Improved Delivery of Nanoscale Zero-Valent Iron Particles and Simplified Design Tools for Effective Aquifer Nanoremediation
Abstract
:1. Introduction
2. Materials and Methods
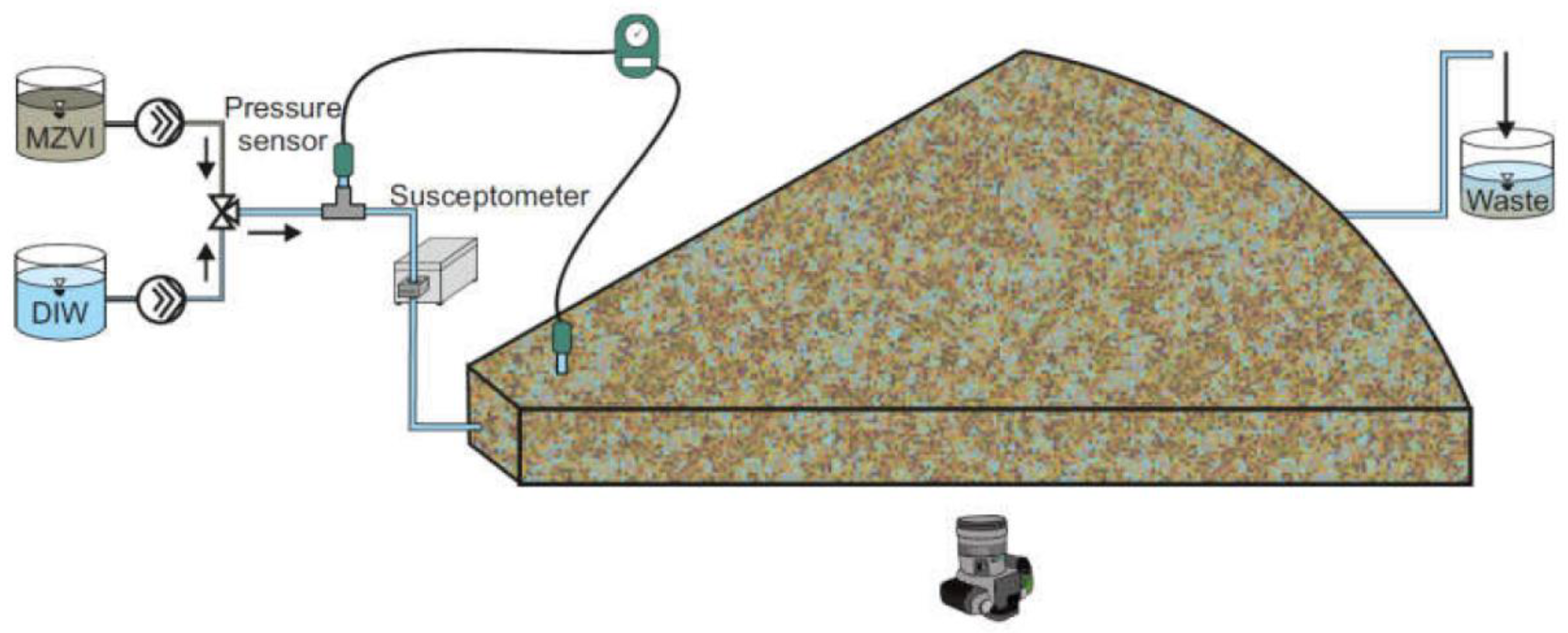
2.1. Experimental Setup and Procedures
2.2. Particle Radial Transport Model
- Equation (1) is a modified advection–dispersion equation that describes the particle transport through a porous medium and their deposition due to physical and physicochemical interactions with the solid matrix;
- Within Equation (1), the liquid–solid phase mass exchange term can assume different forms according to the particle retention mechanism to be taken into account. A single-site linear irreversible deposition kinetics (Equation (2)) was selected for the interpretation of the nZVI test described in Section 2.1;
- Equation (3) expresses the dependency of the particle-attachment coefficient on the slurry and aquifer properties, i.e., the water-flow velocity, the suspension viscosity, the aquifer average grain size [25]. The attachment coefficient also depends on the single collector contact efficiency that, in this study, was calculated using the formulation proposed by Messina et al. (2015, ref. [46]). is a theoretical parameter that, under specific assumptions and simplifications (e.g., single spherical collector, infinite fluid domain, uniform flow field), describes the effect of different deposition mechanisms (i.e., gravitational sedimentation, interception, Brownian diffusion) on the particle transport [47,48].
