Baseline Study on Microplastic Distribution in the Open Surface Waters of the Korean Southwest Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Method
2.2. Microplastic Pretreatment Method
2.2.1. Organic Matter Digestion
2.2.2. Density Separation and Drying
2.3. Identifying the Polymer Types
2.4. Microplastic Shape Classification and Size Measurement
2.5. Quality Assurance/Quality Control (QA/QC)
3. Results
3.1. Spatial Distribution of Water Temperature and Salinity in the Surface Water
3.2. The Numerical Distribution of Microplastic Abundance
3.3. The Distribution of Microplastic Polymer Types
3.4. The Distribution of the Microplastics Shapes
3.5. The Distribution of Microplastics Sizes
4. Discussion
| Investigated Area | n | Investigation | Collected Water Volume | Mesh Size | Numerical Abundance (Particle/L) | Primary Polymer (Mean %) | Primary Shape (Mean %) | Main Size (Mean %) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Republic of Korea | Incheon/ Gyeonggi area | 12 | 2013 | 100 L | 20 μm | mean 152.69 ± 92.38 | - | - | - | [26] |
| Cheonsu Bay | 5 | 2016/2017 | 100 L | 20 μm | mean 0.784 ± 0.272 | PP + PE (54%) | Fragment (81%) | Non-fiber 197 ± 168 μm Fiber 752 ± 711 μm | [27] | |
| Hampyeong | 5 | 2016/2017 | 100 L | 20 μm | mean 1.548 ± 0.211 | PP + PE (84%) | Fragment (81%) | - | ||
| Deukryang Bay | 5 | 2016/2017 | 100 L | 20 μm | mean 1.146 ± 0.423 | PP + PE (82%) | Fragment (81%) | - | ||
| Gwangyang Bay | 5 | 2016/2017 | 100 L | 20 μm | mean 2.362 ± 1.022 | PP + PE (89%) | Fragment (81%) | - | ||
| Ulsan Coast | 5 | 2016/2017 | 100 L | 20 μm | mean 1.764 ± 1.006 | PP + PE (30%) | Fragment (81%) | - | ||
| Yeongil Bay | 5 | 2016/2017 | 100 L | 20 μm | mean 1.688 ± 0.496 | EVA (58%) | Fragment (81%) | - | ||
| Incheon | 5 | 2016/2017 | 100 L | 20 μm | mean 4.064 ± 1.075 | EVA (75%) | Fragment (81%) | - | ||
| Busan | 6 | 2016/2017 | 100 L | 20 μm | mean 2.362 ± 1.022 | EVA (72%) | Fragment (81%) | - | ||
| Yellow Sea | 9 | 2018 | 200 L | 20 μm | mean 0.266 ± 0.459 | - | - | - | [44] | |
| South Sea | 5 | 2018 | 200 L | 20 μm | - | - | - | |||
| East Sea | 8 | 2018 | 200 L | 20 μm | mean 0.289 ± 0.280 | - | - | - | ||
| China | Chinese Coast | 16 | - | 100 L | 50 μm | mean 4.5 | PP, PE (>75%) | Fiber (89.3%) | 500 μm (76%) | [31] |
