An Assessment of the Effects of Light Intensities and Temperature Changes on Cyanobacteria’s Oxidative Stress via the Use of Hydrogen Peroxide as an Indicator
Abstract
:1. Introduction
2. Materials and Methods
2.1. P. ambiguum and P. foetida Cell Cultures
2.2. Experimental Procedure
2.3. Estimation of Total Soluble Protein Content
2.4. Chlorophyll-a Concentration Measurements
2.5. Identification of Cell Growth
2.6. H2O2 Concentration Measurement Assay
2.7. CAT Assay
2.8. Statistical Analysis
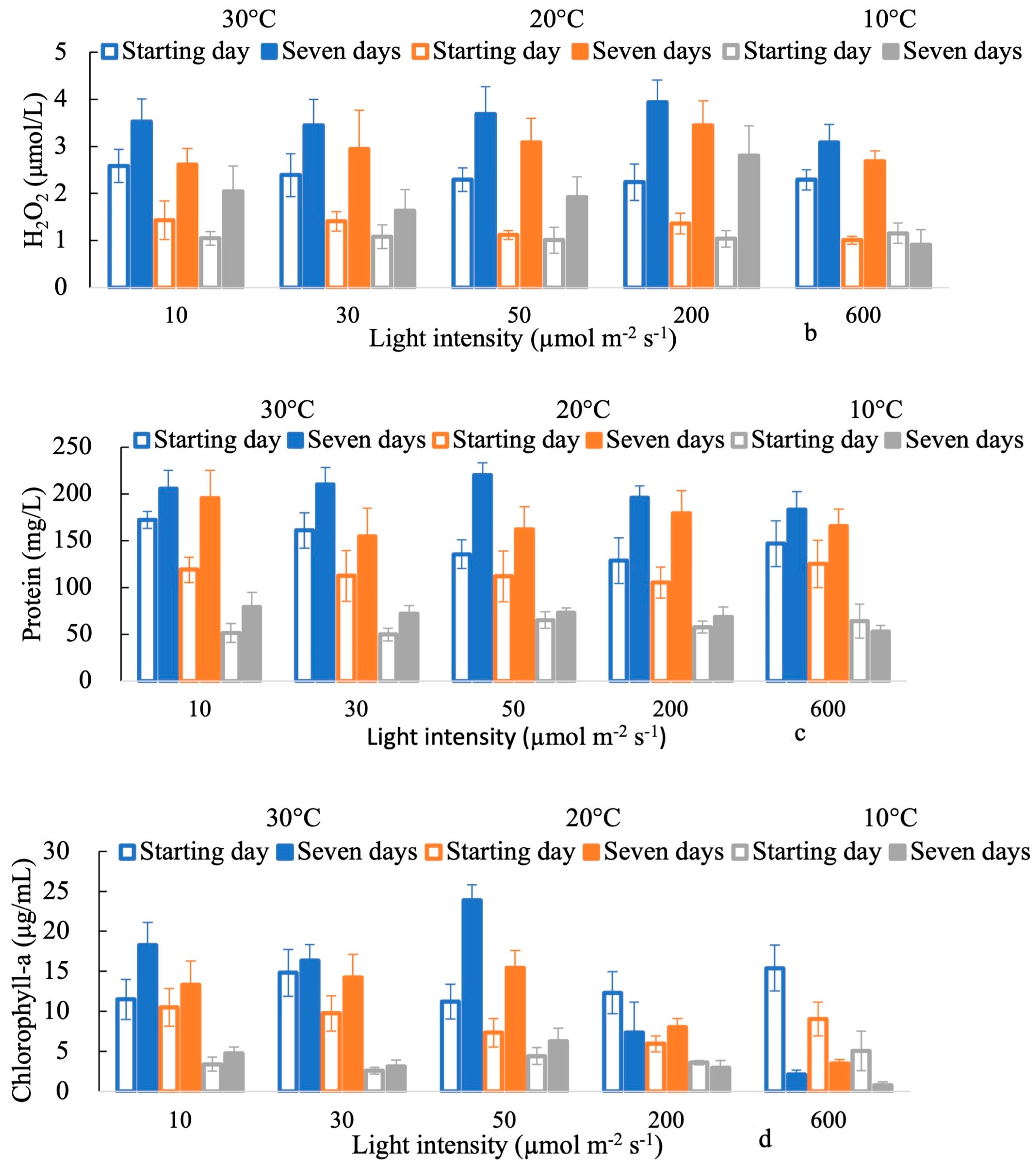
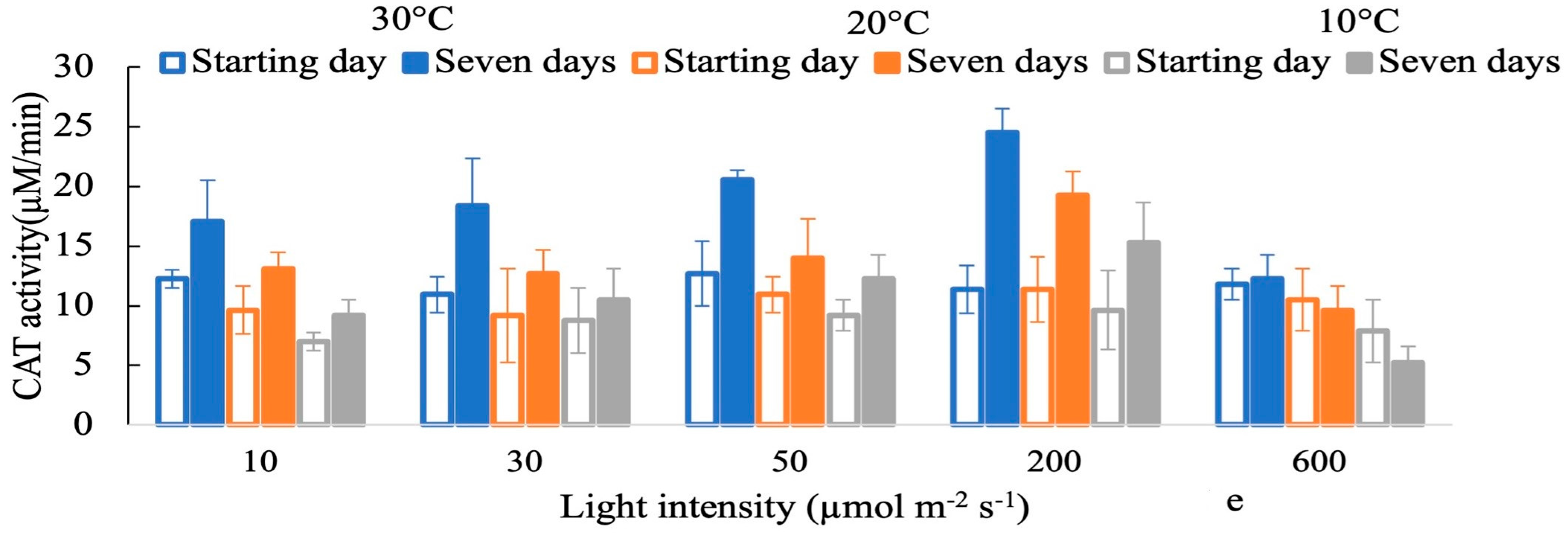
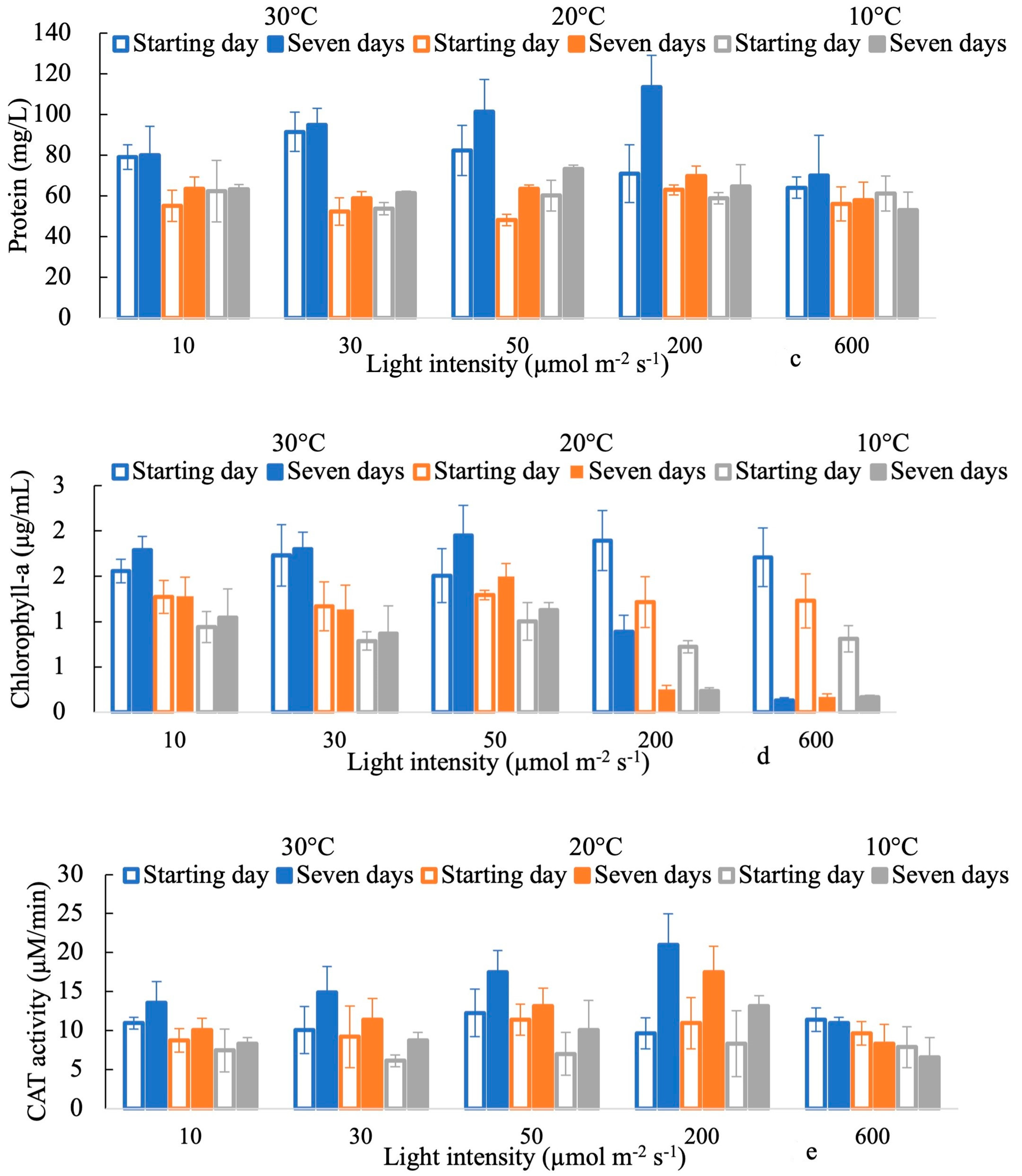
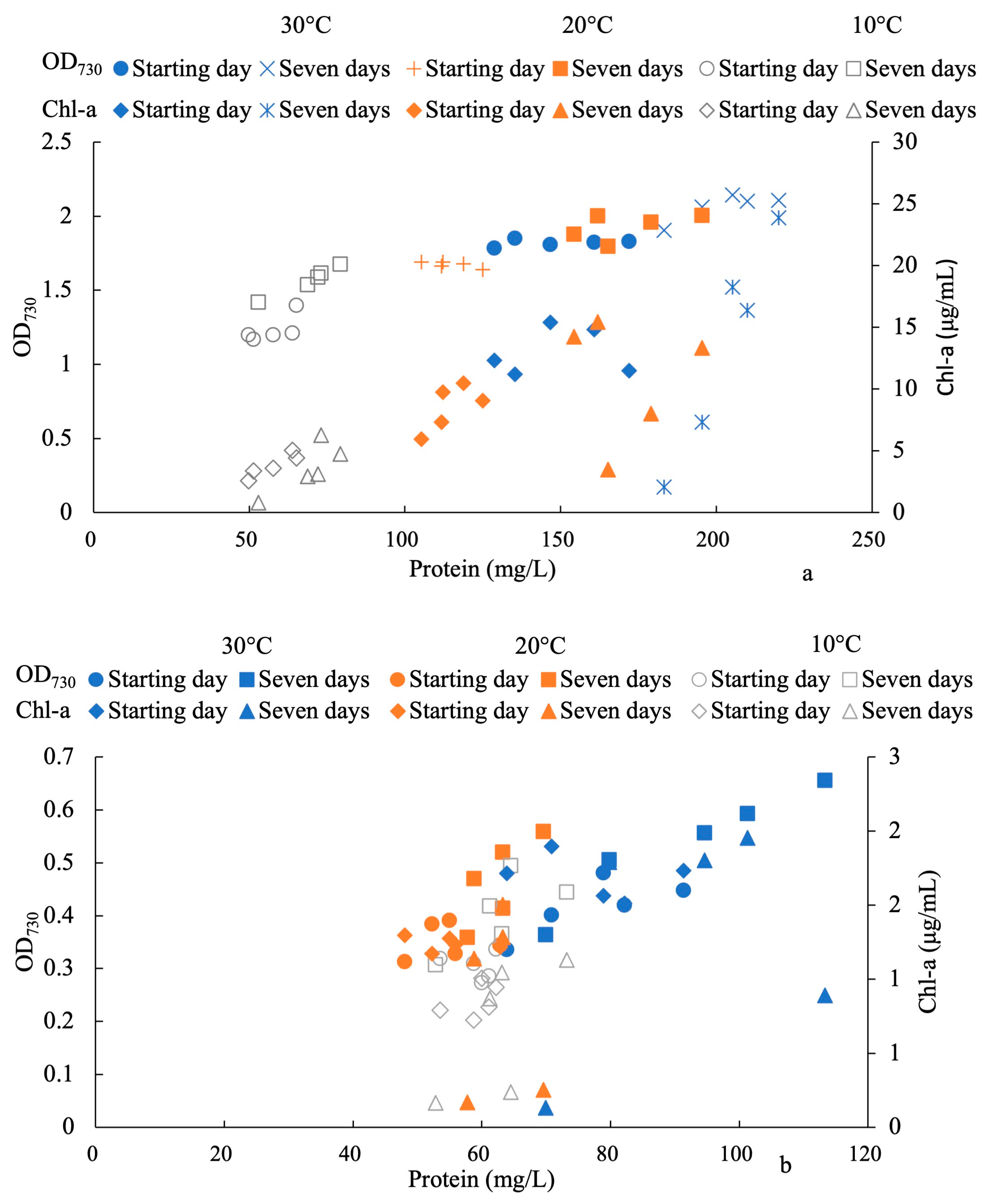
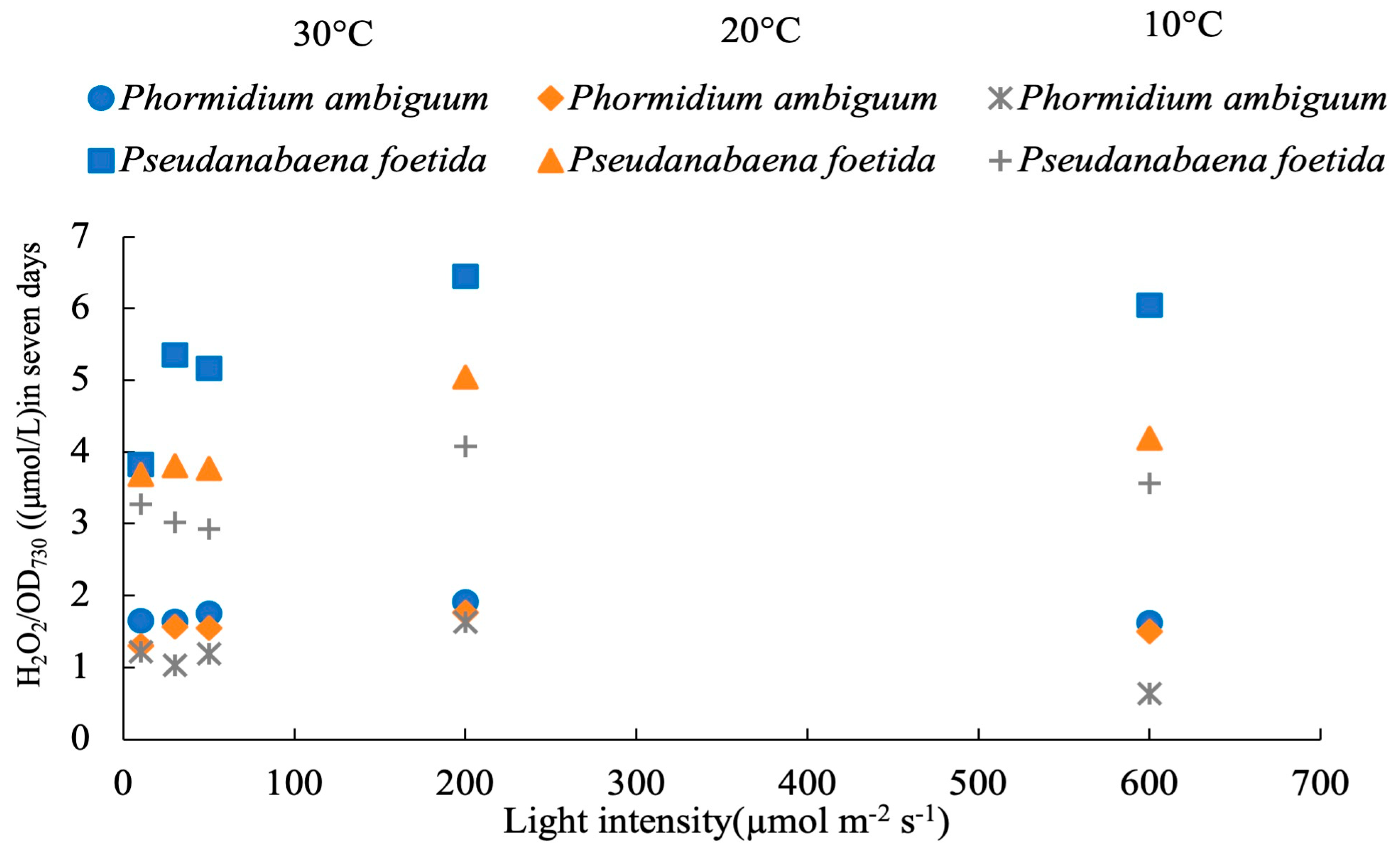
3. Results
4. Discussion
4.1. Effects of Light Intensities on the Response of H2O2
4.2. Antioxidant Activity in Response to Oxidative Stress
4.3. Management of Cyanobacterial Blooms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, Y.; Zhou, M.; Meng, Y.; Liu, J.; Yang, L.; Zuo, Z. Linalool-and α-terpineol-induced programmed cell death in Chlamydomona sreinhardtii. Ecotoxicol. Environ. Saf. 2019, 167, 435–440. [Google Scholar] [CrossRef]
- Carmichael, W.W. Freshwater Blue-Green Algae (Cyanobacteria) Toxins—A Review. Water Environ. 1981, 405, 1–13. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Reich, A.; Zaias, J.; Cheng, Y.S.; Pierce, R.; Naar, J.; et al. Aerosolized Red-Tide Toxins (Brevetoxins) and Asthma. Chest 2007, 131, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.K.; Rai, A.K. Allergenicity of air-borne cyanobacteria Phormidium fragile and Nostoc muscorum. Ecotoxicol. Environ. Saf. 2008, 69, 158–162. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Genitsaris, S.; Kormas, K.A. Airborne Algae and Cyanobacteria Occurrence and Related Health Effects. Front. Biosci. 2011, E3, 772–787. [Google Scholar] [CrossRef] [Green Version]
- Hudnell, H.K. Cyanobacterial harmful algal blooms: State of the science and research needs. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 619. [Google Scholar]
- Butakova, E.A. Specific features of odor-causing compounds (geosmin and 2-methylisoborneol) as secondary metabolites of cyanobacteria. Russ. J. Plant Physiol. 2013, 60, 507–510. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Ding, A.; Hou, Y.; Li, Z.; Niu, X.; Su, Y.; Xu, E. Laws The 2007 water crisis in Wuxi, China: Analysis of the origin. J. Hazard. Mater. 2010, 182, 130–135. [Google Scholar] [CrossRef]