- Equation (4) defines the steady-state flow field resulting from the fluid injection through a single screened well in an infinite, homogeneous and isotropic aquifer system. Under these hypotheses, and assuming negligible influence of the groundwater background velocity on the overall flow field, the Darcy velocity depends only on the injection flow rate and well geometry, and hyperbolically decreases with increasing distance from the injection well. Although previous studies have demonstrated that background flow can have a notable influence on nZVI transport [38,39], in the specific conditions investigated in this study, the assumption of negligible groundwater flow effects can be deemed acceptable. This is due to the fact that the flow field generated by the well during particle injection (with velocities reaching up to 500 m/day) predominates over the natural groundwater velocity, which typically ranges from centimeters to a few meters per day in highly conductive aquifers;
- Equation (5) is a modified Darcy’s law for shear-thinning fluids that expresses the pressure build-up induced by the injection as a function of the porous medium hydraulic conductivity and fluid viscosity .
2.3. Radial Transport Test Interpretation
2.4. Multiparametric Analysis
3. Results
3.1. Slurry Rheology
- the viscosity is in the order of 104 Pa·s in quasi-static conditions (i.e., at shear rates ), which is optimal to guarantee pre-injection stability of the iron suspensions;
- the viscosity decreases to less than 10−3 Pa·s at shear stress values typical of subsurface injections (i.e., at ), thus allowing the expected pressure build-up to be contained.
3.2. Radial Transport Experiment
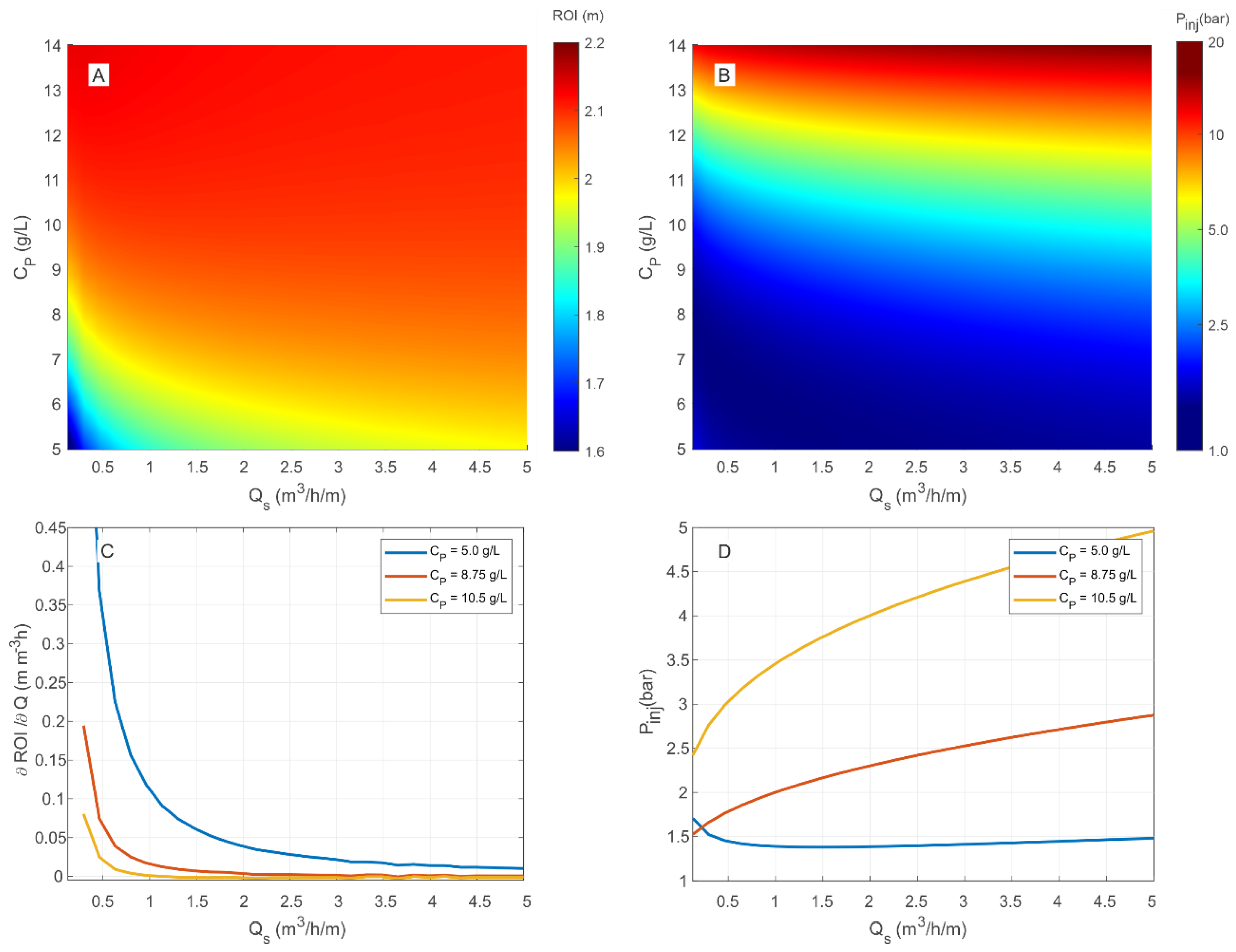
3.3. Predictive Simulations and Implications for Field Applications
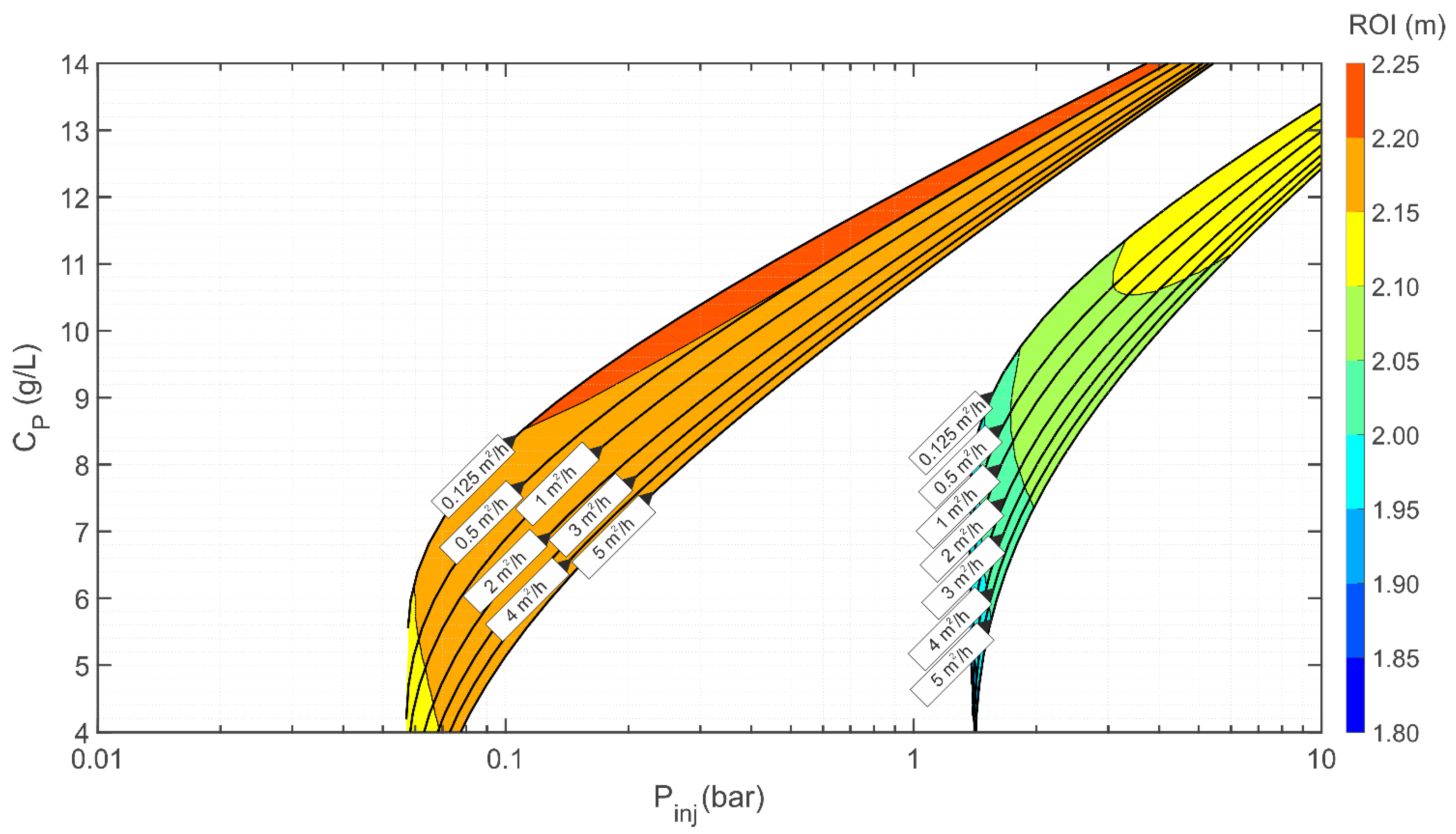
3.4. Multiparametric Graphs
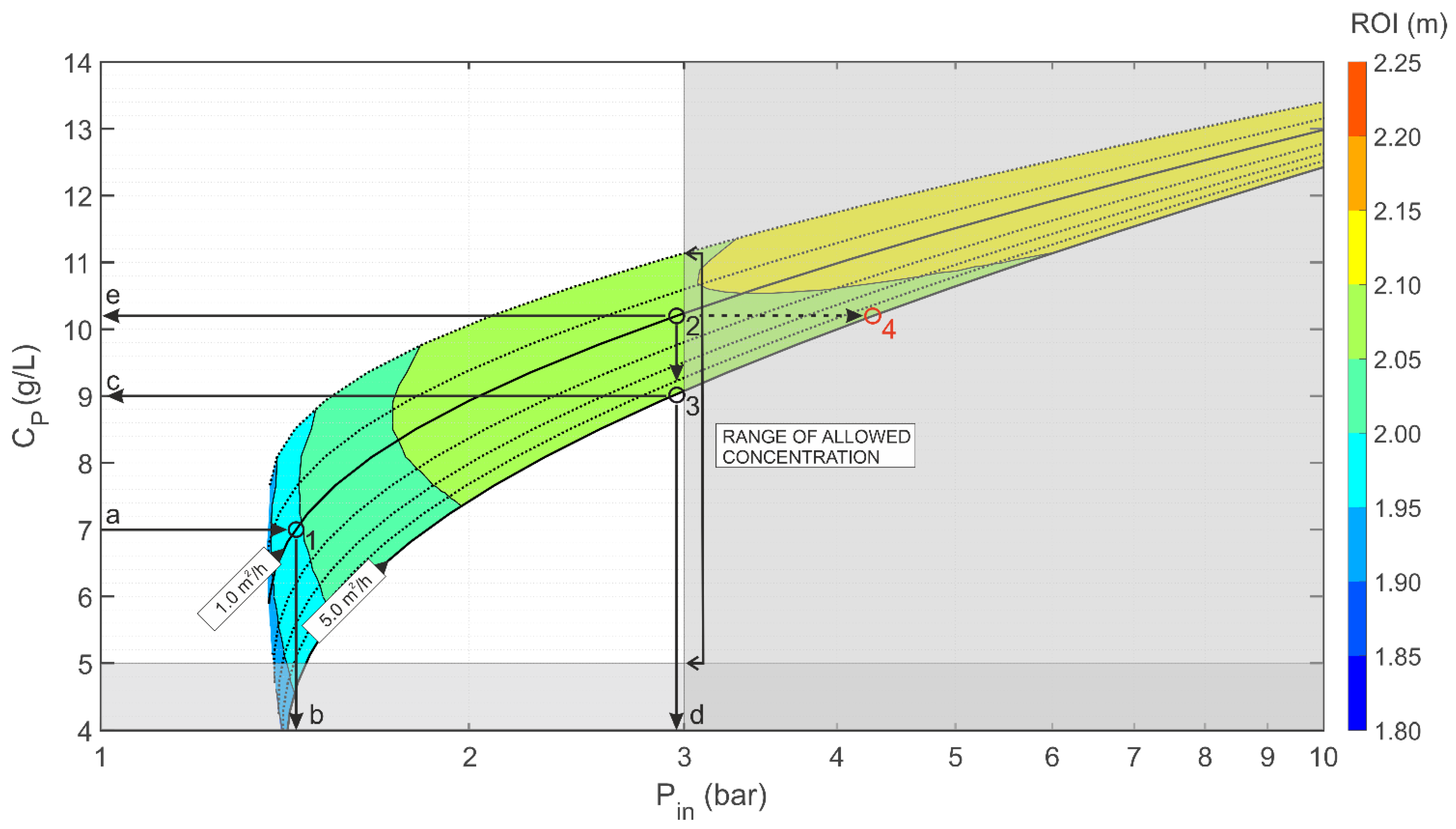
Example of Use of the nZVI Injection Diagrams
- to keep constant the injection flowrate and increase the stabilizer concentration: this can be achieved by going from point 1 to point 2 by moving along the specific flow rate curve of 1 m3/h/m up to the maximum allowed stabilizer concentration of 10.2 g/L; at this new working point it is possible to increase the radius of influence up to 2.10 m with an injection pressure of 2.9 bar;