| Yangtze Estuary | 12–20 L | 32 μm | mean 4.137 ± 2.462 | - | Fiber (79.1%) | >500–1000 μm (67%) | [50] | |||
| North Yellow Sea | 50 | 2016 | 25 L | 30 μm | mean 0.545 ± 0.282 | PE (77.8%) PP (11.1%) | Film (58.1%) Fiber (39.1%) | >500 μm (35.7~83.5%) | [48] | |
| South China Sea | 22 | 2017 | 3 m3 | 20–300 μm | mean 2.569 ± 1.770 | Alkyd (22.5%) PCL (20.9%) PEA (15.5%) PS (14.7%) PTFE (4.7%) | - | - | [49] | |
| Malaysia | Estuaries | 4 9 | 2018 | 2.041 L/s (10 min) | 20 μm | mean 0.422 ± 0.110 | - | Fiber (73.8–80.8%) | - | [47] |
| Sea area | mean 0.211 ± 0.104 | - | - | |||||||
| North Pole | Arctic Central Basin | - | 2016 | 2000 L | 250 μm | 0–0.0075 (8.5 m depth) | Polyester (>80%) | Fiber (94%) | 1000–2000 μm (62%) | [45] |
| Atlantic Ocean | 23 | 2014 | mean 2.6 m3 | 10~50 μm | 0.013–0.501 | PE + PP (48%) | - | >40 μm (64%) | [46] | |
| Southwest sea of Jeju | 23 | 2020 | 30 L | 20 μm | 0.23 ± 0.22 | PP + PE (67%) | Fragment (69%) | 20–300 μm (54.4%) | This study | |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. An Analysis of European Plastics Production, Demand and Waste Data. Plastics Europe. In Plastics–The Facts; Plastics Europe: Brussels, Belgium, 2019. [Google Scholar]
- Zainuddin, A.H.; Aris, A.Z.; Zaki, M.R.M.; Yusoff, F.M.; Wee, S.Y. Occurrence, potential sources and ecological risk estimation of microplastic towards coastal and estuarine zones in Malaysia. Mar. Pollut. Bull. 2022, 174, 113282. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, P.J.; Rochman, C.M. Sources, fate and effects of microplastics in the marine environment: Part 2 of a global assessment. In Reports and Studies—IMO/FAO/Unesco-IOC/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP); International Maritime Organization: London, UK, 2015; Eng No. 93. [Google Scholar]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- De Jesus Piñon-Colin, T.; Rodriguez-Jimenez, R.; Pastrana-Corral, M.A.; Rogel-Hernandez, E.; Wakida, F.T. Microplastics on sandy beaches of the Baja California Peninsula, Mexico. Mar. Pollut. Bull. 2018, 131, 63–71. [Google Scholar] [CrossRef]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Inter. