Status of Research on Greenhouse Gas Emissions from Wastewater Collection Systems
Abstract
1. Introduction
2. Greenhouse Gases in Wastewater Collection Systems
2.1. Greenhouse Gases
2.2. Wastewater Collection Systems
2.3. Greenhouse Gases in Wastewater Collection Systems
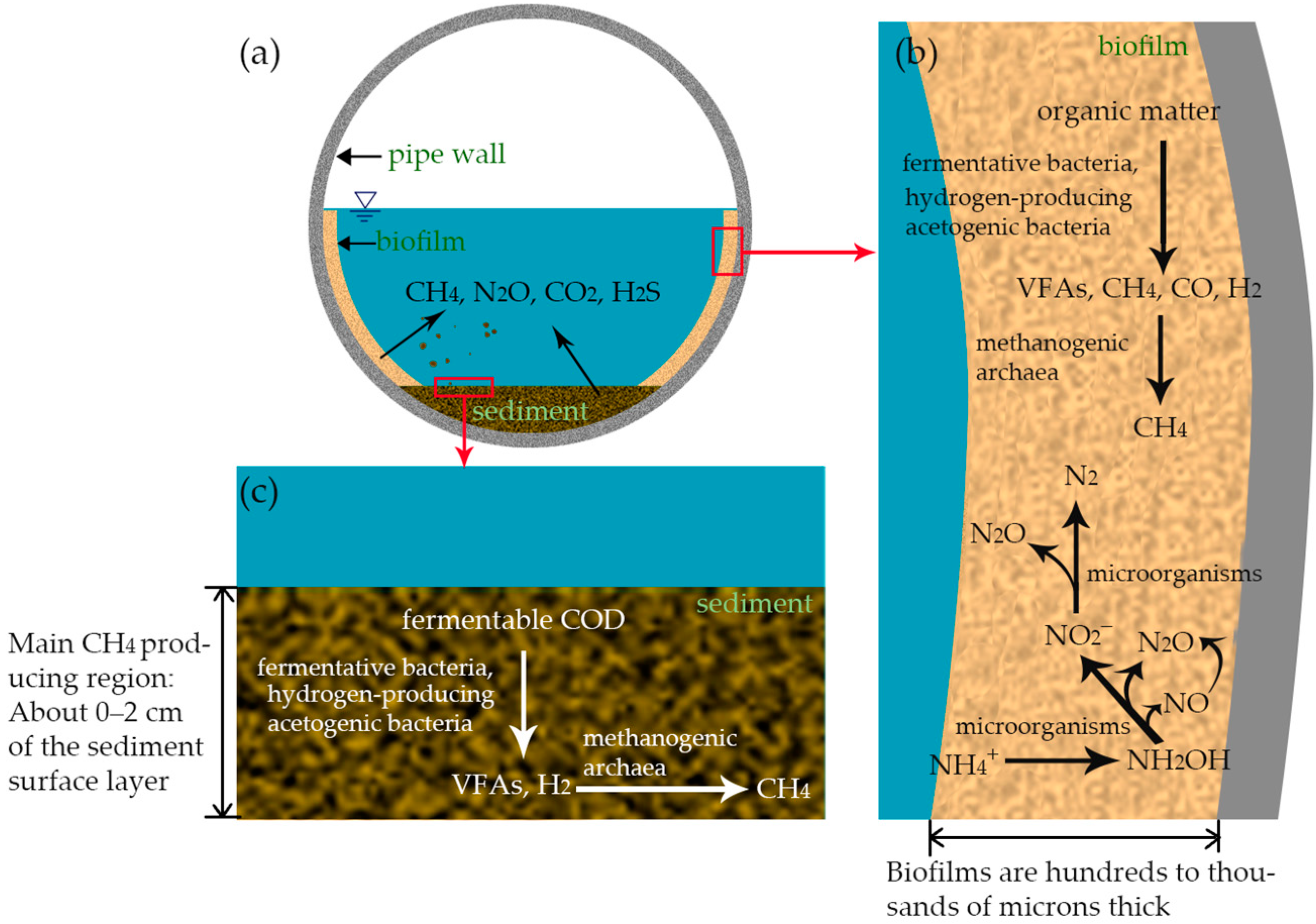
2.4. Mechanism of Greenhouse Gas Production in Wastewater Collection Pipelines
3. Factors Affecting Greenhouse Gas Production in Wastewater Collection Systems
3.1. Factors Affecting CH4 Production
3.1.1. Biofilm
3.1.2. Sediment
3.2. N2O
4. Control of Greenhouse Gases in Wastewater Collection Systems
4.1. CO2
4.2. CH4
4.2.1. O2
4.2.2. Nitrate
4.2.3. Nitrite (Free Nitrous Acid)
4.2.4. Free Ammonia
4.2.5. pH
4.2.6. Iron Salts
4.2.7. Ferrate
4.2.8. Other Substances
4.3. N2O
5. Quantitative Estimates of Greenhouse Gases in Wastewater Collection Systems
5.1. CO2
5.2. CH4
5.3. N2O
6. Modeling
7. Discussion
- Emission data from the literature [84] were multiplied by the ratio of the length of China’s pipeline system to the studied pipeline to calculate emissions.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, B.; Lei, X. Carbon emissions reduction in China’s food industry. Energy Policy 2015, 86, 483–492. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Rafiee, S.; Omid, M.; Mousazadeh, H. Reduction of CO2 emission by improving energy use efficiency of greenhouse cucumber production using DEA approach. Energy 2013, 55, 676–682. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Wang, C. Carbon footprint and predicting the impact of climate change on carbon sequestration ecosystem services of organic rice farming and conventional rice farming: A case study in Phichit province, Thailand. J. Environ. Manag. 2021, 289, 112458. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lin, B. Analysis of carbon emissions reduction of China’s metallurgical industry. J. Clean. Prod. 2018, 176, 1177–1184. [Google Scholar] [CrossRef]
- Ghasemi-Mobtaker, H.; Kaab, A.; Rafiee, S.; Nabavi-Pelesaraei, A. A comparative of modeling techniques and life cycle assessment for prediction of output energy, economic profit, and global warming potential for wheat farms. Energy Rep. 2022, 8, 4922–4934. [Google Scholar] [CrossRef]
