Vicia faba Plant Suitability Assessment for Genotoxicity, Cytotoxicity, and Mutagenicity Testing of Pharmaceutical-Containing Wastewater
Abstract
:1. Introduction
- chromosomal aberrations
- changes in the mitotic index,
- presence of micronuclei.
2. Materials and Methods
2.1. Wastewater Characterization
2.1.1. Reagents
2.1.2. Constructed Wetlands
2.1.3. Chemical Analysis of Wastewater
2.2. Vicia faba Experiment
2.2.1. Germination of Vicia faba Seeds
2.2.2. Micronucleus Assay
- The Mitotic Index (MI) was the number of cells in the dividing process to the total number of observed cells;
- The Micronuclei Index (MN) was the number of cells with micronuclei to the total number of observed cells;
- The number of Chromosomal Aberrations (CA) to the total number of observed cells;
2.3. Antioxidant Enzyme Activity
2.4. Statistical Analysis
3. Results
3.1. Genotoxicity Tests toward Vicia faba
3.2. Activity of Catalase and Superoxide Dismutase
4. Discussion
- Genomic Proximity: The presence of large chromosomes and proximity to cell division stages enables accurate genotoxicity assessment.
- Quantitative Data: The assay delivers quantitative data on the micronucleus frequency and chromosome abnormalities.
- Visual Assessment: Effects on root growth and chromosome structure are readily observable, simplifying result interpretation.
- Simplicity and Accessibility: The broad bean root tip assay is relatively straightforward, requiring no specialized equipment or extensive training. Additionally, the consistent tissue of the broad bean root tip simplifies the maintenance and preparation of microscope slides.
- Disadvantage:
- Time-consuming: The assay takes longer to produce results due to the specific cell cycle stages Vicia faba goes through.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, M.I.; Muscolo, A.; Farooq, M.; Ahmad, W. Sustainable use and management of non-conventional water resources for rehabilitation of marginal lands in arid and semiarid environments. Agric. Water Manag. 2019, 221, 462–476. [Google Scholar] [CrossRef]
- Hashem, M.S.; Qi, X. Treated Wastewater Irrigation—A Review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y. Wastewater irrigation: Past, present, and future. Wiley Interdiscip. Rev. Water 2019, 6, e1234. [Google Scholar] [CrossRef]
