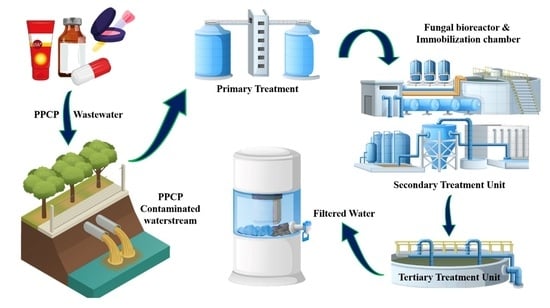
Fungal-Based Remediation in the Treatment of Anthropogenic Activities and Pharmaceutical-Pollutant-Contaminated Wastewater
Abstract
:1. Introduction
2. PPCP as Pollutants
2.1. Sources and Routes
2.2. Mechanism of Action and Hazards Caused to Human Health
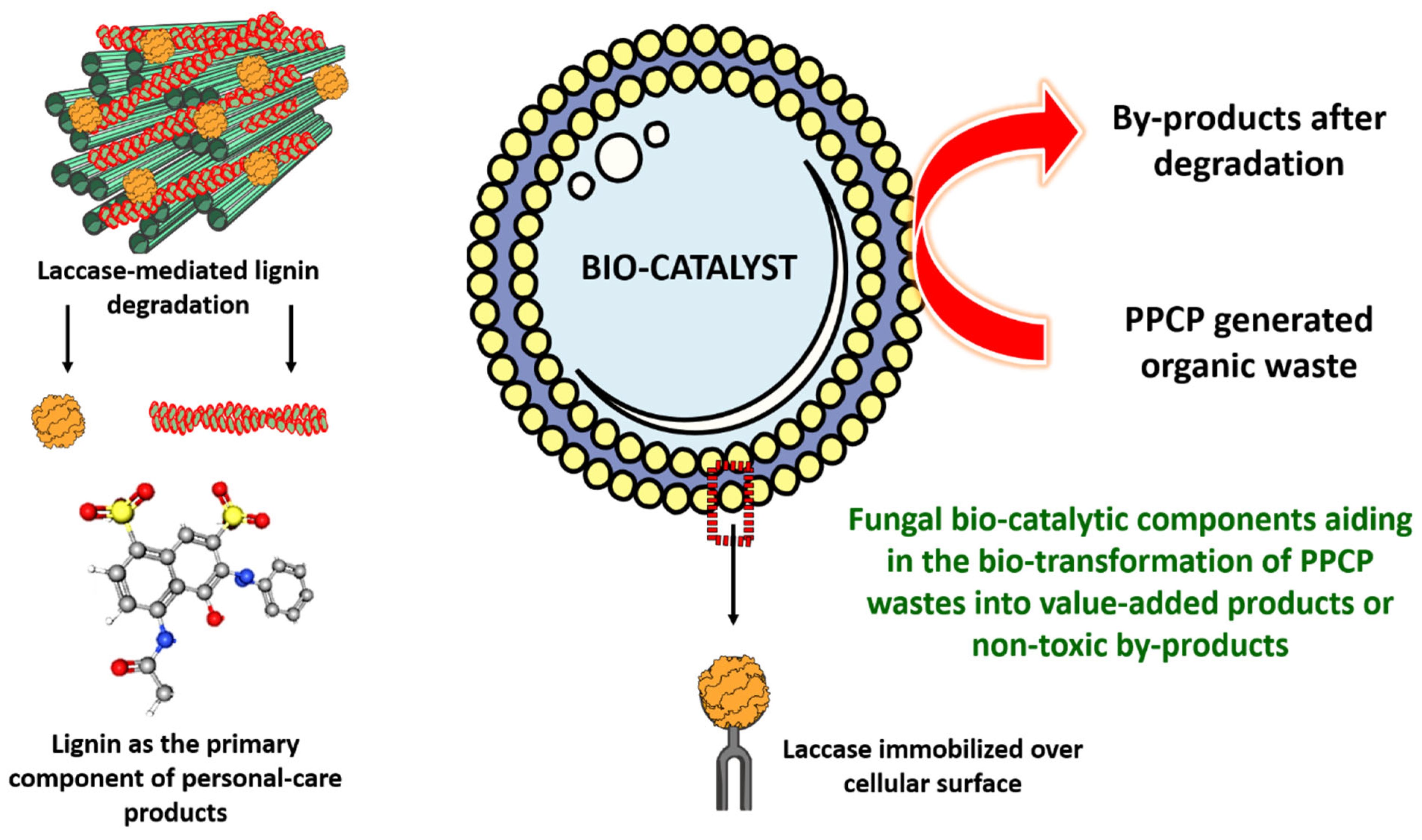
3. Fungal Genera and Enzymes for the Remediation of PPCP
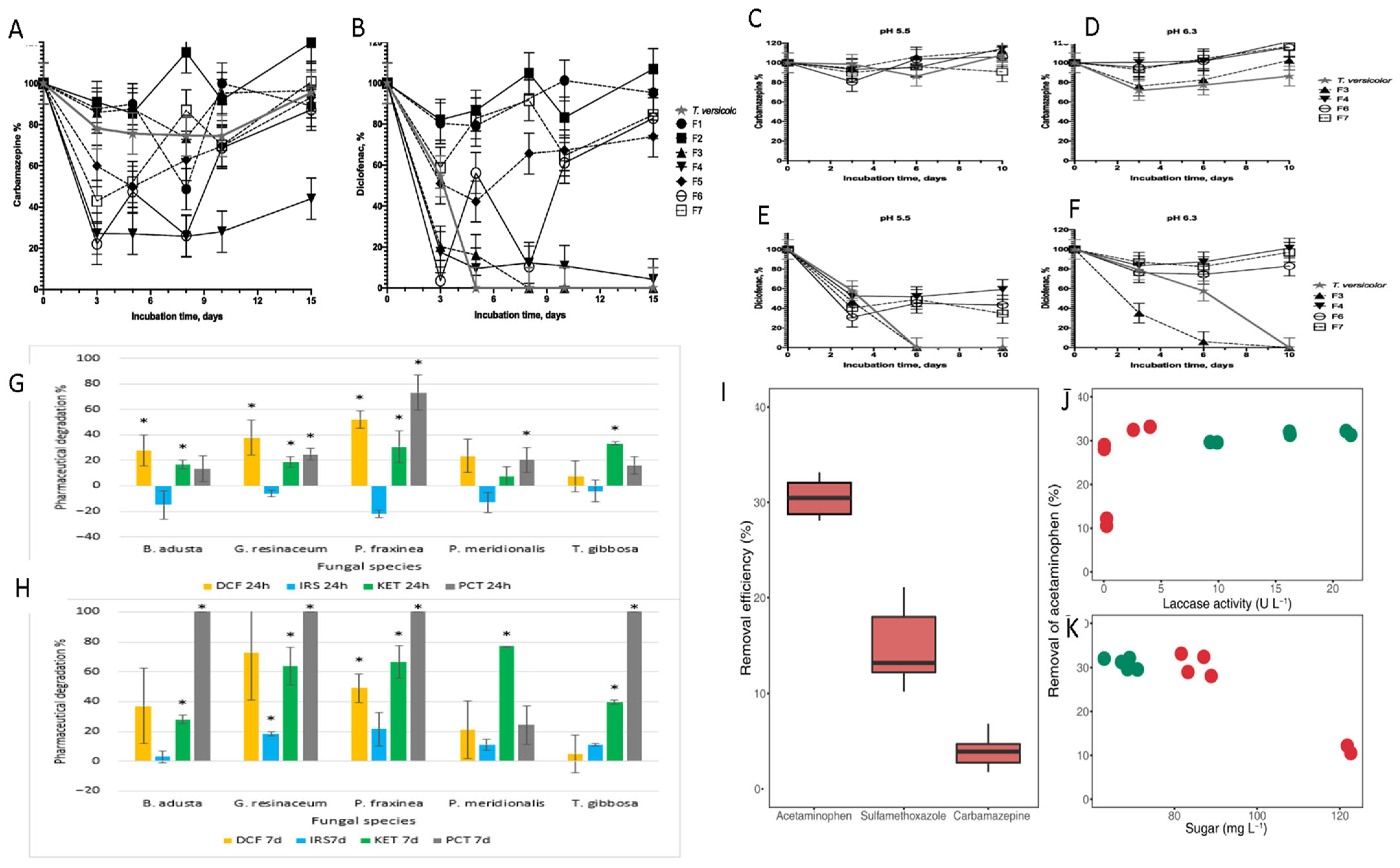
4. Mechanistic Approaches Using Fungal Strains to Remove PPCPs
5. Current Methods of Fungal Biodegradation: Of PPCPs
6. Environmental Regulations and Initiatives in the Removal of PPCP
7. Challenges and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Huang, X.; Witter, J.D.; Spongberg, A.L.; Wang, K.; Wang, D.; Liu, J. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze River, China. Ecotoxicol. Environ. Saf. 2014, 106, 19–26. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Liu, S.; Wang, D.; Zhou, X.; Zhang, M.; Li, L.; Li, X.; Xu, F.; Nie, J. Metal-carbon hybrid materials induced persulfate activation: Application, mechanism, and tunable reaction pathways. Water Res. 2023, 234, 119808. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Fu, J.; Li, X.; Li, B.; Wang, X. Occurrence and fate of PPCPs in typical drinking water treatment plants in China. Environ. Geochem. Health 2019, 41, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Lai, C.; Ma, D.; Yi, H.; Zhang, M.; Xu, F.; Huo, X.; Ye, H.; Li, L.; Yang, L.; Tang, L.; et al. Functional partition of Fe and Ti co-doped g-C3N4 for photo-Fenton degradation of oxytetracycline: Performance, mechanism, and DFT study. Sep. Purif. Technol. 2023, 306, 122546. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Mujawar, M.; Mishra, Y.K.; Hickman, N.; Chavali, M.; Kaushik, A. Bio-inspired graphene-based nano-systems for biomedical applications. Nanotechnology 2021, 32, 502001. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Khunger, A.; Wallen, S.L.; Kaushik, A.; Chaudhary, G.R.; Varma, R.S. Advanced green analytical chemistry for environmental pesticide detection. Curr. Opin. Green Sustain. Chem. 2021, 30, 100488. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Raouf, M.E.A.; Maysour, N.E.; Farag, R.K.; Abdul-Raheim, A.M. Wastewater treatment methodologies, review article. Int. J. Environ. Agri. Sci. 2019, 3, 018. [Google Scholar]
- Rodríguez-Rodríguez, C.E.; Lucas, D.; Barón, E.; Gago-Ferrero, P.; Molins-Delgado, D.; Rodríguez-Mozaz, S.; Eljarrat, E.; Díaz-Cruz, M.S.; Barceló, D.; Caminal, G.; et al. Re-inoculation strategies enhance the degradation of emerging pollutants in fungal bioaugmentation of sewage sludge. Bioresour. Technol. 2014, 168, 180–189. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Baccar, R.; Caminal, G.; Sarrà, M. Can white-rot fungi be a real wastewater treatment alternative for organic micropollutants removal? A review. Water Res. 2018, 138, 137–151. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Singh, L.; Price, W.E.; Nghiem, L.D. Degradation of pharmaceuticals and personal care products by white-rot fungi—A critical review. Curr. Pollut. Rep. 2017, 3, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Lucas, D.; Badia-Fabregat, M.; Vicent, T.; Caminal, G.; Rodríguez-Mozaz, S.; Balcázar, J.L.; Barceló, D. Fungal treatment for the removal of antibiotics and antibiotic resistance genes in veterinary hospital wastewater. Chemosphere 2016, 152, 301–308. [Google Scholar] [CrossRef]
- Stenholm, Å.; Hedeland, M.; Arvidsson, T.; Pettersson, C.E. Removal of diclofenac from a non-sterile aqueous system using Trametes versicolor with an emphasis on adsorption and biodegradation mechanisms. Environ. Technol. 2019, 40, 2460–2472. [Google Scholar] [CrossRef]
- Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Beltrán-Hernández, R.I.; Coronel-Olivares, C.; Vázquez-Rodríguez, G.A. Biosorption of water pollutants by fungal pellets. Water 2020, 12, 1155. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.R.; Kim, M.S.; Lee, T.K. Unveiling of Concealed Processes for the Degradation of Pharmaceutical Compounds by Neopestalotiopsis sp. Microorganisms 2019, 7, 264. [Google Scholar] [CrossRef] [Green Version]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Dhiman, N.; Chaudhary, S.; Singh, A.; Chauhan, A.; Kumar, R. Sustainable degradation of pharmaceutical waste using different fungal strains: Enzyme induction, kinetics and isotherm studies. Environ. Technol. Innov. 2022, 25, 102156. [Google Scholar] [CrossRef]
