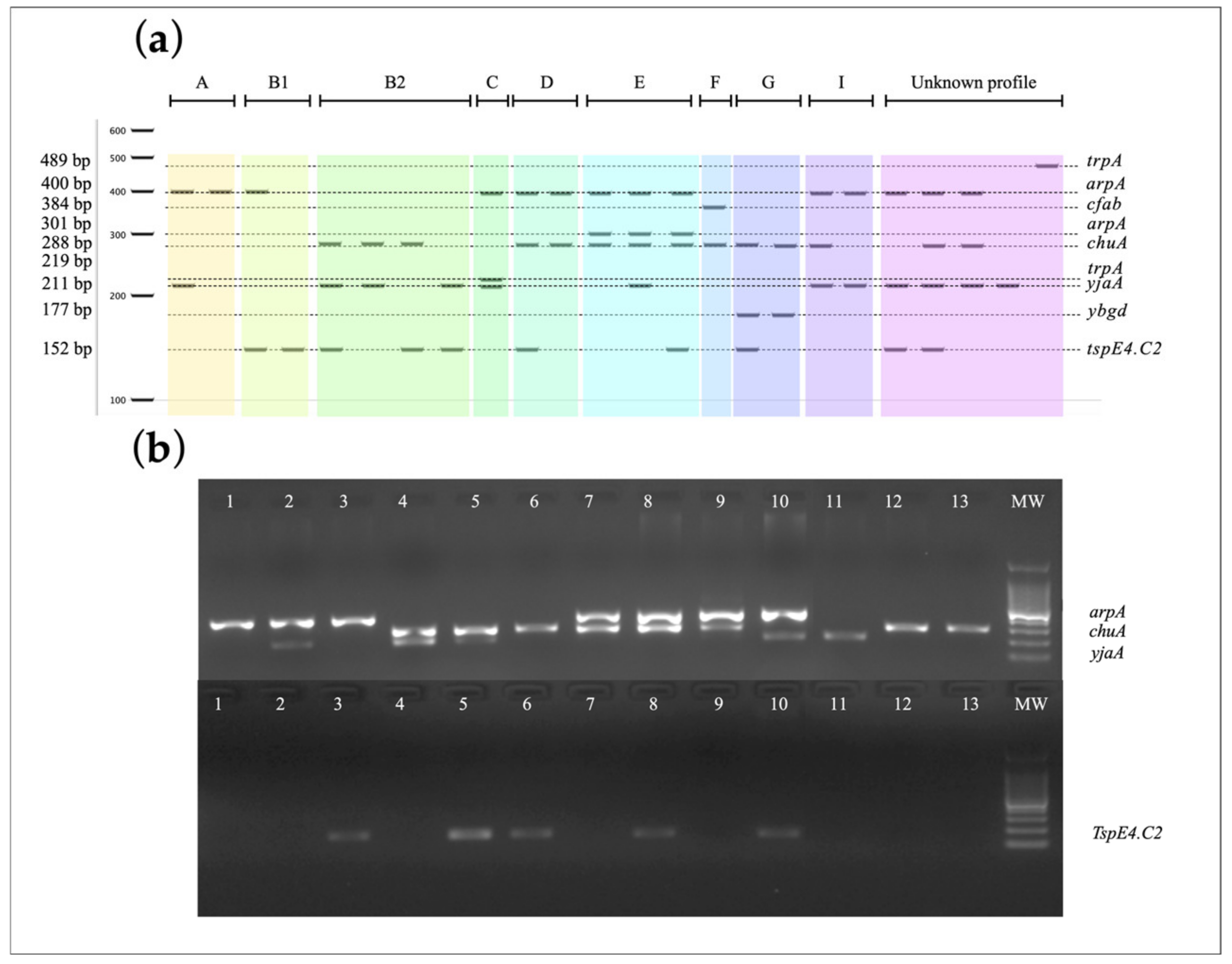

| Code | Gene | PCR Product (bp) | GenBank Accession Number |
|---|---|---|---|
| E01 | arpA | 400 | OQ571720 |
| E03 | arpA | 400 | OQ571721 |
| E04 | chuA | 288 | OQ571722 |
| E05 | chuA | 288 | OQ571723 |
| E06 | chuA | 288 | OQ571724 |
| E07 | yjaA | 211 | OQ571725 |
| E09 | yjaA | 211 | OQ571726 |
| E10 | TspE4.C2 | 152 | OQ571727 |
| E11 | arpA | 301 | OQ571728 |
| E13 | trpA | 489 | OQ571729 |
| E14 | trpA | 489 | OQ571730 |
| E15 | trpA | 489 | OQ571731 |
| E16 | yjaA | 211 | OQ571732 |
| E28 | ybgD | 177 | OQ571733 |
| E30 | cfaB | 384 | OQ57173 |
| Code | Sub-Basin | Coordinates | Municipality | Season/Year | TC | TtC | IE | Putative Source of Contamination According to TtC/IE Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Longitude | Latitude | CFU/100 mL | CFU/100 mL | CFU/100 mL | |||||
| ECO-50 | RC | 87°11′14.443″ W | 14°5′11.112″ N | DC | Rainy/2020 | 4.2 × 104 | 2.2 × 104 | 2.6 × 102 | Human |
| ECO-68 | GUA | 87°11′22.05″ W | 14°5′15.403″ N | DC | Rainy/2021 | 1.31 × 104 | 1.09 × 104 | 4.5 × 103 | Mixed |
| ECO-157 | RC | 87°9′37.871″ W | 14°5′19.319″ N | DC | Rainy/2020 | 1.7 × 104 | 7.90 × 103 | 5.2 × 102 | Human |
| ECO-162 | RH | 87°28′8.722″ W | 14°12′11.512″ N | LP | Rainy/2021 | 1.9 × 104 | 5.1 × 103 | 1.9 × 103 | Mixed |
| ECO-2 | RC | 87°9′30.328″ W | 14°5′10.308″ N | DC | Dry/2021 | 4.2 × 104 | 2.8 × 103 | 1.1 × 102 | Human |
| ECO-76 | RH | 87°18′1.079″ W | 14°8′24.116″ N | DC | Rainy/2021 | 1.0 × 104 | 2.5 × 103 | 8.0 × 102 | Mixed |
| ECO-133 | SJ | 87°14′34.574″ W | 13°57′22.331″ N | SA | Rainy/2020 | 9.0 × 103 | 1.2 × 103 | 1.6 × 102 | Human |
| ECO-126 | SJ | 87°14′34.574″ W | 13°57′22.331″ N | SA | Dry/2022 | 2.0 × 104 | 1.2 × 103 | 2.2 × 104 | Animal |
| ECO-4 | GUA | 87°15′0.273″ W | 14°6′7.35″ N | DC | Dry/2021 | 3.1 × 104 | 1.0 × 103 | 1.1 × 102 | Human |
| ECO-7 | RH | 87°28′8.722″ W | 14°12′11.512″ N | LP | Dry/2021 | 3.00 × 103 | 1.00 × 103 | 1.2 × 102 | Human |
| ECO-112 | YE | 87°3′6.944″ W | 14°2′4.316″ N | SAO | Rainy/2021 | 5.0 × 103 | 9.0 × 102 | 5.3 × 102 | Mixed |
