Investigation of Water Dynamics Nearby Hydroelectric Power Plant of the Gorky Reservoir on Water Environment: Case Study of 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. HPP Operation Mode
2.3. Field and Laboratory Measurements
2.3.1. Meteorology
2.3.2. Hydrophysical Parameters
2.3.3. Hydrobiological Parameters
- (a)
- Phytoplankton
- (b)
- Zooplankton
2.3.4. Hydrochemical Indicators
2.3.5. Bottom Sediments
2.3.6. Concentrations and Fluxes of Methane
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Multi-Scale Variations in Water Discharge
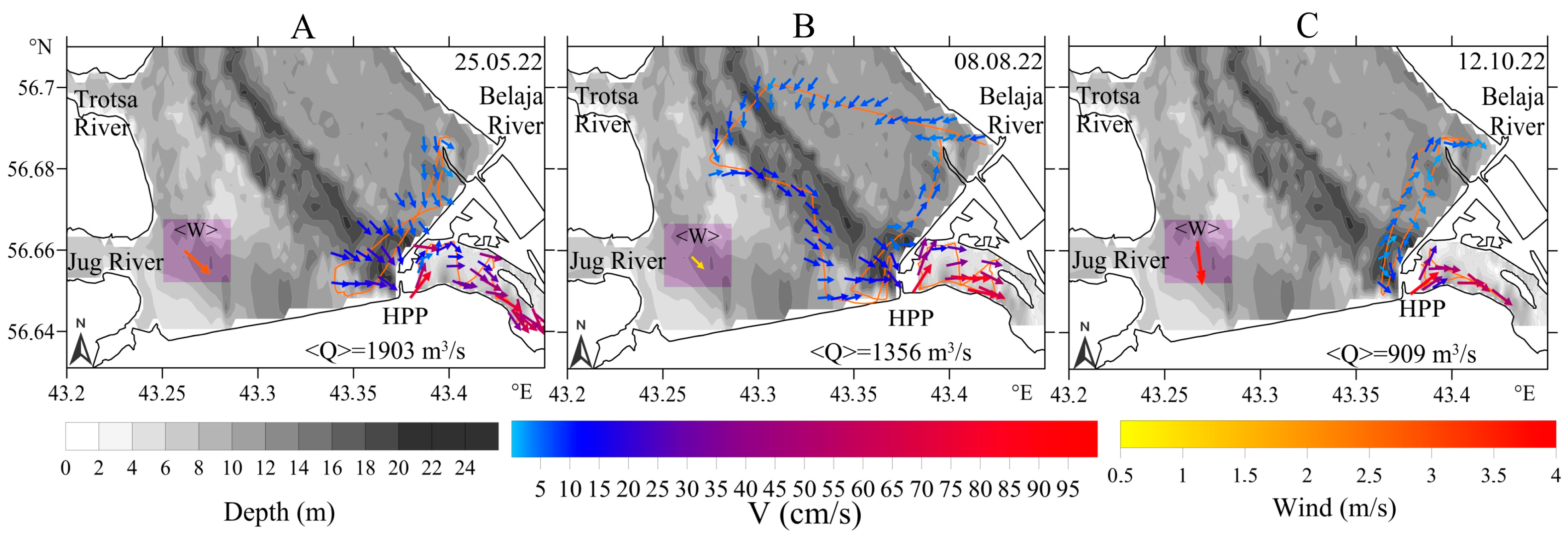
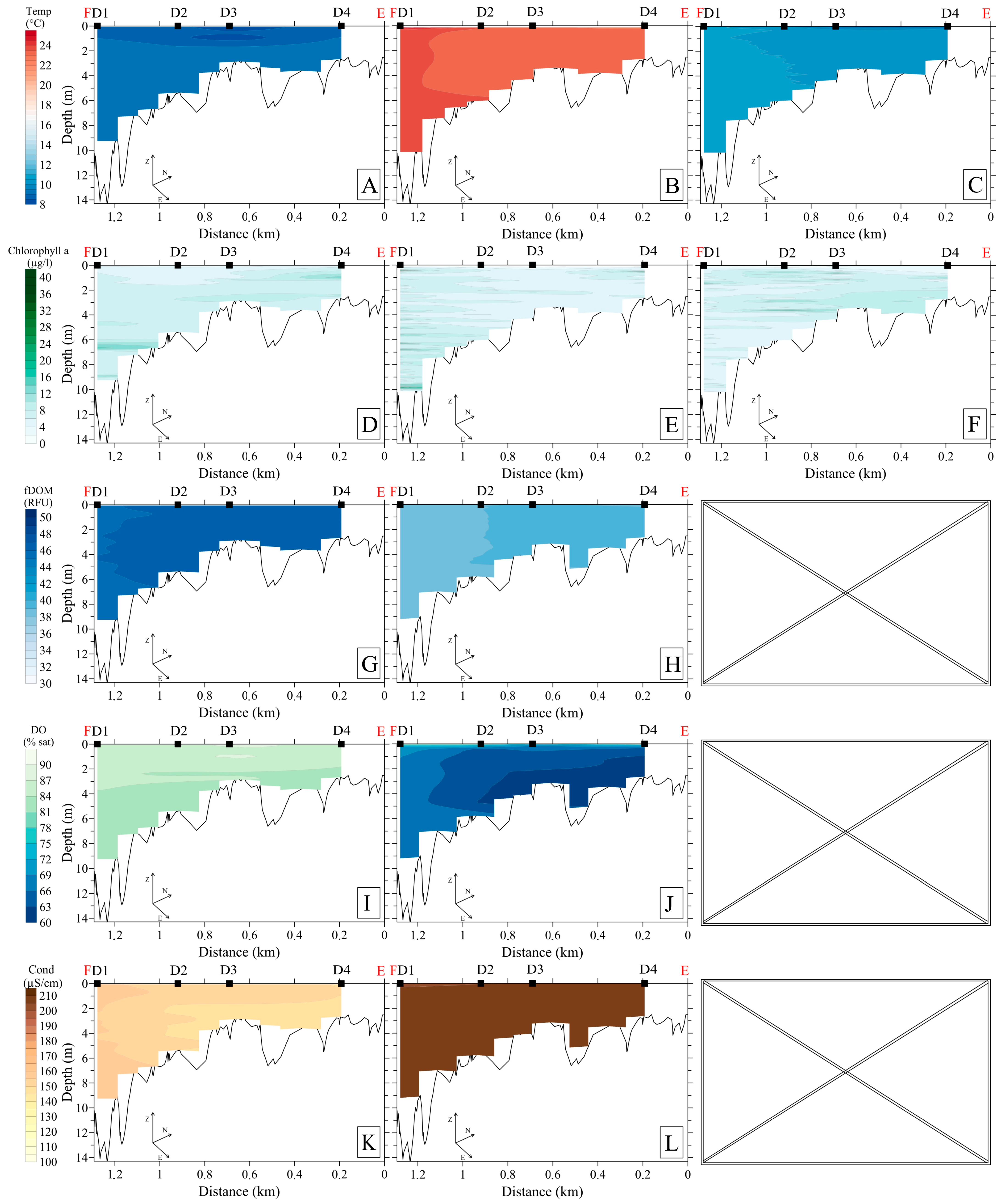
3.2. Hydrophysical Parameters
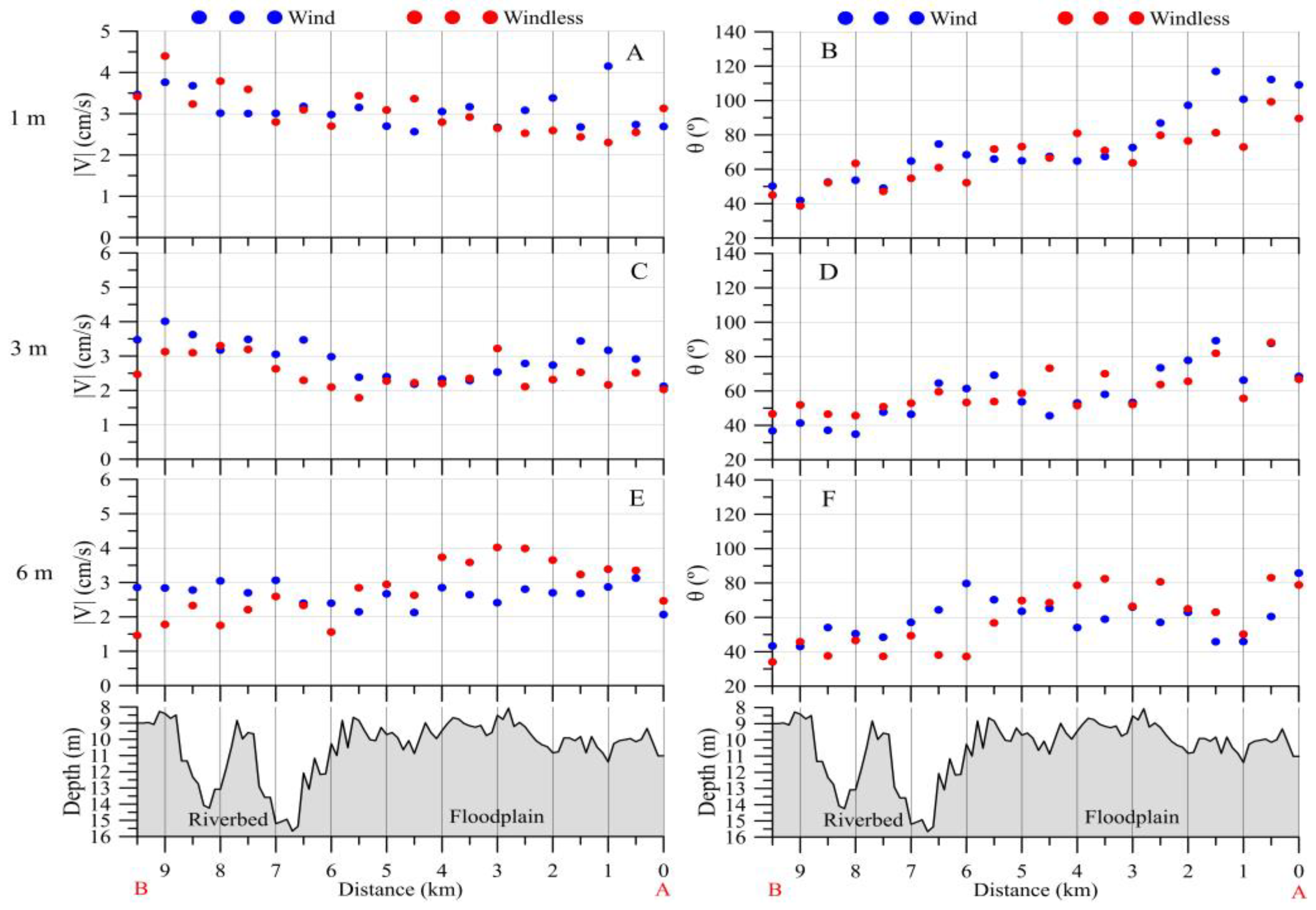
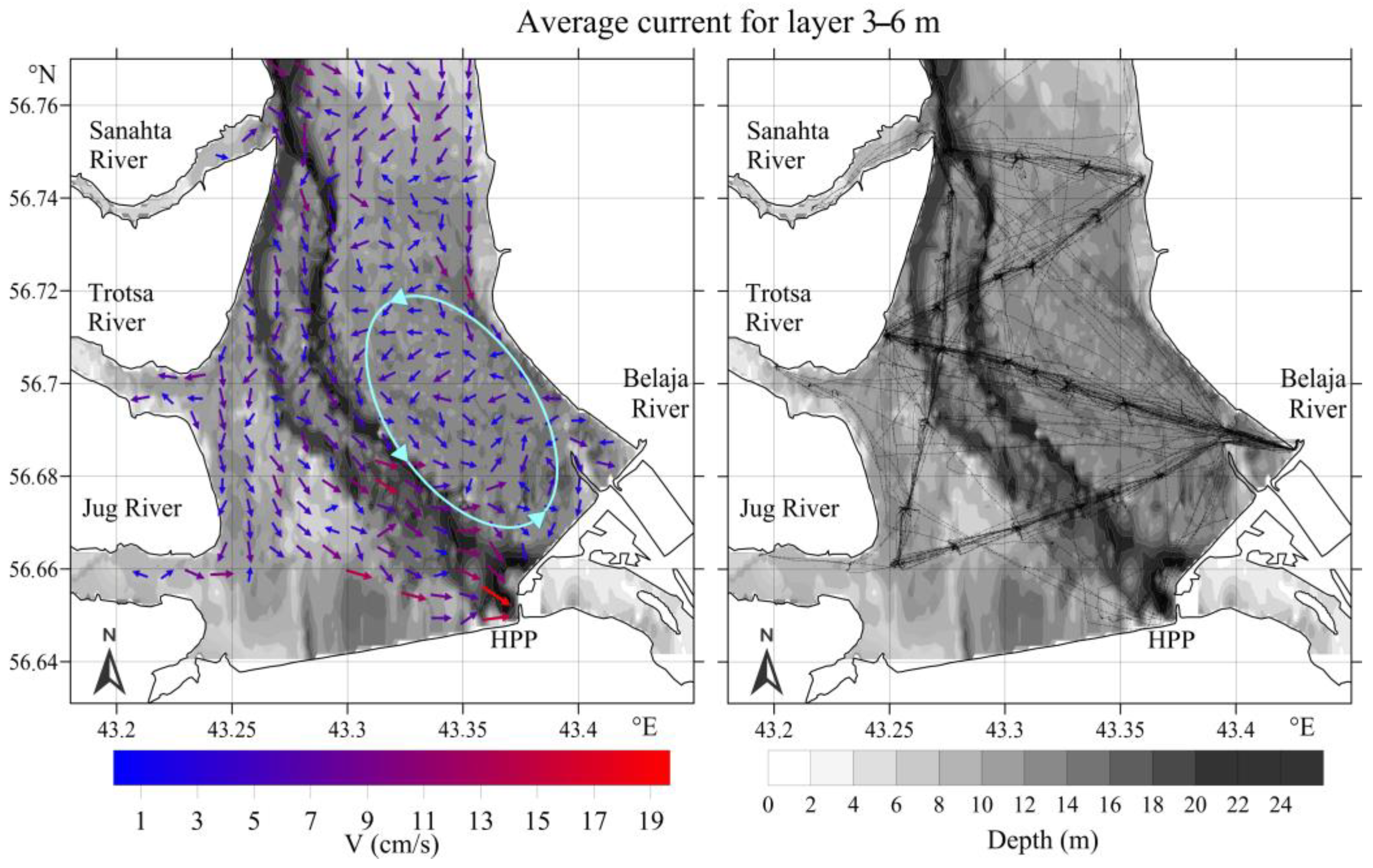
3.2.1. Upstream Area
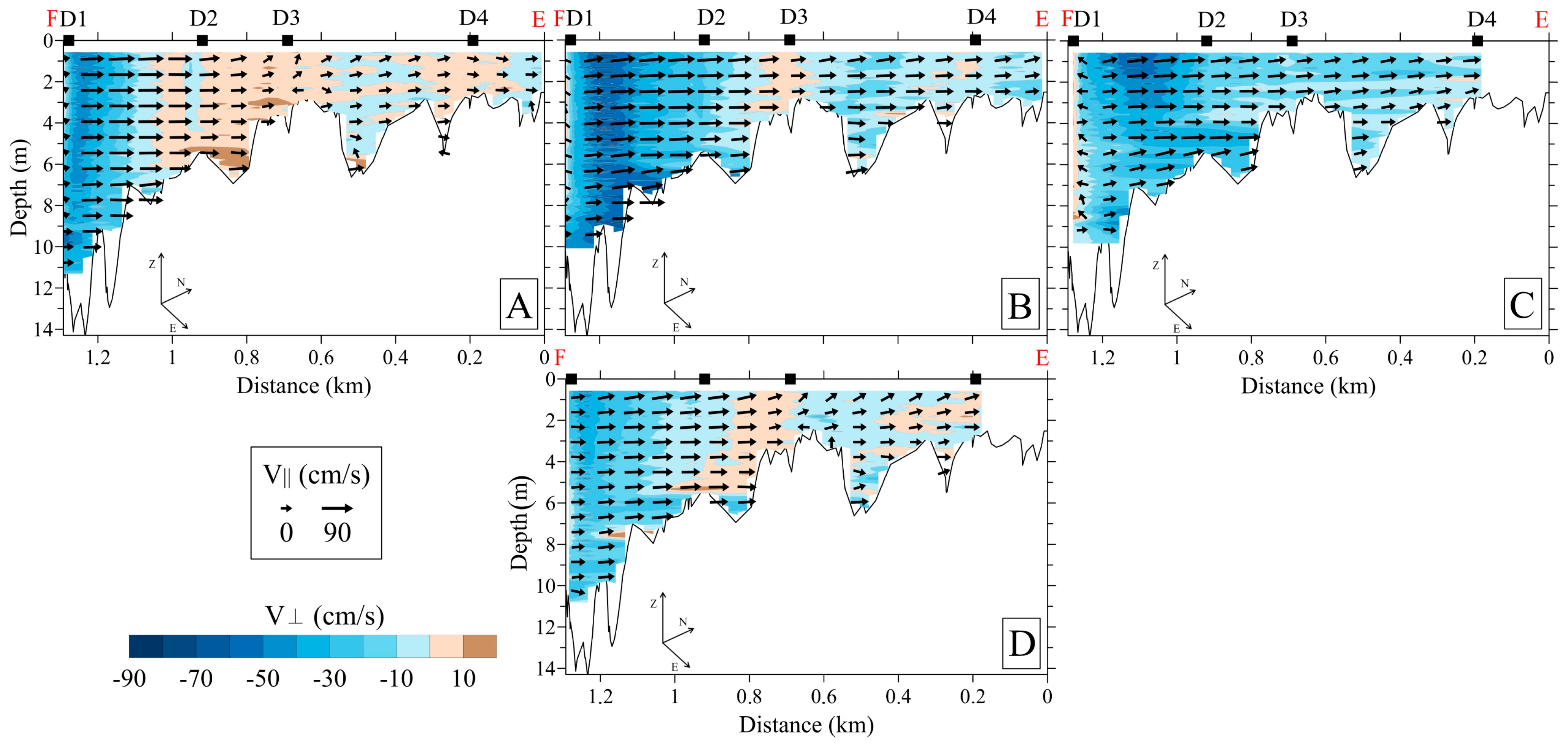
3.2.2. Downstream Area
- (a)
- 800–1000 m3/s: pronounced whirlpool, return current velocity over the floodplain is higher than the velocity over the channel (on average, 8 cm/s and 5 cm/s, respectively);
- (b)
- 3000–3500 m3/s: pronounced channel flow, the current is directed towards the HPP, velocity over the channel is 1.5 times higher than the velocity over the floodplain;
- (c)
- 1000–3000 m3/s: transitional regime: the current velocity increases on the Volga channel, but velocity decreases with increasing water discharge and turns towards the HPP on the floodplain
