Optimization of Adsorption Conditions Using Response Surface Methodology for Tetracycline Removal by MnFe2O4/Multi-Wall Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Experimental Methods
2.2.1. Preparation of MMWCNTs Composites
2.2.2. Batch Adsorption Experiments
2.3. Box–Behnken Design Model
3. Results and Discussion
3.1. Batch Adsorption Experiments
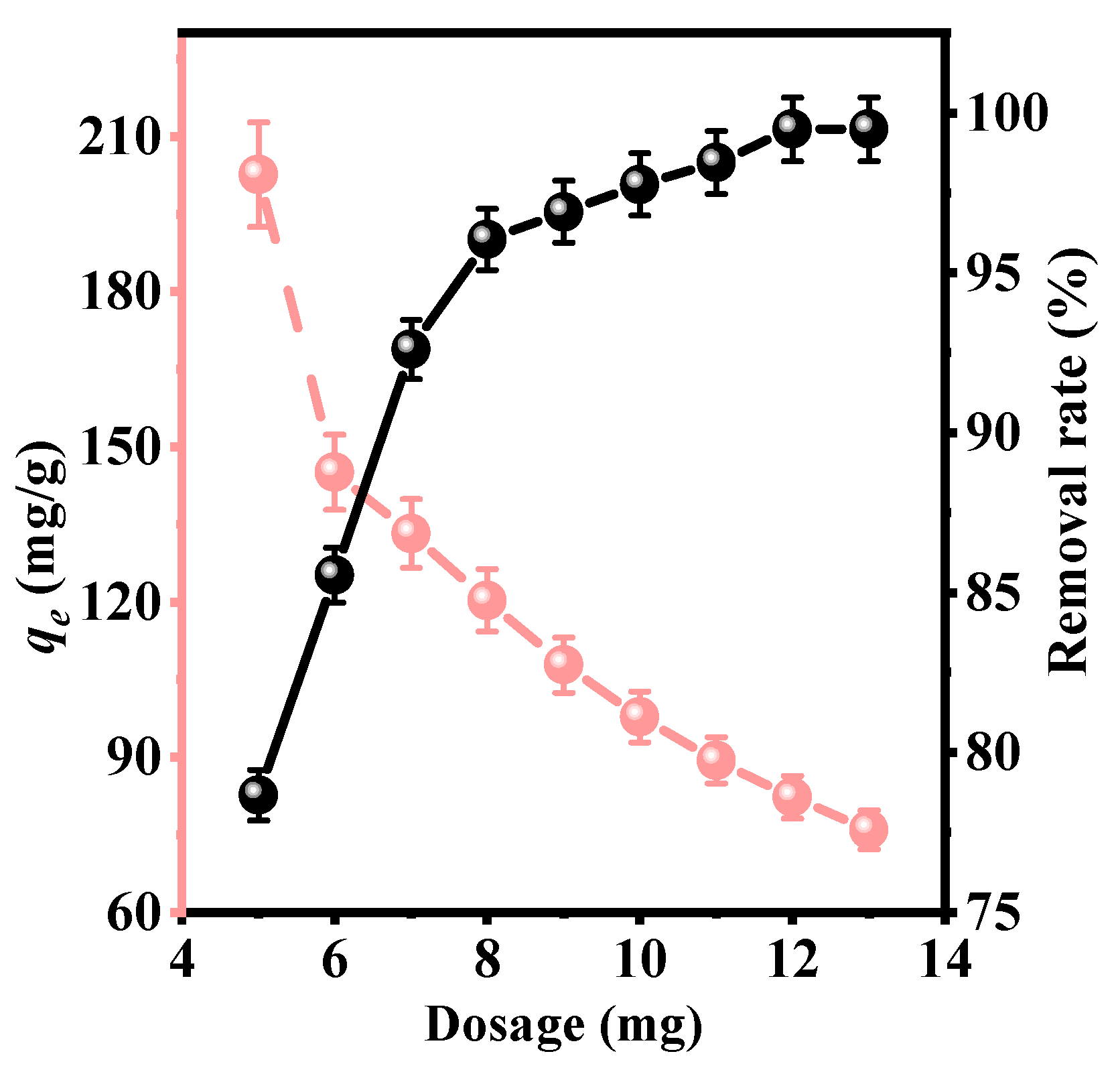
3.1.1. Effect of Dosage
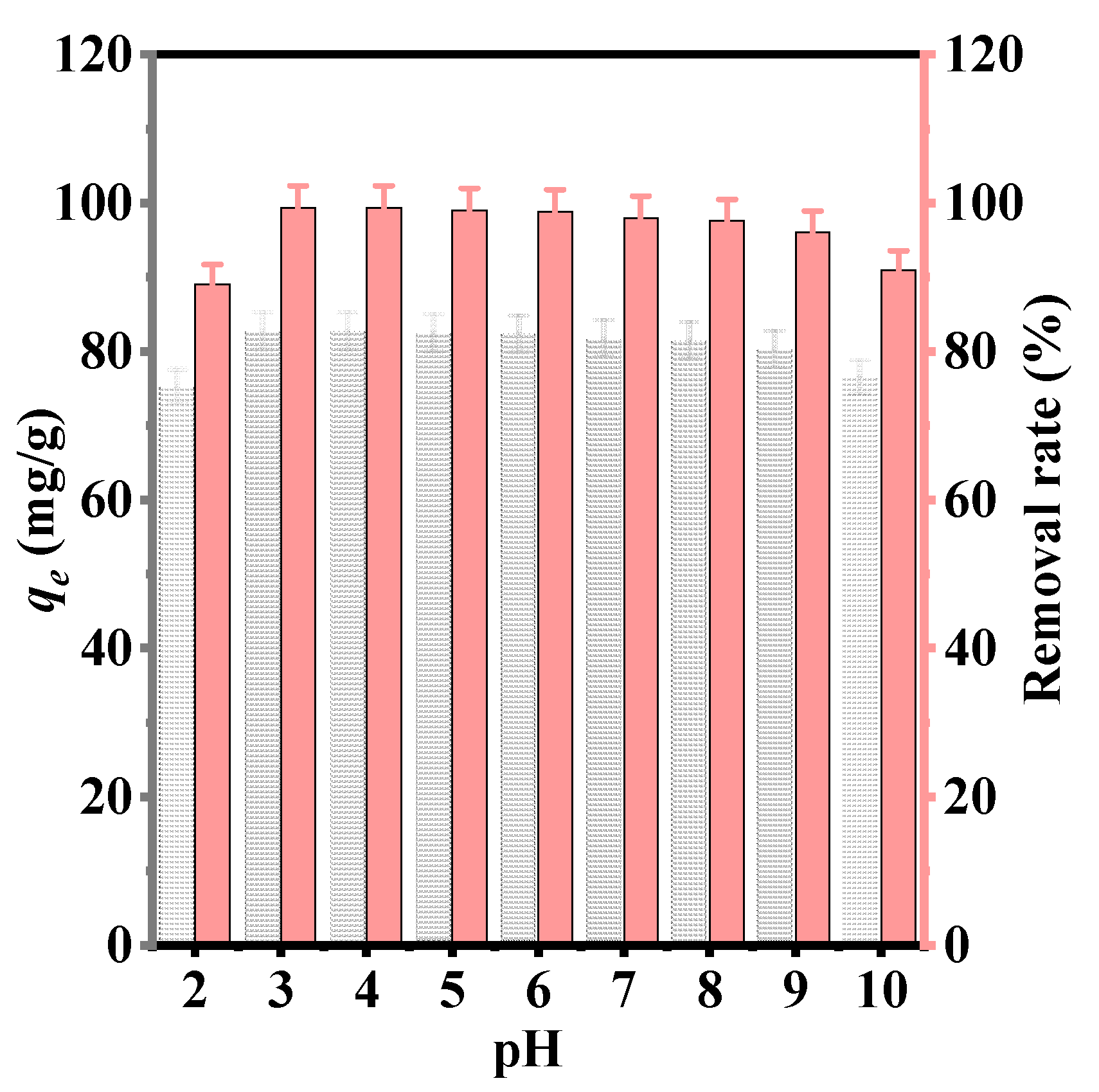
3.1.2. Effect of pH
3.1.3. Effect of Time
3.1.4. Adsorption Kinetics
3.1.5. Mechanism of TC Adsorption by MMWCNTs
3.2. Box–Behnken Design Model
3.2.1. Modeling and Statistical Analysis
3.2.2. Analysis of Variance
3.2.3. Main Effect of Variables
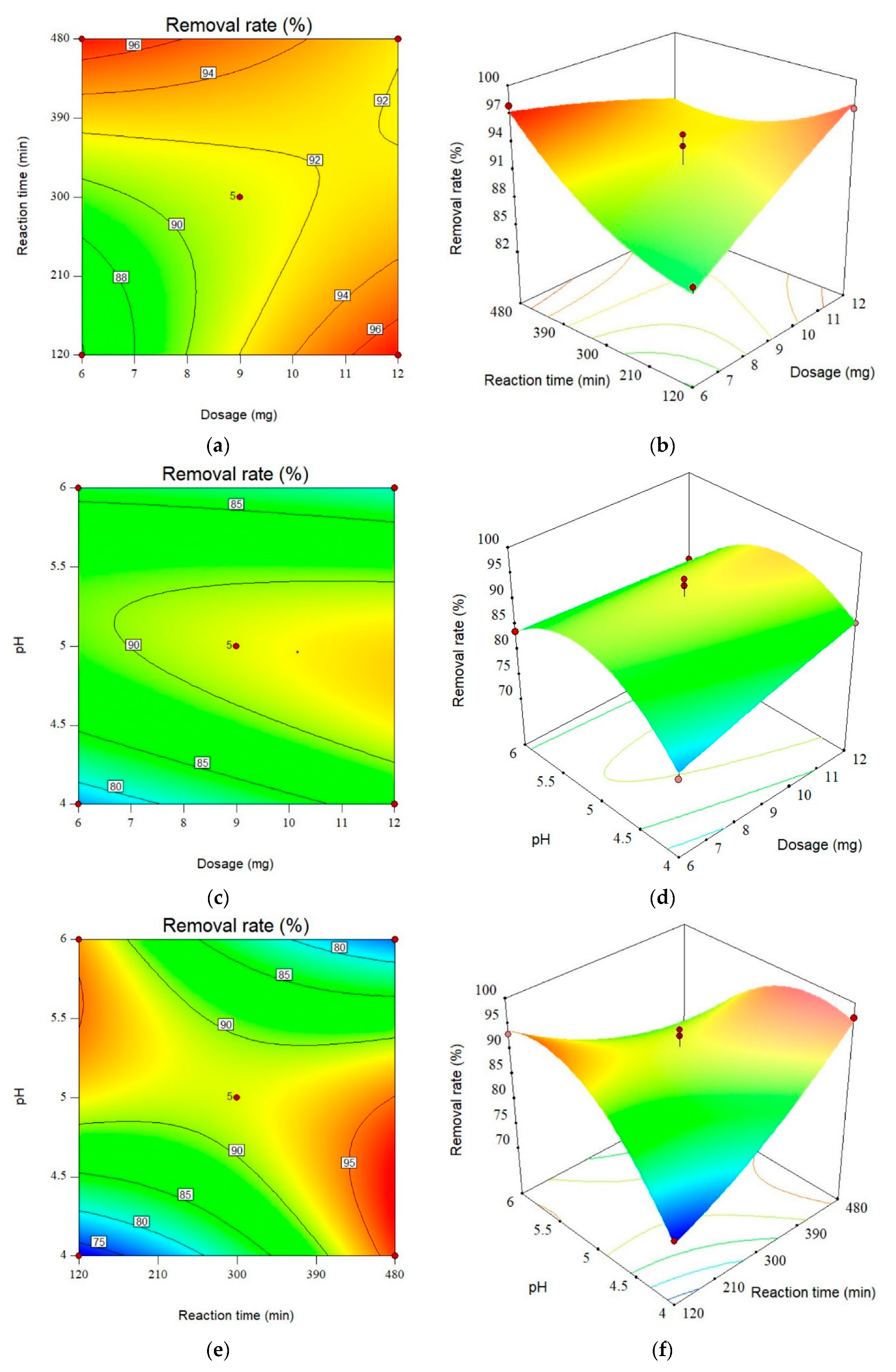
3.2.4. Interaction of Contact Time and Adsorbent Dosage
3.2.5. Interaction of Adsorbent Dosage and pH
3.2.6. Interaction of Contact Time and pH
3.3. Validation of Box–Behnken Design Optimization
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Escobar-Huerfano, F.; Gómez-Oliván, L.M.; Luja-Mondragón, M.; SanJuan-Reyes, N.; Islas-Flores, H.; Hernández-Navarro, M.D. Embryotoxic and teratogenic profile of tretracycline at environmentally relevant concentrations on Cyprinus carpio. Chemosphere 2020, 240, 124969. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, J.; Feng, K.; Xing, D.F. Digestate of Fecal Sludge Enhances the Tetracycline Removal in Soil Microbial Fuel Cells. Water 2022, 14, 2752. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Yang, Z.H.; Xiong, W.P.; Zhou, Y.Y.; Xu, R.; Zhang, Y.R.; Cao, J.; Li, X.; Zhou, C.Y. Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions. Sci. Total Environ. 2019, 650, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.L.; Iqbal, J.R.; Kharbish, S.R.; Ismael, M.; Bhatnagar, A.M. Tuning tetracycline removal from aqueous solution onto activated 2:1 layered clay mineral: Characterization, sorption and mechanistic studies. J. Hazard. Mater. 2020, 384, 121320. [Google Scholar] [CrossRef] [PubMed]
- Naderi, K.; Foroughi, M.; Azqhandi, M.H.A. Tetracycline capture from aqueous solutions by nanocomposite of MWCNTs reinforced with glutaraldehyde cross-linked poly (vinyl alcohol)/chitosan. Chemosphere 2022, 303, 135124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.G.; Tian, Y.M.; Chu, X.X.; Cui, L.; Zhang, H.W.; Li, M.; Zhao, P. Preparation and characteristics of a magnetic carbon nanotube adsorbent: Its efficient adsorption and recoverable performances. Sep. Purif. Technol. 2021, 257, 117917. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zheng, W.F.; Zhang, F.; Li, W.X.; Shen, X.M.; Huang, H.B.; Shi, L.; Shi, R.; Zhang, S.; Lu, M. Sorption Behavior and Prediction of Tetracycline on Sediments from the Yangtze Estuary and Its Coastal Areas. Water 2023, 15, 671. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.Y.; Owens, G.; Chen, Z.L. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef]
