1. Introduction
Water is considered, after air, the most essential material for human survival. It is necessary to sustain life, and thus, an adequate, safe, and accessible supply of water must be available to all; otherwise, life would not exist. In 2010, the General Assembly of the United Nations recognized the significance of equitable access to safe and clean drinking water and sanitation as a human right that is essential for the full enjoyment of life and all human rights [
1,
2,
3,
4,
5].
Although access to water is of major importance for humans, it may not happen (even in developed countries) due to seasonal fluctuations or lack of supplies. However, the problem is of major concern in underdeveloped countries. The disparity in water availability, reliability, and quality between developed and undeveloped countries is remarkable. Around the world, almost one billion people—more than a tenth of the world’s population—lack access to safe drinking water. For example, approximately 780 million people drink water from “unimproved” sources—an unprotected dug well, spring, or a polluted river. According to the World Health Organization (WHO), about two million deaths per year may be attributed to unsafe water [
6,
7,
8,
9,
10].
The use of water is not limited only to the purposes of drinking. It can be classified into three main categories: domestic, industrial, and agricultural. Domestic use includes drinking water, bathing, water used for hygiene purposes, and even watering the yard and garden. Water is used in industries as a cooling or heating medium, for washing, cleaning, blanching, or peeling raw materials, as well as for cleaning and hygiene requirements (equipment, production area, and personnel). Moreover, water can be used in the food industry as an ingredient of the product recipe or the final product itself. Finally, a large portion of water is used for agricultural purposes. Thus, it can be acknowledged that water plays a vital role in many aspects of human life [
11].
Water is obtained from numerous sources and, in its raw state, may contain a variety of pollutants, such as chemical toxic elements or compounds, radiological compounds, and physical pollutants. Consequently, it needs to be treated either on-site or in drinking water treatment plants in order for it not to pose any threat to consumers and to be safe for any of its uses.
The review focuses on drinking water and aims, after careful examination of the current bibliography, to evaluate the different raw water sources, review the existing relative legislation comparatively in terms of quality and safety, and analyze emphatically the main developed treatment methods in drinking water treatment plants (including purification/clarification and chemical disinfection) that render water safe for human consumption. Furthermore, case studies of drinking water treatment plants are presented, and the requirements according to HACCP are highlighted. Additionally, certain novel drinking water treatment methods are proposed. Finally, the case of natural mineral water is examined, and the uses of water in the food industry are discussed. Overall, the novelty of the present work is the study of drinking water in its entire value chain, including its sources of origin, the legislation that governs it, and all of its purification processes, with the aim of fully analyzing all the aspects of an essential component for the sustainment of life. Moreover, the vast majority of review papers regarding water treatment focus on one specific treatment method, while the present review tries to examine and analyze both conventional and novel methods, thus covering the need for thoroughly studying a very important topic like drinking water treatment, safety, and quality.
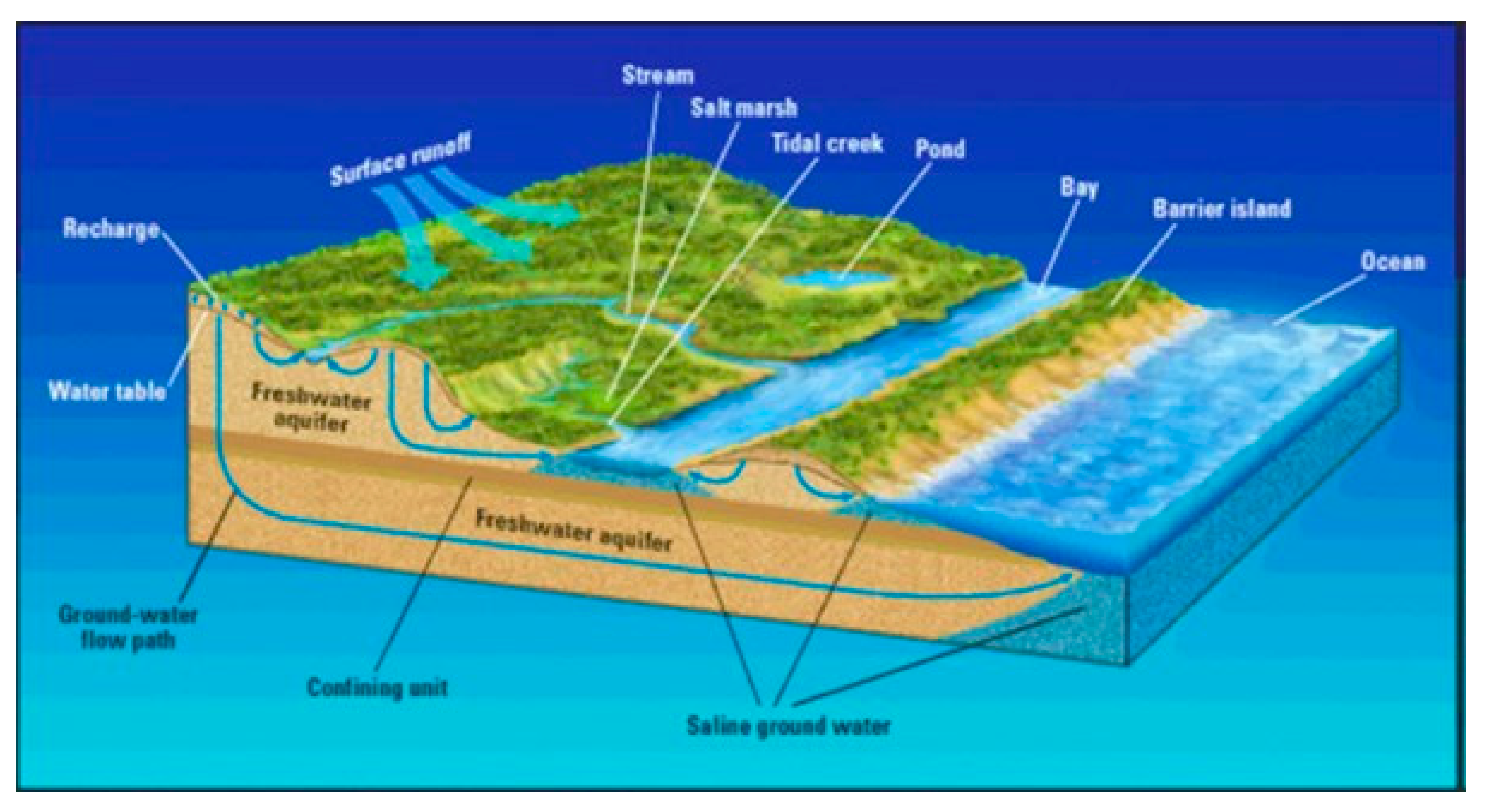
2. Water Sources
Water resources provide water for different types of uses, including the production of some useful goods (i.e., energy production). Their function is to overcome the water desires or demands for various agricultural, industrial, or household purposes. Water resources are nowadays one of the most essential issues among scientists due to climate change impacts and adaptation, since they may be renewable; however, they are finite and scarce. The majority of water (97.22%) is captured in oceans, making it inappropriate for human consumption because of its high salt content. A clean and reliable water supply is critical for domestic use, food and energy production, transportation, recreation, and maintenance of natural ecosystems. Different types of water resources are used for the production of water around the world, categorized either based on their composition and/or on their uses for the benefit of humanity [
12,
13]. In
Figure 1, the various water sources are presented.
The most common classification of water resources includes the following three types: [
14]
- -
Surface water;
- -
Groundwater;
- -
Groundwater under the distinct influence of surface water.
2.1. Surface Water
Surface water is open to the atmosphere and results from overland flow. The main sources of surface water include lakes, rivers, streams, rain catchments, man-made impoundments, and wells that are either shallow or drilled next to or in a stream or river. They are easily located and characterized generally as softer than groundwater. They can easily be contaminated with microorganisms, while their temperature varies depending on the season, making consistent water quality production at drinking water treatment plants (DWTP) challenging. Furthermore, in the winter season, the intake structure may be damaged by ice, or the source may become completely frozen. Additionally, decaying vegetation provides high color in surface waters, while providing the potential to produce high levels of total trihalomethanes (TTHMs). Finally, when containing high precipitate amounts, the levels of turbidity increase, and thus, the treatment cost and operator time increase [
14].
2.2. Groundwater
Groundwater is located between the earth’s crust and 762 m depth, and is considered usable fresh water. It derives from wells and springs that do not communicate with surface water or are not influenced by a local hydrologic event. It must be noted that if the water stored in polar icecaps and glaciers is not considered, then groundwater covers approximately 98% of the global freshwater reserves. It is the most extracted raw material in the world, and its reserves, which are millions of years old, can be present even in arid regions [
14,
15,
16,
17].
Groundwater is generally more difficult to be contaminated, presents stability in its quality throughout the year, and has a lower bacterial count than surface waters. However, if contaminated, it is more difficult to return to its initial stage due to the difficulty of removing the contaminants. Its concentration in minerals is usually higher than in surface waters, leading to increased hardness levels. Moreover, iron and manganese levels are often high, increasing the treatment cost. For groundwater to be extracted, the usage of a pump is necessary; thus, the operation cost further increases. Finally, wells located in coastal areas may be subject to saltwater intrusion and, as a result, contaminated; this contamination is difficult to predict, and the required treatment is costly [
14].
2.3. Groundwater under the Distinct Influence of Surface Water
Groundwater under the distinct influence of surface water is influenced by a neighboring surface water source or a local hydrological event [
14].