3.3. Hydrobiology
3.3.1. Phytoplankton
3.3.2. Zooplankton
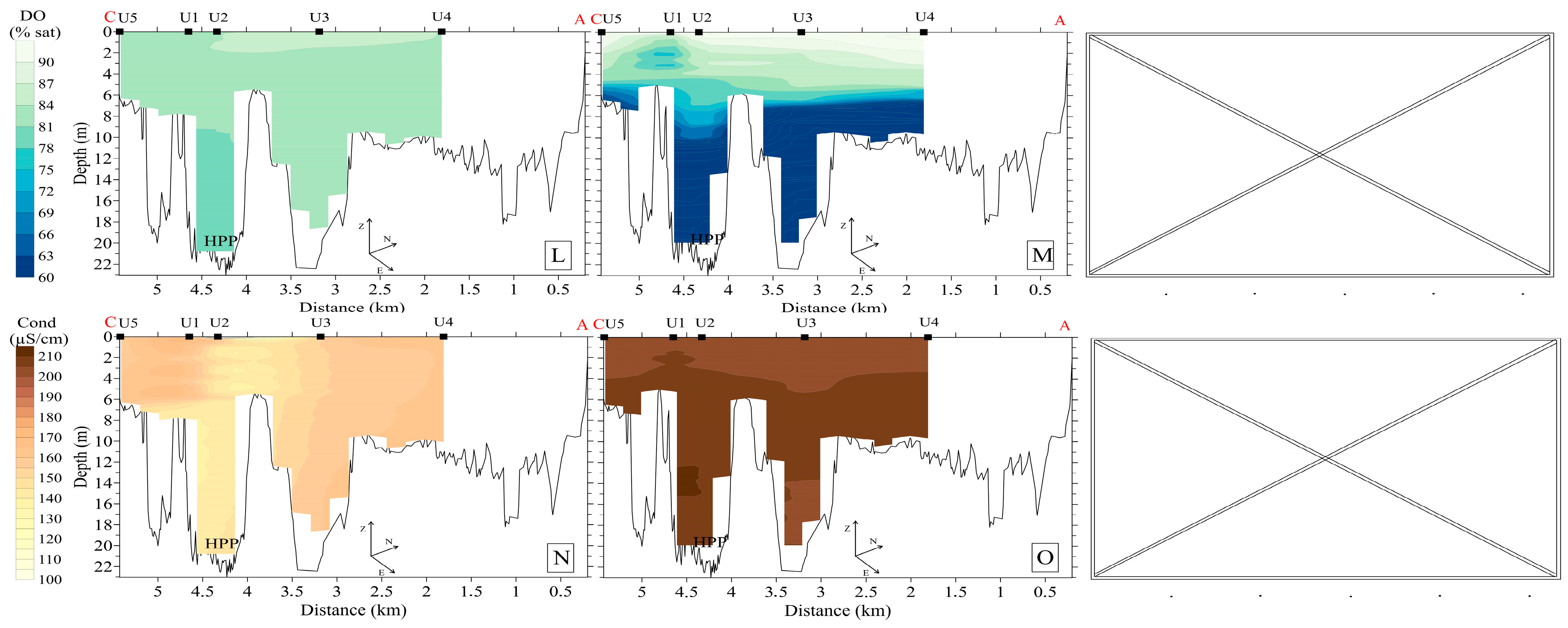
3.4. Hydrochemistry
3.5. Bottom Sediments, Benthos, and Specific Methane Flux
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savichev, O.G.; Krasnoshchèv, S.Y.; Nalivaiko, N.G. River Flow Regulation; Tomsk Polytechnic University: Tomsk, Russia, 2009; p. 114. [Google Scholar]
- Jorgensen, S.E.; Loffler, H.; Rast, W.; Straskraba, M. Lake and Reservoir Management, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; p. 512. [Google Scholar]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and Flow Regulation of the World’s Large River Systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef]
- Palmer, M.; Ruhi, A. Linkages between flow regime, biota, and ecosystem processes: Implications for river restoration. Science 2019, 365, eaaw2087. [Google Scholar] [CrossRef]
- Edelstein, K.K. Reservoirs of Russia: Ecological Problems, Ways of Their Solution; GEOS Publishers: Moscow, Russia, 1998; p. 277. [Google Scholar]
- Goldenfum, J.A. Challenges and solutions for assessing the impact of freshwater reservoirs on natural GHG emissions. Ecohydrol. Hydrobiol. 2012, 12, 115–122. [Google Scholar] [CrossRef]