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High quantities of microplastic in Arctic deep-sea sediments from the HAUSGARTEN observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.D.; Sinclair, M.; Levi, C.J.; Reeves, S.E.; Edgar, G.J. Ubiquity of microplastics in coastal seafloor sediments. Mar. Pollut. Bull. 2017, 121, 104–110. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Lattin, G.L.; Moore, C.J.; Zellers, A.F.; Moore, S.L.; Weisberg, S.B. A comparison of neustonic plastic and zooplankton at different depths near the southern California shore. Mar. Pollut. Bull. 2004, 49, 291–294. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Envrion. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Enviro. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Yang, C.S.; Chang, C.H.; Tsai, P.J.; Chen, W.Y.; Tseng, F.G.; Lo, L.W. Nanoparticle-based in vivo investigation on blood–brain barrier permeability following ischemia and reperfusion. Anal. Chem. 2004, 76, 4465–4471. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Sun, C.; Cao, W.; Wang, M.; Jiang, F.; Ju, P. Comparative study of three sampling methods for microplastics analysis in seawater. Sci. Total Environ. 2021, 765, 144495. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Lee, J.M. Relationship among Size Groups of Plastic Beach Debris as a Tool to Assess Microplastic Pollution in Korea. Ph.D. Dissertation, Department of Ecological Engineering, Pukyong University, Busan, Republic of Korea, 2013. (In Korean). [Google Scholar]
- Chae, D.H.; Kim, I.S.; Kim, S.K.; Song, Y.K.; Shim, W.J. Abundance and distribution characteristics of microplastics in surface seawaters of the Incheon/Kyeonggi coastal region. Arch. Environ. Contam. Toxicol. 2015, 69, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Eo, S.; Jang, M.; Han, G.M.; Isobe, A.; Shim, W.J. Horizontal and vertical distribution of microplastics in Korean coastal waters. Environ. Sci. Technol. 2018, 52, 12188–12197. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Seo, M.H.; Yang, Y.S.; Park, E.O.; Yoon, Y.H.; Kim, D.J.; Jeong, H.G.; Soh, H.Y. Zooplankton and Neustonic Microplastics in the Surface Layer of Yeosu Coastal Areas. Kor. J. Environ. Biol. 2018, 36, 11–20. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Kang, J.H.; Hong, S.H.; Shim, W.J. Spatial distribution of microplastic in the surface waters along the coast of Korea. Mar. Pollut. Bull. 2020, 155, 110729. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, J.W.; Eo, S.; Choi, J.; Song, Y.K.; Cho, Y.; Shim, W.J. Ecological risk assessment of microplastics in coastal, shelf, and deep sea waters with a consideration of environmentally relevant size and shape. Environ. Pollut. 2021, 270, 116217. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Wang, X.; Yang, F.; Chen, M.; Wang, J. Characterization of microplastics in the surface seawater of the South Yellow Sea as affected by season. Sci. Total Environ. 2020, 724, 138375. [Google Scholar] [CrossRef]
- Isobe, A.; Uchhda, K.; Tokai, T.; Iwasaki, S. East Asian seas: A hot spot of pelagic microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef]
- National Institute of Fisheries Science (NFS). Guidelines for Qualitativeness and Quantification of Residual Microplastics in Seawater and Aquatic Life; NIPS: Busan, Republic of Korea, 2021; pp. 1–49. (In Korean) [Google Scholar]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Techical Memorandum; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015; p. 48. [Google Scholar]
- Bimali Koongolla, J.; Andrady, A.L.; Terney Pradeep Kumara, P.B.; Gangabadage, C.S. Evidence of microplastics pollution in coastal beaches and waters in southern Sri Lanka. Mar. Pollut. Bull. 2018, 137, 277–284. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Castillo, A.B.; Al-Maslamani, I.; Obbard, J.P. Prevalence of microplastics in the marine waters of Qatar. Mar. Pollut. Bull. 2016, 111, 260–267. [Google Scholar] [CrossRef]
- Korea Institute of Oceanscience & Technology (KIOST). Guidelines for “Study on Environmental Risk of Marine Microplastics” 01. Guidelines for Collection and Analysis of Floating Microplastics; KIOST: Busan, Republic of Korea, 2017; pp. 1–23. (In Korean) [Google Scholar]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- UNEP. Marine Plastic Debris and Microplstics—Global Lessens and Research to Inspire Action and Guide Policy Change; United Nations Environment Programme: Nairobi, Kenya, 2019. [Google Scholar]