- Li, R.H.; Zhang, Y.C.; Deng, H.X.; Zhang, Z.Q.; Wang, J.J.; Shaheen, S.M.; Xiao, R.; Rinklebe, J.; Xi, B.D.; He, X.S.; et al. Removing tetracycline and Hg (II) with ball-milled magnetic nanobiochar and its potential on polluted irrigation water reclamation. J. Hazard. Mater. 2020, 384, 121095. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, X.X.; Liao, P.; Zhang, C.; Liu, C.X. Enhanced sequestration of tetracycline by Mn (II) encapsulated mesoporous silica nanoparticles: Synergistic sorption and mechanism. Chemosphere 2021, 284, 131334. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Nguyen, T.-B.; Chen, C.-W.; Hung, C.-M.; Vo, T.-D.-H.; Chang, J.-H.; Dong, C.-D. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour. Technol. 2019, 284, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.A.; Polk, J.S.; Datta, T.; Parekh, R.R.; Agga, G.E. Occurrence of Antibiotic Resistant Bacteria in Urban Karst Groundwater Systems. Water 2022, 14, 960. [Google Scholar] [CrossRef]
- Sun, Y.F.; Lyu, H.H.; Cheng, Z.; Wang, Y.Z.; Tang, J.C. Insight into the mechanisms of ball-milled biochar addition on soil tetracycline degradation enhancement: Physicochemical properties and microbial community structure. Chemosphere 2022, 291, 132691. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Huang, D.L.; Zeng, G.M.; Wan, J.; Xue, W.J.; Wen, X.F.; Liu, X.G.; Chen, S.; Li, J.; Liu, C.H.; et al. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Zhang, W.; Zheng, Y.; Zhang, W. Correlation among extracellular polymeric substances, tetracycline resistant bacteria and tetracycline resistance genes under trace tetracycline. Chemosphere 2014, 117, 658–662. [Google Scholar] [CrossRef]
- Yan, L.L.; Liu, Y.; Zhang, Y.D.; Liu, S.; Wang, C.X.; Chen, W.T.; Liu, C.; Chen, Z.L.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakurnar, M.P. Facile synthesis and characterisation of AlNs using Protein Rich Solution extracted from sewage sludge and its application for ultrasonic assisted dye adsorption: Isotherms, kinetics, mechanism and RSM design. J. Environ. Manag. 2018, 206, 215–227. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Moon, C.; Singh, R.; Chaganti, S.R.; Lalman, J.A. Modeling sulfate removal by inhibited mesophilic mixed anaerobic communities using a statistical approach. Water Res. 2013, 47, 2341–2351. [Google Scholar] [CrossRef]
- Chen, F.L.; Zu, Y.G.; Yang, L. A novel approach for isolation of essential oil from fresh leaves of Magnolia sieboldii using microwave-assisted simultaneous distillation and extraction. Sep. Purif. Technol. 2015, 154, 271–280. [Google Scholar] [CrossRef]
