Modeling BTEX Multiphase Partitioning with Soil Vapor Extraction under Groundwater Table Fluctuation Using the TMVOC Model
Abstract
:1. Introduction
2. Methodology
2.1. Background and Conceptual Model of the Field Site
2.2. Simulation Scenarios
2.3. Model Validation
3. Results and Discussion
3.1. Modeling the Natural Spill State
3.2. SVE System Design
3.2.1. Extraction Well Pressure
3.2.2. Influencing Radius
3.2.3. Quantity of Extraction Wells
3.3. Removal Rates of BTEX
3.4. Transformation of BTEX among Gas, Aqueous, and NAPL Phases
3.5. Mass Loss of BTEX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VOCs | volatile organic compounds |
| GT | groundwater table |
| GTF | groundwater table fluctuation |
| SVE | soil vapor extraction |
| BTEX | benzene, toluene, ethylbenzene, o-xylene |
| TMVOC | numerical simulator for three-phase non-isothermal flows of multicomponent hydrocarbon mixtures in saturated–unsaturated heterogeneous media |
| NAPLs | non-aqueous-phase liquids |
| LNAPLs | light non-aqueous-phase liquids |
| XVOCW | the mass fraction distribution of dissolved BTEX |
| FLO (gas) | longitudinal gas flow |
References
- Cavelan, A.; Colfier, F.; Colombano, S.; Davarzani, H.; Deparis, J.; Faure, P. A critical review of the influence of groundwater level fluctuations and temperature on LNAPL contaminations in the context of climate change. Sci. Total Environ. 2022, 806, 150412. [Google Scholar] [CrossRef] [PubMed]
- Onna, C.; Olaobaju, E.A.; Amro, M.M. Experimental and numerical assessment of Light NonAqueous Phase Liquid (LNAPL) subsurface migration behavior in the vicinity of groundwater table. Environ. Technol. Innov. 2021, 23, 101573. [Google Scholar] [CrossRef]
- Zhao, B.; Peng, T.; Hou, R.; Huang, Y.; Zong, W.; Jin, Y.; O’Connor, D.; Sahu, S.; Zhang, H. Manganese stabilization in mine tailings by MgO-loaded rice husk biochar: Performance and mechanisms. Chemosphere 2022, 308, 136292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, A.; Wei, Y.; Miao, Q.; Xu, W.; Zhao, B.; Guo, Y.; Sheng, Y.; Yang, Y. Development of a new methodology for multifaceted assessment, analysis, and characterization of soil contamination. J. Hazard. Mater. 2022, 438, 129542. [Google Scholar] [CrossRef]
- Gribovszki, Z.; Szilágyi, J.; Kalicz, P. Diurnal fluctuations in shallow groundwater levels and stream flow rates and their interpretation—A review. J. Hydrol. 2010, 385, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Zuo, R.; Zheng, S.; Liu, X.; Wu, G.; Wang, S.; Wang, J.; Liu, J.; Huang, C.; Zhai, Y. Groundwater table fluctuation: A driving force affecting nitrogen transformation in nitrate-contaminated groundwater. J. Hydrol. 2023, 621, 129606. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, X.; Zhao, L.; Cao, X. Numerical modeling investigations of colloid facilitated chromium migration considering variable-density flow during the coastal groundwater table fluctuation. J. Hazard. Mater. 2023, 443 Pt B, 130282. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, Y.; Xu, X.; Liu, H.; Xin, P. Groundwater table fluctuations in a coastal unconfined aquifer with depth-varying hydraulic properties. J. Hydrol. 2022, 606, 127407. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, X.; Zhao, L.; Chen, X.; Qiu, H.; Gao, B.; Cao, X. Migration and transformation of chromium in unsaturated soil during groundwater table fluctuations induced by rainfall. J. Hazard. Mater. 2021, 416, 126229. [Google Scholar] [CrossRef]
- Mainhagu, J.; Morrison, C.; Brusseau, M.L. Using vapor phase tomography to measure the spatial distribution of vapor concentrations and flux for vadose-zone VOC sources. J. Contam. Hydrol. 2015, 177–178, 54–63. [Google Scholar] [CrossRef] [Green Version]
- US Environmental Protection Agency. Superfund Remedy Report Thirteenth Edition (EPA-542-R-10-004); Office of Solid Waste and Emergency Response: Washington, DC, USA, 2010.