- to increase both the injection flow rate and the stabilizer concentration: this can be accomplished by imposing equal to 5 m2/h and the injection pressure equal to 2.9 bar, and by deriving from the graph the corresponding maximum dose of stabilizing gel that can be applied to not exceed the pressure threshold (); in these operational conditions, identified by the working point 3, an ROI between 2.05 and 2.10 m is expected. For comparison, if the gel concentration had been increased to 10.2 g/L as in the previous example, the expected injection pressure would have been greater than 4 bar (point 4 in Figure 6), thus leading to potential fracturing of the porous medium.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Acronyms | |
| DIW | Deionized water |
| MNMs | Micro- and Nanoparticle transport, filtration, and clogging Model-Suite |
| mZVI | Microscale zero-valent iron |
| nZVI | Nanoscale zero-valent iron |
| PV | Pore volume |
| ROI | Radius of influence |
| Latin letters | |
| Empirical viscosity correction coefficient (m3/kg) | |
| Specific surface area of the porous medium (m2/m3) | |
| Initial specific surface area of the porous medium (m2/m3) | |
| Specific surface area of the iron particles (m2/m3) | |
| Empirical power law correction coefficient (m3/kg) | |
| length of the well screening (m) | |
| Empirical attachment coefficient (s−1) | |
| Iron particles concentration in the mobile phase (kg/m3) | |
| Polymeric stabilizer concentration (g/L) | |
| Dispersion coefficient (m2/s) | |
| Mean diameter of sand grains (m) | |
| Gravity acceleration (m/s2) | |
| Porous medium permeability (m2) | |
| Intrinsic permeability (m2) | |
| Particle attachment coefficient (s−1) | |
| Power law index (−) | |
| Pressure (Pa) | |
| Threshold fracturing pressure (bar) | |
| Darcy velocity (m/s) | |
| Discharge rate (m3/s) | |
| Unit-length discharge rate (m3/h/m) | |
| Radial distance from well (m) | |
| Iron particles concentration in the solid phase (−) | |
| Depth to water table (m) | |
| Time (s) | |
| Depth from ground level (m) | |
| Greek letters | |
| Shift factor (−) | |
| Shear rate (s−1) | |
| Porosity (−) | |
| Initial porosity (−) | |
| Single collector contact efficiency (−) | |
| Surface increment coefficient (−) | |
| Density reduction coefficient (−) | |
| Fluid viscosity (Pa·s) | |
| Density of sand grains (kg/m3) | |
| Density of iron particles (kg/m3) | |
| Density of injected fluid (kg/m3) | |
| Reference viscosity (Pa·sn) | |
References
- Palma, V.; Accorsi, F.; Casasso, A.; Bianco, C.; Cutrì, S.; Robiglio, M.; Tosco, T. AdRem: An Integrated Approach for Adaptive Remediation. Sustainability 2020, 13, 28. [Google Scholar] [CrossRef]
- Suthersan, S.S.; Horst, J.; Schnobrich, M.; Welty, N.; McDonough, J. Remediation Engineering: Design Concepts; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1-4987-7336-2. [Google Scholar]