- Cao, H.-S.; Tao, Y.; Kong, F.-X.; Yang, Z. Relationship between Temperature and Cyanobacterial Recruitment from Sediments in Laboratory and Field Studies. J. Freshw. Ecol. 2008, 23, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Asaeda, T.; Rahman, M.; Abeynayaka, H.D.L. Hydrogen peroxide can be a plausible biomarker in cyanobacterial bloom treatment. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Welkie, D.; Rubin, B.E.; Diamond, S.; Hood, R.D.; Savage, D.F.; Golden, S.S. A Hard Day’s Night: Cyanobacteria in Diel Cycles. Trends Microbiol. 2019, 27, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.W.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [Green Version]
- Barnuevo, A.; Asaeda, T. Integrating the ecophysiology and biochemical stress indicators into the paradigm of mangrove ecology and a rehabilitation blueprint. PLoS ONE 2018, 13, e0202227. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.; Jones, G.J.; Dunstan, R.H. Effect of irradiance on fatty acid, carotenoid, total protein composition and growth of Microcystis aeruginosa. Phytochemistry 1997, 44, 817–824. [Google Scholar] [CrossRef]
- Hanelt, D.P. Photoinhibition of photosynthesis in marine macroalgae. Sci. Mar. 1996, 60, 243–248. [Google Scholar]
- Hortonn, P.; Ruban, A. Molecular design of the photosystem II light-harvesting antenna: Photosynthesis and photoprotection. J. Exp. Bot. 2005, 56, 365–373. [Google Scholar] [CrossRef]
- Kirilovsky, D. Photoprotection in cyanobacteria: The orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth. Res. 2007, 93, 7–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Zhong, L.; Wiedensohler, A.; Liu, S.; Andreae, M.; Wang, W.; Fan, S. Regional Integrated Experiments on Air Quality over Pearl River Delta 2004 (PRIDE-PRD2004): Overview. Atmos. Environ. 2008, 42, 6157–6173. [Google Scholar] [CrossRef]
- Jones, R.J.; Hoegh-Guldberg, O. Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: Photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ. 2001, 24, 89–99. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ugya, A.Y.; Imam, T.S.; Li, A.; Ma, J.; Hua, X. Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: A mini review. Chem. Ecol. 2020, 36, 174–193. [Google Scholar] [CrossRef]