- Kershaw, P.; Turra, A.; Galgani, F. Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean-GESAMP Reports and Studies No. 99. GESAMP Reports and Studies; United Nations Office: Nairobi, Kenya, 2019; pp. 1–123. [Google Scholar]
- Michida, Y.; Chavanich, S.; Cozar, C.A.; Hagmann, P.; Hinata, H.; Isobe, A.; Kershaw, P.; Kozlovskii, N.; Li, D.; Lusher, A.L.; et al. Guidelines for Harmonizing Ocean Surface Microplastic Monitoring Methods; Ministry of the Environment Japan: Tokyo, Japan, 2019; p. 71. [Google Scholar]
- Jeong, H.; Kusano, T.; Addai-Arhin, S.; Nugraha, W.C.; Novirsa, R.; Phan Dinh, Q.; Shirosaki, T.; Fujita, E.; Kameda, Y.; Cho, H.S.; et al. A Study on the Differences of Microplastics Distributions in the Surface Freshwater Collected by 100- and 355-μm Meshes. Environ. Monitor. Con. Res. 2022, 2, 22–34. [Google Scholar]
- Eo, S.; Hong, S.H.; Song, Y.K.; Han, G.M.; Seo, S.; Shim, W.J. Prevalence of small high-density microplastics in the continental shelf and deep sea waters of East Asia. Water Res. 2021, 200, 117238. [Google Scholar] [CrossRef] [PubMed]
- La Daana, K.K.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef]
- Taha, Z.D.; Amin, R.M.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. MICROPLASTICS in seawater and zooplankton: A CASE study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef]
- Zhu, L.; Bai, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic pollution in North Yellow Sea, China: Observations on occurrence, distribution and identification. Sci. Total Environ. 2018, 636, 20–29. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Liu, M.; Li, S.; Tang, G.; Wang, W.; Cen, Z. Lost but can’t be neglected: Huge quantities of small microplastics hide in the South China Sea. Sci. Total Environ. 2018, 633, 1206–1216. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Khatmullina, L.; Chubarenko, I. Transport of marine microplastic particles: Why is it so difficult to predict? Anthr. Coasts 2019, 2, 293–305. [Google Scholar] [CrossRef]
- Chen, C.T.A. Distributions of nutrients in the East China Sea and the South China Sea connection. J. Ocean. 2008, 64, 737–751. [Google Scholar] [CrossRef]
- Chen, C.T. Chemical and physical fronts in the Bohai, Yellow and East China Seas. J. Mar. Syst. 2009, 78, 394–410. [Google Scholar] [CrossRef]