2.4. Surface Water—Groundwater Properties Comparison
Surface water and groundwater, as mentioned above, present a variety of differences between them. The temperature of surface waters varies with the season, whereas groundwater remains relatively constant. Due to decaying vegetation and the presence of suspended solids, surface water may have high turbidity levels and be colored, while groundwater does not usually have suspended solids or any turbidity. The mineral content is constant and higher in groundwater, whereas in surface waters, it varies depending on the soil, the effluents, and other parameters. In groundwater, dissolved O
2 is usually absent, whereas, in surface waters, it is often near the saturation level. Surface waters contain many living organisms, such as bacteria, viruses, and plankton, whereas, in groundwater, iron bacteria are often present. Additionally, surface waters present a tendency for eutrophic nature that is increased by high temperatures and pollution, whereas eutrophic nature does not exist in groundwater. Finally, groundwater may be less susceptible to contamination than surface waters; however, once it occurs, the contamination lasts for a very long period of time, whereas surface waters can be more easily contaminated, but once the source of contamination is removed, they recover much faster than groundwater [
18,
19,
20,
21].
3. Quality Characteristics of Water
Water quality is mainly referred to as the physicochemical, physical, and chemical properties that must be satisfied so that water is acceptable to consumers. It is connected to the absence of undesirable substances and/or contaminants, and consequently, the water safety frame is comparable to that which normally governs food products. Water, like any other food or drink, has its own sensory characteristics, with appearance, smell, odor, and flavor being the main ones. All these characteristics must meet the consumers’ expectations and be acceptable. Water is a drink characterized by its transparency and absence of any color, taste, and odor; thus, it should have desirable odor, taste, and flavor. The most common problems occurring with the sensory characteristics of water are connected to strong chlorine smell or taste, metallic taste, rotten egg odor, musty or unnatural smells, deviations of color, and turbidity [
22,
23,
24].
Concluding, water, depending on the source or even the treatment, can be contaminated and its quality downgraded. Nonetheless, water quality characteristics have to meet both the legislation standards and the consumers’ expectations. Concerning the bottled water product, it is crucial that no migration of foreign matter to water has occurred in order to prevent any downgrading of its quality.
4. Contamination and Pollution of Water
Contamination and pollution of water are of major concern due to the hazardous effects they can have on ecosystems, humans, and animals. These terms are easily confused in a bibliography, with contamination referred to as the presence of a substance that should not be present naturally or at concentrations above background, and pollution defined as the harm caused to organisms or infrastructures by contaminants. Thus, all pollutants are contaminants, but not all contaminants are pollutants. Due to the complexity of distinguishing them, from here and forth, they will be referred to as one [
25].
Pollution refers to the entry of contaminants into the natural environment that causes disorder, instability, and harm or discomfort to the ecosystem. Pollutants can be either foreign substances or naturally occurring contaminants. Water is mainly referred to as polluted when it is impaired by anthropogenic contaminants and becomes unable to support human use, such as drinking water, and/or undergoes a marked shift in its ability to support its constituent biotic communities, such as fish. It most commonly appears in raw water; however, it can occur due to the application of the water treatment processes (i.e., disinfection by-products). Especially for the disinfection by-products (DBPs), since many of them are known to have negative impacts on human health, tight limitations and legislation limits need to be established. More than 700 DBPs have been detected, with trihalomethanes and haloacetic acids being the most commonly found in drinking water. Up to this moment, only a small fraction of these is subject to legislation [
12,
26,
27,
28].
According to World Health Organization (WHO), pollution of water can be classified into chemical, microbial and radiological aspects; physical pollution of water also occurs in many cases.
4.1. Chemical Aspects
Chemical contaminants may be organic or inorganic. The majority of chemicals are of health concern only after extended exposure, while some of them, particularly emerging ones, have not yet been proven if they cause health problems or not. Rapid changes in water quality occur only if the chemicals are discharged intermittently to water sources; in typical conditions, changes in water occur progressively. Chemical pollution of water, as already referred to, can also occur during its treatment, e.g., during disinfection by DBPs [
12].
4.1.1. Organic Pollutants
Agents that degrade water quality and are organic in nature form big threats to healthy human existence and aquatic life. Such pollutants mainly include [
12]:
- -
Food processing waste;
- -
Petroleum hydrocarbons;
- -
Disinfection by-products (DBPs);
- -
Volatile organic compounds (VOCs);
- -
Insecticides and herbicides;
- -
Tree and bush debris from logging operations;
- -
Chlorinated solvents;
- -
Perchlorate;
- -
Polychlorinated biphenyl (PCB);
- -
Trichloroethylene.
4.1.2. POPs, PBTs, TOMPs
Persistent organic pollutants (POPs) are a class of resistant to environmental degradation pollutants; they have been observed to persist in the environment, to be capable of long-range transport bioaccumulation in human and animal tissue and biomagnifying in food chains, and finally, to have potentially significant impacts on human health and the environment. There are a few natural sources of POPs, but most POPs are human-made in industrial processes, either intentionally or as by-products. Characteristic examples of POPs include carcinogenic polycyclic aromatic hydrocarbons (PAHs) and certain brominated flame retardants, as well as some organometallic compounds, such as tributyltin (TBT). POPs are also classed as PBTs (Persistent, Bioaccumulative, and Toxic) or TOMPs (Toxic Organic Micro Pollutants) [
29,
30].
Disinfection by-products (DBPs) are formed during the disinfection of water with the use of chlorine. Natural organic matter (NOM) is substantially the primary precursor in the formation of DBPs according to the following equation [
12]:
4.1.3. Inorganic Pollutants
Inorganic chemical pollutants are naturally found in the environment, but due to human development, they are often concentrated and released into the environment in urban storm water. The primary inorganic pollutants of concern in urban stormwater are Arsenic (As), Cadmium (Cd), Copper (Cu), Lead (Pb), Zinc (Zn), Mercury (Hg), Nitrogen (N), Phosphorous (P), Nitrate, Nitrite, Ammonia, and Phosphate [
12]. Chemical pollutants can be classified into five categories according to their source [
26]:
- -
Naturally occurring: rocks, soils, and the effects of the geological setting and climate;
- -
Industrial sources and human dwellings: mining, manufacturing, and processing industries, sewage, solid wastes, urban runoff, and fuel leakages;
- -
Agricultural activities: manures, fertilizers, intensive animal practices, and pesticides;
- -
Water treatment or materials in contact with drinking-water: coagulants, DBPs, and piping materials;
- -
Pesticides used in water for public health: larvicides used in the control of insect vectors of disease.
4.1.4. Emerging contaminants
Emerging contaminants in drinking water refer to substances that are not currently regulated or routinely monitored, but have the potential to pose a risk to human health or the environment. These contaminants include various chemicals (endocrine-disrupting chemicals), pharmaceuticals, personal care products, and other various pollutants, such as microplastics, pesticides, and herbicides. The aforementioned contaminants can enter the aquatic environment through wastewater treatment plants, improper disposal, or leaching from landfills [
31].
4.2. Microbial Aspects
The greater risk to public health from microbes (pathogens) in water is associated with the consumption of drinking water contaminated with human and animal excreta, although other sources and routes of exposure may also be significant [
12].
Biological contaminants include bacteria, algae, molds, mildew, cat saliva, viruses, animal dander, house dust, mites, cockroaches, and pollen. They originate from different sources: pollens originate from plants; viruses are transmitted by people and animals; bacteria are carried by people, animals, and soil and plant debris; and household pets are sources of saliva and animal dander. The protein in urine from rats and mice is a potent allergen [
26,
32].
The most hazardous bacteria that raise concern include Campylobacter, E. coli, Salmonella, and the most important protozoan pathogens, Cryptosporidium and Giardia. HACCP, which places a high value on the microbes, has classified pathogens according to their risk and severity in human health.
4.3. Radiological Aspects
It is possible that radioactive substances are present in drinking water. They are categorized as human-made and naturally occurring. They are contained in significantly low concentrations in water, and thus, may generally not pose a threat to human health. Human-made substances are often controllable when entering the aqueous environment, whereas naturally occurring substances can enter at any point and are difficult to control. Additionally, the naturally occurring ones usually result in higher radiation levels than the human-made ones [
33].
4.4. Physical Pollution
Physical pollution of water is described as the presence of naturally occurring contaminants in water sources, including organic material that falls into the water (i.e., leaves), sediments, and even heat. These contaminants can downgrade the quality of water by causing undesirable changes in its color and odor [
34].
4.5. Sensory Characteristics of Water and Pollution
As mentioned above, water must meet the consumers’ expectations regarding its sensorial characteristics. The presence of both organic and inorganic chemical substances in the water may result in a rapid deterioration of its sensorial characteristics and possibly lead to unfavorable effects on human health. For instance, colored water may be an indication of the presence of naturally occurring organic matter. Furthermore, the color of water is greatly affected by the presence of iron and other metals.
The sources of contamination that usually affect the sensory characteristics of water are of biological and chemical origin. Concerning biological sources, decaying vegetation and metabolites of microbiota are believed to be the main causes for causing problems in the sensory characteristics of water. Inorganic contamination derives from human activity, such as agriculture, wastewater discharges, and metallurgical manufacture. On the other hand, organic contamination has roots in soil humus, products of living activity, and decomposition of vegetation and animals. Organic contamination may also derive from household and industrial wastewater.
It should also be noted that during treatment, including the processes of oxidation and disinfection, some by-products formed can have a severe impact on the taste and odor of the water. For example, chlorination can lead to the formation of bromophenols, whereas ozonation results in the formation of intermediate reaction products, which can affect the sensory characteristics of water [
23,
35].
5. Drinking Water Standards
Drinking water standards vary around the world, ranging from complex to simple. Generally, the international drinking water standards are referred to as:
Developed nations that have very high standards of water quality;
The underdeveloped world that continues to struggle with bacterial and chemical contamination.