- Darehshouri, A.; Lütz-Meindl, U. H2O2 localization in the green alga Micrasterias after salt and osmotic stress by TEM-coupled electron energy loss spectroscopy. Protoplasma 2010, 239, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Peng, K.; Shi, M.; Chen, Z.; Zhu, Y.; Bai, Y.; Gao, Y.; Wang, Y. ROS production is associated with glycolate metabolism in Chlamydomonas reinhardtii (Chlorophyceae) under salt stress. Phycologia 2014, 53, 502–507. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Tartoura, K.A.; Youssef, S.A. Stimulation of ROS-scavenging systems in squash (Cucurbita pepo L.) plants by compost supplementation under normal and low temperature conditions. Sci. Hortic. 2011, 130, 862–868. [Google Scholar] [CrossRef]
- He, L.-L.; Wang, X.; Wu, X.-X.; Wang, Y.-X.; Kong, Y.-M.; Liu, B.-M.; Liu, B. Protein damage and reactive oxygen species generation induced by the synergistic effects of ultrasound and methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 361–366. [Google Scholar] [CrossRef]
- Niiyama, Y.; Tuji, A.; Takemoto, K.; Ichise, S. Pseudanabaena foetida sp. nov. and P. subfoetida sp. nov.(Cyanophyta/Cyanobacteria) producing 2-methylisoborneol from Japan. Fottea 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Tavakoli, Y.; Mohammadipanah, F.; Te, S.H.; You, L.; Gin, K.Y.-H. Biodiversity, phylogeny and toxin production profile of cyanobacterial strains isolated from lake Latyan in Iran. Harmful Algae 2021, 106, 102054. [Google Scholar] [CrossRef]
- Abbas, M.; Dia, S.; Deutsch, E.S.; Alameddine, I. Analyzing eutrophication and harmful algal bloom dynamics in a deep Mediterranean hypereutrophic reservoir. Environ. Sci. Pollut. Res. 2023, 30, 37607–37621. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Wilhelm, S.W.; Bullerjahn, G.S.; Paerl, H.W. Climate change and the aquatic continuum: A cyanobacterial comeback story. Environ. Microbiol. Rep. 2023, 15, 3–12. [Google Scholar] [CrossRef]
- Bijekar, S.; Padariya, H.D.; Yadav, V.K.; Gacem, A.; Hasan, M.A.; Awwad, N.S.; Yadav, K.K.; Islam, S.; Park, S.; Jeon, B.-H. The State of the Art and Emerging Trends in the Wastewater Treatment in Developing Nations. Water 2022, 14, 2537. [Google Scholar] [CrossRef]