- Singh, A. A review of wastewater irrigation: Environmental implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, H.; Yin, S.; Sun, L.; Lu, H. Unraveling the toxicity associated with ciprofloxacin biodegradation in biological wastewater treatment. Environ. Sci. Technol. 2022, 56, 15941–15952. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lei, D.; Zhao, X.; Hu, Y.; Yao, S.; Lin, K.; Wang, Z.; Cui, C. Antibiotic residue and toxicity assessment of wastewater during the pharmaceutical production processes. Chemosphere 2022, 291, 132837. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Mackay, N.; Habig, C.; Griffin, J. An ecological risk assessment for triclosan in lotic systems following discharge from wastewater treatment plants in the United States. Environ. Toxicol. Chem. 2022, 21, 2483–2492. [Google Scholar] [CrossRef]
- Hong, B.; Li, Q.; Li, J.; Zhou, M.; Wang, X.; He, B.; Yu, S. Spectrum of pharmaceutical residues in commercial manure-based organic fertilizers from multi-provinces of China mainland in relation to animal farming and possible environmental risks of fertilization. Sci. Total Environ. 2023, 894, 165029. [Google Scholar] [CrossRef]
- Bastos, M.C.; Soubrand, M.; Le Guet, T.; Le Floch, E.; Joussein, E.; Baudu, M.; Casellas, M. Occurrence, fate and environmental risk assessment of pharmaceutical compounds in soils amended with organic wastes. Geoderma 2020, 375, 114498. [Google Scholar] [CrossRef]
- Al-Farsi, R.S.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: A review. Emerg. Contam. 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.M.; Zia, F.; Bakhat, H.F.; Fahad, S.; Ashraf, M.R.; Wilkerson, C.J.; Shah, G.M.; Nasim, W.; Khosa, I.; Shahid, M. Uptake and toxicological effects of pharmaceutical active compounds on maize. Agric. Ecosyst. Environ. 2018, 258, 143–148. [Google Scholar] [CrossRef]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: Concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef]
- Verlicchi, P.; Lacasa, E.; Grillini, V. Quantitative and qualitative approaches for CEC prioritization when reusing reclaimed water for irrigation needs—A critical review. Sci. Total Environ. 2023, 900, 165735. [Google Scholar] [CrossRef]