- Buratti, S.; Rinaldi, F.; Calleri, E.; Bernardi, M.; Oliva, D.; Malgaretti, M.; De Girolamo, G.; Barucco, B.; Girometta, C.E.; Savino, E. Ganoderma resinaceum and Perenniporia fraxinea: Two Promising Wood Decay Fungi for Pharmaceutical Degradation. J. Fungi 2023, 9, 555. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; de Toledo, R.A.; Shim, H. Enhanced removal of naproxen and carbamazepine from wastewater using a novel countercurrent seepage bioreactor immobilized with Phanerochaete chrysosporium under non-sterile conditions. Bioresour. Technol. 2015, 197, 465–474. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Lucas, D.; Pereira, M.A.; Alves, M.; Pennanen, T.; Fritze, H.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Caminal, G. Continuous fungal treatment of non-sterile veterinary hospital effluent: Pharmaceuticals removal and microbial community assessment. Appl. Microbiol. Biotechnol. 2016, 100, 2401–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreve, M.J.; Brockman, A.; Hartleb, M.; Prebihalo, S.; Dorman, F.L.; Brennan, R.A. The white-rot fungus Trametes versicolor reduces the estrogenic activity of a mixture of emerging contaminants in wastewater treatment plant effluent. Int. Biodeterior. Biodegrad. 2016, 109, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Soil fungi for bioremediation of pesticide toxicants: A perspective. Geomicrobiol. J. 2022, 39, 352–372. [Google Scholar] [CrossRef]
- Shourie, A.; Vijayalakshmi, U. Fungal diversity and its role in mycoremediation. Geomicrobiol. J. 2022, 39, 426–444. [Google Scholar] [CrossRef]
- Olicón-Hernández, D.R.; Camacho-Morales, R.L.; Pozo, C.; González-López, J.; Aranda, E. Evaluation of diclofenac biodegradation by the ascomycete fungus Penicillium oxalicum at flask and bench bioreactor scales. Sci. Total Environ. 2019, 662, 607–614. [Google Scholar] [CrossRef]
- Dalecka, B.; Oskarsson, C.; Juhna, T.; Kuttava Rajarao, G. Isolation of fungal strains from municipal wastewater for the removal of pharmaceutical substances. Water 2020, 12, 524. [Google Scholar] [CrossRef] [Green Version]
- Cruz del Álamo, A.; Pariente, M.I.; Sanchez-Bayo, A.; Puyol, D.; Rodríguez, R.; Morales, V.; Bautista, L.F.; Vicente, G.; Melero, J.A.; Molina, R.; et al. Assessment of Trametes versicolor, Isochrysis galbana, and Purple Phototrophic Bacteria for the Removal of Pharmaceutical Compounds in Hospital Wastewater. Adv. Environ. Eng. Res. 2021, 2, 027. [Google Scholar]
- Kasonga, T.K.; Kamika, I.; Ngole-Jeme, V.M. Ligninolytic enzyme activity and removal efficiency of pharmaceuticals in a water matrix by fungus Rhizopus sp. Isolated from cassava. Environ. Technol. 2022, 44, 2157–2170. [Google Scholar] [CrossRef]
- Tomasini, A.; León-Santiesteban, H.H. The role of the filamentous fungi in bioremediation. In Fungal Bioremediation; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–21. [Google Scholar]
- Lu, S.; Yu, Y.; Ren, L.; Zhang, X.; Liu, G.; Yu, Y. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact. Sci. Total Environ. 2018, 621, 1389–1396. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S.S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Krogh, J.; Lyons, S.; Lowe, C.J. Pharmaceuticals and personal care products in municipal wastewater and the marine receiving environment near Victoria Canada. Front. Mar. Sci. 2017, 4, 415. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Bano, F.; Malik, A. Pharmaceuticals and personal care product (PPCP) contamination—A global discharge inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Hopkins, Z.R.; Blaney, L. An aggregate analysis of personal care products in the environment: Identifying the distribution of environmentally-relevant concentrations. Environ. Int. 2016, 92–93, 301–316. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Kaczala, F.; Blum, S.E. The Occurrence of Veterinary Pharmaceuticals in the Environment: A Review. Curr. Anal. Chem. 2016, 12, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Yuan, T.; Li, J.; Shen, Z.; Tian, Y. Occurrence, health risk assessment and water quality criteria derivation of six personal care products (PCPs) in Huangpu River, China. Environ. Monit. Assess. 2022, 194, 577. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.; Malik, S.; Shah, M.P.; Bora, J.; Chaudhary, V.; Kumar, L.; Sayyed, R.Z.; Ranjan, A. A comprehensive review on removal of pollutants from wastewater through microbial nanobiotechnology-based solutions. Biotechnol. Genet. Eng. Rev. 2022, 1–26. [Google Scholar] [CrossRef]
- Malik, S.; Dhasmana, A.; Preetam, S.; Mishra, Y.K.; Chaudhary, V.; Bera, S.P.; Ranjan, A.; Bora, J.; Kaushik, A.; Minkina, T.; et al. Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy. Nanomaterials 2022, 12, 4187. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Kishore, S.; Dhasmana, A.; Kumari, P.; Mitra, T.; Chaudhary, V.; Kumari, R.; Bora, J.; Ranjan, A.; Minkina, T.; et al. A Perspective Review on Microbial Fuel Cells in Treatment and Product Recovery from Wastewater. Water 2023, 15, 316. [Google Scholar] [CrossRef]