- Brusseau, M.L.; Mainhagu, J.; Morrison, C.; Carroll, K.C. The vapor-phase multi-stage CMD test for characterizing contaminant mass discharge associated with VOC sources in the vadose zone: Application to three sites in different lifecycle stages of SVE operations. J. Contam. Hydrol. 2015, 179, 55–64. [Google Scholar] [CrossRef] [Green Version]
- You, K.; Zhan, H. Can atmospheric pressure and water table fluctuations be neglected in soil vapor extraction. Adv. Water Res. 2012, 35, 41–54. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Lv, N.; Wang, H.; Zhang, H. Multiphase migration and transformation of BTEX on groundwater table fluctuation in riparian petrochemical sites. Environ. Sci. Pollut. Res. 2023, 30, 55756–55767. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sheng, Y.; Wang, G.; Guo, L.; Zhang, H.; Zhang, F.; Yang, T.; Huang, D.; Han, X.; Zhou, L. Microbial compositional and functional traits of BTEX and salinity co-contaminated shallow groundwater by produced water. Water Res. 2022, 215, 118277. [Google Scholar] [CrossRef] [PubMed]
- Mckenzie, E.R.; Siegrist, R.L.; Mccray, J.E.; Higgins, C. The influence of a non-aqueous phase liquid (NAPL) and chemical oxidant application on perfluoroalkyl acid (PFAA) fate and transport. Water Res. 2016, 92, 199–207. [Google Scholar] [CrossRef]
- Joun, W.T.; Lee, S.S.; Koh, Y.E.; Lee, K. Impact of Water Table Fluctuations on the Concentration of Borehole Gas from NAPL Sources in the Vadose Zone. Vadose Zone J. 2016, 15, vzj2015-09. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, L.; Yan, Z.; Huang, Y.; Feng, C.; Li, L.; Ling, J. Effective extrapolation models for ecotoxicity of benzene, toluene, ethylbenzene, and xylene (BTEX). Chemosphere 2020, 240, 124906. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Zhou, J.; Xie, X.; Su, Y.; Gu, Q.; Kamon, M. Modelling of benzene distribution in the subsurface of an abandoned gas plant site after a long term of groundwater table fluctuation. Hydrol. Proc. 2013, 27, 3217–3226. [Google Scholar] [CrossRef]