- Sadia, M.; Mahmood, A.; Ibrahim, M.; Irshad, M.K.; Quddusi, A.H.A.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; Show, P.L. Microplastics pollution from wastewater treatment plants: A critical review on challenges, detection, sustainable removal techniques and circular economy. Environ. Technol. Innov. 2022, 28, 102946. [Google Scholar] [CrossRef]
- IPCC. Wastewater Treatment and Discharge. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan, 2006; Volume 5, pp. 6–8. [Google Scholar]
- Foley, J.; Yuan, Z.; Keller, J.; Senante, E.; Chandran, K.; Willis, J.; Shah, A.; van Loosdrecht, M.C.M.; van Voorthuizen, E. N2O and CH4 Emission from Wastewater Collection and Treatment Systems: State of the Science Report and Technical Report; IWA Publishing: London, UK, 2015. [Google Scholar]
- Guisasola, A.; de Haas, D.; Keller, J.; Yuan, Z. Methane formation in sewer systems. Water Res. 2008, 42, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Yuan, Z.; Lant, P. Dissolved methane in rising main sewer systems: Field measurements and simple model development for estimating greenhouse gas emissions. Water Sci. Technol. 2009, 60, 2963–2971. [Google Scholar] [CrossRef]
- Willis, J.; Fillmore, L.; Shah, A.; Yuan, Z.; Sharma, K. Quantifying Methane Evolution from Sewers: Results from WERF/DeKalb Phase 2- Continuous Monitoring. In Proceedings of the Water Environment Federation; Water Environment Federation: Alexandria, VA, USA, 2011; pp. 3851–3858. [Google Scholar]
- Liu, Y.W.; Ni, B.J.; Ganigue, R.; Werner, U.; Sharma, K.R.; Yuan, Z.G. Sulfide and methane production in sewer sediments. Water Res. 2015, 70, 350–359. [Google Scholar] [CrossRef]
- Liu, Y.; Sharma, K.R.; Fluggen, M.; O’Halloran, K.; Murthy, S.; Yuan, Z. Online dissolved methane and total dissolved sulfide measurement in sewers. Water Res. 2015, 68, 109–118. [Google Scholar] [CrossRef]
- IPCC. Wastewater Treatment and Discharge. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Buendia, E.C., Tanabe, K., Kranjc, A., Jamsranjav, B., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019; Volume 5, pp. 7–9. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- CO₂ and Greenhouse Gas Emissions. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 22 March 2023).
- IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis; IPPC: New York, NY, USA, 2013. [Google Scholar]
- Horowitz, C.A. Introductory note to Paris. Int. Leg. Mater. 2016, 55, 740–755. [Google Scholar] [CrossRef]
- Huang, D.; Liu, X.; Jiang, S.; Wang, H.; Wang, J.; Zhang, Y. Current state and future perspectives of sewer networks in urban China. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Ma, Y.; Liu, J. Current status and future prospects of sewer biofilms: Their structure, influencing factors, and substance transformations. Sci. Total Environ. 2019, 695, 133815. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, M.; He, Q.; Sun, X.; Zhou, X.; Su, Z.; Ai, H. Effect of flow rate on growth and oxygen consumption of biofilm in gravity sewer. Environ. Sci. Pollut. Res. 2017, 24, 427–435. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, B.J.; Sharma, K.R.; Yuan, Z. Methane emission from sewers. Sci. Total Environ. 2015, 524–525, 40–51. [Google Scholar] [CrossRef]
- Zan, F.; Tang, W.; Jiang, F.; Chen, G. Diversion of food waste into the sulfate-laden sewer: Interaction and electron flow of sulfidogenesis and methanogenesis. Water Res. 2021, 202, 117437. [Google Scholar] [CrossRef]
- Li, J.; Gao, J.; Thai, P.K.; Shypanski, A.; Nieradzik, L.; Mueller, J.F.; Yuan, Z.; Jiang, G. Experimental Investigation and Modeling of the Transformation of Illicit Drugs in a Pilot-Scale Sewer System. Environ. Sci. Technol. 2019, 53, 4556–4565. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.K.; Jiang, G.; Gernjak, W.; Yuan, Z.; Lai, F.Y.; Mueller, J.F. Effects of sewer conditions on the degradation of selected illicit drug residues in wastewater. Water Res. 2014, 48, 538–547. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Wang, J.; Xu, X.; Shao, Y.; Zhang, Q.; Liu, Y.; Qi, L.; Wang, H. Current status, existent problems, and coping strategy of urban drainage pipeline network in China. Environ. Sci. Pollut. R. 2021, 28, 43035–43049. [Google Scholar] [CrossRef]
- Zuo, Z.; Xing, Y.; Duan, H.; Ren, D.; Zheng, M.; Liu, Y.; Huang, X. Reducing sulfide and methane production in gravity sewer sediments through urine separation, collection and intermittent dosing. Water Res. 2023, 234, 119820. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.U.; Noland, S.S.; Gottlieb, L.J. Bathtub fire: An extraordinary burn injury. J. Burn Care Res. 2006, 27, 97–98. [Google Scholar] [CrossRef]
- Ahrens, M. Worker Casualties involving Wastewater, Sewers or Sewage Treatment Plants and Fire Incidents at Water or Sanitation Utilities; National Fire Protection Association: Quincy, MA, USA, 2012. [Google Scholar]
- Mannina, G.; Butler, D.; Benedetti, L.; Deletic, A.; Fowdar, H.; Fu, G.; Kleidorfer, M.; McCarthy, D.; Steen Mikkelsen, P.; Rauch, W.; et al. Greenhouse gas emissions from integrated urban drainage systems: Where do we stand? J. Hydrol. 2018, 559, 307–314. [Google Scholar] [CrossRef]
- Michael, D.S.; Alexander, D.; Gregory, M.P.; Kirsten, M.; Nicholas, J.A.; Richard, M.S.; William, L.P. Municipal gravity sewers: An unrecognised source of nitrous oxide. Sci. Total Environ. 2014, 468–469, 211–218. [Google Scholar]
- Law, Y.; Ye, L.; Pan, Y.; Yuan, Z. Nitrous oxide emissions from wastewater treatment processes. Philos. Trans. R. Soc. Ser. B Biol. Sci. 2012, 367, 1265–1277. [Google Scholar] [CrossRef]