| ECO-149 | SJ | 87°6′22.6″ W | 13°58′50.117″ N | TAT | Rainy/2020 | 1.1 × 104 | 9.0 × 102 | 0 | N/A |
| ECO-127 | YE | 87°11′10.236″ W | 14°6′27.805″ N | SAO | Rainy/2020 | 1.5 × 103 | 8.0 × 102 | 7.5 × 101 | Human |
| ECO-160 | RC | 87°9′30.328″ W | 14°5′10.308″ N | DC | Rainy/2020 | 2.0 × 104 | 7.0 × 102 | 1.6 × 103 | Animal |
| ECO-145 | RC | 87°12′32.952″ W | 14°6′31.676″ N | DC | Rainy/2020 | 2.3 × 103 | 6.8 × 102 | 1.4 × 102 | Human |
| ECO-71 | CA | 87°6′53.126″ W | 14°16′20.126″ N | DC | Rainy/2020 | 3.4 × 104 | 5.0 × 102 | 6.2 × 102 | Mixed |
| ECO-62 | CA | 87°3′23.589″ W | 14°11′38.021″ N | DC | Dry/2021 | 2.4 × 104 | 5.2 × 102 | 4.0 × 102 | Mixed |
| ECO-153 | RC | 87°12′3.486″ W | 14°6′50.971″ N | DC | Rainy/2020 | 4.3 × 103 | 5.1 × 102 | 7.6 × 101 | Human |
| ECO-13 | GUA | 87°21′18.597″ W | 14°8′35.467″ N | DC | Rainy/2021 | 4.0 × 103 | 4.0 × 102 | 9 | Human |
| ECO-137 | YE | 87°4′9.258″ W | 14°9′26.146″ N | VA | Rainy/2020 | 3.9 × 103 | 3.7 × 102 | 2.9 × 101 | Human |
| ECO-151 | GUA | 87°20′24.475″ W | 14°4′15.533″ N | DC | Rainy/2020 | 1.1 × 103 | 3.7 × 102 | 1 | Human |
| ECO-16 | CA | 87°5′14.859″ W | 14°14′31.612″ N | DC | Dry/2021 | 6.0 × 103 | 3.4 × 102 | 5.2 × 102 | Animal |
| ECO-139 | YE | 87°3′6.944″ W | 14°2′4.316″ N | SAO | Rainy/2020 | 2.6 × 104 | 3.2 × 102 | 5.2 × 101 | Human |
| ECO-92 | RC | 87°10′54.804″ W | 14°5′16.01″ N | DC | Dry/2021 | 6.7 × 102 | 3.1 × 102 | 2.2 × 101 | Human |
| ECO-1 | RH | 87°24′16.359″ W | 14°19′35.199″ N | DC | Dry/2021 | 8.0 × 103 | 3.0 × 102 | 5.6 × 102 | Animal |
| ECO-8 | CA | 86°53′58.031″ W | 14°12′52.217″ N | VSF | Dry/2020 | 6.0 × 104 | 2.5 × 102 | 2.0 × 103 | Animal |
| ECO-66 | RC | 87°11′23.24″ W | 14°5′3.13″ N | DC | Dry/2021 | 3.0 × 103 | 2.5 × 102 | 0 | N/A |
| ECO-104 | RC | 87°10′54.804″ W | 14°5′16.01″ N | DC | Rainy/2021 | 2.7 × 103 | 2.0 × 102 | 1.0 × 102 | Mixed |
| ECO-138 | SJ | 87°24′49.15″ W | 14°4′2.099″ N | LP | Rainy/2020 | 7.0 × 102 | 1.9 × 102 | 2.0 × 102 | Mixed |
| ECO-9 | SJ | 87°23′9.855″ W | 13°58′27.71″ N | DC | Dry/2021 | 5.7 × 102 | 1.4 × 102 | 1.0 × 102 | Human |