Numerous reports convey the drinking water and sanitation problems that the developing world faces. The World Health Organization (WHO) set standards that these countries can use to begin their own safe drinking water programs. However, WHO acknowledges that most developing nations will not be able to meet these drinking water standards. To assist developing nations that may not be able to afford modern treatment technologies, WHO suggests these countries use the standards as guidelines to provide direction about simple filtration and disinfection.
In the U.S., the U.S. Environmental Protection Agency (EPA) is responsible for setting drinking water standards; meanwhile, in the European Union, the water standards are set by the Council Directive 98/83/ED.
Currently, both in the U.S. and European Union, standards exist for inorganic (IOCs), volatile organic (VOCs), semi-volatile organic (SOCs), disinfection by-products (DBPs), microbials, and radiological contaminants. However, not all countries follow the U.S. or EU standards. A comparative presentation of drinking water standards for the majority of contaminants set by the U.S. EPA, E.U., and WHO is shown in
Table 1,
Table 2,
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7 [
11,
26,
36,
37].
Concluding, each organization has established its own regulations and standards, which do not always meet with each other. However, it is of great importance that drinking water complies with the existing standards in order to avoid any risk to human health.
Sensory Standards of Water
The most common standard for the sensory characteristics of water relies on its acceptability by consumers. In the European Union and some member states, regulations relative to water intended for human consumption are for odor and flavor to be acceptable by the consumers and not to present abnormal changes. However, the European Union has set standards for the sensory characteristics of water (EN 1622) that includes the threshold odor number (TON) and the threshold flavor number (TFN). If water is acceptable to the consumers, these tests are not required; otherwise, these tests should be conducted [
38].
TON and TFN standards are referred to a qualitative determination of the sensory characteristics of water; quantitative determination of the responsible compounds is also achievable upon request. Commonly, the compounds that cause the deterioration of sensory characteristics are present in very low concentrations in the water body, and their quantitative analysis can be accomplished using proper and sensitive analytical methods, such as chromatographic methods, colorimetric analysis, and separation techniques [
23].
6. Drinking Water Treatment Methods
Drinking water treatment methods can be classified into two general areas:
- -
Physical methods;
- -
Chemical methods.
Purification of water can be achieved either by a standalone method or by a combination of methods, depending on the regulation standards and the characteristics of raw water. The most commonly used methods will be presented and evaluated in terms of their efficiency, necessity, and applicability.
Table 8 categorizes the most commonly used methods depending on their nature:
6.1. Screening
Raw water often contains large solids and grits that can interfere with the treatment methods, causing severe mechanical wear and increasing the maintenance cost of the water treatment equipment; thus, they have to be removed prior to the main treatment of water. Screening is applied as a preliminary treatment to remove objects such as plastics and metals effectively, preventing any damage to equipment. Screens can be either coarse or fine; coarse screens have larger openings than fine ones, so they are used for removing large solids, whereas fine screens are typically used to remove material that may cause operation and maintenance problems [
39].
Screening systems can be cleaned mechanically or manually. The former has the advantage of reducing labor costs while improving flow conditions and screening capture; therefore, they are more commonly used. On the other hand, the latter does not require excessive equipment maintenance, and thus, they are a good alternative for small-scale drinking water treatment plants. Additionally, manually cleaned screens require frequent raking to avoid clogging and, as a result, have increased labor costs, whereas mechanically cleaned ones may not present this particular problem but have higher equipment costs [
39].
6.2. Coagulation-Flocculation
Raw water often has high turbidity and visible cloudiness as a result of the presence of many particles in the water body; thus, it gets an unwanted appearance and taste. Therefore, it is critical that they are removed so that the water will become acceptable to consumers.
Coagulation is a conventional method for drinking water treatment and is often followed by flocculation. Combining these two methods has proven to be very effective in dealing with the presence of particles in raw water, as they are able to remove suspended particles and dissolved organic and inorganic contaminants. The main goal is the neutralization and destabilization of the small particles, followed by the collisions between the particles in order to form agglomerates [
3,
40].
In most cases, the small particles present in water are negatively charged, repelling each other and making the formation of agglomerates impossible. The neutralization is essential in order to obtain the removal of the stable particles and is achieved by using chemical agents (coagulants). The most common coagulants are aluminum sulfate and iron salts, such as ferric sulfate or ferric chloride. The coagulants carry a positive charge that attracts the negative charge particles leading to the formation of new neutral particles. When a coagulant is added to raw water and mixed rapidly with it, the particles become neutral and unstable. Next, the formation of unstable small agglomerates (flocs) due to the collision of the particles is obtained; as the collisions continue to happen, larger agglomerates begin to exist (flocculation), which can be finally removed by other physical processes, such as clarification [
3,
41,
42,
43].
6.2.1. Types–Dosage of Coagulant and Parameters That Affect Coagulation
The coagulants mainly used in coagulation processes are ferric sulfate [Fe2(SO4)3], ferric chloride [FeCl3], aluminium sulfate [Al2(SO4)3-14H2O], and polyaluminium chloride [Al2(OH)5Cl], and are preferred due to their highly charged counter ions.
In order for the coagulation to be effective, it is important that the proper coagulant dosage is added to raw water. Four different zones of coagulant dosage have been defined.
- -
First Zone: insufficient dosage; therefore, the particles remain negative and stable;
- -
Second Zone: sufficient dosage; therefore, neutralization is achieved, and coagulation is underway;
- -
Third Zone: higher dosage giving charge neutralization and restabilization;
- -
Fourth Zone: even higher dosage that results in the formation of metal hydroxide precipitate.
The dosage of coagulant is highly dependent on the turbidity and total organic carbon (TOC) values in the raw water body. Typically, as the turbidity and TOC grow, higher coagulant dosages are required to achieve high coagulation efficiency [
3,
11,
40,
42,
44].
The majority of coagulants present their counter-ion ability in acidic conditions; therefore, in cases of high alkalinity water, it is necessary to add either a higher dosage of coagulants to obtain the optimum pH level, or acid to lower the required dosage of coagulant. This process, referred to as enhanced coagulation, is effective in removing DBPs precursors such as NOM. Generally, pH controls the coagulation process through its ability to affect the coagulant’s actions. The temperature of the water also plays a key role in coagulation by controlling the solubility of coagulants so that the performance of coagulation decreases in low temperatures. Finally, in order for the coagulation to be effective, rapid mixing with coagulant is required to provide the initial contact between water and chemicals, leading to suitable coagulation conditions and subsequently allowing the flocs to develop during flocculation. Generally, in drinking water and wastewater treatment plants, the effective capacity of coagulation–flocculation systems varies between 5 and 150 m
3/h. [
3,
42].
6.2.2. Sweep Flocculation
Sweep flocculation is a variation of the traditional method of flocculation. It requires a high coagulant dose, lies in the fourth zone of coagulant dosage, and results in the formation of metal hydroxide precipitates which are heavy, sticky, and larger in particle size. They drag the colloidal contaminants and subsequently settle in the suspension. Sweep flocculation should be considered a viable option for water with low particle concentration, because the traditional coagulation–flocculation process would be slow [
3,
42,
45].
6.3. Dissolved Air Flotation (DAF)
Dissolved Air Flotation (DAF) is considered an alternative method to the conventional clarification process. DAF is effectively applied after the coagulation–flocculation processes, especially in waters with high concentrations of oil, grease, and suspended particles. The most important advantage of DAF over conventional clarification is its lower capital cost.
The primary difference between conventional clarification and DAF systems is part of the tank in which the impurities are removed. The main mechanism that takes place in DAF systems is the introduction of air bubbles in the bottom of the tank by pressurizing water, increasing the solubility of air, and thus, large quantities of bubbles can be obtained. The bubbles that are freed in the water body, having a lower density than water, will ascend to the top layer of the tank, colliding with the existing particles. Subsequently, bubble-particle agglomerates will be formed, which will also ascend to the top of the tank if their density is lower than the water. As the introduction of bubbles and ascension of the agglomerates progresses, a sludge blanket at the top of the DAF tank will appear, due to the accumulation of the agglomerates, which is then mechanically removed, allowing the water to pass to the following treatment method.
The most commonly used DAF tanks are either rectangular or circular and have two zones: contact and separation zone; in the former, collision and attachment between the bubbles and the particles happen, whereas, in the latter, the agglomerates ascend to the top layers of the tank and are removed. A typical DAF process flowsheet is presented in
Figure 2. In typical drinking water and wastewater treatment plants, the dimensions of DAF systems vary between 4 and 30 m
3, with water’s flow rate and productivity being 1-200 m
3/h and 500 L/h. DAF is primarily applied in water-containing particles with low density and is able to remove turbidity, natural organic matter, and algae [
3,
11,
42,
46,
47].
Parameters That Affect DAF Systems
For the efficiency of DAF systems, two major factors are determinants:
- -
Sufficient quantity of bubbles;
- -
Conditions of collision and attachment between bubbles and particles.
The efficiency can also be enhanced by other parameters. The generation of small bubbles is preferable over the large bubbles, because small bubbles provide a much bigger surface for collision and attachment, thus enhancing the process. Furthermore, DAF systems are proven to be more efficient with increased contact time and, moreover, enhanced with the use of chemical flocculants, which mainly include salts of iron or aluminum. Finally, depending on the feed water, polymers can be added to enhance the ability of bubbles to capture solids [
48].
6.4. Clarification
Clarification is another physical conventional water treatment method, and usually succeeds coagulation and flocculation and precedes filtration in order to enhance it by removing particles in DWTPs. The driving force of clarification processes is the need to remove suspended particles, such as slit, algae, mud, and agglomerates, from coagulation–flocculation that are usually present in raw water. They are very difficult to settle due to the turbulent motion of water as it flows. When the water enters a sedimentation tank, and the conditions are made more quiescent, the particles will be at rest and will begin to settle out under the influence of gravity.