- Rivett, M.O.; Wealthall, G.P.; Dearden, R.A.; McAlary, T.A. Review of unsaturated-zone transport and attenuation of volatile organic compound (VOC) plumes leached from shallow source zones. J. Contam. Hydrol. 2011, 123, 130–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, G.; Sugiura, N.; Utsumi, M.; Zhang, Z.; Yang, Y. Distribution of petroleum hydrocarbons in soils and the underlying unsaturated subsurface at an abandoned petrochemical site, North China. Hydrol. Proc. 2013, 28, 2185–2191. [Google Scholar] [CrossRef]
- Teramoto, E.H.; Chang, H.K. Field data and numerical simulation of btex concentration trends under water table fluctuations: Example of a jet fuel-contaminated site in Brazil. J. Contam. Hydrol. 2017, 198, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Ugwoha, E.; Andresen, J.M. Sorption and phase distribution of ethanol and butanol blended gasoline vapours in the vadose zone after release. J. Environ. Sci. 2014, 26, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Lari, K.S.; Johnston, C.D.; Davis, G.B. Gasoline Multiphase and Multicomponent Partitioning in the Vadose Zone: Dynamics and Risk Longevity. Vadose Zone J. 2016, 15, vzj2015.07.0100. [Google Scholar] [CrossRef]
- Moshkovich, E.; Ronen, Z.; Gelman, F.; Dahan, O. In Situ Bioremediation of a Gasoline-Contaminated Vadose Zone: Implications from Direct Observations. Vadose Zone J. 2018, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.A.; Albergaria, J.T.; Domingues, V.F.; Alvim-Ferraz, M.; Delerue-Matos, C. Remediation of soils combining soil vapor extraction and bioremediation: Benzene. Chemosphere 2010, 80, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, L.; Yang, Y.; Li, J.; Tang, J.; Bai, S.; Feng, Y. Numerical Simulation of BTEX Migration in Groundwater Table Fluctuation Zone Based on TMVOC. Res. Environ. Sci. 2020, 33, 634–642. [Google Scholar]