- Chen, H.; Ye, J.F.; Zhou, Y.F.; Wang, Z.N.; Jia, Q.L.; Nie, Y.H.; Li, L.; Liu, H.; Benoit, G. Variations in CH4 and CO2 productions and emissions driven by pollution sources in municipal sewers: An assessment of the role of dissolved organic matter components and microbiota. Environ. Pollut. 2020, 263, 114489. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Sudarjanto, G.; Ren, G.; Ganigué, R.; Jiang, G.; Yuan, Z. Assessment of pH shock as a method for controlling sulfide and methane formation in pressure main sewer systems. Water Res. 2014, 48, 569–578. [Google Scholar] [CrossRef]
- Jiang, G.; Sharma, K.R.; Yuan, Z. Effects of nitrate dosing on methanogenic activity in a sulfide-producing sewer biofilm reactor. Water Res. 2013, 47, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Chaosakul, T.; Koottatep, T.; Polprasert, C. A model for methane production in sewers. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, Q.; Li, H.; Yang, C.; Wang, Y.; Ai, H. Modeling of methane formation in gravity sewer system: The impact of microorganism and hydraulic condition. AMB Express 2018, 8, 34. [Google Scholar] [CrossRef]
- Liu, Y.; Tugtas, A.E.; Sharma, K.R.; Ni, B.; Yuan, Z. Sulfide and methane production in sewer sediments: Field survey and model evaluation. Water Res. 2016, 89, 142–150. [Google Scholar] [CrossRef]
- Sun, J.; Ni, B.J.; Sharma, K.R.; Wang, Q.L.; Hu, S.H.; Yuan, Z.G. Modelling the long-term effect of wastewater compositions on maximum sulfide and methane production rates of sewer biofilm. Water Res. 2018, 129, 58–65. [Google Scholar] [CrossRef]
- Zan, F.; Dai, J.; Jiang, F.; Ekama, G.A.; Chen, G. Ground food waste discharge to sewer enhances methane gas emission: A lab-scale investigation. Water Res. 2020, 174, 115616. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Li, J.; Gao, J.; O’Brien, J.W.; Thomas, K.V.; Thai, P.K.; Jiang, G.; Mueller, J.F. Considerations for assessing stability of wastewater-based epidemiology biomarkers using biofilm-free and sewer reactor tests. Sci. Total Environ. 2020, 709, 136228. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.W.; Banks, A.P.W.; Novic, A.J.; Mueller, J.F.; Jiang, G.; Ort, C.; Eaglesham, G.; Yuan, Z.; Thai, P.K. Impact of in-Sewer Degradation of Pharmaceutical and Personal Care Products (PPCPs) Population Markers on a Population Model. Environ. Sci. Technol. 2017, 51, 3816–3823. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, S.; Sharma, K.R.; Ni, B.; Yuan, Z. Stratified microbial structure and activity in sulfide- and methane-producing anaerobic sewer biofilms. Appl. Environ. Microb. 2014, 80, 7042–7052. [Google Scholar] [CrossRef]
- Mohanakrishnan, J.; Gutierrez, O.; Meyer, R.L.; Yuan, Z. Nitrite effectively inhibits sulfide and methane production in a laboratory scale sewer reactor. Water Res. 2008, 42, 3961–3971. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Liu, H.; Nie, Y.; Zhu, Y.; Jia, Q.; Ding, G.; Ye, J. Variable sediment methane production in response to different source-associated sewer sediment types and hydrological patterns: Role of the sediment microbiome. Water Res. 2021, 190, 116670. [Google Scholar] [CrossRef]
- Eijo-Río, E.; Petit-Boix, A.; Villalba, G.; María, E.S.; Desirée, M.; Maria, J.A.; Xavier, A.; Joan, R.; Xavier, G. Municipal sewer networks as sources of nitrous oxide, methane and hydrogen sulphide emissions: A review and case studies. J. Environ. Chem. Eng. 2015, 3, 2084–2094. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, G.; Zhou, Y.; Zhu, D.Z.; Zhang, Y.; Zhang, T. Simultaneous use of nitrate and calcium peroxide to control sulfide and greenhouse gas emission in sewers. Sci. Total Environ. 2023, 855, 158913. [Google Scholar] [CrossRef]