- Roberson, J.A. What’s Next after 40 Years of Drinking Water Regulations? Environ. Sci. Technol. 2011, 45, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Qiao, J.; Zhao, Z.W.; Zhang, S.F.; Qi, L. Fabrication of polymer-modified magnetic nanoparticle based adsorbents for the capture and release of quinolones by manipulating the metal-coordination interaction. J. Sep. Sci. 2018, 41, 2976–2982. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N. Modeling and optimization by particle swarm embedded neural network for adsorption of zinc (II) by palm kernel shell based activated carbon from aqueous environment. J. Environ. Manag. 2018, 206, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.T.; Abdulhameed, A.S.; Jawad, A.H. Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int. J. Biol. Macromol. 2019, 129, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Sarı, A.; Saleh, T.A. Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. J. Environ. Manag. 2018, 206, 170–177. [Google Scholar] [CrossRef]
- Zeng, Q.; Jia, Y.W.; Xu, P.L.; Xiao, M.W.; Liu, Y.M.; Peng, S.L.; Liao, X. Quick and selective extraction of Z-ligustilide from Angelica sinensis using magnetic multiwalled carbon nanotubes. J. Sep. Sci. 2015, 38, 4269–4275. [Google Scholar] [CrossRef]
- Álvarez-Uriarte, J.I.; Iriarte-Velasco, U.; Chimeno-Alanís, N.; González-Velasco, J.R. Application of Principal Component Analysis to the Adsorption of Natural Organic Matter by Modified Activated Carbons. Sep. Sci. Technol. 2011, 46, 2239–2249. [Google Scholar] [CrossRef]
- Duan, X.H.; Liu, X.Y.; Xiao, S.H.; Du, C.; Yan, B.F. Fe-Trimesic Acid/Melamine Gel-Derived Fe/N-Doped Carbon Nanotubes as Catalyst of Peroxymonosulfate to Remove Sulfamethazine. Water 2023, 15, 381. [Google Scholar] [CrossRef]
- Xiang, Y.J.; Xu, Z.Y.; Wei, Y.Y.; Zhou, Y.Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.C.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Zhao, X.N.; Liu, H.Y.; Yan, Z.; Song, C. Aging of Carbon Nanotubes Increases Their Adsorption towards Tetracycline. Water 2022, 14, 2731. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Yetilmezsoy, K.; Salari, M.; Heidarinejad, Z.; Yousefi, M.; Sillanpaa, M. Adsorptive removal of cobalt (II) from aqueous solutions using multi-walled carbon nanotubes and gamma-alumina as novel adsorbents: Modelling and optimization based on response surface methodology and artificial neural network. J. Mol. Liq. 2020, 299, 112154. [Google Scholar] [CrossRef]
- Rogelj, J.; Huppmann, D.; Krey, V.; Riahi, K.; Clarke, L.; Gidden, M.; Nicholls, Z.; Meinshausen, M. A new scenario logic for the Paris Agreement long-term temperature goal. Nature 2019, 573, 357. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.A.; Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Physicochemical modification of chitosan with fly ash and tripolyphosphate for removal of reactive red 120 dye: Statistical optimization and mechanism study. Int. J. Biol. Macromol. 2020, 161, 503–513. [Google Scholar] [CrossRef]