- Zheng, Q.-T.; Yang, C.-B.-X.; Feng, S.-J.; Wu, S.-J.; Zhang, X.-L. Influence mechanism of thermally enhanced phase change on heat transfer and soil vapour extraction. J. Contam. Hydrol. 2023, 257, 104202. [Google Scholar] [CrossRef]
- Liang, C.; Yang, S.-Y. Foam flushing with soil vapor extraction for enhanced treatment of diesel contaminated soils in a one-dimensional column. Chemosphere 2021, 285, 131471. [Google Scholar] [CrossRef]
- Sun, P.; Hua, Y.; Zhao, J.; Wang, C.; Tan, Q.; Shen, G. Insights into the mechanism of hydrogen peroxide activation with biochar produced from anaerobically digested residues at different pyrolysis temperatures for the degradation of BTEXS. Sci. Total Environ. 2021, 788, 147718. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Feng, X. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar]
- Peng, T.; Zhao, B.; O’Connor, D.; Jin, Y.; Lu, Z.; Guo, Y.; Liu, K.; Huang, Y.; Zong, W.; Jiang, J.; et al. Comprehensive assessment of soil and dust heavy metal(loid)s exposure scenarios at residential playgrounds in Beijing, China. Sci. Total Environ. 2023, 887, 164144. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Zhao, L.; Zytner, R.G. Three-dimensional numerical model for soil vapor extraction. J. Contam. Hydrol. 2013, 147, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, Y.; Gao, C.; Du, X.; Qu, T. Developing novel persulfate pellets to remediate BTEXs-contaminated groundwater. J. Water Process Eng. 2023, 52, 103505. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef] [PubMed]
- Perina, T. General well function for soil vapor extraction. Adv. Water Res. 2014, 66, 1–7. [Google Scholar] [CrossRef]
- Suk, H.; Zheng, K.-W.; Liao, Z.-Y.; Liang, C.-P.; Wang, S.-W.; Chen, J.-S. A new analytical model for transport of multiple contaminants considering remediation of both NAPL source and downgradient contaminant plume in groundwater. Adv. Water Resour. 2022, 167, 104290. [Google Scholar] [CrossRef]