- Beryani, A.; Bianco, C.; Casasso, A.; Sethi, R.; Tosco, T. Exploring the Potential of Graphene Oxide Nanosheets for Porous Media Decontamination from Cationic Dyes. J. Hazard. Mater. 2022, 424, 127468. [Google Scholar] [CrossRef]
- Mohammadian, S.; Krok, B.; Fritzsche, A.; Bianco, C.; Tosco, T.; Cagigal, E.; Mata, B.; Gonzalez, V.; Diez-Ortiz, M.; Ramos, V.; et al. Field-Scale Demonstration of in Situ Immobilization of Heavy Metals by Injecting Iron Oxide Nanoparticle Adsorption Barriers in Groundwater. J. Contam. Hydrol. 2021, 237, 103741. [Google Scholar] [CrossRef] [PubMed]
- Czinnerová, M.; Vološčuková, O.; Marková, K.; Ševců, A.; Černík, M.; Nosek, J. Combining Nanoscale Zero-Valent Iron with Electrokinetic Treatment for Remediation of Chlorinated Ethenes and Promoting Biodegradation: A Long-Term Field Study. Water Res. 2020, 175, 115692. [Google Scholar] [CrossRef]
- Mondal, A.; Dubey, B.K.; Arora, M.; Mumford, K. Porous Media Transport of Iron Nanoparticles for Site Remediation Application: A Review of Lab Scale Column Study, Transport Modelling and Field-Scale Application. J. Hazard. Mater. 2021, 403, 123443. [Google Scholar] [CrossRef]
- Wacławek, S.; Nosek, J.; Cádrová, L.; Antoš, V.; Černík, M. Use of Various Zero Valent Irons for Degradation of Chlorinated Ethenes and Ethanes. Ecol. Chem. Eng. S 2015, 22, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Bianco, C.; Tosco, T.; Sethi, R. Zerovalent Iron for the Remediation of Contaminated Aquifers. Geoing. Ambient. E Min. 2018, 155, 5–16. [Google Scholar]
- Tosco, T.; Petrangeli Papini, M.; Cruz Viggi, C.; Sethi, R. Nanoscale Zerovalent Iron Particles for Groundwater Remediation: A Review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Mangayayam, M.C.; Perez, J.P.H.; Alonso-de-Linaje, V.; Dideriksen, K.; Benning, L.G.; Tobler, D.J. Sulfidation Extent of Nanoscale Zerovalent Iron Controls Selectivity and Reactivity with Mixed Chlorinated Hydrocarbons in Natural Groundwater. J. Hazard. Mater. 2022, 431, 128534. [Google Scholar] [CrossRef]
- Němeček, J.; Pokorný, P.; Lacinová, L.; Černík, M.; Masopustová, Z.; Lhotský, O.; Filipová, A.; Cajthaml, T. Combined Abiotic and Biotic In-Situ Reduction of Hexavalent Chromium in Groundwater Using NZVI and Whey: A Remedial Pilot Test. J. Hazard. Mater. 2015, 300, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of Organic Compounds by Nanoscale Zero-Valent Iron and Its Composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- Gallo, A.; Bianco, C.; Tosco, T.; Tiraferri, A.; Sethi, R. Synthesis of Eco-Compatible Bimetallic Silver/Iron Nanoparticles for Water Remediation and Reactivity Assessment on Bromophenol Blue. J. Clean. Prod. 2019, 211, 1367–1374. [Google Scholar] [CrossRef]