- Shahnaz, T.; Sharma, V.; Subbiah, S.; Narayanasamy, S. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process. Eng. 2020, 36, 12. [Google Scholar] [CrossRef]
- Bonetto, L.R.; Crespo, J.S.; Guegan, R.; Esteves, V.I.; Giovanela, M. Removal of methylene blue from aqueous solutions using a solid residue of the apple juice industry: Full factorial design, equilibrium, thermodynamics and kinetics aspects. J. Mol. Struct. 2021, 1224, 14. [Google Scholar] [CrossRef]
- Dil, E.A.; Doustimotlagh, A.H.; Javadian, H.; Asfaram, A.; Ghaedi, M. Nano-sized Fe3O4@SiO2-molecular imprinted polymer as a sorbent for dispersive solid-phase microextraction of melatonin in the methanolic extract of Portulaca oleracea, biological, and water samples. Talanta 2021, 221, 10. [Google Scholar] [CrossRef]
- Rasoulzadeh, H.; Dehghani, M.H.; Mohammadi, A.S.; Karri, R.R.; Nabizadeh, R.; Nazmara, S.; Kim, K.H.; Sahu, J.N. Parametric modelling of Pb (II) adsorption onto chitosan-coated Fe3O4 particles through RSM and DE hybrid evolutionary optimization framework. J. Mol. Liq. 2020, 297, 111893. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J. Hazard. Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon-HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef]
- Gadekar, M.R.; Ahammed, M.M. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manag. 2019, 231, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.F.; Lin, C.H.; Chen, H.W.; Cheng, W.P.; Kao, M.C. Possible control approaches of the Electro-Fenton process for textile wastewater treatment using on-line monitoring of DO and ORP. Chem. Eng. J. 2013, 218, 341–349. [Google Scholar] [CrossRef]
- Turan, N.G.; Mesci, B.; Ozgonenel, O. The use of artificial neural networks (ANN) for modeling of adsorption of Cu(II) from industrial leachate by pumice. Chem. Eng. J. 2011, 171, 1091–1097. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Mehrabpour, M.; Esfandyari, M.; Alidadi, H.; Davoudi, M. Modeling of simultaneous adsorption of dye and metal ion by sawdust from aqueous solution using of ANN and ANFIS. Chemometrics Intell. Lab. Syst. 2018, 181, 72–78. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Karri, R.R.; Tanzifi, M.; Yaraki, M.T.; Sahu, J.N. Optimization and modeling of methyl orange adsorption onto polyaniline nano-adsorbent through response surface methodology and differential evolution embedded neural network. J. Environ. Manag. 2018, 223, 517–529. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Gupta, V.K.; Boopathy, R.; Titus, A.; Sekaran, G. A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: Kinetic and spectroscopic studies. J. Mol. Liq. 2012, 173, 153–163. [Google Scholar] [CrossRef]