- Seung, Y.H. Water masses and circulations around Korean Peninsula, J. Oceanolog. Soc. Kor. 1992, 27, 324–331. (In Korean) [Google Scholar]
- Gong, G.C.; Chen, Y.L.L.; Liu, K.K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Hur, H.B.; Jacobs, G.A.; Teague, W.J. Monthly variations of water masses in the Yellow and East China Seas, November 6. J. Ocean. 1999, 55, 171–184. [Google Scholar] [CrossRef]
- Yoon, S.C.; Youn, S.H.; Whang, J.D.; Suh, Y.S.; Yoon, Y.Y. Long-term Variation in Ocean Environmental Conditions of the Northern East China Sea. J. Kor. Soc. Mar. Environ. Energy 2015, 18, 189–206. (In Korean) [Google Scholar] [CrossRef]
- Zhu, Z.N.; Zhu, X.H.; Zhang, C.; Chen, M.; Zheng, H.; Zhang, Z.; Kaneko, A. Monitoring of Yangtze River Discharge at Datong Hydrometric Station Using Acoustic Tomography Technology. Front. Earth Sci. 2021, 9, 723123. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, C.; Elser, J.J.; Mei, Z.; Hao, Y. Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River–from inland to the sea. Sci. Total Environ. 2019, 659, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cui, Y.; Zhou, H.; Jin, C.; Yu, X.; Xu, Y.; Zhang, C. Microplastic pollution in water, sediment, and fish from artificial reefs around the Ma’an Archipelago, Shengsi, China. Sci. Total Environ. 2020, 703, 134768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Transport of microplastics in coastal seas. Estuar. Coast. Shelf Sci. 2017, 199, 74–86. [Google Scholar] [CrossRef]
- Lin, J.; Xu, X.M.; Yue, B.Y.; Xu, X.P.; Liu, J.Z.; Zhu, Q.; Wang, J.H. Multidecadal records of microplastic accumulation in the coastal sediments of the East China Sea. Chemosphere 2021, 270, 128658. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, H.; Cui, Y.; Wang, C.; Li, Y.; Zhang, D. Microplastics in offshore sediment in the yellow Sea and east China Sea, China. Environ. Pollut. 2019, 244, 827–833. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; El Menif, N.T. Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The colors of the ocean plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]

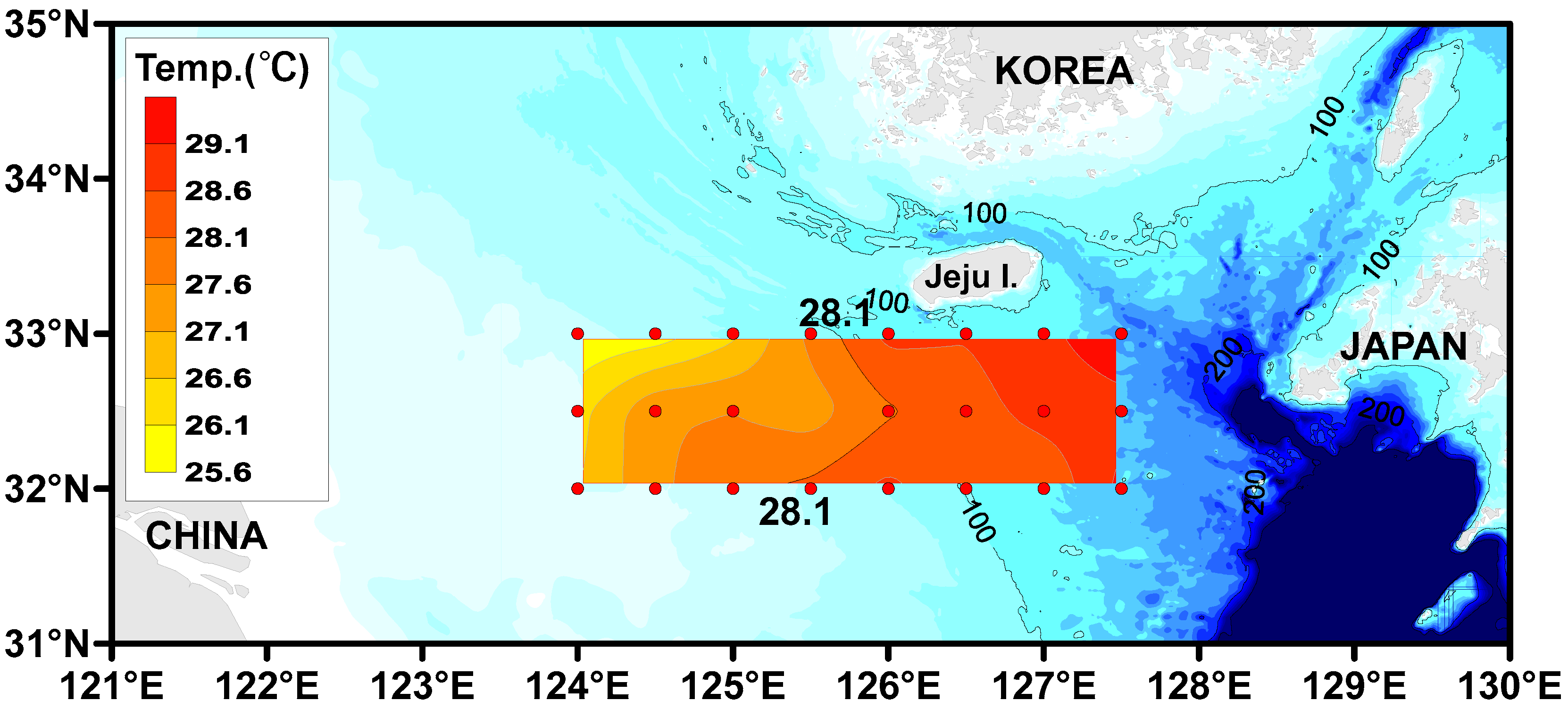