- de Souza, C.P.; Guedes, T.A.; Fontanetti, C.S. Evaluation of herbicides action on plant bioindicators by genetic biomarkers: A review. Environ. Monit. Assess. 2016, 188, 694. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef]
- Dantas, R.F.; Contreras, S.; Sans, C.; Esplugas, S. Sulfamethoxazole abatement by means of ozonation. J. Hazard. Mater. 2008, 150, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Sirés, I.; Oturan, N.; Otyran, M.A. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Minato, Y.; Dawadi, S.; Kordus, S.L.; Sivanandam, A.; Aldrich, C.C.; Baughn, A.D. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Drzymała, J.; Kalka, J. Ecotoxic interactions between pharmaceuticals in mixtures: Diclofenac and sulfamethoxazole. Chemosphere 2020, 259, 127407. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.; Lin, A.Y.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Nopens, I.; Capalozza, C.; Vanrolleghem, P.A. Technical Report: Stability Analysis of a Synthetic Municipal Wastewater; Universiteit Gent: Gent, Belgium, 2001. [Google Scholar]
- Drzymała, J.; Kalka, J.; Sochacki, A.; Felis, E. Towards Sustainable Wastewater Treatment: Bioindication as a Technique for Supporting Treatment Efficiency Assessment. Int. J. Environ. Res. Public Health 2022, 19, 11859. [Google Scholar] [CrossRef]
- ISO 29200:2013; Soil Quality—Assessment of Genotoxic Effects on Higher Plants—Vicia faba Micronucleus Test. ISO: Geneva, Switzerland, 2013.
- OECD. est No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei). In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–152. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zgórska, A.; Borgulat, A. Genotoxicity of wastewater samples from the textile industry detected by broad bean (Vicia faba) micronucleus test assay. Appl. Ecol. Environ. Res. 2020, 18, 5315–5323. [Google Scholar] [CrossRef]
- Oubane, M.; Khadra, A.; Ezzariai, A.; El Fels, L.; Kouisni, L.; Hafidi, M. Micronucleus assay based on Vicia faba roots as a tool to assess the performances of wastewater treatment systems. Environ. Technol. Innov. 2020, 19, 100903. [Google Scholar] [CrossRef]
- Mancini, L.; Lacchetti, I.; Caciolli, S.; Puccinelli, C.; D’Angelo, A.M.; Marchini, S.; Giuseppetti, R.; Pierdominici, E.; Marcheggiani, S.; Carere, M. Wastewater reuse in the industry: An eco-genotoxicological approach. FEB-Fresenius Environ. Bull. 2019, 28, 4974–4978. [Google Scholar]