- Sun, Y.R.; Yang, Y.X.; Yang, M.X.; Yu, F.; Ma, J. Response surface methodological evaluation and optimization for adsorption removal of ciprofloxacin onto graphene hydrogel. J. Mol. Liq. 2019, 284, 124–130. [Google Scholar] [CrossRef]
- Mutalik, S.P.; Mullick, P.; Pandey, A.; Kulkarni, S.S.; Mutalik, S. Box-Behnken design aided optimization and validation of developed reverse phase HPLC analytical method for simultaneous quantification of dolutegravir sodium and lamivudine co-loaded in nano-liposomes. J. Sep. Sci. 2021, 44, 2917–2931. [Google Scholar] [CrossRef]
- Shah, K.J.; Yu, J.C.; Zhang, T.; You, Z.Y. Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics. Water 2023, 15, 1171. [Google Scholar] [CrossRef]
- Dalvand, A.; Nabizadeh, R.; Ganjali, M.R.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Murugesan, A.; Vidhyadevi, T.; Kalaivani, S.S.; Thiruvengadaravi, K.V.; Ravikumar, L.; Anuradha, C.D.; Sivanesan, S. Modelling of lead (II) ion adsorption onto poly (thiourea imine) functionalized chelating resin using response surface methodology (RSM). J. Water Process. Eng. 2014, 3, 132–143. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zheng, Z.H.; Zhang, D.F.; Zhang, X.D. Response surface methodology directed adsorption of chlorate and chlorite onto MIEX resin and study of chemical properties. Environ. Sci.-Wat. Res. Technol. 2020, 6, 2454–2464. [Google Scholar] [CrossRef]
- Demircivi, P.; Bugdayci, M. Synthesis and thermodynamic simulation of Zr Doped SrAl2O4 ceramic powders: Enhanced adsorptive properties for antibiotic tetracycline. Mater. Res. Express. 2019, 6, 12. [Google Scholar] [CrossRef]
- Haghighat, G.A.; Saghi, M.H.; Anastopoulos, I.; Javid, A.; Roudbari, A.; Talebi, S.S.; Ghadiri, S.K.; Giannakoudakis, D.A.; Shams, M. Aminated graphitic carbon derived from corn stover biomass as adsorbent against antibiotic tetracycline: Optimizing the physicochemical parameters. J. Mol. Liq. 2020, 313, 9. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; Ablouh, E.H.; Bouich, A.; Lacherai, A.; Jada, A.; Lima, E.C.; Sher, F. Biosynthesis of SiO2 nanoparticles using extract of Nerium oleander leaves for the removal of tetracycline antibiotic. Chemosphere 2022, 287, 10. [Google Scholar] [CrossRef]
- Gu, S.Y.; Zhang, D.F.; Gao, Y.Q.; Qi, R.Z.; Chen, W.F.; Xu, Z.H. Fabrication of porous carbon derived from cotton/polyester waste mixed with oyster shells: Pore-forming process and application for tetracycline removal. Chemosphere 2021, 270, 12. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Safari, G.H.; Sharafi, K.; Kamani, H.; Jaafari, J. Adsorption of 4-Nitrophenol on calcium alginate-multiwall carbon nanotube beads: Modeling, kinetics, equilibriums and reusability studies. Int. J. Biol. Macromol. 2021, 185, 66–76. [Google Scholar] [CrossRef]
- Islam, A.; Chauhan, A.; Javed, H.; Rais, S.; Ahmad, I. Magnetic Carbon Nanotubes-Silica Binary Composite for Effective Pb(II) Sequestration from Industrial Effluents: Multivariate Process Optimization. Clean-Soil Air Water 2021, 49, 10. [Google Scholar] [CrossRef]