- Auguet, O.; Pijuan, M.; Borrego, C.M.; Gutierrez, O. Control of sulfide and methane production in anaerobic sewer systems by means of Downstream Nitrite Dosage. Sci. Total Environ. 2016, 550, 1116–1125. [Google Scholar] [CrossRef]
- Zuo, Z.; Song, Y.; Ren, D.; Li, H.; Gao, Y.; Yuan, Z.; Huang, X.; Zheng, M.; Liu, Y. Control sulfide and methane production in sewers based on free ammonia inactivation. Environ. Int. 2020, 143, 8. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Park, D.; Sharma, K.R.; Yuan, Z. Effects of long-term pH elevation on the sulfate-reducing and methanogenic activities of anaerobic sewer biofilms. Water Res. 2009, 43, 2549–2557. [Google Scholar] [CrossRef]
- Jiang, G.; Gutierrez, O.; Sharma, K.R.; Yuan, Z. Effects of nitrite concentration and exposure time on sulfide and methane production in sewer systems. Water Res. 2010, 44, 4241–4251. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hu, C.; Zhang, D.; Dai, L.; Duan, N. Impact of a high ammonia-ammonium-pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion. Bioresour. Technol. 2017, 245, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, S.; Wang, Y.; Wang, Y.; Hou, J.; Zhu, T.; Liu, Y. Reduced sulfide and methane in rising main sewer via calcium peroxide dosing: Insights from microbial physiological characteristics, metabolisms and community traits. J. Hazard. Mater. 2023, 451, 131138. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, W.; Xu, D.; Hou, Y.; Ren, B.; Jin, X.; Wang, X.C.; Jin, P. Metagenomics analysis of ecosystem integrating methane and sulfide generation in urban sewer systems. J. Clean. Prod. 2023, 382, 135372. [Google Scholar] [CrossRef]
- Cen, X.; Li, J.; Jiang, G.; Zheng, M. A critical review of chemical uses in urban sewer systems. Water Res. 2023, 240, 120108. [Google Scholar] [CrossRef]
- Ganigué, R.; Yuan, Z. Impact of oxygen injection on CH4 and N2O emissions from rising main sewers. J. Environ. Manag. 2014, 144, 279–285. [Google Scholar] [CrossRef]
- Mohanakrishnan, J.; Gutierrez, O.; Sharma, K.R.; Guisasola, A.; Werner, U.; Meyer, R.L.; Keller, J.; Yuan, Z. Impact of nitrate addition on biofilm properties and activities in rising main sewers. Water Res. 2009, 43, 4225–4237. [Google Scholar] [CrossRef]
- Auguet, O.; Pijuan, M.; Guasch-Balcells, H.; Borrego, C.M.; Gutierrez, O. Implications of Downstream Nitrate Dosage in anaerobic sewers to control sulfide and methane emissions. Water Res. 2015, 68, 522–532. [Google Scholar] [CrossRef]
- Duan, H.; Gao, S.; Li, X.; Ab Hamid, N.H.; Jiang, G.; Zheng, M.; Bai, X.; Bond, P.L.; Lu, X.; Chislett, M.M.; et al. Improving wastewater management using free nitrous acid (FNA). Water Res. 2020, 171, 115382. [Google Scholar] [CrossRef]
- Liu, Y.; Ngo, H.H.; Guo, W.; Peng, L.; Wang, D.; Ni, B. The roles of free ammonia (FA) in biological wastewater treatment processes: A review. Environ. Int. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, L.; Hong, J.; Sun, J.; Jiang, F. Different ferric dosing strategies could result in different control mechanisms of sulfide and methane production in sediments of gravity sewers. Water Res. 2019, 164, 114914. [Google Scholar] [CrossRef]
- Zhang, L.; Keller, J.; Yuan, Z. Inhibition of sulfate-reducing and methanogenic activities of anaerobic sewer biofilms by ferric iron dosing. Water Res. 2009, 43, 4123–4132. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Talaei, M.R.; Rezania, S. An overview on production and application of ferrate (VI) for chemical oxidation, coagulation and disinfection of water and wastewater. J. Environ. Chem. Eng. 2017, 5, 1828–1842. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, B.; Wang, D.; Ma, T.; Yu, D. Variations in distribution and composition of extracellular polymeric substances (EPS) of biological sludge under potassium ferrate conditioning: Effects of pH and ferrate dosage. Biochem. Eng. J. 2016, 106, 37–47. [Google Scholar] [CrossRef]