- Battistelli, A. Modeling Multiphase Organic Spills in Coastal Sites with TMVOC V.2.0. Vadose Zone J. 2008, 7, 316–324. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, Z.; Zhang, C.; Jakada, H. Contamination characteristics of chlorinated hydrocarbons in a fractured karst aquifer using TMVOC and hydro-chemical techniques. Sci. Total Environ. 2021, 794, 148717. [Google Scholar] [CrossRef]
- Vanantwerp, D.J.; Falta, R.W.; Gierke, J.S. Numerical Simulation of Field-Scale Contaminant Mass Transfer during Air Sparging. Vadose Zone J. 2008, 7, 294–304. [Google Scholar] [CrossRef]
- Lekmine, G.; Lari, K.S.; Johnston, C.D.; Bastow, T.P.; Rayner, J.L.; Davis, G.B. Evaluating the reliability of equilibrium dissolution assumption from residual gasoline in contact with water saturated sands. J. Contam. Hydrol. 2017, 196, 30–42. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Xi, B.; Wang, Y.; Tang, J.; Wang, Y.; Zhao, C. Modeling BTEX migration with soil vapor extraction remediation under low-temperature conditions. J. Environ. Manag. 2017, 203, 114–122. [Google Scholar] [CrossRef]
- You, K.; Zhan, H.; Li, J. A new solution and data analysis for gas flow to a barometric pumping well. Adv. Water Res. 2010, 33, 1444–1455. [Google Scholar] [CrossRef]
- Schumacher, B.A.; Minnich, M.M. Extreme Short-Range Variability in VOC-Contaminated Soils. Environ. Sci. Technol. 2000, 34, 3611–3616. [Google Scholar] [CrossRef]
- Davidson, C.J.; Svenson, D.W.; Hannigan, J.H.; Perrine, S.A.; Bowen, S.E. A novel preclinical model of environment-like combined benzene, toluene, ethylbenzene, and xylenes (BTEX) exposure: Behavioral and neurochemical findings. Neurotoxicol. Teratol. 2022, 91, 107076. [Google Scholar] [PubMed]
- Man, J.; Zhou, Q.; Wang, G.; Yao, Y. Modeling and evaluation of NAPL-impacted soil vapor intrusion facilitated by vadose zone breathing. J. Hydrol. 2022, 615 Pt A, 128683. [Google Scholar] [CrossRef]