- De Faria, F.A.; Jaramillo, P.; Sawakuchi, H.O.; Richey, J.E.; Barros, N. Estimating greenhouse gas emissions from future Amazonian hydroelectric reservoirs. Environ. Res. Lett. 2015, 10, 124019. [Google Scholar] [CrossRef]
- Whiting, G.J.; Chanton, J.P. Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration. Tellus B 2001, 53, 521–528. [Google Scholar] [CrossRef]
- Prairie, Y.T.; Alm, J.; Beaulieu, J.; Barros, N.; Battin, T.; Cole, J.; del Giorgio, P.; DelSontro, T.; Guérin, F.; Harby, A.; et al. Greenhouse gas emissions from freshwater reservoirs: What does the atmosphere see? Ecosystems 2018, 21, 1058–1071. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Yin, L.; Zheng, W. Statistical analysis of regional air temperature characteristics before and after dam construction. Urban Clim. 2022, 41, 101085. [Google Scholar] [CrossRef]
- Iakunin, M.; Abreu, E.F.; Canhoto, P.; Pereira, S.; Salgado, R. Impact of a large artificial lake on regional climate: A typical meteorological year Meso-NH simulation results. Int. J. Climatol. 2022, 42, 1231–1252. [Google Scholar] [CrossRef]
- Karadžić, V.; Subakov-Simić, G.; Krizmanić, J.; Natić, D. Phytoplankton and eutrophication development in the water supply reservoirs Garaši and Bukulja (Serbia). Desalination 2010, 255, 91–96. [Google Scholar] [CrossRef]
- Korneva, L.G. Phytoplankton of Volga River Basin Reservoirs; Kostroma Printing House: Kostroma, Russia, 2015; p. 284. [Google Scholar]