- Yan, X.; Sun, J.; Kenjiahan, A.; Dai, X.; Ni, B.; Yuan, Z. Rapid and strong biocidal effect of ferrate on sulfidogenic and methanogenic sewer biofilms. Water Res. 2020, 169, 115208. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tian, L.; Liu, S.; Wang, Y.; Hou, J.; Zhu, T.; Liu, Y. The potent effects of polyoxometalates (POMs) on controlling sulfide and methane production from sewers. Chem. Eng. J. 2023, 453, 139955. [Google Scholar] [CrossRef]
- Sun, Y.; ter Heijne, A.; Rijnaarts, H.; Chen, W. The effect of anode potential on electrogenesis, methanogenesis and sulfidogenesis in a simulated sewer condition. Water Res. 2022, 226, 119229. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhang, Z.; Zhang, T.; Liu, J.; Lu, J. Upstream Natural Pulsed Ventilation: A simple measure to control the sulfide and methane production in gravity sewer. Sci. Total Environ. 2020, 742, 140579. [Google Scholar] [CrossRef]
- Sudarjanto, G.; Gutierrez, O.; Ren, G.; Yuan, Z. Laboratory assessment of bioproducts for sulphide and methane control in sewer systems. Sci. Total Environ. 2013, 443, 429–437. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Kapteijn, F.; Schoffel, K.; Moulijn, J.A. Formation and control of N2O in nitric acid production: Where do we stand today? Appl. Catal. B-Environ. 2003, 44, 35. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, X.; Peng, C.; Wang, S.; Sun, H.; Peng, Y. N2O Production during Nitrogen Removal via Nitrite from Domestic Wastewater: Main Sources and Control Method. Environ. Sci. Technol. 2009, 43, 9400–9406. [Google Scholar] [CrossRef]
- Boiocchi, R.; Gernaey, K.V.; Sin, G. A novel fuzzy-logic control strategy minimizing N2O emissions. Water Res. 2017, 123, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Gu, Y.; Shi, X.; Yang, W. Non-negligible greenhouse gases from urban sewer system. Biotechnol. Biofuels 2019, 12, 100. [Google Scholar] [CrossRef]
- Zhang, Q.; Smith, K.; Zhao, X.; Jin, X.K.; Wang, S.Y.; Shen, J.J.; Ren, Z.J. Greenhouse gas emissions associated with urban water infrastructure: What we have learnt from China’s practice. Wiley Interdiscip. Rev. Water 2021, 8, 1529. [Google Scholar] [CrossRef]
- Guo, D.; Li, B.; Yu, W.; Han, J.; Zhou, Y.; Ye, Z.; Wu, X.; Young, B.; Huang, Y. Revisiting China’s domestic greenhouse gas emission from wastewater treatment: A quantitative process life-cycle assessment. Sci. Total Environ. 2023, 876, 162597. [Google Scholar] [CrossRef]
- Shah, A.; Willis, J.; Fillmore, L. Quantifying Methane Evolution from Sewers: Results from WERF/Dekalb phase 2 Continuous Monitoring at Honey Creek Pumping Station and Force Main. In Proceedings of the Water Environment Federation; Water Environment Federation: Alexandria, VA, USA, 2011; pp. 475–485. [Google Scholar]
- Guo, L.; Porro, J.; Sharma, K.R.; Amerlinck, Y.; Benedetti, L.; Nopens, I.; Shaw, A.; Van Hulle, S.W.H.; Yuan, Z.; Vanrolleghem, P.A. Towards a benchmarking tool for minimizing wastewater utility greenhouse gas footprints. Water Sci. Technol. 2012, 66, 2483–2495. [Google Scholar] [CrossRef]
- Sowby, R.B.; Capener, A. Reducing carbon emissions through water conservation: An analysis of 10 major U.S. cities. Energy Nexus 2022, 7, 100094. [Google Scholar] [CrossRef]