- Malik, S.; Kishore, S.; Bora, J.; Chaudhary, V.; Kumari, A.; Kumari, P.; Kumar, L.; Bhardwaj, A. A Comprehensive Review on Microalgae-Based Biorefinery as Two-Way Source of Wastewater Treatment and Bioresource Recovery. CLEAN–Soil Air Water 2023, 51, 2200044. [Google Scholar] [CrossRef]
- Satpati, G.G.; Dikshit, P.K.; Mal, N.; Pal, R.; Sherpa, K.C.; Rajak, R.C.; Rather, S.-U.; Raghunathan, S.; Davoodbasha, M. A state-of-the-art review on the co-cultivation of microalgae-fungi in wastewater for biofuel production. Sci. Total Environ. 2023, 870, 161828. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S. Derivatization of milk protein using poly (Acrylamide): Its characterization and application. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2018; Volume 381, No. 1; p. 1800120. [Google Scholar]
- Villaverde, J.; Rubio-Bellido, M.; Lara-Moreno, A.; Merchan, F.; Morillo, E. Combined use of microbial consortia isolated from different agricultural soils and cyclodextrin as a bioremediation technique for herbicide-contaminated soils. Chemosphere 2018, 193, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Bora, J.; Imam, S.; Vaibhav, V.; Malik, S. Use of Genetic Engineering Approach in Bioremediation of Wastewater. In Modern Approaches in Waste Bioremediation; Shah, M.P., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Cheng, W.; Li, J.; Wu, Y.; Xu, L.; Su, C.; Qian, Y.; Zhu, Y.-G.; Chen, H. The behavior of antibiotics and antibiotic resistance genes in the eco-agricultural system: A case study. J. Hazard. Mater. 2016, 304, 18–25. [Google Scholar] [CrossRef]
- Cajthaml, T.; Křesinová, Z.; Svobodová, K.; Möder, M. Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 2009, 75, 745–750. [Google Scholar] [CrossRef]
- Akerman-Sanchez, G.; Rojas-Jimenez, K. Fungi for the bioremediation of pharmaceutical-derived pollutants: A bioengineering approach to water treatment. Environ. Adv. 2021, 4, 100071. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [Green Version]
- Haroune, L.; Saibi, S.; Cabana, H.; Bellenger, J.P. Intracellular enzymes contribution to the biocatalytic removal of pharmaceuticals by Trametes hirsuta. Environ. Sci. Technol. 2017, 51, 897–904. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, S.Y.; Kang, M.; Lee, T.K. Removal of pharmaceuticals and personal care products using native fungal enzymes extracted during the ligninolytic process. Environ. Res. 2021, 195, 110878. [Google Scholar] [CrossRef]
- Lucas, D.; Castellet-Rovira, F.; Villagrasa, M.; Badia-Fabregat, M.; Barceló, D.; Vicent, T.; Caminal, G.; Sarrà, M.; Rodríguez-Mozaz, S. The role of sorption processes in the removal of pharmaceuticals by fungal treatment of wastewater. Sci. Total Environ. 2018, 610, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.; Meux, E.; Mathieu, Y.; Thuillier, A.; Chibani, K.; Harvengt, L.; Jacquot, J.-P.; Gelhaye, E. Xenomic networks variability and adaptation traits in wood-decaying fungi. Microb. Biotechnol. 2013, 6, 248–263. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R.Y. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018, 234, 190–213. [Google Scholar] [CrossRef] [PubMed]
- Raychoudhury, T.; Prajapati, S.K. Bioremediation of pharmaceuticals in water and wastewater. In Microbial Bioremediation & Biodegradation; Springer: Singapore, 2020; pp. 425–446. [Google Scholar]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 14613. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 163242. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Orta, M.F.; García-de la Cruz, R.F.; Paz-Maldonado, L.M.T.; Pedraza-González, D.A.; Morales-Avila, M.M.; Balderas-Hernández, V.E.; González-Ortega, O.; Pérez-Martínez, A.S. Assessing three industrially produced fungi for the bioremediation of diclofenac. J. Environ. Sci. Health Part A 2023, 1–10. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial laccase: Recent update on production, properties, and industrial applications. 3 Biotech 2017, 7, 323. [Google Scholar] [CrossRef]
- Holanda, F.H.; Birolli, W.G.; Morais, E.D.S.; Sena, I.S.; Ferreira, A.M.; Faustino, S.M.M.; Solon, L.G.d.S.; Porto, A.L.M.; Ferreira, I.M. Study of biodegradation of chloramphenicol by endophytic fungi isolated from Bertholletia excelsa (Brazil nuts). Biocatal. Agric. Biotechnol. 2019, 20, 101200. [Google Scholar] [CrossRef]