- Gaget, V.; Humpage, A.R.; Huang, Q.; Monis, P.; Brookes, J.D. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Res. 2017, 124, 454–464. [Google Scholar] [CrossRef]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.-C.; Humbert, J.-F.; Guette, C.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Bormans, M.; Lengronne, M.; Brient, L.; Duval, C. Cylindrospermopsin Accumulation and Release by the Benthic Cyanobacterium Oscillatoria sp. PCC 6506 under Different Light Conditions and Growth Phases. Bull. Environ. Contam. Toxicol. 2014, 92, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Fetscher, A.E.; Howard, M.D.; Stancheva, R.; Kudela, R.M.; Stein, E.D.; Sutula, M.A.; Busse, L.B.; Sheath, R.G. Wadeable streams as widespread sources of benthic cyanotoxins in California, USA. Harmful Algae 2015, 49, 105–116. [Google Scholar] [CrossRef]
- Kakimoto, M.; Ishikawa, T.; Miyagi, A.; Saito, K.; Miyazaki, M.; Asaeda, T.; Yamaguchi, M.; Uchimiya, H.; Kawai-Yamada, M. Culture temperature affects gene expression and metabolic pathways in the 2-methylisoborneol-producing cyanobacterium Pseudanabaena galeata. J. Plant Physiol. 2014, 171, 292–300. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp. and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Syst. Ecol. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Ganf, G.G. Rates of oxygen uptake by the planktonic community of a shallow equatorial lake (Lake George, Uganda). Oecologia 1974, 15, 17–32. [Google Scholar] [CrossRef]
- Bryanskaya, A.V.; Orleanskii, V.K.; Dagurova, O.P. A laboratory model of the cyanobacterial mat from the Kotel’nikovskii hot spring (Baikal Region). Microbiology 2008, 77, 490–496. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Certini, G.; De Philippis, R. Cyanobacteria inoculation as a potential tool for stabilization of burned soils. Restor. Ecol. 2020, 28, S106–S114. [Google Scholar] [CrossRef]
- Rangel, L.M.; Soares, M.C.S.; Paiva, R.; Silva, L.H.S. Morphology-based functional groups as effective indicators of phytoplankton dynamics in a tropical cyanobacteria-dominated transitional river–reservoir system. Ecol. Indic. 2016, 64, 217–227. [Google Scholar] [CrossRef]