The most commonly used clarification method is sedimentation, and often these two terms are used as one. Sedimentation involves one or more basins (clarifiers), which are usually large, and open tanks that can be either rectangular or circular. In order for clarification to be effective, the velocity of water has to be significantly reduced so that the predominant force acting on the water body is gravity. Schematically, a horizontal clarifier tank is presented in
Figure 3. A typical horizontal clarifier tank used in drinking water treatment is approximately 63 m
3, and its capacity ranges between 5 and 150 m
3/h. Clarification will be achieved if V
t is higher than V
h and the particles settle before the water body exits the tank [
11,
48,
49,
50,
51].
In the clarification process, the sludge created due to the settling of the particles has to be removed from the basins to be further treated and then discharged.
6.4.1. Parameters That Affect Clarification
Detention time is one parameter that affects clarification; long detention times can allow the operation to adjust to any changes in the quality of treated water. Thus, clarification will be able to provide an acceptable finished water product. The concentration and distribution of particles play an important role in clarification. In the case of high particle concentration, interference from neighboring particles occurs as the settlement progresses, increasing the rate of settling. Temperature is also a factor affecting the performance of clarification, making it more effective as the temperature increases. Another factor is the particle size; the larger the particles are then, the faster the settling will happen. Finally, the movement of water is a major factor; if the water is still, the sedimentation will be faster than when the water is in motion [
50,
51].
6.4.2. Solids Contact Clarification (Sludge Blanket)
Solid contact clarification is a process that enhances the conventional method of sedimentation. Water flows through the tank, and at the point where the settling rate becomes equal to the flowing rate of the water, the suspended agglomerates accumulate, forming a blanket, which has the ability to capture the other suspended particles. Therefore, the process of sedimentation is accelerated by creating a layer of sludge and a layer of clean water; as the accumulation continues, the size of the blanket begins to grow, and thus, a portion of the sludge has to be removed [
11,
48].
6.5. Filtration
Filtration is a conventional physical method of water treatment; it is often the following step of clarification and generally employs the separation of suspended particles from the water body. In filtration, water containing suspended particles passes through a medium or a membrane, in which the particles are retained either on the surface of the medium or within its pores while water (filtrate) is allowed to pass through the medium. It is able to achieve a reduction in water’s turbidity and provides protection against cysts and pathogens.
The filter media, which are porous solid materials, play an important role in this particular method. The holes within the medium create an array of tortuous passages, in which the water flows while the majority of the suspended particles are retained. Typically, the pores of a medium have a size that varies from 500 to 2000 μm, while they are able to retain and remove much smaller particles (0.1–50 μm) due to the collision and sticking between the particles and the filter medium. The filter media are mainly characterized by four physical parameters:
- -
Porosity;
- -
Permeability;
- -
Tortuosity;
- -
Connectivity.
Porosity is the fraction of the medium that contains the voids (a complex network developed by pores that occupy a fraction of the bulk volume of the medium). Effective porosity is the portion of the medium in which the water is flowing. Commonly, the media, according to their structure, can be characterized as unconsolidated or consolidated and ordered or random. The most commonly used media in the filtration processes are sand, crushed anthracite, and garnet.
Permeability refers to the ability of the medium to allow the fluid to pass through it. It depends on the distribution of pore sizes that plays a major role in the flow of water as it can either enhance or reduce the ability of water to flow within the voids. The permeability and porosity of a medium are highly connected. In order for the medium to be permeable, it is necessary to be porous and have certain porosity. However, permeability also depends on tortuosity and connectivity.
Tortuosity refers to the relative average of a flow path, and it considers the changes in the pore sizes along the flow path and its sinuousness. Connectivity refers to the connections or the pores within the porous media. Both characteristics can be measured macroscopically. The combination of tortuosity and connectivity is a useful tool in the determination of permeability, but neither of them alone is able to determine the permeability [
11,
48].
6.5.1. Filtration Mechanism
Filtration is applicable to incompressible (liquids) and compressible (gases) fluids. The compressibility of the fluids determines the physical mechanisms that control the process; as the water flows through the medium, the suspended particles are retained in the feed side, whereas the water is able to pass through the medium and is collected on the other side of the medium.
The filter medium is, in general inhomogeneous; the size of its pores varies, and each pore has a distinct geometry and is randomly distributed.
Filtration is a process that can be applied in the form of two different types:
- -
Cake filtration;
- -
Filter medium filtration.
In cake filtration, the suspended particles accumulate on the surface of the medium, forming a cake. In filter medium filtration, the suspended particles are retained within the pores of the medium, and the media consist either of cartridges or granular media. The cake formation plays a dramatic role in the filtration operation and can influence the flow of water through the medium. The top layers of the formed cake are inhomogeneous, depend on the properties of the medium, and mainly affect the structure of the cake. The cake has its own pores, which are much greater in number than the pores passages of the medium, and thus it can interfere with the medium; the smaller particles are better retained in pores that extend in the whole medium. This is the result of particle bridging or physical adsorption. Furthermore, in cake filtration, it is essential to establish a cake structure that does not have high resistance in flow, which might decrease the effectiveness of filtration [
30,
48].
Filtration must take into consideration two main factors: the source that will force the liquid through the medium and the constructive material of the medium. Typically, there are three cases, depending on the force that is used for the fluid to flow through the medium [
11]:
- -
Small resistance from the medium: the flowing of the fluid is achieved simply by gravity;
- -
Medium resistance from the medium: gravity is ineffective; thus, atmospheric pressure is applied—this is known as a vacuum filter;
- -
High resistance from the medium: an external source of pressure, higher than atmospheric, is applied by a pump.
6.5.2. Parameters That Affect Filtration
Filtration is majorly affected by the pressure differential across the filtering plate and the temperature of the suspension that obviously controls the flow rates. Temperature influences the flow of water through either the cake (if cake filtration is applied) or the filter medium. The inhomogeneity of particles can also affect filtration, as the differences in size between particles can influence the structure of the cake and, subsequently, the filtration process. Another parameter that can interfere with the filtration is the accumulation of small particles inside the porous, which results in a decrease in flow rates. The use of filter aids can reverse this; phenomenon aids are able to retain the particles, whereas the fluid is able to flow. The cake resistance also affects filtration; it can be lowered with the use of coagulating and peptizing agents that are able to change the properties of the formed cake and improve the filtration process [
3].
6.5.3. Contamination of Filter Media
The accumulation of suspended particles on the surface of the medium or within its pores results in medium contamination. As far as cake filtration is concerned, that is the most commonly used method of filtration, the cake (a layer of particles) is formed on the surface of the medium. As the accumulation of these particles continues, the thickness of the cake continues to grow, and upon reaching a certain level, it is necessary to remove it from the medium. The removal of cake can be achieved either by mechanical devices or by backflushing by which the filtrate is flowing backward and towards the feed side. The structure of the cake is influenced by both hydrodynamic and physicochemical factors. The cake can also have an impact on the effectiveness of filtration due to its high resistance; thus, the minimization of its resistance is very important, which can be accomplished by the use of multimedia (most commonly dual media) filter modules or by the use of several enhancements that intensify particle separation. The latter can be achieved by the addition of filter aids, flocculants, or electrolytes to the cake. In this case, the agents added can change the conditions and properties of the suspended particles toward the formation of a small resistance cake [
3,
11,
42,
48].
6.5.4. Backwashing
When the filter media is contaminated, the performance and effectiveness of filtration reduce drastically. This particular reduction can be developed either as a head loss (low flow rates) or as a filter breakthrough (high concentration of particles in the filtrate). The filter medium is restored in its initial conditions by backwashing, where water from the side of the filtrate is forced to flow towards the feed side, and as a result, it drags the entrapped particles and the formed cake, cleaning the filter medium. During backwashing, a solution with a high concentration of particles is formed that has to be disposed of or, depending on the source of water, may need further treatment in order to meet the disposal regulations.
Backwashing may be an effective means of cleaning contaminating filter media, but it can affect the filter’s properties by altering the pore distribution, with the smaller pores at the top of the medium and the larger at the bottom of the medium. Thus, the effectiveness of the medium changes, because the medium presents high-efficiency top layers followed by a development of rapid pressure loss. In order to deal with this specific problem, multi-media filters were developed. In them, each medium has its own characteristics, and therefore after backwashing, the distribution of the pores in the overall media will not alternate [
3,
11,
42,
48].
6.6. Membrane Filtration
Membrane processes can be characterized as a subcategory of the conventional filtration process. Membranes are porous materials and represent permselective barriers that separate almost every pollutant from water, resulting in purified water on the other side of the membrane and in a concentrated solution, which contains the pollutants, on the feed side of the membrane [
3,
5,
11,
48,
52,
53].
6.6.1. Types of Membranes
The most common membranes used in water purification treatment, and in other industrial applications, are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO). Other types of membranes also exist, such as electrodialysis (ED) and forward osmosis (FO), but the previous four types are the ones with the most applications in water treatment.
Membranes are categorized depending on the minimum size of components they have the ability to reject. This is shown in
Table 9 [
52]:
These four types of membranes exhibit continuality in the sizes of the compounds they can reject. Thus, depending on the targeted contaminant that is to be removed, the appropriate type of membrane is selected. MF and UF accomplish separation by size exclusion and require low pressures of operation, whereas NF and RO exhibit different mechanisms of operation. In NF and RO, the physicochemical properties of the components that are allowed to pass and the membrane material determine the mechanism of operation. In these two types of membranes, the pore size does not play such a decisive role as in MF and UF [
5,
53,
54,
55].
6.6.2. Flow Modes
There are two possible modes of operation in membrane processes:
- -
Dead-end filtration;
- -
Crossflow filtration.
Generally, in membrane processes, there are three possible streams:
- -
The feed;
- -
The permeate;
- -
The retentate (existing only in crossflow filtration).