- Moghaddam, A.; Khayatan, D.; Esmaeili Fard Barzegar, P.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Esmaeili Gouvarchin Ghaleh, H.; Tebyaniyan, H. Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: A comprehensive overview. Int. J. Environ. Sci. Technol. 2023, 20, 5659–5696. [Google Scholar] [CrossRef]
- Negi, B.B.; Das, C. Mycoremediation of wastewater, challenges, and current status: A review. Bioresour. Technol. Rep. 2023, 11, 101409. [Google Scholar] [CrossRef]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: Mechanisms, challenges, and future prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion, and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef]
- Vacondio, B.; Birolli, W.G.; Ferreira, I.M.; Seleghim, M.H.; Gonçalves, S.; Vasconcellos, S.P.; Porto, A.L. Biodegradation of pentachlorophenol by marine-derived fungus Trichoderma harzianum CBMAI 1677 isolated from ascidian Didemnun ligulum. Biocatal. Agric. Biotechnol. 2015, 4, 266–275. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Takio, N.; Yadav, M.; Yadav, H.S. Catalase-mediated remediation of environmental pollutants and potential application–A review. Biocatal. Biotransformation 2021, 39, 389–407. [Google Scholar] [CrossRef]
- Chen, Y.; Stemple, B.; Kumar, M.; Wei, N. Cell surface displays fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol A and sulfamethoxazole. Environ. Sci. Technol. 2016, 50, 8799–8808. [Google Scholar] [CrossRef]
- Karigar, C.S.; Rao, S.S. Role of microbial enzymes in the bioremediation of pollutants: A review. Enzym. Res. 2011, 2011, 805187. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The use of algae and fungi for removal of pharmaceuticals by bioremediation and biosorption processes: A review. Water 2019, 11, 1555. [Google Scholar] [CrossRef] [Green Version]
- Hena, S.; Gutierrez, L.; Croué, J.P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Syed, K.; Porollo, A.; Lam, Y.W.; Grimmett, P.E.; Yadav, J.S. CYP63A2 is a catalytically versatile fungal P450 monooxygenase capable of oxidizing higher-molecular-weight polycyclic aromatic hydrocarbons, alkylphenols, and alkanes. Appl. Environ. Microbiol. 2013, 79, 2692–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madadi, R.; Bester, K. Fungi and biochar applications in bioremediation of organic micropollutants from aquatic media. Mar. Pollut. Bull. 2021, 166, 112247. [Google Scholar] [CrossRef]
- Li, F.; Ma, F.; Zhao, H.; Zhang, S.; Wang, L.; Zhang, X.; Yu, H. A lytic polysaccharide monooxygenase from a white-rot fungus drives the degradation of lignin by a versatile peroxidase. Appl. Environ. Microbiol. 2019, 85, e02803-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Morató, C.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D.; Marco-Urrea, E.; Vicent, T.; Sarrà, M. Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor. Water Res. 2013, 47, 5200–5521. [Google Scholar] [CrossRef]
- Jelic, A.; Cruz-Morató, C.; Marco-Urrea, E.; Sarrà, M.; Perez, S.; Vicent, T.; Petrović, M.; Barcelo, D. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012, 46, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.B.; Madhavan, A.; Tarafdar, A.; Sirohi, R.; Anoopkumar, A.N.; Kuriakose, L.L.; Awasthi, M.K.; Binod, P.; Varjani, S.; Sindhu, R. Filamentous fungi for pharmaceutical compounds degradation in the environment: A sustainable approach. Environ. Technol. Innov. 2023, 31, 103182. [Google Scholar]
- Aranda, E.; Godoy, P.; Reina, R.; Badia-Fabregat, M.; Rosell, M.; Marco-Urrea, E.; García-Romera, I. Isolation of Ascomycota fungi with capability to transform PAHs: Insights into the biodegradation mechanisms of Penicillium oxalicum. Int. Biodeterior. Biodegrad. 2017, 122, 141–150. [Google Scholar] [CrossRef]
- Blánquez, P.; Sarrà, M.; Vicent, T. Development of a continuous process to adapt the textile wastewater treatment by fungi to industrial conditions. Process Biochem. 2008, 43, 1–7. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Operation of stirred tank reactors (STRs) and fixed-bed reactors (FBRs) with free and immobilized Phanerochaete chrysosporium for the continuous removal of pharmaceutical compounds. Biochem. Eng. J. 2012, 66, 38–45. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of three active pharmaceutical compounds by the fungus Phanerochaete chrysosporium in a fed-batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef]
- Bashir, S.M.; Kimiko, S.; Mak, C.-W.; Fang, J.K.-H.; Gonçalves, D. Personal Care and Cosmetic Products as a Potential Source of Environmental Contamination by Microplastics in a Densely Populated Asian City. Front. Mar. Sci. 2021, 8, 683482. [Google Scholar] [CrossRef]
- Saharan, V.K.; Rizwani, M.A.; Malani, A.A.; Pandit, A.B. Effect of the geometry of hydrodynamically cavitating device on the degradation of orange-G. Ultrason. Sonochemistry 2013, 20, 345–353. [Google Scholar] [CrossRef]