- Huaccallo-Aguilar, Y.; Alvarez-Torrellas, S.; Martinez-Nieves, J.; Delgado-Adamez, J.; Gil, M.V.; Ovejero, G.; Garcia, J. Magnetite-Based Catalyst in the Catalytic Wet Peroxide Oxidation for Different Aqueous Matrices Spiked with Naproxen-Diclofenac Mixture. Catalysts 2021, 11, 514. [Google Scholar] [CrossRef]
- Aliyu, A. Synthesis, electron microscopy properties and adsorption studies of Zinc (II) ions (Zn2+) onto as-prepared Carbon Nanotubes (CNTs) using Box-Behnken Design (BBD). Sci. Afr. 2019, 3, e00069. [Google Scholar] [CrossRef]
- Thiyagarajan, P.; Selvam, K.; Sudhakar, C.; Selvankumar, T. Enhancement of Adsorption of Magenta Dye by Immobilized Laccase on Functionalized Biosynthesized Activated Carbon Nanotubes. Water Air Soil Pollut. 2020, 231, 364. [Google Scholar] [CrossRef]
- Osaghi, B.; Safa, F. Cloud Point Extraction in Presence of Multi-walled Carbon Nanotubes for Removal of Direct Green 26 from Aqueous Solutions: Optimization by Box-Behnken Design and Desirability Functions. J. Anal. Chem. 2020, 75, 802–811. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Smektala, P.; Zglobicka, I.; Michalski, J.; Kurzydlowski, K.J.; Krzeminski, P. Escudero-Onate, C. Non-woven polypropylene fabric modified with carbon nanotubes and decorated with nanoakaganeite for arsenite removal. Int. J. Environ. Sci. Technol. 2018, 15, 1831–1842. [Google Scholar] [CrossRef]
- Safa, F.; Osaghi, B. Adsorption onto MWCNTs Coupled with Cloud Point Extraction for Dye Removal from Aqueous Solutions: Optimization by Experimental Design. Comb. Chem. High Throughput Screen. 2021, 24, 246–258. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Hussein, D.S.; Farouq, R. Optimization of photocatalytic degradation of methylene blue dye using titanate nanotube. J. Nanophotonics 2020, 14, 026008. [Google Scholar] [CrossRef]
- Cao, J.Y.; Xiong, Z.K.; Lai, B. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: Adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.J.; Shah, S.M.; Su, X.G. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Zeng, Z.T.; Ye, S.J.; Wu, H.P.; Xiao, R.; Zeng, G.M.; Liang, J.; Zhang, C.; Yu, J.F.; Fang, Y.L.; Song, B. Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution. Sci. Total Environ. 2019, 648, 206–217. [Google Scholar] [CrossRef]
- Jun, L.Y.; Karri, R.R.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Mohammad, K.; Jagadish, P.; Abdullah, E.C. Modeling and optimization by particle swarm embedded neural network for adsorption of methylene blue by jicama peroxidase immobilized on buckypaper/polyvinyl alcohol membrane. Environ. Res. 2020, 183, 109158. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Corrigendum to “Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review” (Sci. Total Environ. 2018, 640, 772). Sci. Total Environ. 2019, 650, 696. [Google Scholar] [CrossRef]