In dead-end operations, the feed stream flows perpendicular to the surface of the membrane, whereas in crossflow, the flow is parallel (tangential) to the membrane surface.
Figure 4 presents the two possible modes of operation. As a result, in dead-end filtration, there are only two possible streams, the feed stream, and the permeate stream. In dead-end mode, the rejected compounds accumulate on the surface of the membrane; thus, a cake is formed, and the phenomenon of membrane clogging is developed, resulting in a decrease in the effectiveness of the process due to the permeate flow decrease. Crossflow mode is able to overcome this problem where the feed stream is flowing parallel to the membrane surface, and the permeate flows through the membrane in a perpendicular direction. Therefore, the feed stream is able to drag the rejected compounds in a rejection stream (retentate), and a much thinner cake than that in dead-end is formed; thus, a decrease in the flux of permeate is more difficult to present. Consequently, the most commonly used mode in water treatment is crossflow. Dead-end systems are applied in water with low concentrations of solids, making the formation of cake more difficult, and thus the process can continue unhindered. A typical membrane filtration system used in drinking water treatment is approximately 7 m
3, and its productivity rises up to 10 m
3/h [
11,
53].
The flow of the fluid across the membrane is achieved due to the transmembrane pressure (TMP). At every rate of flow, there is a critical value in the flux of permeate in which there will be no accumulation of compounds and no formation of cake. In order to keep a steady flux of permeate flow, the TMP must be constant [
5].
6.6.3. Membrane Materials and Manufacturing Methods
Membranes can be manufactured by a variety of materials, and the choice of the proper material depends on the following characteristics: processing requirements, thermal stability, chemical stability, and fouling tendency. Generally, the membrane materials are:
- -
Organic membranes;
- -
Inorganic membranes.
Organic membranes are polymeric with an asymmetric (anisotropic) or symmetric (microporous) structure. Inorganic membranes are either ceramic or metallic. Membranes from inorganic materials are not easily contaminated and are not affected by chemical additions. However, due to their higher costs of manufacturing in water treatment, organic membranes are used in the majority of applications [
5,
56,
57,
58].
Organic membranes can be manufactured by a variety of methods. Depending on the applied method, the manufactured membrane will have different characteristics, such as pore size, distribution, and porosity. The most common methods for manufacturing inorganic membranes are:
- -
Phase inversion: CA, PA, PP, PS;
- -
Interfacial Polymerization: PA—thin film composite;
- -
Stretching sheets of partially crystalline polymers: PTFE;
- -
Irradiation and etching: polycarbonate, polyester.
Respectively inorganic membranes are manufactured by the method of moulding and sintering of fine-grain powders. With this method, ceramic and metal oxide membranes are made [
11].
6.6.4. Organic Membranes
Organic membranes can have an asymmetric or symmetric structure. Usually, in UF, NF, and RO applications, only asymmetric membranes are used, but MF applications use both asymmetric and symmetric membranes. In RO and NF applications, the most commonly used membranes are homogenous asymmetric CA and PA, combining the high efficiency of rejection and high-water flux. However, the dominant membranes now are the thin film composite (TFC), which exhibits a greater range of pH and more permeability to water. However, they are more prone to fouling and can be affected by some oxidizing agents that are used in disinfection (chlorine, etc.). UF polymeric membranes usually are made by PS, CA, PES, or regenerated cellulose and are mainly manufactured by the process of phase inversion. Membranes that are cellulose-based present higher fluxes in equivalent rejections and are less prone to fouling, but are not as stable as the non-cellulosic membranes. PTFE, PVDF, PP, PS, and cellulosic are among the most common polymeric materials by which the MF membranes are manufactured. Again, the cellulosic-based membranes are less prone to fouling, but present less stability than the others, especially PTFE, PVDF, and PP [
5].
6.6.5. Inorganic Membranes
There are four types of inorganic membranes:
- -
Ceramic;
- -
Sintered materials;
- -
Glass;
- -
Zeolite.
The polymeric, organic membranes are much less expensive than the inorganic, but the inorganic membranes are much more chemically and thermally stable. The major applications of inorganic membranes include ceramic MF and UF membranes, which are manufactured by sintering and sole gel processes. Organo–mineral membranes stand in the middle ground of ceramic and polymeric. This type of membrane has the advantage of higher flux over polymeric membranes [
59].
6.6.6. Membrane Fouling
The accumulation of foulants present in the feed water stream, at the membrane surface, or within the membrane’s pores, known as membrane fouling, has a negative impact on the membrane processes by reducing the flux and/or decreasing the effluent quality. Membrane fouling can occur either in the membrane surface or within its pores. Generally, there are four fouling modes [
11,
53]:
- -
Complete pore blocking;
- -
Internal pore blocking;
- -
Partial pore blocking;
- -
Cake filtration.
The rate of membrane fouling depends on a variety of conditions and characteristics, such as [
3]:
- -
Nature of foulants;
- -
Concentration of foulants;
- -
Membrane material;
- -
Membrane type;
- -
Characteristics of membrane’s pores;
- -
Surface characteristics of the membrane.
Foulants can be classified depending on their characteristics [
52]:
- -
Solids (slits, colloids, organic material);
- -
Scalants (low solubility salts);
- -
Microorganisms (can survive the processes that precede the membrane process);
- -
Organics (proteins, carbohydrates, and NOM).
The adhesion of foulants on the membrane is caused by repulsive and attractive forces, so their relative magnitude determines the occurrence of fouling. Attractive forces include van der Waals, hydrophobic, and polymer binding interactions, whereas repulsive forces include hydration and steric forces. Electrostatic interactions can be either attractive or repulsive [
3,
52,
53,
60].
Furthermore, there are some other contributing factors that affect the extent of fouling. These are presented in
Table 10 [
5]:
6.6.7. Membrane Cleaning—Prevention of Fouling
When fouling occurs, it is necessary for the membranes to be cleaned in order for the process to continue without a decrease in flux or in the effluent quality. Cleaning can be mostly conducted by backflushing or by chemical cleaning. In some cases, it may be achieved by hydraulic energy in the form of high-flow velocity in the feed channel [
3,
60].
The optimum condition in the membrane processes is to prevent the occurrence of fouling. Prevention of fouling can be accomplished by [
52]:
- -
Hydrodynamic and physical methods;
- -
Operation in optimal conditions;
- -
Appropriate selection of the membrane;
- -
Optimization of the treatments that precede membrane processing.
6.6.8. Membrane Filtration Applications
MF and UF membranes operate at low pressures, whereas NF and RO operate at high pressures; thus, the operating cost of NF and RO membranes is much higher than MF and UF. RO membranes have the ability to remove natural organic matter, dissolved contaminants, and a great proportion of dissolved salts. These dissolved salts do not pose a threat to human consumption and, in combination with the generation of a highly rejected stream, have resulted in a decrease in the use of RO in drinking water treatment. RO membranes are mainly applied in desalination processes. NF membranes are a viable option for removing natural organic matter, hardness, pesticides, and color. NF is also able to cope with emerging contaminants such as endocrine-disrupting compounds (EDCs), pharmaceutically active compounds (PhACs), and personal care products (PCPs).
On the other hand, MF and UF membranes are not able to remove the same extent of pollutants as NF and RO do. However, they remove particulate matter, and MF has the ability to retain viruses completely. MF and UF combination with other treatment techniques, such as coagulation, adsorption, and ion exchange, will provide effective means for removing the pollutants NF membranes are able to remove [
11,
42,
53].
6.7. Adsorption
Adsorption is a process with the ability to effectively replace chemical coagulation and clarification. In adsorption processes, the three main elements that need to be defined are:
- -
Liquid phase: the water which contains the pollutants that are to be removed;
- -
Adsorbates: the pollutants that need to be separated from the water body;
- -
Adsorbents: the material (most commonly carbon) that the adsorbates will be adsorbed.
The main purpose of the method is to achieve the separation of the adsorbates from the liquid phase and their adsorption onto the adsorbents, and it can be either physical or chemical. In the former, the pollutants are attached to the surface of the adsorbent by van der Waals forces, and the density of electrons remains stable in the system. In the latter, an extended rearrangement of electron density is observed, and a chemical bond is created; the nature of the chemical bond can range from virtually complete ionic to virtually complete covalent character [
61,
62]. The process progresses until the rate of adsorption becomes equal to the rate of desorption, thus ending the accumulation of the pollutants at the adsorbent’s surface.
Figure 5 demonstrates the process of adsorption. A typical adsorption system used in drinking water treatment is approximately 7 m
3, and its productivity rises up to 10 m
3/h [
3,
42,
48].
6.7.1. Activated Carbon
Activated carbon is the most common adsorbent used in adsorption processes. Raw carbonaceous materials, such as coal, lignite, wood, etc., by their nature, possess some adsorbing abilities, which are greatly enhanced by the activation process that mainly involves thermal treatment. The purpose of this process is the creation of a huge internal area, in which the pollutants will be adsorbed. Activated carbon has the ability to remove synthetic organic chemicals and natural organic compounds that are disinfection byproducts precursors, algal toxins, and unpleasant tastes and odors. Two types of activated carbon are majorly applied in the adsorption treatment technique:
- -
Granular Activated Carbon (GAC);
- -
Powdered Activated Carbon (PAC).
Generally, GAC particles may be bigger than PAC’s; however, the difference in size does not affect the adsorbing efficiency, since the pores and not the external surface determine the quantity of the pollutants that will be adsorbed [
3,
11,
12,
42,
48].