- Lim, F.Y.; Ong, S.L.; Hu, J. Recent Advances in the Use of Chemical Markers for Tracing Wastewater Contamination in Aquatic Environment: A Review. Water 2017, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Monitoring the occurrence of pharmaceuticals in soils irrigated with reclaimed wastewater. Environ. Pollut. 2018, 235, 312–321. [Google Scholar] [CrossRef]
- Rashid, S.S.; Liu, Y.Q. Comparison of life cycle toxicity assessment methods for municipal wastewater treatment with the inclusion of direct emissions of metals, PPCPs, and EDCs. Sci. Total Environ. 2021, 756, 143849. [Google Scholar] [CrossRef] [PubMed]
- Tarpani, R.R.Z.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and their effect on the environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G. A critical review of the current technologies in wastewater treatment plants by using hydrodynamic cavitation process: Principles and applications. J. Environ. Health Sci. Eng. 2020, 18, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Govindwar, S.P.; Kurade, M.B.; Jeon, B.H. (Eds.) Current Developments in Bioengineering and Biotechnology: Advances in Eco-Friendly and Sustainable Technologies for the Treatment of Textile Wastewater; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in the environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present, and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.; Kahissay, M.H.; Hailu, A.D. Pharmaceuticals wastage and pharmaceuticals waste management in public health facilities of Dessie town, North East Ethiopia. PLoS ONE 2021, 16, e0259160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Abdollahi, M. Environmental Distribution of Personal Care Products and Their Effects on Human Health. Iran. J. Pharm. Res. 2021, 20, 216–253. [Google Scholar] [CrossRef]
- Checcucci, A.; Trevisi, P.; Luise, D.; Modesto, M.; Blasioli, S.; Braschi, I.; Mattarelli, P. Exploring the Animal Waste Resistome: The Spread of Antimicrobial Resistance Genes through the Use of Livestock Manure. Front. Microbiol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environ. Pollut. 2018, 241, 1153–1166. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Islas-Flores, H.; Dublán-García, O.; SanJuan-Reyes, N. Amoxicillin in the Aquatic Environment, Its Fate and Environmental Risk. In Environmental Health Risk—Hazardous Factors to Living Species; Larramendy, M., Soloneski, S., Eds.; InTech: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [Green Version]
- Qandil, A.M.; Al-Zoubi, L.O.; Al-Bakri, A.G.; Amawi, H.A.; Al-Balas, Q.A.; Alkatheri, A.M.; Albekairy, A.M. Synthesis, Antibacterial Evaluation, and QSAR of α-Substituted-N4-Acetamides of Ciprofloxacin and Norfloxacin. Antibiotics 2014, 3, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. CLEAN—Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Peng, X.; Ou, W.; Wang, C.; Wang, Z.; Huang, Q.; Jin, J.; Tan, J. Occurrence and ecological potential of pharmaceuticals and personal care products in groundwater and reservoirs in the vicinity of municipal landfills in China. Sci. Total Environ. 2014, 490, 889–898. [Google Scholar] [CrossRef]
- Kung, T.A.; Lee, S.H.; Yang, T.C.; Wang, W.H. Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan. Sci. Total Environ. 2018, 635, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.P.; Kogut, K.R.; Bradman, A.; She, J.; Gavin, Q.; Zahedi, R.; Parra, K.L.; Harley, K.G. Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carmona, Y.; Ashrap, P.; Calafat, A.M.; Ye, X.; Rosario, Z.; Bedrosian, L.D.; Huerta-Montanez, G.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; et al. Determinants and characterization of exposure to phthalates, DEHTP, and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 56–69. [Google Scholar] [CrossRef]
- Herzog, B.; Amorós-Galicia, L.; Sohn, M.; Hofer, M.; Quass, K.; Giesinger, J. Analysis of photokinetics of 2′-ethylhexyl-4-methoxycinnamate in sunscreens. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2019, 18, 1773–1781. [Google Scholar] [CrossRef]
- Le Thi Minh, T.; Nguyen Phuoc, D.; Dinh Quoc, T.; Ngo, H.H.; Do Hong Lan, C. Presence of e-EDCs in surface water and effluents of pollution sources in Sai Gon and Dong Nai river basin. Sustain. Environ. Res. 2016, 26, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Jjemba, P.K. Pharma-Ecology: The Occurrence and Fate of Pharmaceuticals and Personal Care Products in the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [Green Version]

| Authors and Date | Timeline of Proposed Hypothesis and Research | Proposed Hypothesis and Significant Findings | The Significant Contribution of Studies in Current Articles from the Review |