- Sappa, G.; Barbieri, M.; Viotti, P.; Tatti, F.; Andrei, F. Assessment of Zerovalent Iron Nanoparticle (NZVI) Efficiency for Remediation of Arsenic-Contaminated Groundwater: Two Laboratory Experiments. Water 2022, 14, 3261. [Google Scholar] [CrossRef]
- Micić, V.; Bossa, N.; Schmid, D.; Wiesner, M.R.; Hofmann, T. Groundwater Chemistry Has a Greater Influence on the Mobility of Nanoparticles Used for Remediation than the Chemical Heterogeneity of Aquifer Media. Environ. Sci. Technol. 2020, 54, 1250–1257. [Google Scholar] [CrossRef]
- Schöftner, P.; Waldner, G.; Lottermoser, W.; Stöger-Pollach, M.; Freitag, P.; Reichenauer, T.G. Electron Efficiency of NZVI Does Not Change with Variation of Environmental Parameters. Sci. Total Environ. 2015, 535, 69–78. [Google Scholar] [CrossRef]
- Zhang, W.; Elliott, D.W. Applications of Iron Nanoparticles for Groundwater Remediation. Remediat. J. 2006, 16, 7–21. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and Sedimentation of Aqueous Nanoscale Zerovalent Iron Dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Z.; Yi, Y.; Tsang, E.P.; Fang, Z. Increasing the Electron Selectivity of Nanoscale Zero-Valent Iron in Environmental Remediation: A Review. J. Hazard. Mater. 2022, 421, 126709. [Google Scholar] [CrossRef]
- Xiao, S.; Jin, Z.; Dong, H.; Xiao, J.; Li, Y.; Li, L.; Li, R.; Chen, J.; Tian, R.; Xie, Q. A Comparative Study on the Physicochemical Properties, Reactivity and Long-Term Performance of Sulfidized Nanoscale Zerovalent Iron Synthesized with Different Kinds of Sulfur Precursors and Procedures in Simulated Groundwater. Water Res. 2022, 212, 118097. [Google Scholar] [CrossRef] [PubMed]
- Brumovský, M.; Oborná, J.; Micić, V.; Malina, O.; Kašlík, J.; Tunega, D.; Kolos, M.; Hofmann, T.; Karlický, F.; Filip, J. Iron Nitride Nanoparticles for Enhanced Reductive Dechlorination of Trichloroethylene. Environ. Sci. Technol. 2022, 56, 4425–4436. [Google Scholar] [CrossRef]
- Brumovský, M.; Micić, V.; Oborná, J.; Filip, J.; Hofmann, T.; Tunega, D. Iron Nitride Nanoparticles for Rapid Dechlorination of Mixed Chlorinated Ethene Contamination. J. Hazard. Mater. 2023, 442, 129988. [Google Scholar] [CrossRef]
- Phenrat, T.; Lowry, G.V. (Eds.) Nanoscale Zerovalent Iron Particles for Environmental Restoration: From Fundamental Science to Field Scale Engineering Applications; Springer: Cham, Switzerland, 2019; ISBN 978-3-319-95340-3. [Google Scholar]