- De la Escalera, G.M.; Kruk, C.; Segura, A.M.; Nogueira, L.; Alcántara, I.; Piccini, C. Dynamics of toxic genotypes of Microcystis aeruginosa complex (MAC) through a wide freshwater to marine environmental gradient. Harmful Algae 2017, 62, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Majedi, S.M.; Pavagadhi, S.; Te, S.H.; Boo, C.Y.; Gin, K.Y.-H.; Swarup, S. Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria. Metabolites 2022, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhu, G.; Xu, H.; Zhu, M.; Zhang, Y.; Qin, B. Temporal dependence of chlorophyll a–nutrient relationships in Lake Taihu: Drivers and management implications. J. Environ. Manag. 2022, 306, 114476. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.Y.; Tran, T.T.; Nguyen, T.M.Y.; Ngo, X.Q.; Nguyen, X.D.; Pham, T.L. Seasonal changes in phytoplankton assemblages and environmental variables in highly turbid tropical estuaries of the Mekong River, Vietnam. Environ. Monit. Assess. 2022, 194 (Suppl. S2), 776. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Dickson, S.; Hobson, P.; Howard, C.; Zander, A.; Burch, M. Quantification of the ultrasound induced sedimentation of Microcystis aeruginosa. Ultrason. Sonochem. 2014, 21, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Asaeda, T.; Senavirathna, M.J.; Vamsi Krishna, L. Evaluation of habitat preferences of invasive macrophyte Egeria densa in different channel slopes using hydrogen peroxide as an indicator. Front. Plant Sci. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Asaeda, T.; Rashid, H.; Schoelynck, J. Tissue Hydrogen Peroxide Concentration Can Explain the Invasiveness of Aquatic Macrophytes: A Modeling Perspective. Front. Environ. Sci. 2021, 8, 516301. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Liping, X.; Schoelynck, J. Hydrogen Peroxide Variation Patterns as Abiotic Stress Responses of Egeria densa. Front. Plant Sci. 2022, 13, 855477. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Abeynayaka, H.D.L.; Asaeda, T.; Kaneko, Y. Buoyancy Limitation of Filamentous Cyanobacteria under Prolonged Pressure due to the Gas Vesicles Collapse. Environ. Manag. 2017, 60, 293–303. [Google Scholar] [CrossRef]
- Abeynayaka, H.D.L.; Asaeda, T.; Rashid, M.H. Effects of elevated pressure on Pseudanabaena galeata Böcher in varying light and dark environments. Environ. Sci. Pollut. Res. 2018, 25, 21224–21232. [Google Scholar] [CrossRef] [PubMed]
- ISO 10260:1992; Water Quality—Measurement of Biochemical Parameters—Spectrometric Determination of the Chlorophyll—A Concentration. International Organization for Standardization: Geneva, Switzerland, 1992.