6.7.2. Parameters That Affect Adsorption
The main parameter that affects the method of adsorption is the quality of the used adsorbent. For adsorption to be effective, the adsorbent must have a big surface area (higher rate of adsorption) and a large internal area (larger adsorbed quantity of pollutants) [
63,
64]. Furthermore, when adsorption is planned, it is necessary to know the characteristics of the liquid phase, such as temperature and pH, and the properties of the targeted pollutants. The exhaustion of activated carbon also affects adsorption; when the quality of water meets the minimum standards after the adsorption treatment, the carbon needs to be removed and regenerated by thermal treatment, in which the organic matter in the pores of carbon is oxidized and subsequently removed. The regenerated activated carbon is again functional; however, its adsorbing capacity is slightly less than that of virgin one, and eventually, after repeated regenerations, activated carbon needs to be replaced and disposed of [
3,
11,
42].
6.8. Ion Exchange (IX)
Ion exchange is a new method that has recently been applied in drinking water treatment and is often used as an enhancement to other processes, such as coagulation. The main advantage that it offers is the removal of very significant quantities of NOM, which is the main precursor for the formation of DBPs. Ion exchange is mainly applied when softening of water is required due to its ability to remove calcium and magnesium.
Ion exchange employs the use of a resin and includes cation and anion exchange, depending on the surface charge of the resin. In cation exchange, positively charged ions are exchanged with positively charged ions, whereas in anion exchange, negatively charged ions exchange with other negatively charged ions available on the resin surface. The process progresses until all the available ions on the surface of the resin are exchanged, and when the resin becomes exhausted can once again become operational by regeneration. Regeneration restores the resin to its initial conditions, and thus ion exchange can be applied. The most common regenerant is a saturated brine solution, but sodium chloride solutions are also used.
Figure 6 presents the operation of an ion exchange mixed resin. A typical ion exchange system equipped with a mixed resin used in drinking water treatment is approximately 6 m
3, and its productivity rises up to 10 m
3/h.
After the regeneration, the solution used as the regenerant will contain the ions that are removed during the operation and the regenerant ions. Therefore, this particular solution will have high levels of total dissolved solids (TDS) and will be necessary to be transferred to different facilities in order to be treated and discharged [
11,
42,
65,
66].
Parameters That Affect Ion Exchange
The main factor that influences the effectiveness of ion exchange is the contaminant’s properties. Ions in the treated water compete for the available sites of the resin; ions with greater affinity for ion exchange resins will be more effectively removed. Affinity is greatly influenced by the atomic weight and the valence of the ions. Another parameter that affects the performance of the ion exchange resins is their nature. Generally, anionic exchange resins are applied for the removal of NOM and some hazardous anions, whereas cationic resins are mainly used for water softening. Therefore, the type of resin that will be used should be closely examined in order to estimate the efficiency of the targeted pollutants removal [
3,
65,
66,
67].
6.9. Advanced Oxidation Processes (AOPs)
Advanced oxidation processes (AOPs) are another applied technological method for water purification. The main purpose of this particular technology is the degradation of organic and inorganic compounds present in water. AOPs are able to degrade emerging contaminants, such as pharmaceuticals and endocrine-disrupting compounds, and compounds that cause unpleasant taste and odor. The degradation is a result of continuous oxidation processes that are carried by hydroxyl radicals (*OH) formed by AOPs. Hydroxyl radicals are powerful, nonselective chemical oxidants, which oxidize rapidly the majority of the compounds present in water by two mechanisms. According to the first mechanism, the radical breaks the double bond between two atoms of carbon and is added to them, whereas according to the second mechanism, the radical acts by abstracting hydrogen bonded with carbon. Despite the differences between the two mechanisms, they share the same result: the formation of carbon-centered radicals that react with dissolved oxygen and then a series of reactions leading to carbon dioxide and water [
3,
67,
68,
69].
The generation of hydroxyl radicals can be made by a variety of advanced oxidation processes (AOPs) that can be classified as photochemical or as non-photochemical methods. They are presented in
Table 11 [
3,
67,
70]:
6.9.1. Parameters of AOPs That Need Assessment
AOPs need to be assessed prior to their applications on a large scale in order to evaluate their efficiency, as well as their ability to remain efficient in removing the targeted contaminants. Another parameter that needs to be assessed is the ability of AOPs to adapt to possible changes in the process, such as the characteristics (NOM levels, alkalinity, etc.) of water. Therefore, careful designing of AOPs is required that should take into consideration many safety factors in order to provide sufficient efficiency. Finally, AOPs are rarely used as the only method for purifying water; thus, they must be integrated with other techniques. Therefore, the appropriate choice of AOP and its ease of incorporation into the overall treatment process is of major importance [
3,
11,
67,
69,
70,
71].
6.9.2. Considerations of AOPs
AOPs are proven effective means of dealing with emerging contaminants due to their ability to degrade organic and inorganic compounds; however, the products formed should be taken into close consideration. The degradation of compounds by reacting with *OH results in the formation of oxygen-rich compounds, which are generally more hydrophilic. Therefore, they present a decrease in their ability to penetrate through cell membranes and a loss in their baseline toxicity. Studies have shown a decrease in estrogenic and antimicrobial activities of estrogenic compounds and antibiotics, respectively, after AOP treatment [
3,
11,
67,
69,
70,
72,
73].
Additionally, the products generated from AOPs may be, in some cases, more toxic than the original compounds, due to the transformation of the chemical structure of the compound, which leads to the potential of the formation of a new toxic compound (i.e., the formation of N-nitrosodimethylamine from the ozonation of dimethyl sulfoxide) [
10,
11,
48].
6.10. Disinfection
Raw water may contain a variety of hazardous microorganisms, which include bacteria, viruses, and protozoa, that can be the cause of waterborne diseases. It also contains organic and inorganic chemical compounds that may be harmful to human health. Disinfection aims to eliminate these threats in order to provide safe water to consumers. It can be either chemical or physical, and the main methods applied for disinfection are presented in
Table 12 [
11,
48,
74]:
Disinfection’s effectiveness is mainly governed by two factors [
11,
48]:
- -
The dosage of the disinfectant agent;
- -
The contact time between the disinfectant agent and water.
6.10.1. Chlorine
Chlorine is a very powerful oxidant and the most commonly used disinfectant agent, and its dosage depends on the water characteristics, as well as the standards applied. It has the ability to effectively cope with both microorganisms, as well as with chemical compounds by oxidizing chemical compounds and emerging contaminants and transforming them into other chemicals that are not harmful to human health. The ability of chlorine to eliminate microorganisms results from HOCl, which is generated when chlorine is added to water according to the following reaction:
HOCl reacts with the cell structure of microorganisms and is able to inactivate required life processes.
When chlorine remains in the water for long periods, a residual is formed that can act as a protector of water against pathogen contamination in the distribution system. However, chlorine is a very toxic substance and has a sensorial impact; thus, chlorine’s residual should be closely monitored. If it exceeds the limits, the process of dechlorination is necessary to be applied by adding sulfur dioxide or sodium bisulfite [
10,
11,
48,
74].
6.10.2. Chlorine’s Compounds
The compounds of chlorine mainly used as disinfectant agents are chloramines and chlorine dioxide. Chloramines are formed by mixing chlorine with ammonia, and are not as effective as chlorine, but are mainly used in distribution systems due to their elevated stability. On the other hand, chlorine dioxide is a more effective disinfectant than chlorine, causing severe damage to microorganisms and rapidly oxidizing organic and inorganic compounds [
10].
6.10.3. Ozone
Ozone is a very powerful oxidant and can be used as a disinfectant. It was developed as an effective alternative to conventional chlorine disinfection due to concerns about chlorine toxicity. It is formed by combining oxygen molecules with oxygen atoms, and as a strong oxidant can rapidly react with chemical compounds and emerging contaminants, transforming them into different chemical compounds. It is also an effective means of coping with the pathogens present in water. Its main advantage is the ability to remain unaffected by pH changes, whereas chlorine’s efficiency depends highly on pH. The main concern about ozone is that it is difficult to be transferred to the water treatment plant because of its high reactivity and unstable nature. Therefore, ozone, when deployed as a disinfectant, is produced on-site in the water treatment plant. Finally, the main disadvantage of ozone compared to chlorine is the elevated capital and energy costs [
3,
11,
74].
6.10.4. UV Light
UV is a physical method of disinfection that has the ability to penetrate the cells of microorganisms and photochemically adjust their DNA, inactivating them and making them unable to reproduce. This process is reversible, and if the microorganisms are exposed to visible light again, they will reactivate and begin reproduction. UV also has the ability to cope with chemical compounds with a mechanism similar to AOPs. The energy of UV creates hydroxyl radicals (*OH), that subsequently oxidize the chemical compounds and transform them to other compounds. UV treatment can be combined with ozone, elevating the effectiveness of the overall process more than the effectiveness of the ones when applied separately. Finally, it may be necessary that after the UV treatment, chlorine residual is added to the water so that it will be further protected against microorganism growth in the distribution system [
11,
74].
6.10.5. Disinfection Byproducts (DBPs)
Disinfection is a necessary step in the drinking water treatment process, and thus, it cannot be left out of the whole process. Disinfection byproducts (DBPs) are the main concern about the disinfection process, since they are generated by the reaction of chemical disinfectants with natural organic matter (NOM) and inorganic ions present in water. NOM is a major precursor of DBPs as it reacts with hypochlorous acid (in chlorine disinfection) in the presence of bromide ions according to the following equation [
3,
75,
76]:
Generally, each chemical disinfectant agent has its own residual and DBPs. These are presented in
Table 13 [
3,
22,
24,
38]:
UV can also generate some DBPs, but as of today, they still have not been fully assessed and evaluated.
DBPs are bioaccumulative and can have a severe impact on human health. THMs are linked with bladder cancer, whereas chlorate is known to be the cause of anuria, abdominal pain, and renal failure. Some DBPs can produce reproductive and developmental effects at high doses. Finally, high concentrations of some inorganic byproducts can result in hemolytic anemia [
77,
78,
79].