| ECO-77 | CA | 86°51′52.369″ W | 14°16′37.795″ N | CA | Rainy/2021 | 2.0 × 102 | 1.3 × 102 | 1.3 × 102 | Mixed |
| ECO-75 | CA | 86°54′45.999″ W | 14°6′12.513″ N | MO | Dry/2021 | 2.96 × 104 | 1.1 × 102 | 3.7 × 102 | Animal |
| ECO-29 | GUA | 87°17′8.841″ W | 14°3′54.693″ N | DC | Rainy/2021 | 2.0 × 102 | 1.0 × 102 | 5 | Human |
| ECO-70 | GUA | 87°14′52.816″ W | 14°4′16.653″ N | DC | Dry/2021 | 7.0 × 103 | 1.0 × 102 | 8.0 × 101 | Mixed |
| ECO-101 | RC | 87°7′34.822″ W | 14°4′34.016″ N | DC | Rainy/2021 | 5.0 × 103 | 1.0 × 102 | 2.5 × 101 | Human |
| ECO-105 | CA | 86°51′52.369″ W | 14°16′37.795″ N | CA | Dry/2021 | 5.0 × 102 | 1.0 × 102 | 0 | N/A |
| ECO-159 | RC | 87°6′48.804″ W | 14°6′50.428″ N | DC | Rainy/2020 | 1.9 × 103 | 9.5 × 101 | 6.2 × 102 | Animal |
| ECO-14 | CA | 87°4′50.448″ W | 14°20′0.282″ N | TAL | Rainy/2021 | 5.0 × 102 | 9.0 × 101 | 2.8 × 102 | Animal |
| ECO-98 | CA | 87°4′50.448″ W | 14°20′0.282″ N | TAL | Rainy/2021 | 5.0 × 102 | 9.0 × 101 | 2.8 × 102 | Animal |
| ECO-58 | RC | 87°11′22.05″ W | 14°5′15.403″ N | DC | Rainy/2020 | 1.9 × 104 | 6.7 × 101 | 3.8 × 102 | Animal |
| ECO-119 | RC | 87°6′48.804″ W | 14°6′50.428″ N | SL | Rainy/2021 | 1.4 × 103 | 6.4 × 101 | 1.3 × 102 | Human |
| ECO-61 | GUA | 87°19′9.788″ W | 14°4′45.484″ N | DC | Dry/2021 | 2.3 × 104 | 6.0 × 101 | 5.0 × 101 | Mixed |
| ECO-102 | GUA | 87°13′56.103″ W | 14°4′25.433″ N | DC | Rainy/2021 | 2.0 × 102 | 6.0 × 101 | 2.6 × 101 | Mixed |
| ECO-113 | SJ | 87°21′9.426″ W | 14°1′0.002″ N | OJ | Rainy/2021 | 1.0 × 103 | 5.6 × 101 | 3.3 × 101 | Mixed |
| ECO-85 | SJ | 87°21′9.426″ W | 14°1′0.002″ N | OJ | Dry/2021 | 3.2 × 102 | 5.1 × 101 | 1.4 × 101 | Mixed |
| ECO-55 | RC | 87°18′53.046″ W | 14°4′44.041″ N | DC | Rainy/2021 | 5.3 × 102 | 5.0 × 101 | 0 | N/A |
| ECO-164 | SJ | 87°25′56.462″ W | 14°3′2.87″ N | LP | Rainy/2020 | 6.0 × 102 | 3.4 × 101 | 2.9 × 102 | Animal |
| ECO-108 | GUA | 87°13′18.651″ W | 14°5′30.667″ N | DC | Rainy/2021 | 3.0 × 103 | 3.3 × 101 | 6.2 × 101 | Animal |
| ECO-89 | CA | 87°6′53.126″ W | 14°16′20.126″ N | CA | Dry/2021 | 1.0 × 103 | 3.0 × 101 | 1.2 × 101 | Human |