- Gregor, J.; Maršálek, B. Freshwater phytoplankton quantification by chlorophyll a: A comparative study of in vitro, in vivo and in situ methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Axler, R.P.; Owen, C.J. Measuring chlorophyll and phaeophytin: Whom should you believe? Lake Reserv. Manag. 1994, 8, 143–151. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Queval, G.; Hager, J.; Gakière, B.; Noctor, G. Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J. Exp. Bot. 2008, 59, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Dautania, G.K.; Singh, G.P. Role of Light and Dark Cycle and Different Temperatures in the Regulation of Growth and Protein Expression in Oscillatoria agardhii Strain. Braz. Arch. Biol. Technol. 2014, 57, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Vesterkvist, P.S.M.; Misiorek, J.O.; Spoof, L.E.M.; Toivola, D.M.; Meriluoto, J.A.O. Comparative Cellular Toxicity of Hydrophilic and Hydrophobic Microcystins on Caco-2 Cells. Toxins 2012, 4, 1008–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Sainz, M.; Díaz, P.; Monza, J.; Borsani, O. Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought-heat stressed Lotus japonicus. Physiol. Plant. 2010, 140, 46–56. [Google Scholar] [CrossRef]
- Machová, K.; Elster, J.; Adamec, L. Xanthophyceaen assemblages during winter–spring flood: Autecology and ecophysiology of Tribonema fonticolum and T. monochloron. Hydrobiologia 2008, 600, 155–168. [Google Scholar] [CrossRef]
- Harel, Y.; Ohad, I.; Kaplan, A. Activation of Photosynthesis and Resistance to Photoinhibition in Cyanobacteria within Biological Desert Crust. Plant Physiol. 2004, 136, 3070–3079. [Google Scholar] [CrossRef] [Green Version]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Athukorala, D.A.; Amarasinghe, U.S. Status of the fisheries in two reservoirs of the Walawe river basin, Sri Lanka: A case of participation of fishers in management. Asian Fish. Sci. 2010, 23, 284–300. [Google Scholar] [CrossRef]
- McLellan, N.L.; Manderville, R.A. Toxic mechanisms of microcystins in mammals. Toxicol. Res. 2017, 6, 391–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leunert, F.; Eckert, W.; Paul, A.; Gerhardt, V.; Grossart, H.-P. Phytoplankton response to UV-generated hydrogen peroxide from natural organic matter. J. Plankton Res. 2014, 36, 185–197. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Mahvi, A.H.; Ehrampoush, M.H.; Ghaneian, M.T.; Yousefinejad, S.; Faramarzian, M.; Mazloomi, S.M.; Dehghani, M.; Fallahzadeh, H. Evaluation of kenaf fibers as moving bed biofilm carriers in algal membrane photobioreactor. Ecotoxicol. Environ. Saf. 2018, 152, 1–7. [Google Scholar] [CrossRef]