In recent years, legislation and standards for DBPs have tightened, and nowadays, close monitoring of them is applied. This is essential in order not only for disinfection to be effective but also to provide a final product that is not harmful and dangerous to the consumers.
7. Drinking Water Treatment Methods Combination and Comparison
In drinking water treatment, the proper selection of the treatment method or the proper combination of methods is of major importance in order to achieve purified water that will not pose a threat to human health as the final product. Each method has its own advantages and disadvantages, while its effectiveness depends on the characteristics of the raw water body. Thus, the correct evaluation and analysis of the characteristics of the raw water body is of vital significance.
Generally, each pollutant can be dealt with effectively with a particular method. Natural organic matter, which is a precursor for the formation of DBPs, can be treated effectively by coagulation–flocculation, membrane filtration, and advanced oxidation processes. Advanced oxidation processes or ozonation in combination with granular activated carbon adsorption are useful tools in dealing with pesticides. Algae and turbidity are more effectively removed by coagulation-flocculation, whereas microfiltration or ultrafiltration can remove protozoa and bacteria. Ultrafiltration is also effective in removing viruses. Taste and odor, which give the water unpleasant sensorial characteristics, can be removed by the combination of adsorption in activated carbon and membrane filtration. Dissolved organic carbon has been found to be more effectively removed by membrane filtration or advanced oxidation processes. Concerning the inorganic ions removal, membrane filtration is the most successful method, whereas adsorption can also effectively remove arsenic and fluoride. Oxidation processes combined with membrane filtration can also remove iron and manganese. Finally, emerging contaminants, such as pesticides, endocrine disrupting compounds, pharmaceutically active compounds, and personal care products, require efficient removal by advanced oxidation processes, membrane filtration (nanofiltration), or by a combination of adsorption in activated carbon and membrane filtration (microfiltration or ultrafiltration) [
3,
11,
52,
68].
Table 14 presents the purpose, the parameters, the evaluation of effectiveness, and the disadvantages or setbacks of each method that has been analyzed previously.
Drinking Water Treatment Plants
A drinking water treatment plant (DWTP) is a facility where the raw water body undergoes a variety of treatments in order to eliminate and remove hazardous contaminants and unaesthetic sensorial characteristics. The final product of a DWTP must be purified water that does not pose any threat to human health. Depending on the characteristics of the raw water body, the processes that a DWTP includes must be altered in order to achieve an acceptable final product.
The most common treatment methods that a DWTP includes are coagulation, flocculation, clarification, filtration, and at the final step, disinfection, as referred. A conventional DWTP is schematically shown in
Figure 7. In a typical DWTP, the rapid mix and the flocculation–sedimentation tanks are approximately 25 m
3 each, while the filtration unit covers about 10–12 m
2 of space [
32].
In Greece, one of the biggest DWTPs is the plant in Aspropyrgos, which is relatively elementary, and the whole processing includes screening, coagulation, flocculation, sedimentation, clarification, filtration, and disinfection. The flowchart of the plant is presented in detail in
Figure 8 [
80].
In recent years, due to the rise and growth of emerging contaminants, such as PhACs, EDCs, and PCPs, new models of DWTPs that are more complex than conventional ones have been studied. The study of new treatment plants is primarily on a pilot scale and has to be evaluated on a full scale. A typical example of a pilot scale DWTP that is planned to remove pharmaceuticals is presented in
Figure 9 [
81].
8. Novel Methods of Drinking Water Treatment
Drinking water treatment is a field that constantly evolves; thus, new drinking water treatment methods are developed either on a pilot scale or on DWTPs. These novel methods aim to enhance the effectiveness of DWTPs by either combining them with conventional ones or even replacing some of them [
82,
83]. The most significant novel methods include [
84]:
- -
Membrane Distillation;
- -
Forward Osmosis (FO);
- -
Hydrate-based Desalination (HBD);
- -
Ceramic Ultrafiltration and Microfiltration;
- -
Electrodeionization;
- -
Electrocoagulation;
- -
Ion Exchange;
- -
Advanced Oxidation Processes.
The processes of ion exchange, advanced oxidation processes, and ceramic Ultrafiltration and Nanofiltration have been analyzed previously in this text.
Membrane distillation and FO are two methods that represent substantially desalination technologies and are currently under active development. Membrane distillation is a thermally driven separation process that utilizes a vapor pressure gradient between the feed solution and the distillate; thus, mass is transferred through a hydrophobic, microporous membrane [
85]. Membrane distillation is the only membrane process that is not affected by any changes in the characteristics of the feed [
84,
85,
86,
87].
Forward Osmosis (FO) is an innovative water recovery process that requires low- to no-hydraulic pressure to recover water. Water is spontaneously drawn from a feed solution into a more concentrated draw solution (DS) (
Figure 10). To date, many different FO draw solutions have been tested, including inorganic salts (i.e., NaCl or MgCl
2), thermolytic salts (i.e., aqueous carbonated ammonia or aqueous carbonated trimethylamine), sucrose, polyelectrolytes, and magnetic nanoparticles [
85,
88,
89,
90]. The feed and the draw solutions are separated by a semi-permeable membrane that selectively allows water molecules to pass through its pores. The membranes that are used in FO are composed of a hydrophilic active layer cast onto a supportive structure [
91]. The driving force for the transmembrane movement of water is the osmotic pressure difference between the aforementioned solutions and the tendency of water to equilibrate its chemical potential in both solutions [
84,
92].
Applications of FO include desalination, power generation, food processing, and wastewater treatment. Standalone FO is limited to certain applications, mainly in the food and wine industry, as it results in a diluted draw solution (consisting of nutrient compounds) instead of fresh water [
85,
91]. If clean water recovery is the goal, an additional separation step is necessary to reclaim water from the dilute draw solution. This additional separation step requires energy and results in a hybrid FO process, whereby water is initially drawn from the feed into the concentrated draw solution and is subsequently recovered from the diluted draw solution [
92,
93,
94,
95].
Various hybrid FO processes have been tested, including FO–RO, FO–LPRO (Low-Pressure Reverse Osmosis), FO–UF, FO–NF, FO–MD, FO–Thermal Distillation, and FO–Freeze Concentration [
96,
97]. The main advantages of FO are associated with its low energy consumption, high rejection of a wide variety of contaminants, and the low fouling propensity of the FO membrane. In addition, the membrane requires less cleaning than RO membranes, resulting in a significant reduction in maintenance and operating cost. The energy requirements depend on the transmembrane flux, the draw solution (DS) concentration, and the recovery rate [
85,
91]. However, it has been proven that the theoretical minimum desalination energy requirement is higher with FO, than without FO. This is attributed to the energy penalty associated with the recovery of the draw solutes and the regeneration of the concentrated draw solution, which is one of the biggest challenges in FO, and is defined by the draw solute used [
86,
95,
98,
99,
100,
101].
Hydrate-based desalination (HBD) is another promising technology, capable of recovering clean water from both low- and high-salinity streams in the form of gas hydrates (
Figure 11) [
102,
103]. The latter are ice-like structures, composed of water molecules that physically encompass gaseous molecules [
104]. The formation of gas hydrates occurs at specific temperature-pressure conditions and is selective: water molecules form cages, as the result of hydrogen bonds between them, which encapsulate gas molecules, excluding dissolved ions and salts from the hydrate crystals [
105]. The gases stabilize the cage lattices, thus leading to the formation of stable crystals. Salts are considered thermodynamic inhibitors, and therefore, their presence in solution leads to a reduction of the driving force, which eventually leads to slower kinetics [
10,
102,
103,
106]. However, despite the shift in the hydrate phase equilibrium to lower temperatures and higher pressures, spectroscopic studies proved that salts and dissolved ions are excluded from the crystal structures [
10,
102,
103,
107]. Therefore, gas hydrate crystals consist of water and gas, and when melted, they produce clean water, while the gaseous component can be recovered and reused in the process [
102,
103]. Although the potential of HBD to recover high-yield and high-purity water is strong, there are some major challenges associated with the process, which are primarily associated with the slow kinetics of hydrate formation [
102,
103].
Electrocoagulation is an alternative method to conventional coagulation and can be applied both in the treatment of drinking water, as well as in wastewater treatment [
108]. It has the ability to generate coagulant species in situ by the electrolytic oxidation of sacrificial materials, which is triggered by the application of an electric current through the electrodes. The electrodes are mainly made of Al or Fe materials because of their availability, lack of toxicity, and high valence [
108,
109]. This method presents a variety of advantages over conventional coagulation. It combines oxidation, coagulation, and precipitation in one method, reduces the need for chemical reagents, presents a lower risk of secondary pollution, and has lower energy requirements, thus reducing the operation cost [
110,
111,
112].
Electrodeionization is another desalination method that combines conventional electrodialysis with ion exchange technology, and has the ability to produce ultrapure water by putting a mixed-bed ion exchange resin into the dilated cell of a conventional electrodialysis cell unit [
113]. This particular arrangement increases the conductivity in the substantially non-conductive water, and thus, the water of ultrapure quality is produced [
114]. The main advantage of the method is the fact that the resin can be regenerated without chemical agents, but via water splitting under current, thus decreasing the energy consumption for the treating of low salt solutions [
3,
84].
These novel methods are either applied on the pilot scale or are not yet fully established in DWTPs. It is essential to be thoroughly studied prior to their application on a large scale in order to be an effective and viable alternative to conventional drinking water methods.
9. HACCP in Drinking Water Treatment Plants
DWTPs must guarantee the safety of the final product, and the distributed water after the treatment in the plant must be safe for human consumption without posing any threat to the consumer’s health. The Hazard Analysis Critical Control Points system (HACCP) is a food safety management system that uses the approach of controlling critical points in food and drink production. In the European Union, the application of HACCP in the food industry is mandatory according to Regulation (EC) No 852/2004 [
80,
115,
116].