- Mineeva, N.; Lazareva, V.; Livinov, A.; Stepanova, I.; Chuiko, G.; Papchenkov, V.; Korneva, L.; Shcherbina, G.; Pryanichnikova, E.; Perova, S.; et al. Chapter 2. The Volga River. In Rivers of Europe, 2nd ed.; Tockner, K., Zarfl, C., Robinson, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–79. [Google Scholar]
- Genkal, S.I.; Okhapkin, A.G.; Vodeneeva, E.L. Planktonic Centric Diatoms (Bacillariophyta, Coscinodiscaceae) of the Cheboksary Reservoir, Russia. Inland Water Biol. 2022, 15, 750–759. [Google Scholar] [CrossRef]
- Molkov, A.; Fedorov, S.; Pelevin, V. Toward Atmospheric Correction Algorithms for Sentinel-3/OLCI Images of Productive Waters. Remote Sens. 2022, 14, 3663. [Google Scholar] [CrossRef]
- Dobrokhotova, D.V.; Kapustin, I.A.; Molkov, A.A.; Leshchev, G.V. A study of the effect of hydropower operation regime on the redistribution of phytoplankton in the upper water layer in the dam section of the Gorki Reservoir. Sovrem. Probl. Distantsionnogo Zondirovaniya Zemli Iz Kosmosa 2023, 20, 242–252. [Google Scholar] [CrossRef]
- Litvinov, A.C. About measurement of currents in reservoirs by EPV-2r recorders. Proc. Inst. Biol. Inland Waters USSR Acad. Sci. 1968, 16, 259–268. [Google Scholar]
- Hydrometeorological Regime of Lakes and Reservoirs of the USSR. Reservoirs of the Upper Volga; Gidrometeoizdat: Leningrad, Russia, 1975; p. 292.