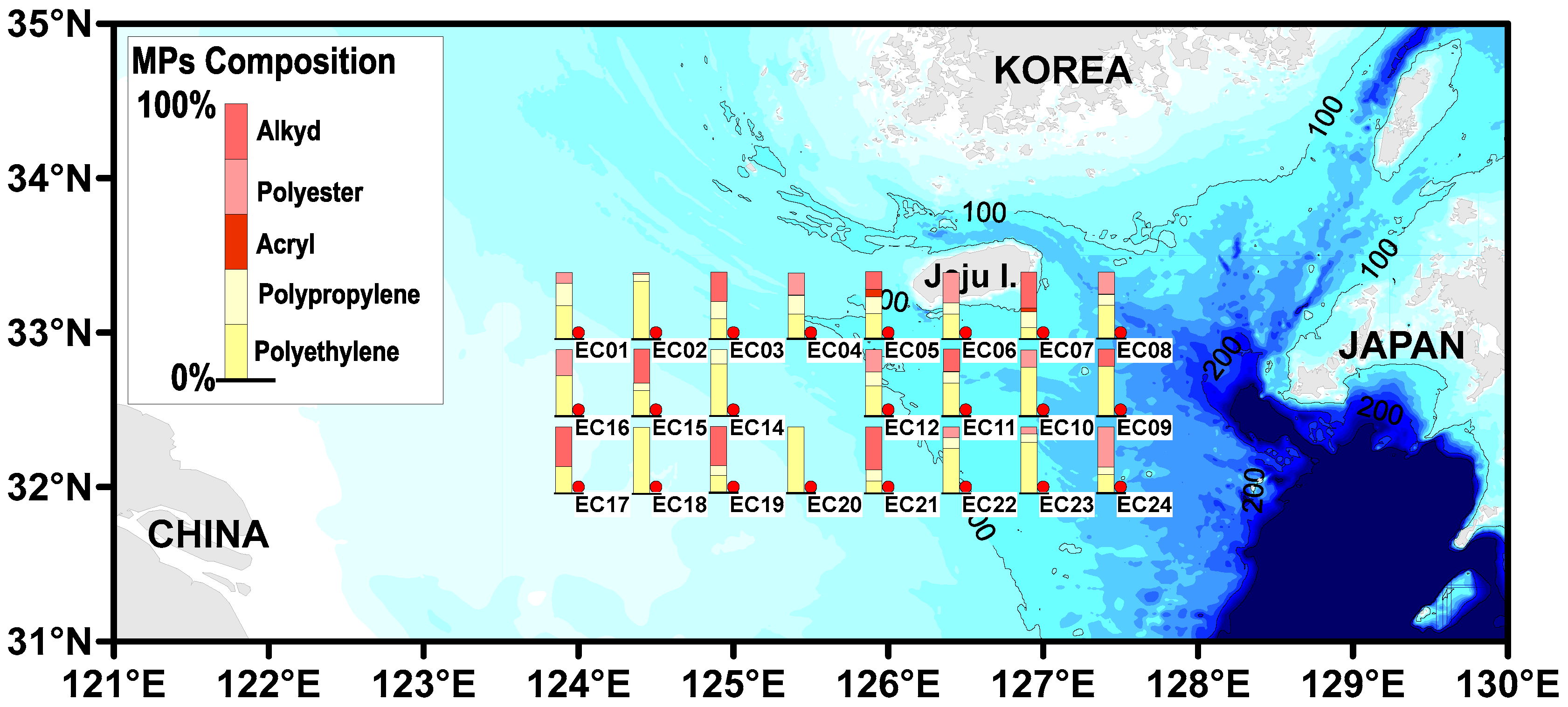
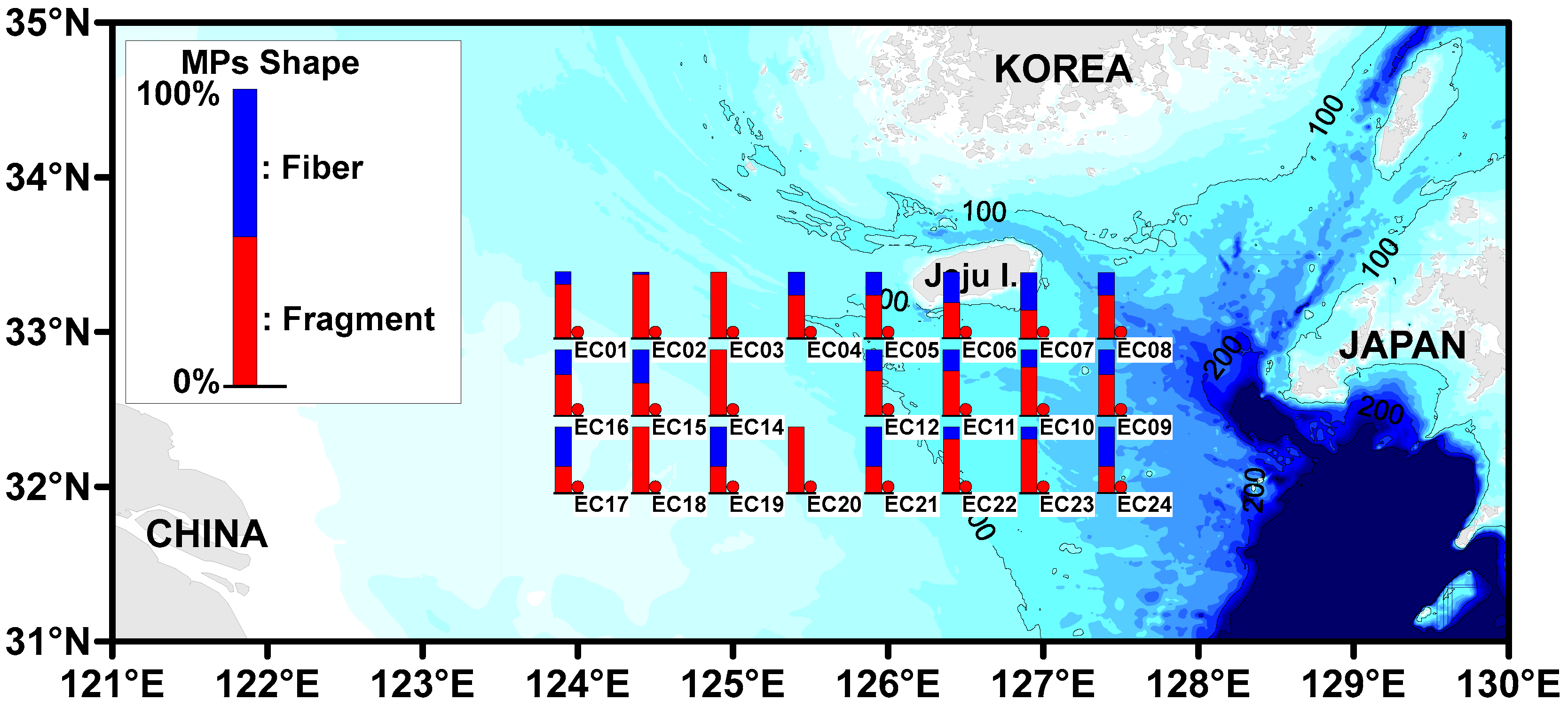
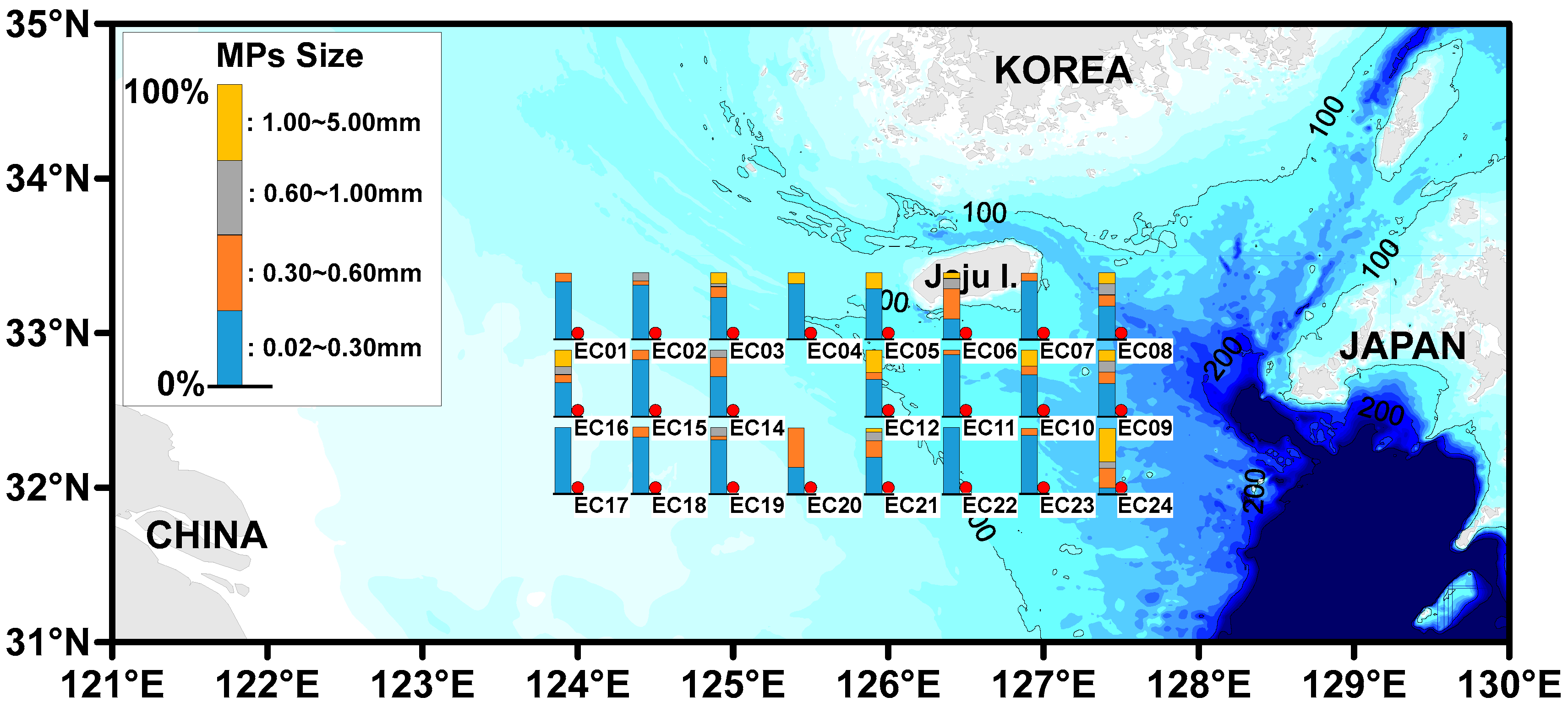
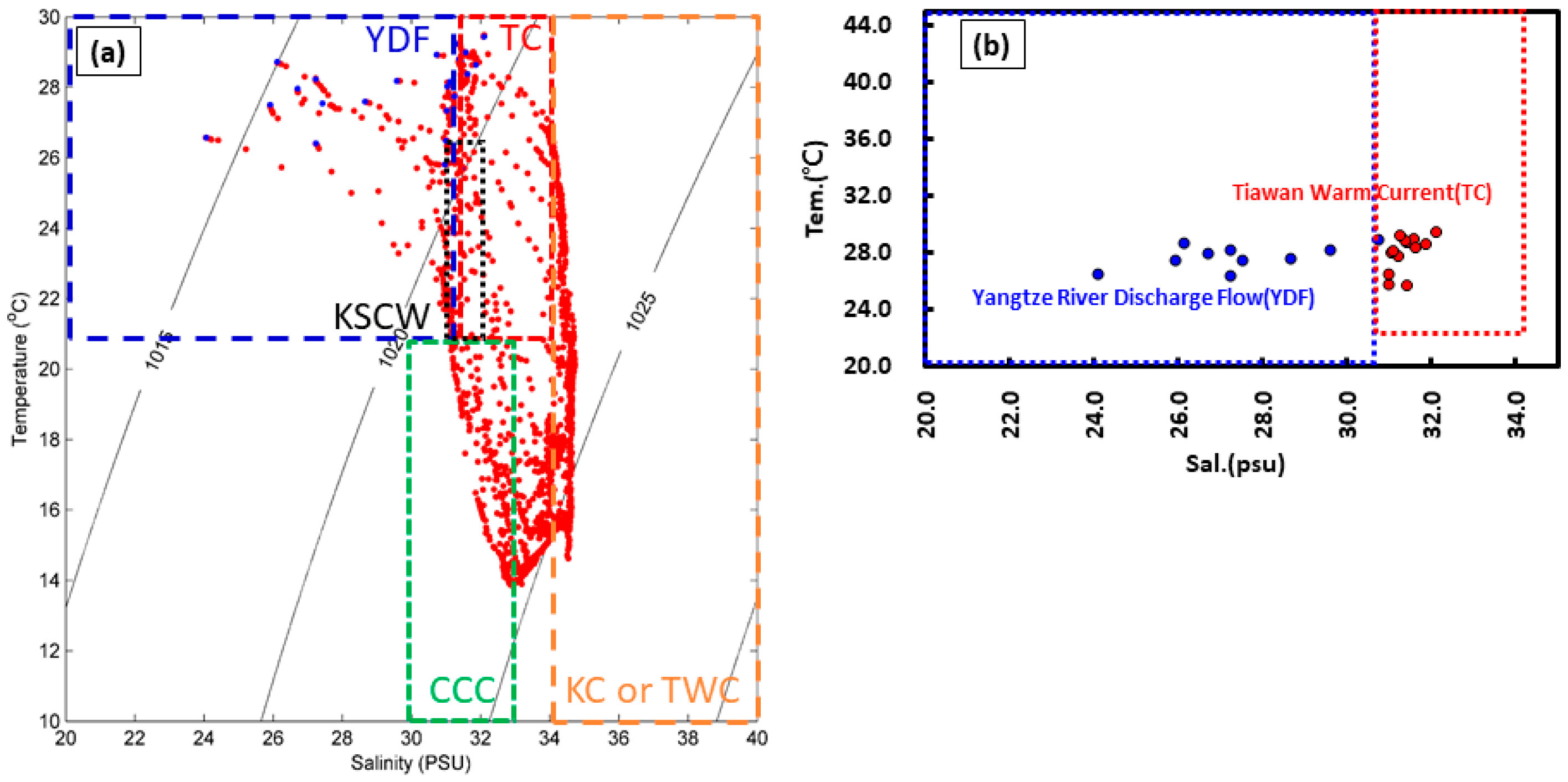
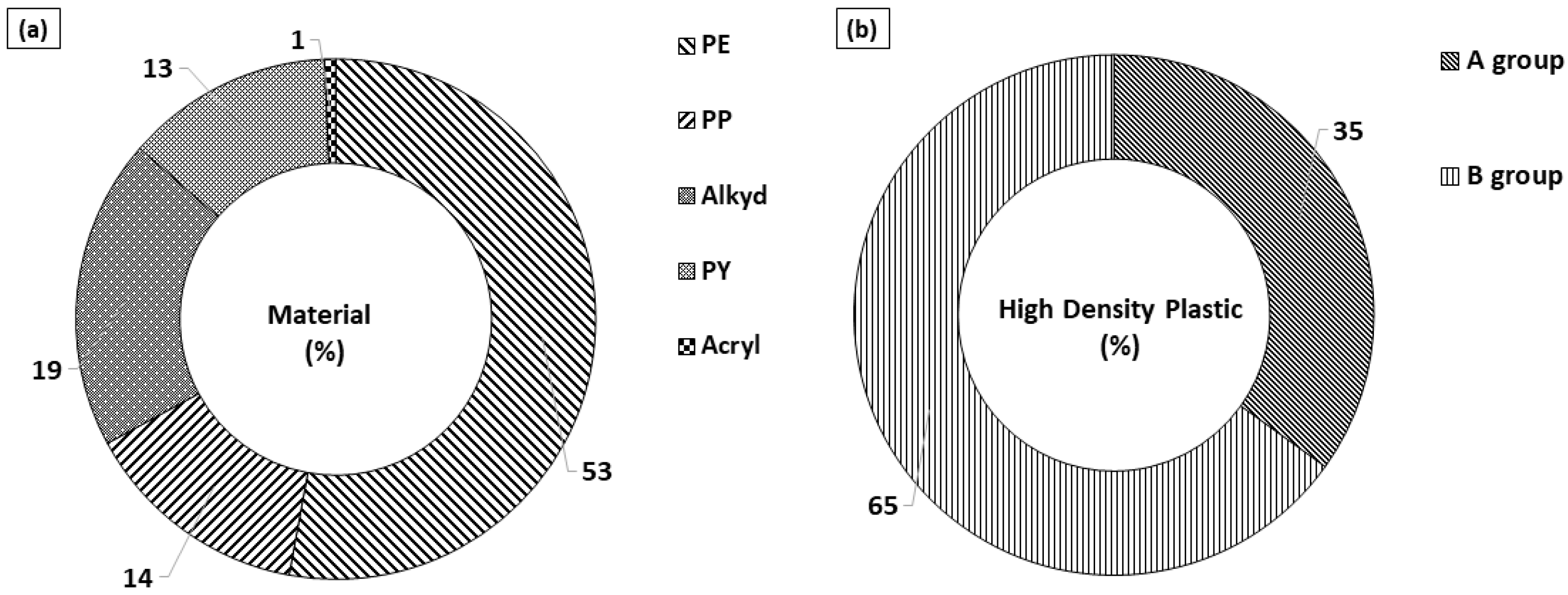

| Site | MP Abundance (Particles/L) (a) | MP Size (%) | MP Shape (%) (b) | Tem. (°C) | Sal. (psu) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | PP | PY | Acryl | Alkyd | Total | 0.02–0.3 mm | 0.3–0.6 mm | 0.6~1.0 mm | 1.0–5.0 mm | Fragment | Fiber | |||
| EC01 | 0.33 | 0.17 | N.D | N.D | 0.10 | 0.60 | 87.5 | 12.5 | N.D | N.D | 83.3 | 16.7 | 25.69 | 31.44 |
| EC02 | 1.20 | 0.13 | 0.03 | N.D | N.D | 1.37 | 82.9 | 4.9 | 12.2 | N.D | 97.6 | 2.4 | 25.81 | 30.97 |
| EC03 | 0.23 | 0.20 | N.D | N.D | 0.33 | 0.77 | 63.2 | 15.8 | 5.3 | 15.8 | 100.0 | 0.0 | 26.48 | 30.98 |
| EC04 | 0.20 | 0.17 | 0.20 | N.D | N.D | 0.57 | 88.2 | 11.8 | N.D | N.D | 64.7 | 35.3 | 27.74 | 31.24 |
| EC05 | 0.10 | 0.07 | N.D | 0.03 | 0.07 | 0.27 | 75.0 | N.D | N.D | 25.0 | 62.5 | 37.5 | 28.74 | 31.43 |
| EC06 | 0.17 | 0.07 | 0.20 | N.D | N.D | 0.43 | 30.8 | 46.2 | 15.4 | 7.7 | 53.8 | 46.2 | 28.63 | 31.87 |
| EC07 | 0.17 | 0.23 | N.D | 0.03 | 0.50 | 0.93 | 88.9 | 11.1 | N.D | N.D | 42.9 | 57.1 | 28.99 | 31.57 |
| EC08 | 0.10 | 0.03 | 0.07 | N.D | N.D | 0.20 | 50.0 | 16.7 | 16.7 | 16.7 | 66.7 | 33.3 | 29.44 | 32.10 |
| EC09 | 0.20 | 0.07 | N.D | N.D | 0.17 | 0.43 | 50.0 | 16.7 | 16.7 | 16.7 | 61.5 | 38.5 | 28.92 | 30.73 |
| EC10 | 0.20 | N.D | 0.07 | N.D | N.D | 0.27 | 62.5 | 12.5 | N.D | 25.0 | 75.0 | 25.0 | 28.81 | 31.38 |
| EC11 | 0.27 | 0.10 | N.D | N.D | 0.17 | 0.53 | 93.8 | 6.3 | N.D | N.D | 68.8 | 31.3 | 28.38 | 31.62 |
| EC12 | 0.13 | 0.07 | 0.10 | N.D | N.D | 0.30 | 55.6 | 11.1 | N.D | 33.3 | 66.7 | 33.3 | 28.03 | 31.04 |
| EC14 | 0.27 | 0.07 | N.D | N.D | N.D | 0.33 | 60.0 | 30.0 | 10.0 | N.D | 100.0 | 0.0 | 27.59 | 28.66 |
| EC15 | 0.27 | 0.07 | N.D | N.D | 0.33 | 0.67 | 86.4 | 13.6 | N.D | N.D | 50.0 | 50.0 | 27.55 | 27.50 |
| EC16 | 0.17 | N.D | 0.10 | N.D | N.D | 0.27 | 50.0 | 12.5 | 12.5 | 25.0 | 62.5 | 37.5 | 26.41 | 27.24 |
| EC17 | 0.13 | N.D | N.D | N.D | 0.20 | 0.33 | 100.0 | N.D | N.D | N.D | 40.0 | 60.0 | 26.57 | 24.06 |
| EC18 | 0.23 | N.D | N.D | N.D | N.D | 0.23 | 85.7 | 14.3 | N.D | N.D | 100.0 | 0.0 | 27.49 | 25.92 |