- Foo, S.C.; Chapman, I.J.; Hartnell, D.M.; Turner, A.D.; Franklin, D.J. Effects of H2O2 on growth, metabolic activity and membrane integrity in three strains of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2020, 27, 38916–38927. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.N.; Purdie, D.A. Effect of elevated temperature, darkness and hydrogen peroxide treatment on oxidative stress and cell death in the bloom-forming toxic cyanobacterium Microcystis aeruginosa. J. Phycol. 2011, 47, 1316–1325. [Google Scholar] [CrossRef]
- Drábková, M.; Admiraal, W.; Maršálek, B. Combined exposure to hydrogen peroxide and light selective effects on cyanobacteria, green algae, and diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.C.; Souza, E.D.S.; Dousseau, S.; de Castro, E.M.; Magalhães, P.C. Seedlings of Garcinia brasiliensis (Clusiaceae) subjected to root flooding: Physiological, morphoanatomical, and antioxidant responses to the stress. Aquat. Bot. 2013, 111, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Kura-Hotta, M.; Satoh, K.; Katoh, S. Relationship between Photosynthesis and Chlorophyll Content during Leaf Senescence of Rice Seedlings. Plant Cell Physiol. 1987, 28, 1321–1329. [Google Scholar] [CrossRef]
- Tandeau de Marsac, N. Occurrence and nature of chromatic adaptation in cyanobacteria. J. Bacteriol. 1977, 130, 82–91. [Google Scholar] [CrossRef] [Green Version]
- López, C.V.G.; García, M.D.C.C.; Fernández, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Liu, X.; Huang, T.; Ma, B.; Li, N.; Yang, W.; Li, H.; Zhao, K. Novel insights in seasonal dynamics and co-existence patterns of phytoplankton and micro-eukaryotes in drinking water reservoir, Northwest China: DNA data and ecological model. Sci. Total. Environ. 2023, 857, 159160. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, X.; Peijnenburg, W.; Zhang, M.; Sun, L.; Zhai, Y.; Yu, Q.; Wu, J.; Lu, T.; Qian, H. Alteration of dominant cyanobacteria in different bloom periods caused by abiotic factors and species interactions. J. Environ. Sci. 2021, 99, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Chang, K.