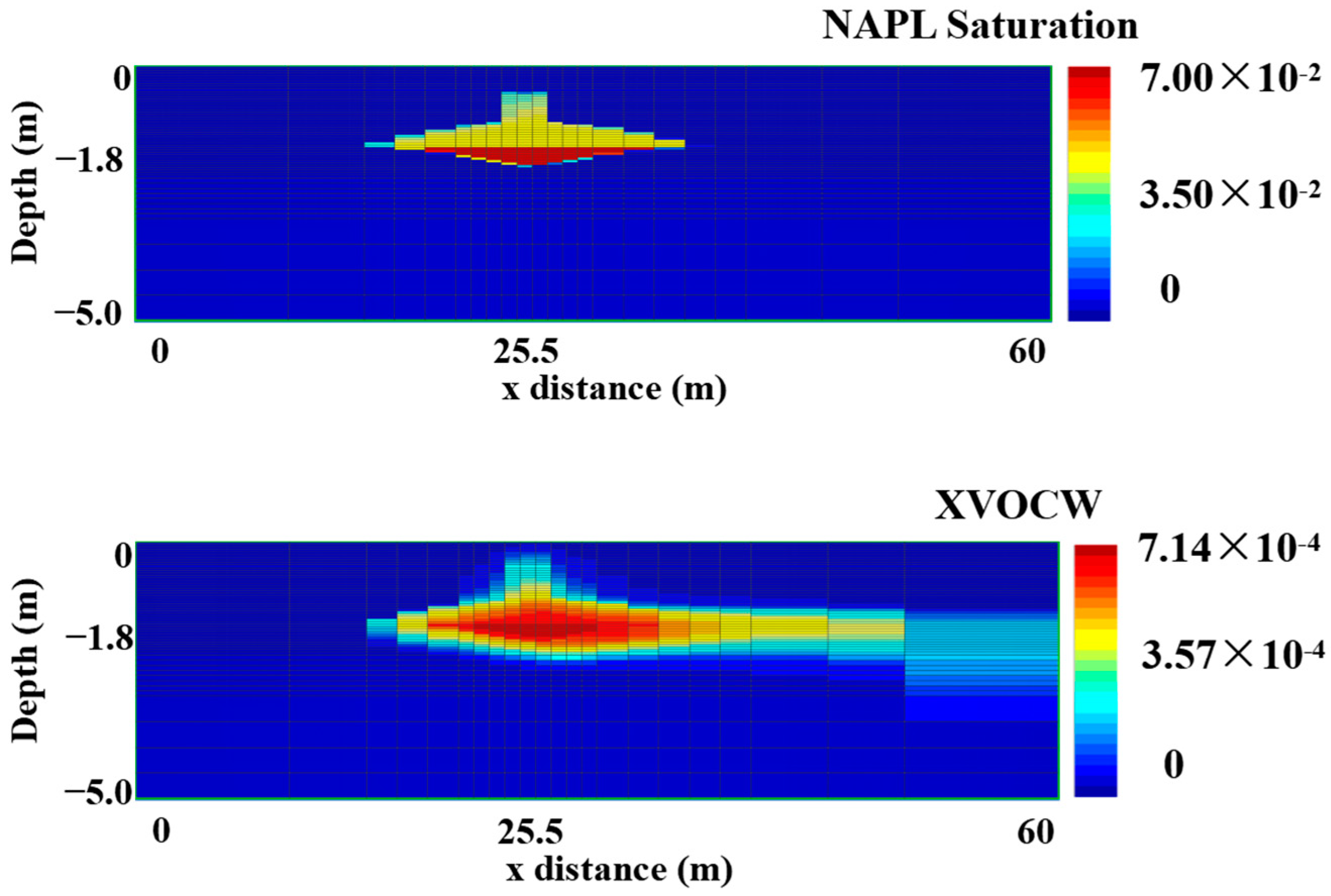
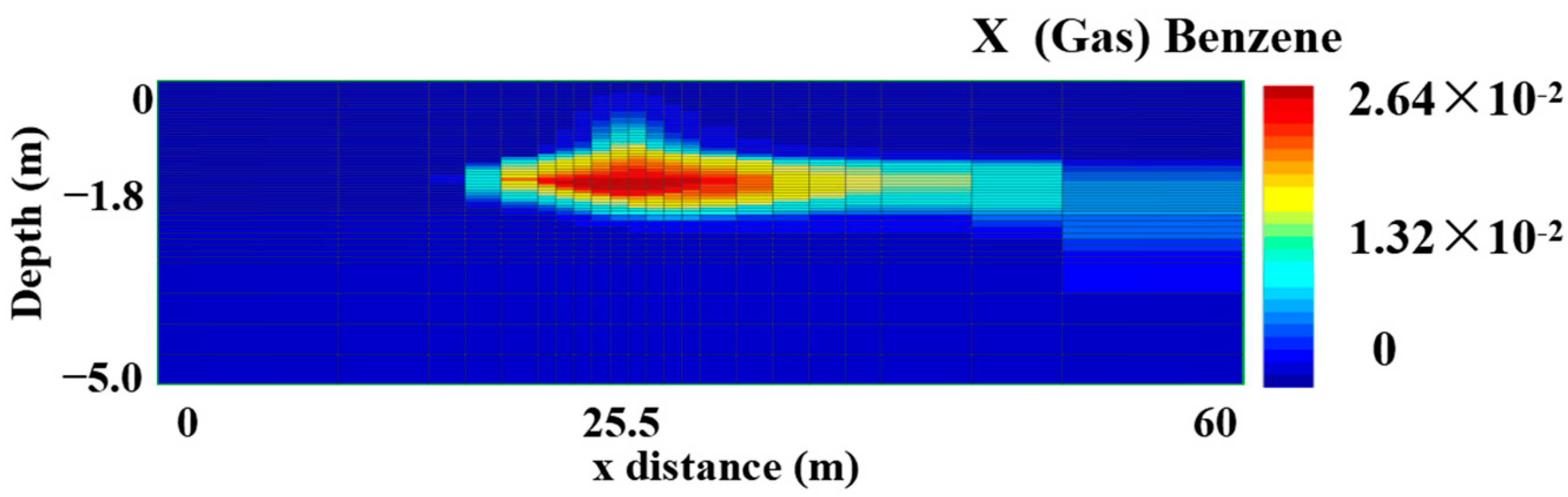
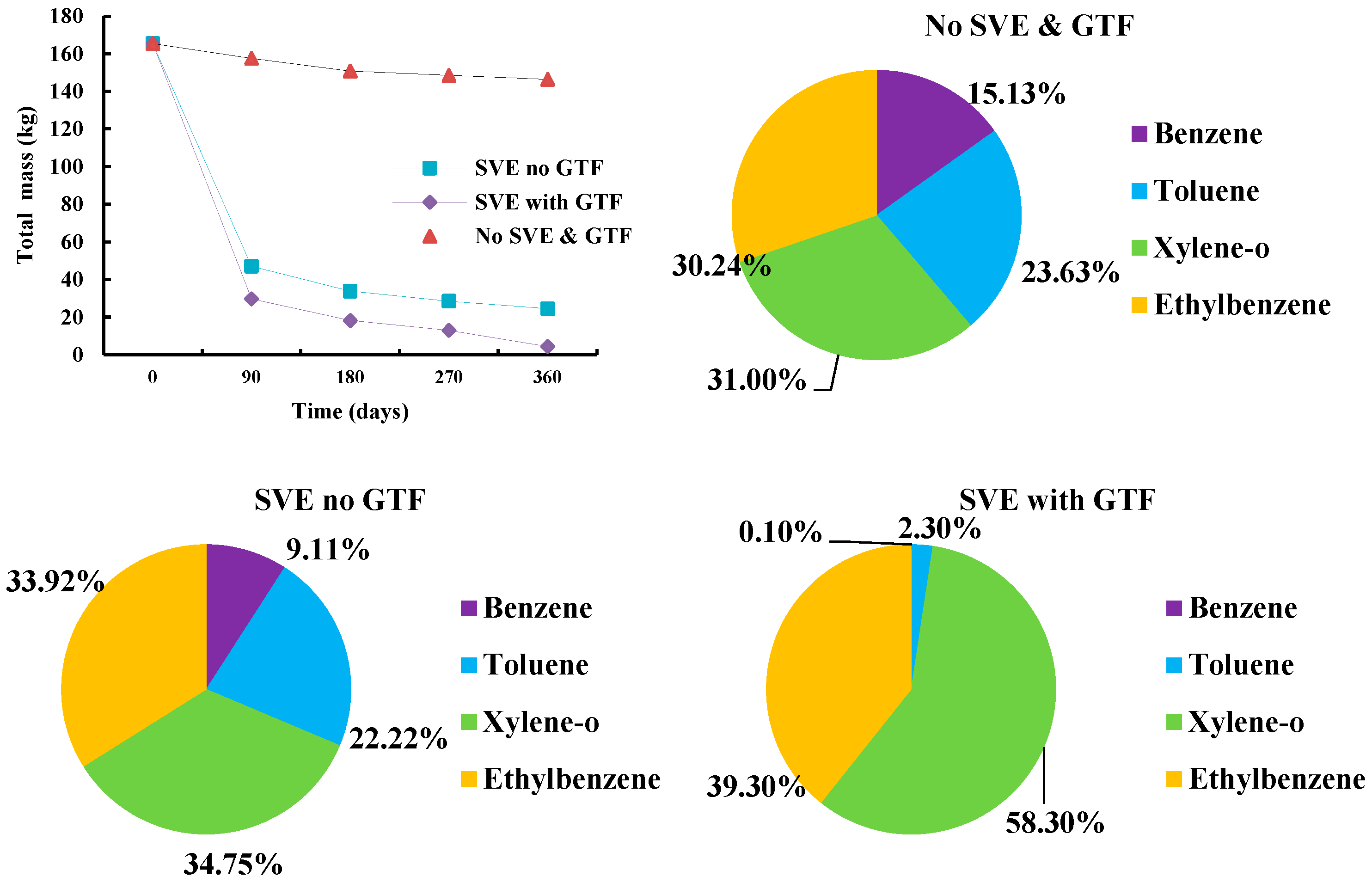



| Scenarios | 0 Day | 90 Days | 180 Days | 270 Days | 360 Days | Removal Rate (%) * | |
|---|---|---|---|---|---|---|---|
| Total mass (kg) | SVE no GTF | 165.48 | 47.07 | 33.86 | 28.55 | 24.55 | 85.16% |
| SVE with GTF | 165.48 | 29.77 | 18.25 | 13.06 | 4.42 | 97.33% | |
| No SVE & GTF | 165.48 | 157.58 | 150.79 | 148.55 | 146.47 | 11.49% | |
| Benzene mass (kg) | SVE no GTF | 28.55 | 6.42 | 4.31 | 3.07 | 2.24 | 92.17% |
| SVE with GTF | 28.55 | 1.45 | 0.30 | 0.07 | 0.00 | 100.00% | |
| No SVE & GTF | 28.55 | 25.75 | 23.35 | 22.72 | 22.15 | 22.42% | |
| Toluene mass (kg) | SVE no GTF | 40.12 | 10.35 | 7.89 | 6.50 | 5.46 | 86.40% |
| SVE with GTF | 40.12 | 5.97 | 3.16 | 1.82 | 0.11 | 99.74% | |
| No SVE & GTF | 40.12 | 37.79 | 35.75 | 35.16 | 34.62 | 13.71% | |
| Ethylbenzene mass (kg) | SVE no GTF | 47.96 | 14.79 | 10.69 | 9.37 | 8.33 | 82.63% |
| SVE with GTF | 47.96 | 10.65 | 6.99 | 5.19 | 1.74 | 96.37% | |
| No SVE & GTF | 47.96 | 46.52 | 45.28 | 44.78 | 44.30 | 7.63% | |
| Xylene-o mass (kg) | SVE no GTF | 48.85 | 15.51 | 10.98 | 9.60 | 8.53 | 82.53% |
| SVE with GTF | 48.85 | 11.70 | 7.80 | 5.98 | 2.58 | 94.72% | |
| No SVE & GTF | 48.85 | 47.53 | 46.41 | 45.89 | 45.41 | 7.05% | |
| Scenarios | Days | Gas Phase (kg) | Aqueous Phase (kg) | NAPL Phase (kg) | Total Mass (kg) | |
|---|---|---|---|---|---|---|
| No SVE and GTF | Benzene | 0 | 0.0237 | 3.14 | 25.39 | 28.55 |
| 360 | 0.0206 | 3.19 | 18.94 | 22.15 | ||
| Removal rate (%) * | 13.18% | −1.71% | 25.41% | 22.42% | ||
| Toluene | 0 | 0.0169 | 1.26 | 38.84 | 40.12 | |
| 360 | 0.0203 | 1.40 | 33.19 | 34.62 | ||
| Removal rate (%) * | −20.13% | −11.26% | 14.54% | 13.71% | ||
| Ethylbenzene | 0 | 0.0117 | 0.45 | 47.50 | 47.96 | |
| 360 | 0.0181 | 0.54 | 43.74 | 44.30 | ||
| Removal rate (%) * | −54.34% | −19.61% | 7.91% | 7.63% | ||