| EC19 | 0.13 | 0.07 | N.D | N.D | 0.30 | 0.50 | 81.3 | 6.3 | 12.5 | N.D | 40.0 | 60.0 | 27.97 | 26.71 |
| EC20 | 0.17 | N.D | N.D | N.D | N.D | 0.17 | 40.0 | 60.0 | N.D | N.D | 100.0 | 0.0 | 28.23 | 27.23 |
| EC21 | 0.07 | 0.07 | N.D | N.D | 0.23 | 0.37 | 56.3 | 25.0 | 12.5 | 6.3 | 36.4 | 63.6 | 28.73 | 26.12 |
| EC22 | 0.27 | 0.07 | N.D | N.D | N.D | 0.33 | 100.0 | N.D | N.D | N.D | 83.3 | 16.7 | 28.18 | 29.60 |
| EC23 | 0.23 | 0.03 | N.D | N.D | N.D | 0.27 | 88.9 | 11.1 | N.D | N.D | 88.9 | 11.1 | 28.16 | 31.11 |
| EC24 | 0.10 | 0.03 | 0.20 | N.D | N.D | 0.33 | 10.0 | 30.0 | 10.0 | 50.0 | 40.0 | 60.0 | 29.24 | 31.25 |
| Min | 0.07 | 0.03 | 0.03 | 0.03 | 0.07 | 0.17 | 10.0 | 4.9 | 5.3 | 6.3 | 36.4 | 0.0 | 25.69 | 24.06 |
| Max | 1.20 | 0.23 | 0.20 | 0.03 | 0.50 | 1.37 | 100.0 | 60.0 | 16.7 | 50.0 | 100.0 | 63.6 | 29.44 | 32.10 |
| Mean | 0.23 | 0.09 | 0.12 | 0.03 | 0.24 | 0.46 | 69.0 | 16.0 | 5.4 | 9.6 | 68.9 | 31.1 | 27.90 | 29.64 |
| SD | 0.22 | 0.06 | 0.06 | 0.00 | 0.12 | 0.27 | 23.2 | 14.0 | 6.5 | 13.6 | 21.4 | 21.4 | 1.05 | 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, B.K.; Jeong, H.H.; Ju, M.J.; Ko, U.; Dae, K.H.; Kim, H.J.; Cho, C.R.; Soh, H.Y.; Ishibashi, Y.; Cho, H.S. Baseline Study on Microplastic Distribution in the Open Surface Waters of the Korean Southwest Sea. Water 2023, 15, 2393. https://doi.org/10.3390/w15132393
Min BK, Jeong HH, Ju MJ, Ko U, Dae KH, Kim HJ, Cho CR, Soh HY, Ishibashi Y, Cho HS. Baseline Study on Microplastic Distribution in the Open Surface Waters of the Korean Southwest Sea. Water. 2023; 15(13):2393. https://doi.org/10.3390/w15132393
Chicago/Turabian StyleMin, Byeong Kyu, Hui Ho Jeong, Mi Jo Ju, Uni Ko, Keum Hyang Dae, Hyun Jung Kim, Chon Rae Cho, Ho Young Soh, Yasuhiro Ishibashi, and Hyeon Seo Cho. 2023. "Baseline Study on Microplastic Distribution in the Open Surface Waters of the Korean Southwest Sea" Water 15, no. 13: 2393. https://doi.org/10.3390/w15132393
APA StyleMin, B. K., Jeong, H. H., Ju, M. J., Ko, U., Dae, K. H., Kim, H. J., Cho, C. R., Soh, H. Y., Ishibashi, Y., & Cho, H. S. (2023). Baseline Study on Microplastic Distribution in the Open Surface Waters of the Korean Southwest Sea. Water, 15(13), 2393. https://doi.org/10.3390/w15132393