- Kapustin, I.A.; Molkov, A.A. Structure of currents and depth in the lake part of the Gorky Reservoir. Russ. Meteorol. Hydrol. 2019, 7, 110–117. [Google Scholar]
- Kapustin, I.A.; Ermakov, S.A.; Smirnova, M.V.; Vostryakova, D.V.; Molkov, A.A.; Cheban, E.Y.; Leshchev, G.V. On the formation of an isolated lens of a river runoff by a whirlpool in the Gorky reservoir. Sovrem. Probl. Distantsionnogo Zondirovaniya Zemli Iz Kosmosa 2021, 18, 214–221. [Google Scholar] [CrossRef]
- Kapustin, I.A.; Vostryakova, D.V.; Molkov, A.A.; Danilicheva, O.A.; Leshchev, G.V.; Ermakov, S.A. Field observations of convergent currents in the near-surface layer of water using foam patterns in quasi-synchronous satellite optical images. Sovrem. Probl. Distantsionnogo Zondirovaniya Zemli Iz Kosmosa 2021, 18, 188–196. [Google Scholar] [CrossRef]
- Schlezinger, D.R.; Taylor, C.D.; Howes, B.L. Assessment of zooplankton injury and mortality associated with underwater turbines for tidal energy production. Mar. Technol. Soc. J. 2013, 47, 142. [Google Scholar] [CrossRef]
- Bickel, S.L.; Hammond, J.D.M.; Tang, K.W. Boat-generated turbulence as a potential source of mortality among copepods. J. Exp. Mar. Biol. Ecol. 2011, 401, 105. [Google Scholar] [CrossRef]
- Dubovskaya, O.P. Natural Mortality of Zooplankton in the Reservoirs of the Yenisei Basin; Author’s Abstract of Ph.D. in Biological Sciences; The Zoological Institute RAS: St. Petersburg, Russia, 2006. [Google Scholar]
- Zakonnov, V.V.; Zakonnova, A.V.; Tsvetkov, A.I.; Sherysheva, N.G. Hydrodynamic processes and their role in formation of bottom sediments in reservoirs of the Volga—Kama cascade. Trans. IBIW RAS 2018, 81, 35–46. [Google Scholar]
- Labay, V.S. Benthos distribution in the lower rithral of the poronai river under the impact of some abiotic environmental factors. Trans. “SakhNIRO 2007, 9, 184–206. [Google Scholar]
- Kemenes, A.; Melack, J.; Forsberg, B. Downstream emissions of CH4 and CO2 from hydroelectric reservoirs(Tucuruí, Samuel, and Curuá-Una) in the Amazon basin. Columbia Inland Waters 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Butorin, N.V.; Ziminova, N.A.; Kurdin, V.P. Bottom Sediments of Upper Volga Reservoirs; Nauka: Leningrad, Russia, 1975; p. 157. [Google Scholar]
- RusHydro. Hydroelectric Power Station Reservoir Levels. Available online: https://rushydro.ru/informer/ (accessed on 14 July 2023).
- Weather Archive in the Volga Hydro-Meteorological Observatory. Available online: https://rp5.ru/%D0%90%D1%80%D1%85%D0%B8%D0%B2_%D0%BF%D0%BE%D0%B3%D0%BE%D0%B4%D1%8B_%D0%B2_%D0%92%D0%BE%D0%BB%D0%B6%D1%81%D0%BA%D0%BE%D0%B9_%D0%93%D0%9C%D0%9E (accessed on 14 July 2023).
- Vodeneeva, E.L.; Kulizin, P.V. Algae of the Mordovian Nature Reserve (Annotated List of Species); Flora and Fauna of Nature Reserves: Moscow, Russia, 2019; Volume 134, p. 62. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2021; Available online: http://www.algaebase.org (accessed on 14 July 2023).
- Zhikharev, V.; Vodeneeva, E.; Kudrin, I.; Gavrilko, D.; Startseva, N.; Kulizin, P.; Erina, O.; Tereshina, M.; Okhapkin, A.; Shurganova, G. The Species Structure of Plankton Communities as a Response to Changes in the Trophic Gradient of the Mouth Areas of Large Tributaries to a Lowland Reservoir. Water 2023, 15, 74. [Google Scholar] [CrossRef]