|---|---|---|---|
| [1,2,4,5,6,7,11,33,34,35,36,37,38,39,40] | 2012–2021 | Presence of PPCPs and merging resources as sources of contamination in effluent and water bodies at the global level in waterbodies. | In total, 28.5% of studies show PPCPs and other sources as contamination of wastewater. |
| [3,41,42,43,44,45,46,47,48] | 2018–2023 | Use of physical, chemical, and biological methods (bioremediation) through general microbes and nanotechnology. | In total, 2.5% of data show the use of chemical treatment, bioremediation, and green nanobiotechnology in wastewater treatment. |
| [15,16,18,19,20,21,22,23,24,25,26,29,31,32,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] | 2008–2023 | PPCP degradation through bioremediation using white-rot fungal strains through fungal-based enzymes (oxidoreductase, lignocellulolytic, and laccases), endophytic fungi, Neopestalotiopsis spp., Rhizopus, Phanerochaete chrysosporium, Trichoderma harzianum, and hybrid process with both the chemical and biological process. | In total, 32.7% of derived data explain the function of various fungal enzymes in bioremediation and hybrid bioremediation. |
| [30,55,75,76,77,78,79,80,81] | 2011–2021 | PPCP degradation through combinatorial bioremediation using enzymatic algal and fungal strains (white rot), only algal strains and fungal + biochar, and fungus + bacteria. | In total, 9.2% of data explain the role of only pure fungal strains and mixed cultures in wastewater treatment. |
| [14,17,82,83,84,85,86] | 2012–2014 | Biodegradation through Trametes versicolor and Penicillium oxalicum fungal strains using bioreactors. | In total, 10% of data reveal involvement and culturing of specific fungal enzymes with the latest techniques using fluidized bed reactors (FBR). |
| [23,87,88] | 2012–2015 | Biodegradation through Phanerochaete chrysosporium fungal strains using bioreactors (fed-batch stirred reactor). | |
| [6,8,9,10,33,51,89,90,91,92,93,94,95,96,97] | 2018–2023 | Advanced techniques, devices, and life cycle toxicity assessment methods for treating industrial wastewater contaminated by PPCP pollutants. Use of chemical methods, chemical markers, and dermal contact in tracing and monitoring aquatic contamination, bisphenols, and triclosan in soils irrigated wastewater. | In total, 11.76% of studies focus on life cycle assessment techniques for wastewater treatment. The data show methods of monitoring and tracing contaminated wastewater. |
| Pollutant | Use | Source | Hazard Response | References |
|---|---|---|---|---|
| Pharmaceutical Drugs | ||||
| Penicillin G | STDs such as syphilis/gonorrhea. Bacterial pneumonia, wounds, and other infections, meningitis, and anthrax | Manure (primarily liquid) | Allergic reactions, diarrhea, nausea/vomiting, neurotoxicity/seizure, fever, and angioedema | [99] |
| Cephalosporin | UTI, septic shock, infection of bones and joints, and OBGYN infections | Water (surface and sewage) | Anaphylaxis during surgery, skin rashes/ urticaria, and positive Coomb’s test | [104] |
| Amoxicillin | UTI and infection of genitals, tonsillitis, LRTI, and otolaryngological infections | STP and hospital effluents, surface water, and WWTPs | Hypersensitivity of all types, including anaphylaxis, and GI disturbances | [1,105] |
| Tetracycline | Parapharyngeal infections, UTI, and GI infections | Manure and groundwater | Bloating, black hairy tongue, throat infection, and migraine | [106] |
| Ciprofloxacin | UTI, neonatal sepsis, typhoid, and cystic fibrosis | River water and domestic water supply | Nausea, fatigue, malaise, pale skin, aberrant liver function, and headache | [1,98] |
| Norfloxacin | Genitourinary tract infection | Tap water and river water | Pain in joints and muscles, rectal pain, stomach upset, dizziness, nausea, and headache | [1,107] |
| Sulfamethoxazole | Shigellosis, URTI, LRTI, and UTI | Plant leaves, sewage, and river and ground water | Neuropsychological symptoms, pruritis, and migraine | [1,6,98] |
| Erythromycin | RTI, bronchitis, lung infection, diphtheria, and pertussis | Tap, river, and sewage water | Severe GI symptoms, including abdominal cramping | [1,6,108] |
| Trimethoprim | Cystitis | Groundwater | Severe GI manifestations including change in taste and apathy for food | [109] |
| Sulphadiazine | UTI, Toxoplasma gondii encephalitis, malaria, infection of the ear, and chancroid | Water (ground) | Loss of appetite, emesis, diarrhea, and headache | [98] |
| Ibuprofen/ Diclofenac | NSAID | Groundwater and seawater | Bioaccumulation and toxicity | [1] |
| Metformin | Antidiabetic (antihyperglycemic agent) | Seawater | Bioaccumulation and toxicity | [1] |