- Kwaśniewska, J.; Bara, A.W. Plant Cytogenetics in the Micronuclei Investigation—The Past, Current Status, and Perspectives. Int. J. Mol. Sci. 2022, 23, 1306. [Google Scholar] [CrossRef]
- Drzymała, J.; Kalka, J. Genotoxicity, mutagenicity, and cytotoxicity assessment of diclofenac and sulfamethoxazole in environmental concentrations toward Vicia faba. Ecotoxicology 2023, accepted. [Google Scholar]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Kookana, R.S.; Boxall, A.B. Fate and uptake of pharmaceuticals in soil-plant systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Wuu, K.D.; Grant, W.F. Morphological and somatic chromosomal aberration and induced by pesticides in barley (Hordeum vulgare). Can. J. Genet. Cytol. 1966, 8, 481–501. [Google Scholar] [CrossRef]
- Bolle, P.; Mastrangelo, S.; Tucci, P.; Evandri, M.G. Clastogenicity of atrazine assessed with the Allium cepa test. Environ. Mol. Mutagen. 2004, 43, 137–141. [Google Scholar] [CrossRef]
- Srivastava, K.; Mishra, K. Cytogenetic effects of commercially formulated atrazine on the somatic cells of Allium cepa and Vicia faba. Pestic. Biochem. Physiol. 2009, 93, 8–12. [Google Scholar] [CrossRef]
- Wu, S.; Wu, E.; Qiu, L.; Zhong, W.; Chen, J. Effects of phenanthrene on the mortality, growth, and anti-oxidant system of earthworms (Eisenia fetida) under laboratory conditions. Chemosphere 2011, 83, 429–434. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, N. Defense signaling in plants against micro-creatures: Do or die. Indian Phytopathol. 2020, 73, 605–613. [Google Scholar] [CrossRef]
- Fatima, R.A.; Ahmad, M. Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci. Total Environ. 2005, 346, 256–273. [Google Scholar] [CrossRef]
- Cai, H.; Liang, J.; Ning, X.A.; Lai, X.; Li, Y. Algal toxicity induced by effluents from textile-dyeing wastewater treatment plants. J. Environ. Sci. 2020, 91, 199–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Zhou, J.; Masunaga, S.; Ma, F. Reduction in toxicity of wastewater from three wastewater treatment plants to alga (Scenedesmus obliquus) in northeast China. Ecotoxicol. Environ. Saf. 2015, 119, 132–139. [Google Scholar] [CrossRef] [PubMed]



| Tested Compound | Formula | CAS Number | Molar Mass, g mol−1 | pKa | logKOW, pH 8 |
|---|---|---|---|---|---|