- Okhapkin, A.; Sharagina, E.; Kulizin, P.; Startseva, N.; Vodeneeva, E. Phytoplankton community structure in highly-mineralized small gypsum karst lake (Russia). Microorganisms 2022, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Dubovskaya, O.P. Non-predatory mortality of the crustacean zooplankton, and its possible causes (a review). Biol. Bull. Rev. 2009, 70, 168–192. [Google Scholar]
- Dzyuban, A.N. Experiment in evaluating methane emissions in water bodies of urbanized territories in the Rybinsk reservoir basin. Water Resour. 2010, 37, 583–585. [Google Scholar] [CrossRef]
- Sweerts, J.; Bar-Gilissen, M.; Cornelse, A.; Cappenberg, T.E. Oxygen-consuming processes at the profundal and littoral sediment-water interface of a small meso-eutrophic lake. Limnol. Oceanogr. 1991, 36, 1124–1133. [Google Scholar] [CrossRef]
- Elrod, V.A.; Berelson, W.M.; Coale, K.N.; Johnson, K.S. The flux of iron from continental schelf sediments: A missing sourse of global budgets. Geophys. Res. Lett. 2004, 31, L12307. [Google Scholar] [CrossRef]
- Martynova, M.V. Bottom Sediments as a Component of Limnic Ecosystems; Nauka: Moscow, Russia, 2010; p. 243. [Google Scholar]
- Bastviken, D.; Santoro, A.; Marotta, H. Methane emissions from Pantanal, South America, during the low water season: Toward more comprehensive sampling. Environ. Sci. Technol. 2010, 44, 5450–5455. [Google Scholar] [CrossRef]
- Bastviken, D.; Cole, J.; Pace, M.; Tranvik, L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biochem. Cycles 2004, 18, GB4009. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F. Cyanobacterial blooms in a temperate river-floodplain ecosystem: The importance of hydrological extremes. Aquat. Ecol. 2011, 45, 335–349. [Google Scholar] [CrossRef]
- Rakhuba, A.V. Assessment of the Influence Exercised by the Hydrodynamic Regime on the Phytoplankton Development and the Water Quality of the Kuibyshev Reservoir. Uchenye Zap. Kazan. Univ. Seriya Estestv. Nauk. 2020, 162, 430–444. [Google Scholar] [CrossRef]
- Miracle, M.R.; Vicente, E.; Pedrós-Alió, C. Biological studies of Spanish meromictic and stratified karstic lakes. Limnetica 1992, 8, 59–77. [Google Scholar] [CrossRef]
- Frolova, E.A.; Bayanov, N.G.; Minin, A.E.; Minina, L.M. Macrozoobenthos of the Gorky reservoir. Taxonomic structure and quantitative development. Proc. Mordovian State Nat. Reserve 2020, 25, 381–392. [Google Scholar]







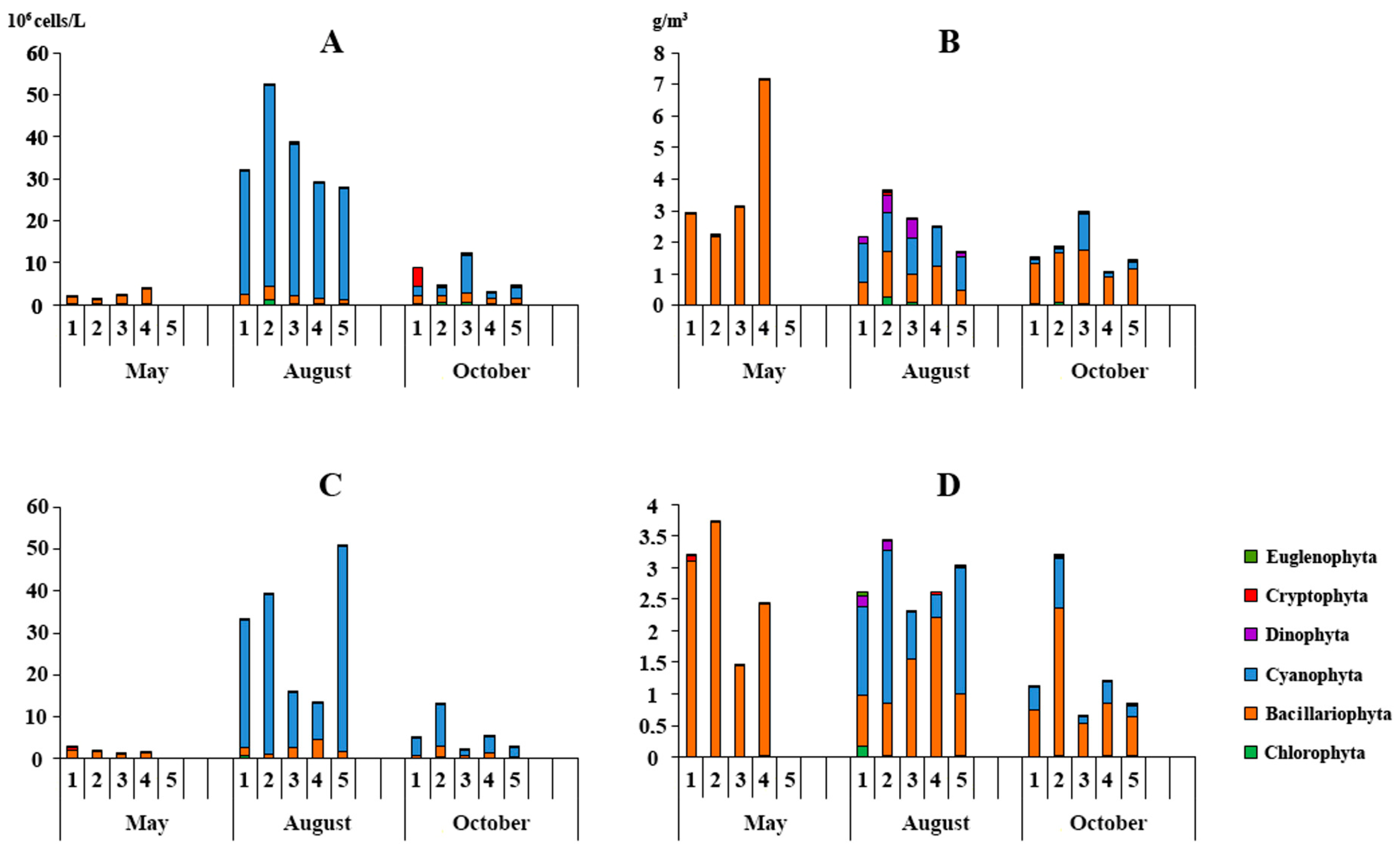


| Stations | 25 May 2022 | 8 August 2022 | 12 October 2022 | |||
|---|---|---|---|---|---|---|
| N | B | N | B | N | B | |
| U1 | 1.66 | 2.91 | 31.71 | 2.19 | 8.76 | 1.48 |
| U2 | 1.02 | 2.20 | 52.31 | 3.61 | 4.2 | 1.84 |
| U3 | 1.99 | 3.12 | 38.45 | 2.77 | 11.91 | 2.93 |
| U4 | 3.73 | 7.15 | 29.02 | 2.48 | 2.88 | 1.04 |
| U5 | – | – | 27.67 | 1.68 | 4.18 | 1.39 |
| D1 | 2.56 | 3.21 | 33.22 | 2.62 | 5.04 | 1.12 |
| D2 | 1.75 | 3.73 | 39.25 | 3.42 | 13.04 | 3.18 |
| D3 | 1.14 | 1.44 | 15.71 | 2.31 | 1.99 | 0.64 |
| D4 | 1.35 | 2.43 | 13.26 | 2.61 | 5.32 | 1.19 |
| D5 | 1.14 | 1.64 | 50.56 | 3.03 | 2.69 | 0.83 |
| Date | Upstream | Downstream | ||
|---|---|---|---|---|
| N Surface * (N Integral) | B Surface (B Integral) | N Surface * (N Integral) | B Surface (B Integral) | |
| 25 May 2022 (spring) | 2.10 ± 0.58 (1.85 ± 0.33) | 3.85 ± 1.12 (3.81 ± 1.26) | 1.70 ± 0.31 (1.29 ± 0.10) | 2.70 ± 0.50 (2.41 ± 0.40) |
| 8 August 2022 (summer) | 35.83 ± 4.52 (45.05 ± 9.14) | 2.55 ± 0.32 (2.87 ± 0.19) | 30.43 ± 7.10 (21.95 ± 7.91) | 2.80 ± 0.19 (1.78 ± 0.32) |
| 12 October 2022 (autumn) | 6.39 ± 1.70 (4.28 ± 0.95) | 1.74 ± 0.32 (1.59 ± 0.24) | 5.62 ± 1.96 (4.72 ± 0.72) | 1.39 ± 0.46 (1.71 ± 0.21) |