| Xylene-o | 0 | 0.0094 | 0.55 | 48.30 | 48.85 | |
| 360 | 0.0146 | 0.65 | 44.74 | 45.41 | ||
| Removal rate (%) * | −55.23% | −19.40% | 7.36% | 7.05% | ||
| SVE no GTF | Benzene | 0 | 0.0237 | 3.14 | 25.39 | 28.55 |
| 360 | 0.0042 | 0.29 | 1.94 | 2.24 | ||
| Removal rate (%) * | 82.41% | 90.75% | 92.35% | 92.17% | ||
| Toluene | 0 | 0.0169 | 1.26 | 38.84 | 40.12 | |
| 360 | 0.0035 | 0.14 | 5.32 | 5.46 | ||
| Removal rate (%) * | 79.41% | 89.17% | 86.31% | 86.40% | ||
| Ethylbenzene | 0 | 0.0117 | 0.45 | 47.50 | 47.96 | |
| 360 | 0.0024 | 0.06 | 8.27 | 8.33 | ||
| Removal rate (%) * | 79.25% | 87.16% | 82.59% | 82.63% | ||
| Xylene-o | 0 | 0.0094 | 0.55 | 48.30 | 48.85 | |
| 360 | 0.0021 | 0.07 | 8.46 | 8.53 | ||
| Removal rate (%) * | 78.01% | 86.94% | 82.48% | 82.53% | ||
| SVE with GTF | Benzene | 0 | 0.0237 | 3.14 | 25.39 | 28.55 |
| 360 | 7.55 × 10−6 | 3.86 × 10−4 | 2.24 × 10−6 | 3.96 × 10−4 | ||
| Removal rate (%) * | 99.97% | 99.99% | 99.99% | 99.99% | ||
| Toluene | 0 | 0.0169 | 1.26 | 38.84 | 40.12 | |
| 360 | 0.0012 | 0.02 | 0.08 | 0.11 | ||
| Removal rate (%) * | 92.88% | 98.03% | 99.79% | 99.74% | ||
| Ethylbenzene | 0 | 0.0117 | 0.45 | 47.50 | 47.96 | |
| 360 | 0.0067 | 0.08 | 1.65 | 1.74 | ||
| Removal rate (%) * | 42.53% | 81.20% | 96.53% | 96.37% | ||
| Xylene-o | 0 | 0.0094 | 0.55 | 48.30 | 48.85 | |
| 360 | 0.0063 | 0.12 | 2.45 | 2.58 | ||
| Removal rate (%) * | 32.65% | 77.82% | 94.92% | 94.72% | ||
| Scenario 1: No SVE and GTF | |||||
|---|---|---|---|---|---|
| Days | Gas Phase (kg) | Aqueous Phase (kg) | NAPL Phase (kg) | Total Mass (kg) | |
| 0 | 0.07 | 6.10 | 159.31 | 165.48 | |
| 90 | 0.42 | 6.24 | 150.93 | 157.58 | |
| 180 | 0.08 | 5.87 | 144.84 | 150.79 | |
| 270 | 0.03 | 6.43 | 142.08 | 148.55 | |
| 360 | 0.08 | 6.55 | 139.85 | 146.47 | |
| Mass fractions (%) * | 0 days | 0.04% | 3.69% | 96.27% | / |
| 360 days | 0.27% | 3.96% | 95.78% | / | |
| Scenario 2: SVE No GTF | |||||