- Zib, L.; Byrne, D.M.; Marston, L.T.; Chini, C.M. Operational carbon footprint of the U.S. water and wastewater sector’s energy consumption. J. Clean. Prod. 2021, 321, 128815. [Google Scholar] [CrossRef]
- Eva, R.; Oriol, G.; Philippe, R.; Boutin, C.; Corominas, L. Life cycle assessment of urban wastewater systems: Quantifying the relative contribution of sewer systems. Water Res. 2015, 77, 35–48. [Google Scholar]
- Su, X.; Shao, W.; Liu, J.; Jiang, Y.; Wang, J.; Yang, Z.; Wang, N. How does sponge city construction affect carbon emission from integrated urban drainage system? J. Clean. Prod. 2022, 363, 132595. [Google Scholar] [CrossRef]
- Kyung, D.; Kim, D.; Yi, S.; Choi, W.; Lee, W. Estimation of greenhouse gas emissions from sewer pipeline system. Int. J. Life Cycle Assess. 2017, 22, 1901–1911. [Google Scholar] [CrossRef]
- Clemens, J.; Haas, B. Nitrous oxide emissions in sewer systems. Acta Hydrochim. Hydrobiol. 1997, 25, 96–99. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Zheng, F.F.; Maier, H.R.; Ostfeld, A.; Creaco, E.; Savic, D.; Langeveld, J.; Kapelan, Z. Water quality modeling in sewer networks: Review and future research directions. Water Res. 2021, 202, 117419. [Google Scholar] [CrossRef]
- Guisasola, A.; Sharma, K.R.; Keller, J.; Yuan, Z. Development of a model for assessing methane formation in rising main sewers. Water Res. 2009, 43, 2874–2884. [Google Scholar] [CrossRef]
- Short, M.D.; Daikeler, A.; Wallis, K.; Peirson, W.L.; Peters, G.M. Dissolved methane in the influent of three Australian wastewater treatment plants fed by gravity sewers. Sci. Total Environ. 2017, 599, 85–93. [Google Scholar] [CrossRef]
- Beelen, B.; Parker, W. A probabilistic approach to the quantification of methane generation in sewer networks. J. Environ. Manag. 2022, 320, 115775. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment of China. Third National Communication on Climate Change of the People’s Republic of China; China Environmental: Beijing, China, 2018; pp. 20–22.
- Liu, H.; Jia, Y.; Niu, C. “Sponge city” concept helps solve China’s urban water problems. Environ. Earth Sci. 2017, 76, 473. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Ding, X.; Shao, W.; Mei, C.; Li, Z.; Wang, K. Assessing the mitigation of greenhouse gas emissions from a green infrastructure-based urban drainage system. Appl. Energy 2020, 278, 115686. [Google Scholar] [CrossRef]
| Factor | Effects |
|---|---|
| Temperature | Production of CH4 is higher in summer than in winter [8,13,36]. |
| Flow velocity | Production of CH4 in biofilms in gravity pipelines has an optimum flow rate (water flow shear force ≈ 1.45 Pa) [37]. An increase in the flow rate increases the generation of CH4 in sediments when the flow rate is less than 0.31 m/s [38]. |
| COD | Production of CH4 increases with an increase in sCOD [23,33,39,40]. |
| Biomass | Production of CH4 in biofilms increases with an increase in biomass [37]. |
| Pipeline type | Production of CH4 in pressure pipelines is higher than in gravity pipelines [25,41,42]. |
| HRT and A/V | The concentration of CH4 in solution is positively correlated with HRT and the A/V ratio of the pipeline biofilm [9,10,13,36]. |
| NO3− | Increasing the nitrate concentration inhibits the production of CH4 [35]. |
| SO42− | Production of CH4 decreases with an increase in sulfate concentration (>40 mg S/L) [23,33,40]; however, another study found that changes in sulfate concentration (5–30 mg S/L) did not significantly affect methane production [12,39]. |