- Luna, M.; Gastone, F.; Tosco, T.; Sethi, R.; Velimirovic, M.; Gemoets, J.; Muyshond, R.; Sapion, H.; Klaas, N.; Bastiaens, L. Pressure-Controlled Injection of Guar Gum Stabilized Microscale Zerovalent Iron for Groundwater Remediation. J. Contam. Hydrol. 2015, 181, 146–158. [Google Scholar] [CrossRef]
- Tosco, T.; Gastone, F.; Sethi, R. Guar Gum Solutions for Improved Delivery of Iron Particles in Porous Media (Part 2): Iron Transport Tests and Modeling in Radial Geometry. J. Contam. Hydrol. 2014, 166, 34–51. [Google Scholar] [CrossRef] [Green Version]
- Strutz, T.J.; Hornbruch, G.; Dahmke, A.; Köber, R. Effect of Injection Velocity and Particle Concentration on Transport of Nanoscale Zero-Valent Iron and Hydraulic Conductivity in Saturated Porous Media. J. Contam. Hydrol. 2016, 191, 54–65. [Google Scholar] [CrossRef]
- Johnson, R.L.; Nurmi, J.T.; O’Brien Johnson, G.S.; Fan, D.; O’Brien Johnson, R.L.; Shi, Z.; Salter-Blanc, A.J.; Tratnyek, P.G.; Lowry, G.V. Field-Scale Transport and Transformation of Carboxymethylcellulose- Stabilized Nano Zero-Valent Iron. Environ. Sci. Technol. 2013, 47, 1573–1580. [Google Scholar] [CrossRef]
- Raychoudhury, T.; Tufenkji, N.; Ghoshal, S. Aggregation and Deposition Kinetics of Carboxymethyl Cellulose-Modified Zero-Valent Iron Nanoparticles in Porous Media. Water Res. 2012, 46, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, M.; Zhang, W.; Ahmad, S.; Wang, L.; Tang, J. Synthesis of Carboxymethyl Cellulose Stabilized Sulfidated Nanoscale Zero-Valent Iron (CMC-S-NZVI) for Enhanced Reduction of Nitrobenzene. Sep. Purif. Technol. 2023, 315, 123704. [Google Scholar] [CrossRef]
- Tiraferri, A.; Chen, K.L.; Sethi, R.; Elimelech, M. Reduced Aggregation and Sedimentation of Zero-Valent Iron Nanoparticles in the Presence of Guar Gum. J. Colloid Interface Sci. 2008, 324, 71–79. [Google Scholar] [CrossRef]
- Sakulchaicharoen, N.; O’Carroll, D.M.; Herrera, J.E. Enhanced Stability and Dechlorination Activity of Pre-Synthesis Stabilized Nanoscale FePd Particles. J. Contam. Hydrol. 2010, 118, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mosaferi, M.; Nemati, S.; Khataee, A.; Nasseri, S.; Hashemi, A.A. Removal of Arsenic (III, V) from Aqueous Solution by Nanoscale Zero-Valent Iron Stabilized with Starch and Carboxymethyl Cellulose. J. Environ. Health Sci. Eng. 2014, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Gastone, F.; Tosco, T.; Sethi, R. Guar Gum Solutions for Improved Delivery of Iron Particles in Porous Media (Part 1): Porous Medium Rheology and Guar Gum-Induced Clogging. J. Contam. Hydrol. 