| DCF | C14H11Cl2NO2 | 15307-86-5 | 296.15 | 4.15 | 4.51 |
| SMX | C10H11N3O3S | 723-46-6 | 253.28 | 5.6–5.7 | 0.89 |
| Columns Description | Frequency of Wastewater Dosing | Presence of DCF and SMX | Presence of M. giganteus | |
|---|---|---|---|---|
| Rack 1 | R1-CTRL | 2 times a week in a volume of 2.5 L, HLR 1 = 80 L d−1 m−2 | – | + |
| R1-PhC | + | |||
| Rack 2 | R2-CTRL | 5 times a week in a volume of 1.0 L, HLR = 32 L d−1 m−2 | – | + |
| R2-PhC | + | |||
| Types of Columns | Removal Efficiency, R % | |||
|---|---|---|---|---|
| TOC | N-NH4 | DCF | SMX | |
| R1-CTRL | 87.8 ± 3.9 | 19.9 ± 7.3 | ||
| R1-PhC | 87.3 ± 1.9 | 18.0 ± 11.4 | 68.8 ± 8.2 | 79.1 ± 4.3 |
| R2-CTRL | 93.3 ± 1.4 | 45.8 ± 11.9 | ||
| R2-PhC | 92.3 ± 1.1 | 58.9 ± 10.0 | 86.8 ± 9.7 | 98.0 ± 0.8 |
| Samples | Hydroponic Culture | Soil Culture | ||
|---|---|---|---|---|
| MI, % | dMI, % | MI, % | dMI, % | |
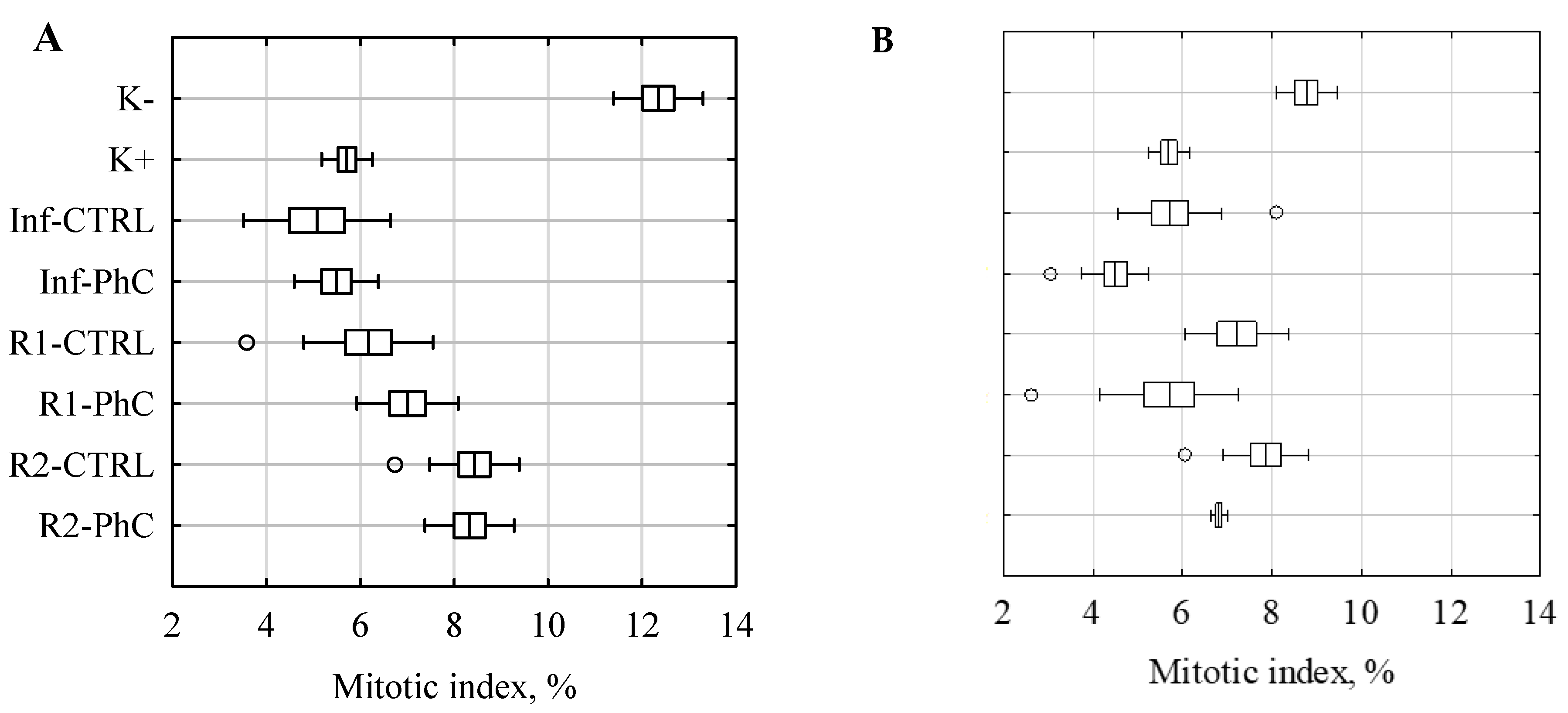
| Negative control (K−) | 12.3 ± 1.0 abd | - | 8.8 ± 0.7 abd | - |
| Positive control (K+) | 5.7 ± 0.5 ab | 53.7 | 5.7 ± 0.5 ab | 35.0 |
| Inf-CTRL | 5.1 ± 1.6 ac | 58.9 | 5.7 ± 1.2 ac | 34.9 |
| Inf-PhC | 5.5 ± 0.9 ac | 55.5 | 4.5 ± 0.7 abc | 48.7 |
| R1-CTRL | 6.2 ± 1.4 ae | 50.0 | 7.2 ± 1.2 abcef | 17.8 |
| R1-PhC | 7.0 ± 1.1 abcde | 43.2 | 5.7 ± 1.6 acdef | 25.0 |
| R2-CTRL | 8.4 ± 1.0 abce | 31.8 | 7.9 ± 0.9 abcef | 10.4 |
| R2-PhC | 8.3 ± 1.0 abcde | 32.5 | 6.8 ± 0.2 abcdef | 22.2 |
| Samples | Hydroponic Culture | Soil Culture |
|---|---|---|
| Micronuclei, ‰ | ||
| K− | 0.00 ± 0.00 ab | 0.00 ± 0.00 ab |
| K+ | 0.77 ± 0.29 abd | 0.14 ± 0.07 abd |
| Inf-CTRL | 0.01 ± 0.02 b | 0.00 ± 0.00 b |
| Inf-PhC | 0.01 ± 0.02 b | 0.00 ± 0.01 b |
| R1-CTRL | 0.00 ± 0.00 b | 0.00 ± 0.01 b |
| R1-PhC | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| R2-CTRL | 0.00 ± 0.01 b | 0.00 ± 0.00 b |
| R2-PhC | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Samples | Hydroponic Culture | Soil Culture |
|---|---|---|
| Chromosomal Aberrations, ‰ | ||
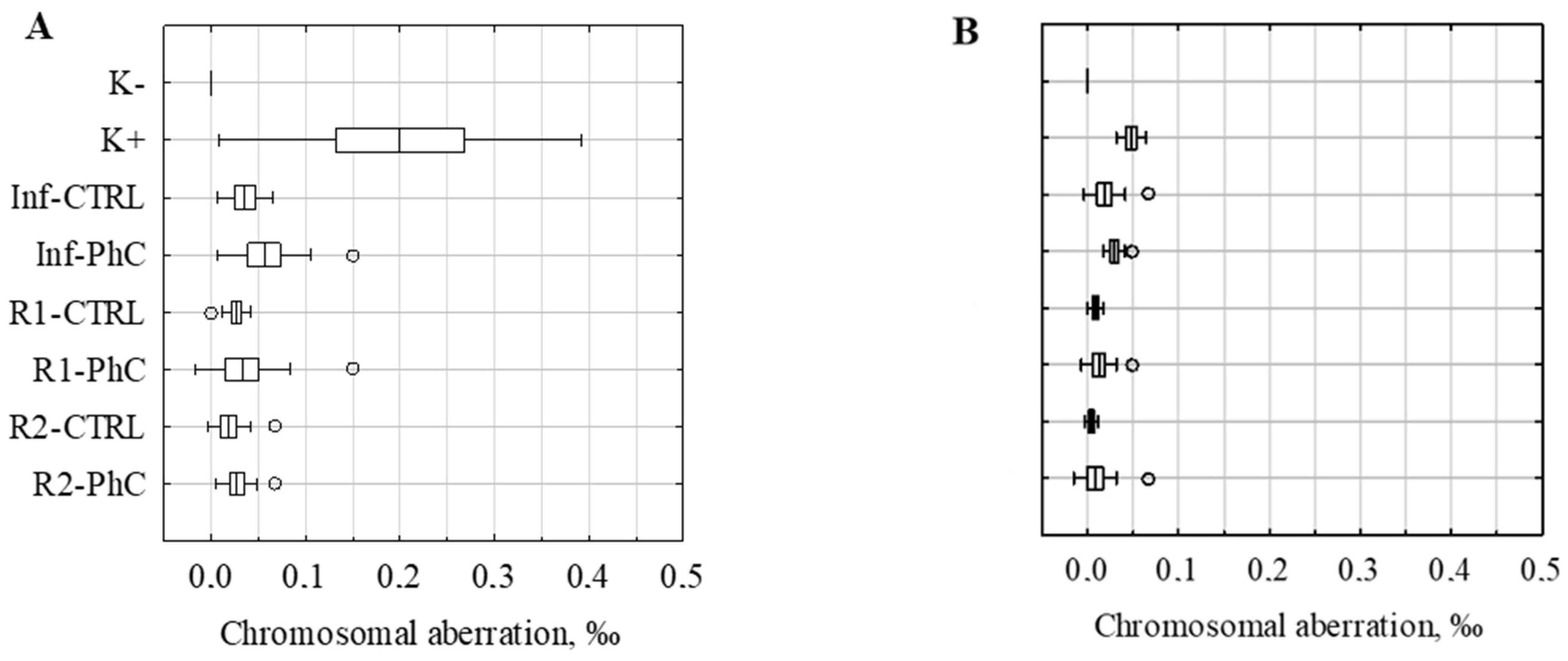
| K− | 0.00 ± 0.00 ab | 0.00 ± 0.00 ab |
| K+ | 0.20 ± 0.19 abd | 0.05 ± 0.02 abd |
| Inf-CTRL | 0.04 ± 0.03 ab | 0.02 ± 0.02 abc |
| Inf-PhC | 0.06 ± 0.05 a | 0.03 ± 0.01 abc |
| R1-CTRL | 0.03 ± 0.02 abd | 0.01 ± 0.01 bd |
| R1-PhC | 0.03 ± 0.05 ab | 0.01 ± 0.02 b |
| R2-CTRL | 0.02 ± 0.02 ab | 0.00 ± 0.01 b |