| pH | The optimal pH is about 7, and an increase in pH inhibits the production of CH4 [34]. |
| DO | DO reduces CH4 production by affecting the anaerobic environment of microorganisms in sewage. However, studies have shown that DO is completely consumed above the surface biofilm and sediments, so anaerobic conditions are widespread throughout the biofilm and sediment, even if the DO in the water is high [38,43,44]. |
| Factor | Effects |
|---|---|
| Microorganisms | Microorganisms in sediments are the key producers of CH4; deactivated sediments have low production of CH4 [33]. |
| Wastewater | Fresh sediments with no sewage can produce only a small amount of CH4, while the organic matter in sewage significantly increases sediment CH4 emissions [33]. |
| Biomass | The rate of methane generation is relatively insensitive to the concentration of MA in the sediments. Lower concentrations of MA in sediments can lead to deeper penetration of the substrate, allowing the MA in deeper sediments to use the substrate to produce CH4, resulting in relatively small changes in the overall methane production rate [38]. |
| Sediment type | The location of the sediment, the age of a WCS, and the characteristics of the wastewater discharged into a WCS account for the production of sediments with differing physical and biological properties, affecting CH4 production rates [38,45]. |
| Sediment depth | CH4 production in sediments is a surficial process, mainly occurring at depths of 0–2 cm. Due to the limited permeability of fermentable COD, CH4 production in deeper layers of the sediment (2–3.5 cm) is very low, consistent with the distribution of MA in sediments with depth. Therefore, CH4 production is largely unaffected by the total sediment depth since substrate penetration into sediments is relatively shallow, up to a few millimeters [38]. |
| GHG | Source | WCS Location | Literature Data | Literature Results | Emissions in China |
|---|---|---|---|---|---|
| CH4 | [84] | Daejeon, South Korea | pipeline length, 1940 km | 1254 t CH4/y 1 | 525,337 t CH4/y |
| [75] | Xi`an, China | population, 8,705,600 | 7.96 t CH4/d | 495,232 t CH4/y | |
| [11] | Dekalb County, USA | population, 600,000 | 56.86 t CH4/y | 140,623 t CH4/y | |
| [88] | New South Wales, Australia 2 | population | 77.47 gCH4/person·y | 114,957 t CH4/y | |
| [8] | Queensland, Australia 3 | concentration | 4.50 mg/L | 303,806 t CH4/y | |
| [89] | Ontario, Canada | concentration | 2.1–3.0 mg/L | 141,776–202,537 t CH4/y | |
| N2O | [31] | New South Wales, Australia 4 | population | 1.63 gN2O/person·y | 2419 t N2O/y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, D.; Liu, Y.; Zhao, W.; Qiu, S.; Cui, N.; Hu, X.; Zhao, P. Status of Research on Greenhouse Gas Emissions from Wastewater Collection Systems. Water 2023, 15, 2512. https://doi.org/10.3390/w15142512
Gu D, Liu Y, Zhao W, Qiu S, Cui N, Hu X, Zhao P. Status of Research on Greenhouse Gas Emissions from Wastewater Collection Systems. Water. 2023; 15(14):2512. https://doi.org/10.3390/w15142512
Chicago/Turabian StyleGu, Dongmei, Yiwen Liu, Weigao Zhao, Shuntian Qiu, Nuo Cui, Xinyue Hu, and Peng Zhao. 2023. "Status of Research on Greenhouse Gas Emissions from Wastewater Collection Systems" Water 15, no. 14: 2512. https://doi.org/10.3390/w15142512
APA StyleGu, D., Liu, Y., Zhao, W., Qiu, S., Cui, N., Hu, X., & Zhao, P. (2023). Status of Research on Greenhouse Gas Emissions from Wastewater Collection Systems. Water, 15(14), 2512. https://doi.org/10.3390/w15142512