- Khodaie, M.; Ghasemi, N.; Moradi, B.; Rahimi, M. Removal of Methylene Blue from Wastewater by Adsorption onto ZnCl2 Activated Corn Husk Carbon Equilibrium Studies. J. Chem. 2013, 2013, 383985. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Akinlabi, A.K. Congo Red Biosorption on Palm Kernel Seed Coat. Ind. Eng. Chem. Res. 2009, 48, 6188–6196. [Google Scholar] [CrossRef]
- Huang, B.Y.; Liu, Y.G.; Li, B.; Liu, S.B.; Zeng, G.M.; Zeng, Z.W.; Wang, X.H.; Ning, Q.M.; Zheng, B.H.; Yang, C.P. Effect of Cu (II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite. Carbohydr. Polym. 2017, 157, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Z.; Li, Y.F.; Cao, Y.Y.; Han, L.J. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Yue, Y.M.; Zhang, P.X.; Wang, W.; Cai, Y.C.; Tan, F.T.; Wang, X.Y.; Qiao, X.L.; Wong, P.K. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. J. Hazard. Mater. 2020, 384, 121302. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gu, P.; Li, X.Y.; Zhang, G.H. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Jing, X.-R.; Wang, Y.-Y.; Liu, W.-J.; Wang, Y.-K.; Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 2014, 248, 168–174. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Liu, X.C.; Xiang, Y.J.; Wang, P.; Zhang, J.C.; Zhang, F.F.; Wei, J.H.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.H.; Zhu, W.S.; Wu, X.Y.; Hou, F.F.; Xun, S.H.; Wu, P.W.; Ji, H.Y.; Xu, H.; Li, H.M. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Ji, L.L.; Chen, W.; Duan, L.; Zhu, D.Q. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Anfar, Z.; Ahsaine, H.A.; El Alem, N.; Ezahri, M. Acridine orange adsorption by zinc oxide/almond shell activated carbon composite: Operational factors, mechanism and performance optimization using central composite design and surface modeling. J. Environ. Manag. 2018, 206, 383–397. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Cheng, Z.J. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Danmaliki, G.I.; Saleh, T.A.; Shamsuddeen, A.A. Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon. Chem. Eng. J. 2017, 313, 993–1003. [Google Scholar] [CrossRef]
- Zhao, W.X.; Ma, A.Q.; Ji, J.H.; Chen, X.; Yao, T. Multiobjective Optimization of a Double-Side Linear Vernier PM Motor Using Response Surface Method and Differential Evolution. IEEE Trans. Ind. Electron. 2020, 67, 80–90. [Google Scholar] [CrossRef]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A.; Agarwal, S.; Gupta, V.K. Modeling and optimization of Direct Red 16 adsorption from aqueous solutions using nanocomposite of MnFe2O4/MWCNTs: RSM-CCRD model. J. Mol. Liq. 2017, 233, 370–377. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A.; Dil, E.A. Screening and optimization of highly effective ultrasound-assisted simultaneous adsorption of cationic dyes onto Mn-doped Fe3O4-nanoparticle-loaded activated carbon. Ultrason. Sonochem. 2017, 34, 1–12. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef] [PubMed]