HACCP Principles
Codex Alimentarius has established the seven main principles of HACCP (adopted by EC) in order for the application of the system to be practical and successful. These seven main principles are detailed below [
6,
117]:
- -
Principle 1: perform a hazard analysis;
- -
Principle 2: determination of the Critical Control Points (CCPs);
- -
Principle 3: establishment of one or several limits;
- -
Principle 4: establishment of a CCP monitoring system;
- -
Principle 5: establishment of corrective actions if monitoring indicates that a specific CCP is no longer under control;
- -
Principle 6: establishment of procedures of verification to confirm a successful operation of the HACCP system;
- -
Principle 7: introduction of a documentation system taking into action that takes into account all processes and records in accordance with the principles and their application.
Critical Control Points are determined by a decision tree, which is provided by Codex Alimentarius. When establishing a HACCP plan for a DWTP, it is essential that all the seven main principles are followed intact in order to assure the quality and safety of the final product. In DWTPs, the potential CCPs based on the decision tree are [
117]:
- -
The source of water;
- -
An integrated water treatment process;
- -
The handling or storage of the water;
- -
The use of water in the production process steps;
- -
Any other risk of contamination of the water supply for the actual process.
Generally, potential CCPs involve all the processes that have an impact on the characteristics and composition in an unforeseen way. CCPs can be processes that are designed to remove certain chemical or microbiological hazards (i.e., membrane filtration, disinfection, etc.), or processes whose efficiency has to be monitored and controlled. Furthermore, CCPs may be certain processes, such as disinfection, in which toxic substances may be formed; thus, the efficiency of post-sanitizing has to be evaluated.
The corrective actions that a HACCP system can provide are numerous and vary depending on the event that has occurred. For example, a simple action is a spot dosage to prevent any alternative supplies, whereas, in events with greater significance, the public notification may be unavoidable [
80,
118].
10. Natural Mineral Waters and Spring Waters
According to Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009, natural mineral water is defined as microbiologically wholesome water originating in an underground water table or deposit and emerging from a spring tapped at one or more natural or bore exits. Natural mineral water can be clearly distinguished from ordinary drinking water by:
- -
Its nature, which is mineral content, trace elements, or other constituents and, where appropriate, by certain effects;
- -
Its original purity.
Both characteristics have been preserved intact because of the underground origin of water, which has been protected from all hazards of pollution [
119,
120].
10.1. Types of Natural Mineral Water
Generally, at source or after bottling, effervescent natural mineral waters give off carbon dioxide spontaneously and in a clearly visible manner under normal conditions of pressure and temperature. The Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 determine three different types of natural mineral water [
121]:
- -
Naturally Carbonated Natural Mineral Water: the carbon dioxide content after decanting and bottling is the same as at the source.
- -
Natural Mineral Water Fortified with Gas from the Spring: the carbon dioxide content after decanting and bottling is greater than at the source.
- -
Carbonated Natural Mineral Water: carbon dioxide has been added to an origin other than the water table or deposit from which the water comes.
10.2. Natural Mineral Water Treatment
The treatment of natural mineral water is governed by strict regulations that are established in Directive 2009/54/EC of the European Parliament. According to this Directive, natural mineral water, in its state at source, may not be subjected to any treatment other than:
- -
The separation of its unstable elements, such as iron and sulfur compounds, by filtration or decanting, possibly precedes oxygenation.
- -
The separation of iron, manganese and sulfur compounds, and arsenic from certain natural mineral waters by treatment with ozone-enriched air.
- -
The separation of other undesirable constituents.
- -
The total or partial elimination of free carbon dioxide by exclusively physical methods.
In the treatment of natural mineral water, only physical methods are applied, while chemical treatment is forbidden by the legislation. It is also established that the above treatment methods must not alter the composition of the water as regards the essential constituents that give the natural mineral water its properties. Thus, minimal treatment may include successive filtration or decanting for the separation of unstable elements, such as iron and sulfur compounds; also, treatment with ozone-enriched air can be applied for the separation of iron, manganese, and sulfur compounds and arsenic from certain natural mineral waters. Finally, any disinfection treatment or the addition of bacteriostatic elements or any other treatment likely to alternate the viable colony count of the natural mineral water shall be prohibited [
121].
10.3. Natural Mineral Water Regulations
The European Union, with the COMMISSION DIRECTIVE 2003/40/EC of 16 May 2003, has established the concentration limits and standards for natural mineral waters. These standards are presented in
Table 15 [
122]:
In addition to the above guidelines, the COMMISSION DIRECTIVE 2003/40/EC of 16 May 2003 has also established the standards concerning the maximum limits for residues from the treatment of natural mineral waters and spring waters by ozone-enriched air. These standards are presented in
Table 16 [
122]:
11. Water Use in Food Industry
Water is not only an essential element in life, but it is also a necessary “material” in the industry. The majority of industries require the use of water as a vital tool for their well-being and productivity. In particular, for the food industry, water is an important facility necessary for various uses. It may be an ingredient of the product formulation or can be required for preliminary operations of raw material (i.e., cleaning and blanching of fruits or vegetables), for boiler feed, for cooling or heating purposes (i.e., in plate heat exchangers), and finally for sanitary operations and purposes (i.e., plant cleaning, CIP). Many preliminary or main processes applied to food industries demand high water amounts, such as soaking, pasteurizing, conservation, and many as well as other complementary operations such as cleaning, washing hygiene, and sanitizing programs. As a result, due to large amounts of water applied in food industries, the water supply is, in some cases, a main factor for the selection of the factory place [
123,
124,
125].
Water can be supplied in the food industries by a municipal drinking water treatment plant as distributed to consumers. However, some food industries can have their own water drilling facilities and, therefore, their own water treatment plants in order to treat the water according to their internal standards. Finally, it must be noted that even if the food industry is supplied water by municipal drinking water treatment plants, it may need further treatment in order to meet the expectations or internal standards of the industry. A typical example is the beverage industry, which requires specific standards in order for the water not to cause any deviations in the sensorial quality of the final product [
126,
127].
11.1. Water Treatment in Food Industries
One of the most important things in the use or treatment of water in the food industry is the proper selection of the streams that will be used. Prior to the selection of the stream, it is essential to evaluate the level of chemical and microbial contamination of it; streams with low levels of contamination do not require excessive treatment and present a lower risk of perception and, thus, they are the most preferable [
124,
126,
127].
Due to the scarcity of water and the quality demands of it in the industry, water has to be either reconditioned and then reused or treated before its application in the food industry while simultaneously recovering valuable food components. Water intended for reuse should be reconditioned by properly designed means to reduce or eliminate microbiological, chemical, and physical contaminants, according to its intended use. Water treatment in the food industry consists of physical, chemical, or a combination of both types of methods. In some cases, pretreatment is necessary in order to boost the overall efficiency of the final treatment. The most commonly applied chemical method is by using chlorine, chlorine compounds, or ozone compounds that possess the ability to lower the levels of hazardous chemicals and microbial load. Furthermore, the remaining disinfectant dosage can protect the water from further contamination. On the other hand, membrane filtration is the most applicable physical method and, depending on the membrane type used, can recover some valuable by-products from the waste streams. In some food preservations methods, highly concentrated solutions are used, i.e., osmotic solutions, brines, etc., which require regeneration after each use. In such cases, similar methods applied to water treatment may be used in order for the water to be reused [
123,
124,
126,
127].
Some characteristics and examples of treatments for processed water from the food industry are presented in
Table 17 [
123].
11.2. Regulations of Water Used in the Food Industry
The quality of water used in the food industry is also governed by legislation and standards. Generally, the water used in the food industry can be classified as water that is used for food-contact applications and water that is used for non-food-contact applications. The quality of the first must be at least respective to potable water, whereas other qualities of water are accepted on the latter occasion [
123].
In the European Union, several directives have been established concerning the use of water in the food industry. The Council directive 98/83/EC provides the choice of selection for the use of water of alternative qualities when the water quality does not affect the wholesomeness of the product. The responsibility of evaluating the possibility of the use of different water qualities and of the consumers’ protection is in the hands of competent national authorities. In the USA, the US Environmental Protection Agency and the US Department of Agriculture provide support guidelines for the food industry. Generally, the reuse of water is allowed under the condition that it will not adulterate the product and not create unsanitary conditions [
33,
128,
137,
138].
12. Conclusions
This review provides full and concise coverage concerning drinking and natural mineral water. Access to safe and clean drinking water is established as a human right, and thus this particular study focuses on the need and the means that render water safe for human consumption. There are different raw water sources, and the legislation concerning both drinking and natural mineral water plays a major role in the final quality of water; thus, they are thoroughly presented. Moreover, all the necessary insight into the conventional drinking water treatment methods is provided, while simultaneously introducing novel drinking water treatment methods, which, if applied after their evaluation, will enhance the productivity of DWTPs. Additionally, it is stated that HACCP in DWTPs plays a major role in the quality and safety of drinking water; thus, its flawless application is of critical concern. The role of water in the food industry is analyzed, and it is stated that it is a major element in it. This review article calls for public awareness concerning both the need to provide safe and adequate amounts of drinking water to the entirety of human beings as well as the means and treatment methods to achieve it. Research needs to focus on the improvement of the already existing drinking water treatment methods and the establishment of novel ones in order to render water safe for consumption.
From the critical review carried out in the present work, it is clear that the treatment of drinking water is a complex process, in which important parameters must be taken into account, such as the source of the water, the contaminants present in it, the compliance of the final product with the existing regulations, and the cost and energy consumption necessary for water purification. From this perspective, it is vital to move towards the future of drinking water treatment, which should include advanced treatment technologies as described in the present study, smart water treatment systems, water reuse, and resource recovery plans and strategies, and strengthening and updating on the existing regulations, which will play a crucial role in driving advancements in drinking water treatment.