| Acetaminophen/ Paracetamol | Antipyretic/analgesic | Sea and surface water, hospital, municipal effluents and sediments, and WWTPs | Bioaccumulation and toxicity | [1] |
| Caffeine/Nicotine | Neuro-stimulant | Sea, river, treated, and untreated WWTPs | Bioaccumulation and toxicity | [1,6,98] |
| Naproxen/ Indomethacin | NSAID | Groundwater and river water | Bioaccumulation and toxicity | [1,109] |
| Triclocarban/ Triclosan | Antimicrobial | WWTP (influent) | Bioaccumulation and toxicity | [1] |
| Diltiazem | Calcium channel blockers | Surface water and sediment | Bioaccumulation and toxicity | [1] |
| Carbamazepine | Anticonvulsant | Surface water and sediment | Bioaccumulation and toxicity | |
| Morphine/ Dihydrocodeine | Analgesics (opioid) | River water and treated and untreated WWTPs | Bioaccumulation and toxicity | [98] |
| Atenolol | Antihypertensive | Surface water, seawater, and sediment | Bioaccumulation and toxicity | [1] |
| 17α- ethinylestradiol/17 β-estradiol | Estrogen (endocrine-disrupting compounds) | Sediment and sludge/WWTPs | Bioaccumulation and toxicity | [39] |
| Methylparaben/ Ethylparaben | Preservative (endocrine disrupter) | River water and reservoirs | Bioaccumulation and toxicity | [109] |
| Clofibric acid/ Benzafibrate/ Gemfibrozil | Lipid regulator | Groundwater | Bioaccumulation and toxicity | [98] |
| Clotrimazole/ Econazole/ Miconazole/ Tebuconazole/ Ketoconazole | Antifungal and pesticides | Reservoirs and groundwater | Bioaccumulation and toxicity | [109] |
| Personal care products | ||||
| Alkylphenol polyethoxylated (APEOs): NP, NPEOs, OP, 4OP, 4tOP, and OPEs | Detergents, disinfectants, and surface cleaners | Wastewater treatment plants (WWTPs), sludge and sediments, landfill leaks, and surface water | Endocrine disruption interferes with human reproduction, inhibits progesterone/androstenedione, induces testosterone/17β-estradiol production, decreases the human sperm count and motility, alters hormone metabolism and aberrant hypothalamus–pituitary–adrenal axis activity, ulcerative colitis, hepatic infection, and various carcinomas | [102,110] |
| Antimicrobials: triclocarban, Triclosan, 1,4-dichlorobenzene, and ortho-phenylphenol | Detergents, toothpaste, soaps, and perfumes | Solid, sediment, effluent, raw wastewater, drinking water, and surface and groundwater | ROS generation, reduced GSH/GSSG ratio, and altered mRNA expressions, aberrant energy production and cell cycle regulation, apoptosis, lipid accumulation, decreased sperm count and motility, PCOS, aberrant thyroid function, autoimmunity, and congenital disabilities | [111,112] |
| Bisphenols: Bisphenol A, B, F, AF, and S (BPA/B/F/AF/S) | Shampoos and conditioners, sun protection lotion, washing detergents, nail polishes, and shaving creams | Freshwater, tap water, river and marine surface water, and sediments | Adverse reproductive health, hampered pregnancy, fetal growth, preterm birth, uterine leiomyoma, risk of oxidative damage to nucleic acids, and risk of diabetes | [40,112] |
| D3, 4, 5, and 6 | Antiperspirants, lotions, oils, shampoos, creams for baby care, deodorants, fragrances, hair care products, lotions, nail colors, and skin cleansers | Air and sewage systems | Pulmonary toxicity, estrogenic activity, and breast carcinogenesis | [102,112] |
| Ethanolamines: MEA, DEA, and TEA | Cleaners, detergents, shampoos, and dyes | Soil and groundwater | Allergic irritation, contact dermatitis, bronchoconstriction and asthma, scalp irritation, hair loss, cuticle damage, and protein loss | |
| Fragrances: ATII, DPMI, ABDI, AHDI, HHCB, and AHTN | Room fresheners, body lotions, cleansing lotions, deodorants, fabric softeners, detergents, and other cosmetics | Air and sewage systems and river water | Atopic asthma, phototoxicity, photoallergy, hand eczema and allergy, contact dermatitis, antiprogestogenic effects, and human ovarian cancer | |
| Glycol ethers: ME, BE, IPE, and EE | Face wash, lotions, polish/wax, and shaving cream | Indoor air | Longer pregnancy time, reduced motile sperm count, lethal for developing children, asthma, eczema, allergic rhinosinusitis, IgE sensitization, skin erythema, and contact dermatitis | |
| Insect repellents: DEET, BR, IR3535, ID, and PBO | Insect repellents and cosmetics | River water and groundwater | Manageable minor toxicities, erythematous–edematous dermatitis, urticaria, and carcinogenic potential | |
| Parabens: BePB, EP, MP, PP, and BP | Blush, cosmetics, foundation, mascara, and sunscreen | River water and soil | Breast tumors, endocrine disruptors to birth outcomes, decreased neonates’ body length, and impaired child cognitive abilities | |