| R2-PhC | 0.03 ± 0.02 abd | 0.01 ± 0.02 bcd |
| Samples | Hydroponic Culture | Soil Culture | ||
|---|---|---|---|---|
| Catalase, µmol H2O2 min−1mg Protein−1 | iCAT, % | Catalase, µmol H2O2 min−1mg Protein−1 | iCAT, % | |
| K− | 11.8 ± 0.9 ab | - | 9.9 ± 2.9 ab | - |
| K+ | 18.9 ± 2.9 ab | 60.5 | 15.8 ± 2.6 ab | 60.1 |
| Inf-CTRL | 17.3 ± 2.5 ac | 47.4 | 16.9 ± 1.4 ac | 71.3 |
| Inf-PhC | 19.4 ± 1.4 ac | 65.0 | 18.2 ± 0.9 ac | 84.3 |
| R1-CTRL | 13.1 ± 3.8 bc | 11.6 | 12.5 ± 3.2 bc | 26.3 |
| R1-PhC | 14.9 ± 1.7 bc | 26.8 | 13.3 ± 3.8 bc | 34.8 |
| R2-CTRL | 12.8 ± 2.3 bc | 8.9 | 11.1 ± 2.0 bc | 12.0 |
| R2-PhC | 14.1 ± 3.0 bc | 19.8 | 11.5 ± 2.3 bc | 16.5 |
| Samples | Hydroponic Culture | Soil Culture | ||
|---|---|---|---|---|
| Superoxide Dismutase, Umin−1mg Protein−1 | iSOD, % | Superoxide Dismutase, Umin−1mg Protein−1 | iSOD, % | |
| K− | 1.1 ±0.2 ab | - | 1.2 ± 0.0 ab | - |
| K+ | 2.2 ± 0.2 ab | 106.6 | 2.0 ± 0.3 ab | 66.2 |
| Inf-CTRL | 3.3 ± 0.8 abcd | 211.8 | 2.5 ± 0.8 acd | 109.6 |
| Inf-PhC | 3.9 ± 0.6 abcd | 267.3 | 3.0 ± 1.0 abcd | 156.5 |
| R1-CTRL | 1.5 ± 0.2 abcf | 38.2 | 1.5 ± 0.3 bc | 23.7 |
| R1-PhC | 1.8 ± 0.1 abcef | 70.8 | 1.6 ± 0.5 c | 32.1 |
| R2-CTRL | 1.4 ± 0.2 abcf | 29.9 | 1.3 ± 0.4 bc | 10.9 |
| R2-PhC | 1.6 ± 0.2 abcef | 53.4 | 1.5 ± 0.5 c | 23.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalka, J.; Drzymała, J. Vicia faba Plant Suitability Assessment for Genotoxicity, Cytotoxicity, and Mutagenicity Testing of Pharmaceutical-Containing Wastewater. Water 2023, 15, 3044. https://doi.org/10.3390/w15173044
Kalka J, Drzymała J. Vicia faba Plant Suitability Assessment for Genotoxicity, Cytotoxicity, and Mutagenicity Testing of Pharmaceutical-Containing Wastewater. Water. 2023; 15(17):3044. https://doi.org/10.3390/w15173044
Chicago/Turabian StyleKalka, Joanna, and Justyna Drzymała. 2023. "Vicia faba Plant Suitability Assessment for Genotoxicity, Cytotoxicity, and Mutagenicity Testing of Pharmaceutical-Containing Wastewater" Water 15, no. 17: 3044. https://doi.org/10.3390/w15173044
APA StyleKalka, J., & Drzymała, J. (2023). Vicia faba Plant Suitability Assessment for Genotoxicity, Cytotoxicity, and Mutagenicity Testing of Pharmaceutical-Containing Wastewater. Water, 15(17), 3044. https://doi.org/10.3390/w15173044