| ECO-128 | GUA | 87°19′9.788″ W | 14°4′45.484″ N | DC | Dry/2022 | 1.6 × 102 | 3.0 × 101 | 1.0 × 101 | Mixed |
| ECO-79 | RC | 87°9′29.49″ W | 14°6′41.027″ N | DC | Rainy/2021 | 7.0 × 102 | 2.6 × 101 | 0 | N/A |
| ECO-80 | GUA | 86°54′45.999″ W | 14°6′12.513″ N | DC | Rainy/2021 | 6.0 × 102 | 2.6 × 101 | 5.7 × 101 | Animal |
| ECO-5 | RC | 87°7′40.775″ W | 14°4′5.857″ N | DC | Dry/2021 | 2.1 × 104 | 2.5 × 101 | 3.4 × 102 | Animal |
| ECO-121 | YE | 87°2′3.604″ W | 14°10′27.206″ N | VA | Dry/2022 | 2.1 × 101 | 1.7 × 101 | 4.9 × 101 | Animal |
| ECO-12 | CA | 86°56′47.027″ W | 14°8′48.662″ N | VSF | Rainy/2020 | 7.2 × 102 | 1.5 × 101 | 5.5 × 101 | Animal |
| ECO-125 | CA | 87°3′23.589″ W | 14°11′38.021″ N | DC | Dry/2022 | 3.7 × 101 | 1.5 × 101 | 4.8 × 101 | Animal |
| ECO-131 | RC | 87°9′43.072″ W | 14°5′16.712″ N | DC | Rainy/2020 | 3.7 × 101 | 1.5 × 101 | 4.8 × 101 | Animal |
| ECO-52 | RC | 87°10′7.136″ W | 14°4′58.629″ N | DC | Rainy/2020 | 7.4 × 101 | 1.4 × 101 | 0 | N/A |
| ECO-93 | CA | 86°54′45.999″ W | 14°6′12.513″ N | MO | Dry/2021 | 1.2 × 104 | 1.4 × 101 | 1.3 × 102 | Animal |
| ECO-120 | YE | 87°2′43.977″ W | 13°59′40.757″ N | TAT | Dry/2022 | 4.0 × 101 | 1.2 × 101 | 3.8 × 101 | Animal |
| ECO-135 | CA | 87°2′27.391″ W | 14°19′42.839″ N | CA | Rainy/2020 | 1.6 × 103 | 1.2 × 101 | 2.5 × 101 | Animal |
| ECO-25 | RC | 87°11′28.989″ W | 14°5′58.559″ N | DC | Rainy/2021 | 6 | 1.0 × 101 | 0 | N/A |
| ECO-54 | RC | 87°12′30.01″ W | 14°6′24.68″ N | DC | Rainy/2020 | 3.2 × 101 | 1.0 × 101 | 0 | N/A |
| ECO-60 | RC | 87°9′29.49″ W | 14°6′41.027″ N | DC | Dry/2021 | 4.3 × 101 | 1.0 × 101 | 0 | N/A |
| ECO-67 | RC | 87°11′11.883″ W | 14°6′2.284″ N | DC | Rainy/2020 | 1.8 × 101 | 1.0 × 101 | 0 | N/A |
| ECO-74 | YE | 87°0′58.187″ W | 13°55′36.551″ N | MA | Rainy/2020 | 1.8 × 102 | 1.0 × 101 | 6.0 × 101 | Animal |
| ECO-81 | SJ | 87°20′43.432″ W | 13°58′7.944″ N | DC | Rainy/2020 | 5.1 × 102 | 1.0 × 101 | 1.2 × 102 | Animal |
| ECO-84 | GUA | 87°15′54.488″ W | 14°3′2.404″ N | DC | Rainy/2020 | 3.6 × 101 | 1.0 × 101 | 0 | N/A |