2014, 166, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Fopa, R.D.; Bianco, C.; Archilha, N.L.; Moreira, A.C.; Pak, T. A Pore-Scale Investigation of the Effect of Nanoparticle Injection on Properties of Sandy Porous Media. J. Contam. Hydrol. 2023, 253, 104126. [Google Scholar] [CrossRef]
- Schiefler, A.A.; Bruns, S.; Müter, D.; Uesugi, K.; Sørensen, H.O.; Tobler, D.J. Retention of Sulfidated NZVI (S-NZVI) in Porous Media Visualized by X-ray μ-CT–the Relevance of Pore Space Geometry. Environ. Sci. Nano 2022, 9, 3439–3455. [Google Scholar] [CrossRef]
- Chen, B.; Lv, N.; Xu, W.; Gong, L.; Sun, T.; Liang, L.; Gao, B.; He, F. Transport of Nanoscale Zero-Valent Iron in Saturated Porous Media: Effects of Grain Size, Surface Metal Oxides, and Sulfidation. Chemosphere 2023, 313, 137512. [Google Scholar] [CrossRef] [PubMed]
- Mondino, F.; Piscitello, A.; Bianco, C.; Gallo, A.; de Folly D’Auris, A.; Tosco, T.; Tagliabue, M.; Sethi, R. Injection of Zerovalent Iron Gels for Aquifer Nanoremediation: Lab Experiments and Modeling. Water 2020, 12, 826. [Google Scholar] [CrossRef] [Green Version]
- Andrei, F.; Sappa, G.; Boni, M.R.; Mancini, G.; Viotti, P. Mobility of NZVI in a Reconstructed Porous Media Monitored by an Image Analysis Procedure. Water 2021, 13, 2797. [Google Scholar] [CrossRef]
- Viotti, P.; Sappa, G.; Tatti, F.; Andrei, F. NZVI Mobility and Transport: Laboratory Test and Numerical Model. Hydrology 2022, 9, 196. [Google Scholar] [CrossRef]
- Tatti, F.; Papini, M.P.; Sappa, G.; Raboni, M.; Arjmand, F.; Viotti, P. Contaminant Back-Diffusion from Low-Permeability Layers as Affected by Groundwater Velocity: A Laboratory Investigation by Box Model and Image Analysis. Sci. Total Environ. 2018, 622–623, 164–171. [Google Scholar] [CrossRef]
- Jaberi, N.; Linley, S.; Thomson, N.R. Targeted Delivery of Nanoparticles to a Heterogeneous Crude Oil Zone in an Unsaturated Porous Medium. Environ. Sci. Water Res. Technol. 2023, 9, 1518–1530. [Google Scholar] [CrossRef]
- Bianco, C.; Tosco, T.; Sethi, R. A 3-Dimensional Micro- and Nanoparticle Transport and Filtration Model (MNM3D) Applied to the Migration of Carbon-Based Nanomaterials in Porous Media. J. Contam. Hydrol. 2016, 193, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Bianco, C.; Tosco, T.; Sethi, R. MNMs 2021 Software (Micro- and Nanoparticle Transport, Filtration, and Clogging Model-Suite). Available online: www.polito.it/groundwater/software/mnms/ (accessed on 9 September 2022).