-H.; Kusaba, M.; Nakano, S.-I. Temperature-dependent dominance of Microcystis (Cyanophyceae) species: M. aeruginosa and M. wesenbergii. J. Plankton Res. 2009, 31, 171–178. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Mallery, K.; Hong, J.; Hondzo, M. Temperature effects on growth and buoyancy of Microcystis aeruginosa. J. Plankton Res. 2018, 40, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Shi, X.; Chen, C.; Yu, L.; Sun, C. Responses of Microcystis Colonies of Different Sizes to Hydrogen Peroxide Stress. Toxins 2017, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Chalanika De Silva, H.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017, 12, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Heo, W.M.; Kim, B. The effect of artificial destratification on phytoplankton in a reservoir. Hydrobiologia 2004, 524, 229–239. [Google Scholar] [CrossRef]
- Havens, K.E.; Phlips, E.J.; Cichra, M.F.; Li, B.-L. Light availability as a possible regulator of cyanobacteria species composition in a shallow subtropical lake. Freshw. Biol. 1998, 39, 547–556. [Google Scholar] [CrossRef]
- Asaeda, T.; Imberger, J. Structure of bubble plumes in linearly stratified environments. J. Fluid Mech. 1993, 249, 35–57. [Google Scholar] [CrossRef]
- Imteaz, M.A.; Asaeda, T. Artificial mixing of lake water by bubble plume and effects of bubbling operations on algal bloom. Water Res. 2000, 34, 1919–1929. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Asaeda, T.; Abeynayaka, H.D.L.; Fukahori, K. An Assessment of the Effects of Light Intensities and Temperature Changes on Cyanobacteria’s Oxidative Stress via the Use of Hydrogen Peroxide as an Indicator. Water 2023, 15, 2429. https://doi.org/10.3390/w15132429
Rahman M, Asaeda T, Abeynayaka HDL, Fukahori K. An Assessment of the Effects of Light Intensities and Temperature Changes on Cyanobacteria’s Oxidative Stress via the Use of Hydrogen Peroxide as an Indicator. Water. 2023; 15(13):2429. https://doi.org/10.3390/w15132429
Chicago/Turabian StyleRahman, Mizanur, Takashi Asaeda, Helayaye Damitha Lakmali Abeynayaka, and Kiyotaka Fukahori. 2023. "An Assessment of the Effects of Light Intensities and Temperature Changes on Cyanobacteria’s Oxidative Stress via the Use of Hydrogen Peroxide as an Indicator" Water 15, no. 13: 2429. https://doi.org/10.3390/w15132429