| Phthalate: BBP, DEHP, DEHTP, DMP, DiBP, DBP, and DiNP | Several cosmetics, antiperspirants, baby care products, perfumes, hair gels/sprays, and mousses | River water, fresh water, air, and soil | Skin irritation, rhinitis, and eczema, asthma, altered liver and kidney function, metabolic disorder, cardiological problems, obesity, lipid accumulation, and behavioral problems among children | [113] |
| UV filters: BP1, BP2, BP3, BP4, 3BC, BS, 4DHB, EHMC, PEG25-PABA, Et-PABA. HMS, 4HB, IMC, 4MBC OC, OD-PABA, OMC, OS, PS, TiO2, and ZnO | Cosmetics | Groundwater, surface water, drinking water, wastewater effluents, and seawater | Skin sensitization, impaired reproductive health, and Hirschsprung’s disease | [37,102,111,112,114] |
| Fungal Strain | Enzyme(s) Involved | PPCP | Reactor Type | Culture Type | Initial Concentration (mg/L) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| Trametes versicolor | Laccase, LiP, and MnP |
| Fluidized bed | Fed-batch |
|
| [82] |
| Trametes versicolor | Laccase, LiP, and MnP |
| Fluidized bed | Fed batch |
|
| [83] |
| Trametes versicolor | Laccase, LiP, and MnP |
| Fixed bed | Continuous |
|
| [86] |
| Trametes versicolor | Laccase, LiP, and MnP |
| Fluidized bed | Continuous |
|
| [61] |
| Trametes versicolor | Laccase, LiP, and MnP |
| Memnbrane reactor | Continuous |
|
| [60] |
| Trametes versicolor | Laccase, LiP, and MnP |
| Erlenmeyer flask | Fed-batch |
|
| [50] |
| Phanerochaet chrysosporium | LiP and MnP |
| Stirred tank | Fed-batch |
|
| [12] |
| LiP and MnP |
| Stirred tank | Continuous |
|
| ||
| Pleurotus ostreatus | Laccase and MnP |
| Erlenmeyer flask | Fed-batch |
|
| [50] |
| Dichomitus squalene |
| Erlenmeyer flask | Fed-batch |
|
| ||
| Bjerkandera adusta |
| Erlenmeyer flask | Fed-batch |
|
|
| Name of the Physiological Parameter | Type of Physiological Parameter | Abbreviation | Source | Used for the Bioremediation of | References |
|---|---|---|---|---|---|
| Laccase | Enzyme | Lac | Cerrena unicolor, Trametes hispida, Daedalea quercina, Coriolus versicolor, Trametes versicolor | 2,4- Dichlorophenol, pentachlorophenol TNT PAH Anthracene and benzo pyrene Delor 106 (PCB) PCP Atrazine (herbicide) Di(2-ethylhexyl) phthalate, heavy metals Pesticides Phenols PCB Phenylurea herbicide diuron Gasoline Anthracene Naphthalene Organic pollutants | [60] |
| Tyrosinase | Enzyme | Tyros | Agaricus bisporus | Phenolic compounds | [95] |
| Lignin Peroxidase | Enzyme | LiP | Phanerochaete chrysosporium | Bentazon (herbicide) Trichlorophenol Poly aromatic hydrocarbon (PAH) Delor 106 (Polychlorinated biphenyl (PCB)) Phencyclidine (PCP) Remazol Brilliant Blue R PAH | [81] |
| Versatile Peroxidase | Enzyme | VP | Pleurotus eryngii, Bjerkandera adusta | Phenolic as well as non-phenolic compounds | [75] |
| Manganese peroxidase | Enzyme | MnP | Phlebia radiata, Lentinula edodes, Pleurotus ostreatus, Phanerochaete chrysosporium | Trichlorophenol PAH P. Delor 106 (PCB) Remazol Brilliant Blue R PCP PAH Di(2-ethylhexyl) phthalate, heavy metals, PAH Reactive black 5, Veratryl alcohol | [95] |
| Dye-decolorizing peroxidases | Enzyme | DyPs | Irpex lacteus | Non-phenolic lignin model compounds Organic compounds | [63] |
| Unspecific peroxygenases | Enzyme | UPO | Agrocybe aegerita, Marasmius rotula | Non-phenolic lignin model compounds Organic compounds | |
| Catalase | Enzyme | Cat | Neurospora crassa | Heavy metals | [73] |
| Cytochrome P450 | Cytochrome | Cyt | Phanerochaete chrysosporium, Saccharomyces cerevisiae | Mutagenic/carcinogenic fused-ring high molecular weight PAHs (HMW-PAHs) Crude oil aliphatic hydrocarbon n-alkanes Endocrine-disrupting long-chain alkylphenols (APs) | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; Bora, J.; Nag, S.; Sinha, S.; Mondal, S.; Rustagi, S.; Hazra, R.; Kumar, H.; Rajput, V.D.; Minkina, T.; et al. Fungal-Based Remediation in the Treatment of Anthropogenic Activities and Pharmaceutical-Pollutant-Contaminated Wastewater. Water 2023, 15, 2262. https://doi.org/10.3390/w15122262
Malik S, Bora J, Nag S, Sinha S, Mondal S, Rustagi S, Hazra R, Kumar H, Rajput VD, Minkina T, et al. Fungal-Based Remediation in the Treatment of Anthropogenic Activities and Pharmaceutical-Pollutant-Contaminated Wastewater. Water. 2023; 15(12):2262. https://doi.org/10.3390/w15122262
Chicago/Turabian StyleMalik, Sumira, Jutishna Bora, Sagnik Nag, Sweta Sinha, Sagar Mondal, Sarvesh Rustagi, Richismita Hazra, Harshavardhan Kumar, Vishnu D. Rajput, Tatiana Minkina, and et al. 2023. "Fungal-Based Remediation in the Treatment of Anthropogenic Activities and Pharmaceutical-Pollutant-Contaminated Wastewater" Water 15, no. 12: 2262. https://doi.org/10.3390/w15122262