- Pulido-Reyes, G.; Magherini, L.; Bianco, C.; Sethi, R.; von Gunten, U.; Kaegi, R.; Mitrano, D.M. Nanoplastics Removal during Drinking Water Treatment: Laboratory- and Pilot-Scale Experiments and Modeling. J. Hazard. Mater. 2022, 436, 129011. [Google Scholar] [CrossRef] [PubMed]
- Tosco, T.; Sethi, R. Transport of Non-Newtonian Suspensions of Highly Concentrated Micro- and Nanoscale Iron Particles in Porous Media: A Modeling Approach. Environ. Sci. Technol. 2010, 44, 9062–9068. [Google Scholar] [CrossRef] [Green Version]
- Messina, F.; Marchisio, D.L.; Sethi, R. An Extended and Total Flux Normalized Correlation Equation for Predicting Single-Collector Efficiency. J. Colloid Interface Sci. 2015, 446, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Tufenkji, N.; Elimelech, M. Correlation Equation for Predicting Single-Collector Efficiency in Physicochemical Filtration in Saturated Porous Media. Environ. Sci. Technol. 2004, 38, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, X.; Ghoshal, S. Correlation Equation for Predicting the Single-Collector Contact Efficiency of Colloids in a Horizontal Flow. Langmuir 2015, 31, 7210–7219. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.; Bonn, D.; Meunier, J. Viscous Fingering in a Shear-Thinning Fluid. Phys. Fluids 2000, 12, 256–261. [Google Scholar] [CrossRef]


| (A) Particle transport | |
| Modified advection-dispersion equation | |
| (1) | |
| nZVI Deposition kinetics | |
| (2) | |
| Velocity dependency | |
| (3) | |
| (B) Non-Newtonian fluid flow | |
| (4) | |
| (5) | |
| (C) Fluid rheology | |
| (6) | |
| (7) | |
| (8) | |
| (9) | |
| (D) Porous medium clogging | |
| (10) | |
| (11) | |
| (12) |
| Parameter | Values | Units |
|---|---|---|
| Simulation radius | 0.9 | m |
| Cell number | 300 | - |
| Time step | 2 | s |
| Pore Volume () | 8.13 | L |
| Injection flow rate () | 7 | L/h |
| 1 | m3/h/m | |
| Injected volume | 6.5 | L |
| nZVI concentration | 10 | g/L |
| Polymer concentration | 7 | g/L |
| Sand bulk density () | 1.46 · 103 | kg/m3 |
| Sand specific surface area () | 2.14 · 10−4 | m2/m3 |
| Sand hydraulic conductivity () 1 | 2 · 10−4 | m/s |
| Sand dispersivity () 1 | 4.2 · 10−3 | m |
| Sand specific storage () 1 | 1 · 10−5 | m−1 |
| Sand porosity () | 0.48 | - |
| Empirical attachment coefficient () 1 | 0.15 | s−1 |
| Density reduction coefficient () 1 | 0.35 | - |
| Surface increment coefficient () 1 | 0.85 | - |
| Empirical viscosity correction coefficient () 2 | 0.75 | m3/kg |
| Empirical power law correction coefficient () 2 | 6.3 · 10−2 | m3/kg |
| Lithology | (−) | (m/s) | (m2) | (kg/m3) | (m) | (m) |
|---|---|---|---|---|---|---|
| Gravelly sand | 0.25 | 2.5 · 10−2 | 2.55 · 10−9 | 1961 | 1.58 · 10−3 | 2.8 |
| Medium sand | 0.27 | 3.0 · 10−3 | 3.06 · 10−10 | 1736 | 5.48 · 10−4 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, C.; Mondino, F.; Casasso, A. Improved Delivery of Nanoscale Zero-Valent Iron Particles and Simplified Design Tools for Effective Aquifer Nanoremediation. Water 2023, 15, 2303. https://doi.org/10.3390/w15122303
Bianco C, Mondino F, Casasso A. Improved Delivery of Nanoscale Zero-Valent Iron Particles and Simplified Design Tools for Effective Aquifer Nanoremediation. Water. 2023; 15(12):2303. https://doi.org/10.3390/w15122303
Chicago/Turabian StyleBianco, Carlo, Federico Mondino, and Alessandro Casasso. 2023. "Improved Delivery of Nanoscale Zero-Valent Iron Particles and Simplified Design Tools for Effective Aquifer Nanoremediation" Water 15, no. 12: 2303. https://doi.org/10.3390/w15122303
APA StyleBianco, C., Mondino, F., & Casasso, A. (2023). Improved Delivery of Nanoscale Zero-Valent Iron Particles and Simplified Design Tools for Effective Aquifer Nanoremediation. Water, 15(12), 2303. https://doi.org/10.3390/w15122303