| ECO-88 | RC | 87°9′29.49″ W | 14°6′41.027″ N | DC | Rainy/2020 | 1.1 × 101 | 1.0 × 101 | 0 | N/A |
| ECO-90 | GUA | 87°21′18.164″ W | 14°3′33.726″ N | DC | Rainy/2021 | 2.0 × 103 | 1.0 × 101 | 1.7 × 102 | Animal |
| ECO-103 | SJ | 87°13′56.159″ W | 13°56′30.157″ N | SA | Rainy/2021 | 2.0 × 103 | 1.0 × 101 | 4.5 × 102 | Animal |
| ECO-111 | GUA | 87°17′14.495″ W | 14°8′9.232″ N | DC | Rainy/2021 | 2.5 × 101 | 1.0 × 101 | 3.3 × 101 | Animal |
| ECO-116 | CA | 87°9′58.941″ W | 14°5′10.581″ N | DC | Rainy/2021 | 1.7 × 102 | 1.0 × 101 | 2.8 × 101 | Animal |
| ECO-118 | SJ | 87°12′42.381″ W | 13°56′39.729″ N | SA | Rainy/2021 | 2.3 × 101 | 1.0 × 101 | 0 | N/A |
| ECO-122 | YE | 86°58′27.391″ W | 13°55′5.266″ N | GA | Dry/2022 | 5.6 × 102 | 1.0 × 101 | 1.3 × 102 | Animal |
| ECO-124 | CA | 86°55′1.54″ W | 14°6′15.122″ N | MO | Dry/2022 | 4.6 × 101 | 1.0 × 101 | 6 | Mixed |
| ECO-129 | GUA | 87°17′8.841″ W | 14°3′54.693″ N | DC | Dry/2022 | 4.0 × 101 | 1.0 × 101 | 1.3 × 101 | Mixed |
| ECO-21 | RC | 87°11′31.419″ W | 14°5′13.637″ N | DC | Rainy/2020 | 8.4 × 101 | 7 | 0 | N/A |
| ECO-65 | CA | 86°51′52.369″ W | 14°16′37.795″ N | CA | Dry/2021 | 1.0 × 102 | 7 | 4.1 × 101 | Animal |
| ECO-86 | SJ | 87°14′34.574″ W | 13°57′22.331″ N | SA | Dry/2021 | 1.2 × 102 | 7 | 2.7 × 101 | Animal |
| ECO-114 | GUA | 87°15′54.488″ W | 14°3′2.404″ N | DC | Rainy/2021 | 2.0 × 101 | 6 | 0 | N/A |
| ECO-132 | RC | 87°8′22.844″ W | 14°7′21.464″ N | DC | Rainy/2020 | 1.4 × 101 | 5 | 4 | Mixed |
| ECO-158 | YE | 87°3′43.358″ W | 14°2′25.498″ N | SAO | Rainy/2020 | 4.8 × 101 | 4 | 1.6 × 101 | Animal |
| ECO-64 | CA | 86°55′1.54″ W | 14°6′15.122″ N | MO | Dry/2021 | 1.0 × 103 | 3 | 9 | Animal |
| ECO-17 | GUA | 87°13′3.67″ W | 14°4′47.649″ N | DC | Dry/2021 | 3 | 2 | 0 | N/A |
| ECO-23 | RC | 87°9′22.173″ W | 14°5′32.643″ N | DC | Rainy/2020 | 1.1 × 102 | 2 | 0 | N/A |
| ECO-63 | CA | 87°0′13.144″ W | 14°9′31.227″ N | VA | Dry/2021 | 6.0 × 102 | 2 | 1.9 × 101 | Animal |
| ECO-141 | SJ | 87°21′9.426″ W | 14°1′0.002″ N | OJ | Rainy/2020 | 1.2 × 102 | 2 | 0 | N/A |
| ECO-144 | CA | 87°6′45.806″ W | 14°6′58.371″ N | SL | Rainy/2020 | 3.3 × 102 | 2 | 0 | N/A |