| Seasonal average | 15.68 ± 4.49 (17.06 ± 6.01) | 2.63 ± 0.34 (2.76 ± 0.47) | 13.36 ± 4.31 (9.32 ± 3.44) | 2.27 ± 0.28 (1.97 ± 0.19) |
| Concentration. mg/L | Station U1 (in Front of the Entrance Channel) | Station U3 (500 m to the Left) | Station U4 (3 km to the Left) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 May 2022 | 8 August 2022 | 12 October 2022 | 25 May 2022 | 8 August 2022 | 12 October 2022 | 25 May 2022 | 8 August 2022 | 12 October 2022 | |
| Iron | 0.05 ± 0.01 | 0.09 ± 0.02 | 0.16 ± 0.04 | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.15 ± 0.04 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.02 |
| Magnesium | <0.05 | <0.05 | 7.5 ± 1.2 | <0.05 | <0.05 | 6.8 ± 1.0 | <0.05 | <0.05 | 6.1 ± 0.9 |
| Phosphorus | 21.5 ± 3.2 | 0.19 ± 0.02 | 0.08 ± 0.02 | 21.5 ± 3.2 | 0.36 ± 0.04 | 0.09 ± 0.03 | 19.0 ± 2.9 | 0.28 ± 0.03 | 0.07 ± 0.02 |
| Calcium | 0.02 ± 0.01 | 21.9 ± 3.3 | 20 ± 3 | 0.02 ± 0.01 | 22.9 ± 3.4 | 17 ± 3 | 0.03 ± 0.01 | 23.1 ± 3.5 | 20 ± 3 |
| Chlorides | 5.1 ± 0.5 | 4.4 ± 1.1 | 5.0 ± 1.0 | 3.5 ± 0.8 | 4.6 ± 1.1 | 4.5 ± 1.1 | 3.8 ± 0.9 | 4.7 ± 1.1 | 5.3 ± 0.5 |
| Nitrites | 0.10 ± 0.01 | 0.4 ± 0.1 | <0.2 | 0.02 ± 0.01 | 0.13 ± 0.04 | <0.2 | 0.10 ± 0.01 | 0.18 ± 0.05 | <0.2 |
| Sulphates | 106.0 ± 10.6 | 127.9 ± 12 | 17.7 ± 1.2 | 97.3 ± 9.7 | 125.7 ± 12 | 14.6 ± 1.5 | 106.4 ± 10.6 | 135.1 ± 13 | 16.2 ± 1.6 |
| Nitrates | 2.8 ± 0.6 | 0.32 ± 0.09 | 2.7 ± 0.5 | 0.9 ± 0.2 | 1.0 ± 0.2 | 2.3 ± 0.5 | 3.1 ± 0.6 | 0.33 ± 0.09 | 2.2 ± 0.4 |
| Phosphates | 0.05 ± 0.001 | 0.57 ± 0.11 | 0.23 ± 0.01 | 0.10 ± 0.01 | 1.1 ± 0.2 | 0.26 ± 0.05 | 0.10 ± 0.01 | 0.86 ± 0.17 | 0.21 ± 0.01 |
| Ammonium ion | 0.73 ± 0.11 | 0.14 ± 0.03 | 0.28 ± 0.11 | 0.75 ± 0.11 | 0.15 ± 0.03 | 0.29 ± 0.1 | 0.68 ± 0.10 | 0.13 ± 0.03 | 0.30 ± 0.12 |
| Hydrocarbonates | 85.4 ± 14.5 | 109.8 ± 13 | 104 ± 18 | 73.2 ± 12.5 | 103.7 ± 12 | 107 ± 18 | 79.3 ± 13.5 | 115.9 ± 14 | 110 ± 18 |
| pH. pH units | 6.5 ± 0.2 | 8.2 ± 0.2 | 7.1 ± 0.2 | 6.8 ± 0.2 | 8.14 ± 0.2 | 7.1 ± 0.2 | 7.3 ± 0.2 | 7.9 ± 0.2 | 7.2 ± 0.2 |
| Total mineralisation | 162 ± 31 | 156 ± 30 | 110 ± 20 | 130 ± 25 | 148 ± 28 | 115 ± 22 | 144 ± 27 | 146 ± 28 | 115 ± 22 |
| Concentration. mg/L | Station D1 (below the Entrance Channel) | Station D3 (500 m to the Left) | Station D4 (1 km to the Left) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 May 2022 | 8 August 2022 | 12 October 2022 | 25 May 2022 | 8 August 2022 | 12 October 2022 | 25 May 2022 | 8 August 2022 | 12 October 2022 | |
| Iron | 0.07 ± 0.02 | 0.10 ± 0.02 | 0.098 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.02 | 0.13 ± 0.03 | 0.10 ± 0.02 | 0.09 ± 0.02 | 0.22 ± 0.05 |
| Magnesium | <0.05 | <0.05 | 5.3 ± 0.8 | <0.05 | <0.05 | 7.4 ± 1.1 | <0.05 | <0.05 | 10.5 ± 1.6 |
| Phosphorus | 18.9 ± 2.8 | 0.23 ± 0.02 | 0.07 ± 0.02 | 18.1 ± 2.7 | 0.27 ± 0.03 | 0.09 ± 0.03 | 18.3 ± 2.8 | 0.21 ± 0.02 | 0.08 ± 0.02 |
| Calcium | 0.5 ± 0.2 | 21.6 ± 3.2 | 16.5 ± 2.5 | 0.02 ± 0.01 | 21.8 ± 3.3 | 21 ± 3 | <0.02 | 22.8 ± 3.4 | 41 ± 5 |
| Chlorides | 2.6 ± 0.6 | 5.7 ± 1.4 | 5.7 ± 0.6 | 3.6 ± 0.9 | 4.4 ± 1.1 | 4.9 ± 1.2 | 4.1 ± 1.0 | 4.5 ± 1.1 | 5.2 ± 0.5 |
| Nitrites | 0.4 ± 0.1 | 0.41 ± 0.12 | <0.2 | 0.06 ± 0.01 | 0.39 ± 0.11 | <0.2 | 0.08 ± 0.01 | 0.41 ± 0.12 | ˂ 0.2 |
| Sulphates | 172.2 ± 17.2 | 121 ± 12 | 16.0 ± 1.6 | 83.0 ± 8.3 | 117 ± 12 | 14.9 ± 1.5 | 109.3 ± 10.9 | 132 ± 13 | 17.3 ± 1.7 |