| Days | Gas phase (kg) | Aqueous phase (kg) | NAPL phase (kg) | Total mass (kg) | |
| 0 | 0.07 | 6.10 | 159.31 | 165.48 | |
| 90 | 0.03 | 0.89 | 46.15 | 47.07 | |
| 180 | 0.02 | 0.74 | 33.09 | 33.86 | |
| 270 | 0.01 | 0.68 | 27.85 | 28.55 | |
| 360 | 0.01 | 0.64 | 23.90 | 24.55 | |
| Mass fractions (%) * | 0 days | 0.04% | 3.69% | 96.27% | / |
| 360 days | 0.06% | 1.89% | 98.05% | / | |
| Scenario 3: SVE with GTF | |||||
| Days | Gas phase (kg) | Aqueous phase (kg) | NAPL phase (kg) | Total mass (kg) | |
| 0 | 0.07 | 6.10 | 159.31 | 165.48 | |
| 90 | 0.06 | 0.96 | 28.75 | 29.77 | |
| 180 | 0.02 | 0.27 | 17.96 | 18.25 | |
| 270 | 0.01 | 0.20 | 12.85 | 13.06 | |
| 360 | 0.01 | 0.23 | 4.18 | 4.42 | |
| Mass fractions (%) * | 0 days | 0.04% | 3.69% | 96.27% | / |
| 360 days | 0.19% | 3.23% | 96.58% | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zheng, J.; Li, J.; Huan, H.; Zhao, X.; Lv, N.; Ma, Y.; Zhang, H. Modeling BTEX Multiphase Partitioning with Soil Vapor Extraction under Groundwater Table Fluctuation Using the TMVOC Model. Water 2023, 15, 2477. https://doi.org/10.3390/w15132477
Yang Y, Zheng J, Li J, Huan H, Zhao X, Lv N, Ma Y, Zhang H. Modeling BTEX Multiphase Partitioning with Soil Vapor Extraction under Groundwater Table Fluctuation Using the TMVOC Model. Water. 2023; 15(13):2477. https://doi.org/10.3390/w15132477
Chicago/Turabian StyleYang, Yang, Jingwei Zheng, Juan Li, Huan Huan, Xiaobing Zhao, Ningqing Lv, Yan Ma, and Hao Zhang. 2023. "Modeling BTEX Multiphase Partitioning with Soil Vapor Extraction under Groundwater Table Fluctuation Using the TMVOC Model" Water 15, no. 13: 2477. https://doi.org/10.3390/w15132477
APA StyleYang, Y., Zheng, J., Li, J., Huan, H., Zhao, X., Lv, N., Ma, Y., & Zhang, H. (2023). Modeling BTEX Multiphase Partitioning with Soil Vapor Extraction under Groundwater Table Fluctuation Using the TMVOC Model. Water, 15(13), 2477. https://doi.org/10.3390/w15132477