| ECO-56 | YE | 87°2′3.604″ W | 14°10′27.206″ N | VA | Rainy/2021 | 2 | 1 | 1 | Mixed |
| ECO-97 | GUA | 87°16′10.496″ W | 14°7′49.125″ N | DC | Rainy/2021 | 1.5 × 101 | 1 | 4 × 101 | Animal |
| ECO-152 | RC | 87°6′38.747″ W | 14°10′50.657″ N | DC | Rainy/2020 | 4 | 1 | 3 | Animal |
| ECO-168 | RC | 87°11′40.086″ W | 14°5′9.171″ N | DC | Rainy/2020 | 6 | 1 | 0 | N/A |
| ECO-6 | SJ | 87°12′31.039″ W | 14°1′24.918″ N | DC | Rainy/2021 | 8 | 0 | 0 | N/A |
| ECO-20 | SJ | 87°18′27.629″ W | 13°55′53.561″ N | OJ | Dry/2021 | 6 | 0 | 0 | N/A |
| ECO-38 | CA | 86°55′1.54″ W | 14°6′15.122″ N | MO | Rainy/2021 | 2 | 0 | 0 | N/A |
| ECO-73 | CA | 86°51′58.996″ W | 14°7′34.236″ N | MO | Rainy/2020 | 4.8 × 103 | 0 | 0 | N/A |
| ECO-140 | RC | 87°11′23.24″ W | 14°5′3.13″ N | DC | Rainy/2020 | 1.0 × 101 | 0 | 0 | N/A |
| ECO-163 | RC | 87°12′0.902″ W | 14°5′16.38″ N | DC | Dry/2021 | 1.3 × 101 | 0 | 0 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, K.; Ortiz, B.; Rivera, L.; Peña, T.; Chirinos-Escobar, M.; Enríquez, L.; Maldonado, V.; Fontecha, G. Monitoring of Microbial Contamination of Groundwater in the Upper Choluteca River Basin, Honduras. Water 2023, 15, 2116. https://doi.org/10.3390/w15112116
Mendoza K, Ortiz B, Rivera L, Peña T, Chirinos-Escobar M, Enríquez L, Maldonado V, Fontecha G. Monitoring of Microbial Contamination of Groundwater in the Upper Choluteca River Basin, Honduras. Water. 2023; 15(11):2116. https://doi.org/10.3390/w15112116
Chicago/Turabian StyleMendoza, Keylin, Bryan Ortiz, Luis Rivera, Tania Peña, Marcio Chirinos-Escobar, Lourdes Enríquez, Victoria Maldonado, and Gustavo Fontecha. 2023. "Monitoring of Microbial Contamination of Groundwater in the Upper Choluteca River Basin, Honduras" Water 15, no. 11: 2116. https://doi.org/10.3390/w15112116
APA StyleMendoza, K., Ortiz, B., Rivera, L., Peña, T., Chirinos-Escobar, M., Enríquez, L., Maldonado, V., & Fontecha, G. (2023). Monitoring of Microbial Contamination of Groundwater in the Upper Choluteca River Basin, Honduras. Water, 15(11), 2116. https://doi.org/10.3390/w15112116
