| Run Order | Dosage (mg) | Time (min) | pH | TC Removal (%) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | 9 | 300 | 5 | 89.56 | 91.28 |
| 2 | 9 | 300 | 5 | 94.55 | 91.28 |
| 3 | 9 | 300 | 5 | 90.32 | 91.28 |
| 4 | 12 | 300 | 6 | 82.29 | 81.01 |
| 5 | 6 | 480 | 5 | 97.88 | 97.31 |
| 6 | 12 | 480 | 5 | 91.65 | 92.28 |
| 7 | 9 | 300 | 5 | 88.65 | 91.28 |
| 8 | 9 | 480 | 4 | 97.28 | 96.57 |
| 9 | 9 | 120 | 6 | 93.19 | 93.90 |
| 10 | 6 | 120 | 5 | 86.52 | 85.89 |
| 11 | 9 | 300 | 5 | 93.34 | 91.28 |
| 12 | 6 | 300 | 6 | 83.93 | 83.85 |
| 13 | 9 | 480 | 6 | 75.54 | 76.19 |
| 14 | 12 | 120 | 5 | 96.97 | 97.54 |
| 15 | 9 | 120 | 4 | 73.35 | 72.70 |
| 16 | 6 | 300 | 4 | 76.01 | 77.29 |
| 17 | 12 | 300 | 4 | 86.68 | 86.76 |
| Run Order | Parameter | Experimental (%) | Predicted (%) | Expectation | Deviation (%) | ||
|---|---|---|---|---|---|---|---|
| Dosage (mg) | Time (min) | pH | |||||
| 1 | 12.0 | 120 | 5.4 | 98.86 | 99.16 | 0.984 | −0.300 |
| 2 | 12.1 | 120 | 5.5 | 98.12 | 98.99 | 0.981 | −0.870 |
| 3 | 12.2 | 123 | 5.5 | 98.11 | 98.92 | 0.980 | −0.810 |
| 4 | 11.8 | 120 | 5.4 | 98.00 | 98.96 | 0.968 | −0.960 |
| 5 | 11.9 | 120 | 5.1 | 97.98 | 98.15 | 0.965 | −0.170 |
| 6 | 12.0 | 136 | 5.5 | 97.98 | 97.87 | 0.959 | 0.110 |
| 7 | 12.1 | 480 | 4.2 | 97.96 | 97.32 | 0.948 | 0.640 |
| 8 | 12.3 | 479 | 4.3 | 97.83 | 97.27 | 0.947 | 0.560 |
| 9 | 11.9 | 479 | 4.2 | 97.06 | 97.32 | 0.945 | −0.260 |
| 10 | 11.9 | 479 | 4.2 | 97.01 | 97.33 | 0.943 | −0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Hao, C.; Guo, Y.; Shao, W.; Tian, Y.; Zhao, P. Optimization of Adsorption Conditions Using Response Surface Methodology for Tetracycline Removal by MnFe2O4/Multi-Wall Carbon Nanotubes. Water 2023, 15, 2392. https://doi.org/10.3390/w15132392
Zhao W, Hao C, Guo Y, Shao W, Tian Y, Zhao P. Optimization of Adsorption Conditions Using Response Surface Methodology for Tetracycline Removal by MnFe2O4/Multi-Wall Carbon Nanotubes. Water. 2023; 15(13):2392. https://doi.org/10.3390/w15132392
Chicago/Turabian StyleZhao, Weigao, Chenjie Hao, Yiping Guo, Wanfei Shao, Yimei Tian, and Peng Zhao. 2023. "Optimization of Adsorption Conditions Using Response Surface Methodology for Tetracycline Removal by MnFe2O4/Multi-Wall Carbon Nanotubes" Water 15, no. 13: 2392. https://doi.org/10.3390/w15132392
APA StyleZhao, W., Hao, C., Guo, Y., Shao, W., Tian, Y., & Zhao, P. (2023). Optimization of Adsorption Conditions Using Response Surface Methodology for Tetracycline Removal by MnFe2O4/Multi-Wall Carbon Nanotubes. Water, 15(13), 2392. https://doi.org/10.3390/w15132392