| Nitrates | 6.9 ± 0.7 | 1.1 ± 0.2 | 1.8 ± 0.4 | 0.9 ± 0.1 | 1.8 ± 0.4 | 2.7 ± 0.5 | 2.7 ± 0.6 | 0.53 ± 0.11 | 1.8 ± 0.4 |
| Phosphates | 1.5 ± 0.2 | 0.72 ± 0.14 | 0.21 ± 0.01 | 0.10 ± 0.01 | 0.84 ± 0.17 | 0.26 ± 0.05 | 0.01 ± 0.001 | 0.63 ± 0.13 | 0.20 ± 0.01 |
| Ammonium ion | 0.61 ± 0.09 | 0.16 ± 0.03 | 0.30 ± 0.12 | 0.66 ± 0.09 | 0.16 ± 0.03 | 0.31 ± 0.12 | 0.74 ± 0.11 | 0.13 ± 0.03 | 0.32 ± 0.13 |
| Hydrocarbonates | 75.6 ± 12.9 | 109.8 ± 13 | 107 ± 18 | 72.0 ± 12.2 | 97.6 ± 11.7 | 104 ± 18 | 73.2 ± 12.5 | 115.9 ± 13 | 107 ± 18 |
| pH. pH units | 7.2 ± 0.2 | 7.8 ± 0.2 | 7.2 ± 0.2 | 7.1 ± 0.2 | 7.1 ± 0.2 | 7.2 ± 0.2 | 6.9 ± 0.2 | 7.2 ± 0.2 | 7.2 ± 0.2 |
| Total mineralization | 148 ± 28 | 150 ± 28 | 112 ± 23 | 130 ± 25 | 158 ± 30 | 114 ± 24 | 126 ± 24 | 144 ± 28 | 128 ± 24 |
| Station | Depth. m | Tsurf-Tbot. °C | Hygroscopic Humidity. % | OM. % | Diffusion Flux CH4 from BS mgC/m2 day | CH4. mkl/l (Surface/Bottom) | Specific Flux CH4. mgC/m2 day | CBW. mgO/m2 day | Aerobic Destruction. mgC/m2 day | Total Destruction. mgC/m2 day | Turbidity. NTU (Surface/Bottom) | pH (Surface/Bottom) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25–26 May 2022 | ||||||||||||
| U3 | 8 | 0.5 | 2.8 | 6.7 | nd | 2.6/1.6 | nd | 25 | nd | nd | 14/7 | 8.4/8.2 |
| U2 | 8 | 0.1 | 1.3 | 4.0 | 0.03 | 1.5/1.3 | nd | 32 | 58 | 140 | 6/10 | 8.1/8.0 |
| U11 | 11 | 1.4 | 7.9 | 23.3 | 0.20 | 3.7/2.7 | 2.5–8.0 | 84 | 177 | 181 | 6/nd | 8.1/nd |
| U9 | 16 | 1.1 | 7.1 | 17.4 | 1.89 | 1.5/2.0 | 0.25 | 108 | 135 | 193 | 6/12 | 8.1/8.1 |
| U13 | 9 | 1 | 5.5 | 11.8 | 0.14 | 3.1/3.1 | 1.9 | 98 | 213 | 272 | 5/5 | 8.1/8.1 |
| 24–25 August 2022 | ||||||||||||
| U1 | 20 | 0.9 | 0.7 | 1.9 | nd | 0.5/1.4 | 0.5 | nd | nd | nd | nd/4.8 | 8.5/8.3 |
| U3 | 8.5 | 2.0 | 1.6 | 8.6 | 1.89 | 0.4/0.5 | 1.8 | 235 | 262 | 418 | nd/5.5 | 8.4/8.1 |
| U5 | 14.5 | 2.2 | 3.1 | 7.1 | 3.34 | 0.5/4.3 | 468 | 176 | 88 | 913 | nd/10.7 | 8.2/7.8 |
| U6 | 9.5 | 2.8 | 8.5 | 13.9 | 0.35 | 0.6/3.3 | 0.1 | 309 | 221 | 974 | nd/5.4 | 8.3/8.0 |
| U12 | 18.5 | 3.2 | 6.8 | 17.1 | 2.45 | 0.4/61.9 | 1.7 | 349 | 334 | 366 | nd/6 | 8.7/8.1 |
| U11 | 9 | 2.9 | nd | nd | nd | 0.9/15.9 | 974 | 315 | 254 | 312 | nd/6.3 | 8.9/8.4 |
| U7 | 9.5 | 2.9 | 5.0 | 10.3 | 0.06 | 0.9/0.7 | nd | 362 | 317 | 515 | nd/4.6 | 8.9/8.1 |
| U14 | 18 | 3.1 | 7.1 | 16.2 | 0.95 | 0.2/0.2 | 1.9 | 424 | 352 | 229 | nd 6/24 | 8.6/8.1 |
| U8 | 17.5 | 3.1 | 6.6 | 14.9 | 8.13 | 0.2/1.1 | 3.1 | 235 | 262 | 418 | 11.4/30.9 | 9.0/7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molkov, A.; Kapustin, I.; Grechushnikova, M.; Dobrokhotova, D.; Leshchev, G.; Vodeneeva, E.; Sharagina, E.; Kolesnikov, A. Investigation of Water Dynamics Nearby Hydroelectric Power Plant of the Gorky Reservoir on Water Environment: Case Study of 2022. Water 2023, 15, 3070. https://doi.org/10.3390/w15173070
Molkov A, Kapustin I, Grechushnikova M, Dobrokhotova D, Leshchev G, Vodeneeva E, Sharagina E, Kolesnikov A. Investigation of Water Dynamics Nearby Hydroelectric Power Plant of the Gorky Reservoir on Water Environment: Case Study of 2022. Water. 2023; 15(17):3070. https://doi.org/10.3390/w15173070
Chicago/Turabian StyleMolkov, Aleksandr, Ivan Kapustin, Maria Grechushnikova, Daria Dobrokhotova, George Leshchev, Ekaterina Vodeneeva, Ekaterina Sharagina, and Anton Kolesnikov. 2023. "Investigation of Water Dynamics Nearby Hydroelectric Power Plant of the Gorky Reservoir on Water Environment: Case Study of 2022" Water 15, no. 17: 3070. https://doi.org/10.3390/w15173070






