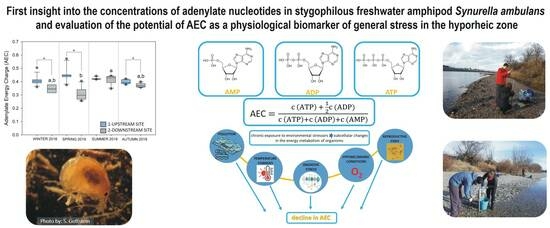
Energy Status of Stygophilous Amphipod Synurella ambulans as a Promising Biomarker of Environmental Stress in the Hyporheic Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Amphipod Sampling
2.3. Chemicals
2.4. Preparation of Synuella ambulans Samples
2.5. Separation and Quantification of Adenylates and AEC Calculation
2.6. Data Analyses
3. Results
3.1. Concentrations of Adenine Nucleotides and Adenylate Energy Charge in S. ambulans
3.2. Effects of Environmental Variables and Metals Accumulated in the Organism on AEC of S. ambulans as Dependent Variable
4. Discussion
4.1. Energy Status of S. ambulans
4.2. Effects of Environmental Variables and Metals Accumulated in the Organism on Energy Status of S. ambulans
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, D.E. Energy charge of adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef] [PubMed]
- Ivanovici, A.M. Application of adenylate energy charge to problems of environmental impact assessment in aquatic organisms. Helgoland Mar. Res. 1980, 33, 556–565. [Google Scholar] [CrossRef]
- Koop, J.H.E.; Winkelmann, C.; Becker, J.; Hellmann, C.; Ortmann, C. Physiological indicators of fitness in benthic invertebrates: A useful measure for ecological health assessment and experimental ecology. Aquat. Ecol. 2011, 45, 547–559. [Google Scholar] [CrossRef]
- Marazza, D.; Bornens, P.; Le Gal, Y. Effect of ammonia on survival and adenylate energy charge in the shrimp Palaemonetes varians. Ecotox. Environ. Saf. 1996, 34, 103–108. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Jayasundara, N.; Ren, X.; Gao, B.; Liu, P.; Li, J.; Meng, X. Physiological and molecular responses in the gill of the swimming crab Portunus trituberculatus during long-term ammonia stress. Front. Mar. Sci. 2021, 8, 797241. [Google Scholar] [CrossRef]
- Morris, S.; van Aardt, W.J.; Ahern, M.D. The effect of lead on the metabolic and energetic status of the Yabby, Cherax destructor, during environmental hypoxia. Aquat. Toxicol. 2005, 75, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Shofer, S.L.; Tjeerdema, R.S. Effects of hypoxia and toxicant exposure on adenylate energy charge and cytosolic ADP concentrations in abalone. Comp. Biochem. Physiol. 1998, 119, 51–57. [Google Scholar] [CrossRef]
- Werner, I.; Viant, M.R.; Rosenblum, E.S.; Gantner, A.S.; Tjeerdema, R.S.; Johnson, M.L. Cellular response to temperature stress in steelhead trout (Onchorynchus mykiss) parr with different rearing histories. Fish Physiol. Biochem. 2006, 32, 261–273. [Google Scholar] [CrossRef]
- Babarro, J.M.F.; Labarta, U.; Fernández Reiriz, M.J. Energy metabolism and performance of Mytilus galloprovincialis under anaerobiosis. J. Mar. Biol. Ass. UK 2007, 87, 941–946. [Google Scholar] [CrossRef]
- Isani, G.; Serra, R.; Cattani, O.; Cortesi, P.; Carpene, E. Adenylate energy charge and metallothionein as stress indices in Mytilus galloprovincialis exposed to cadmium and anoxia. J. Mar. Biol. Ass. UK 1997, 77, 1187–1197. [Google Scholar] [CrossRef]
- Dehn, P.F.; Haya, K.; Aiken, D.E. Adenylate energy charge, arginine phosphate and ATPase activity in juvenile Homarus americanus during the molt cycle. Comp. Biochem. Physiol. 1985, 81, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.L.; Suárez, M.P.; San Juan, F. Seasonal changes of nucleotides in mussel (Mytilus galloprovincialis) mantle tissue. Comp. Biochem. Physiol. 2006, 143, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.E. Adenylate control and the adenylate energy charge. In Cellular Energy Metabolism and Its Regulation; Atkinson, D.E., Ed.; Academic Press Inc.: New York, NY, USA, 1977; pp. 85–107. [Google Scholar]
- Thébault, M.T.; Biegniewska, A.; Raffin, J.P.; Skorkowski, E.F. Short term cadmium intoxication of the shrimp Palaemon serratus: Effect on adenylate metabolism. Comp. Biochem. Phys. 1996, 113, 345–348. [Google Scholar] [CrossRef]
- Louis, F.; Rocher, B.; Barjhoux, I.; Bultelle, F.; Dedourge-Geffard, O.; Gaillet, V.; Bonnard, I.; Delahaut, L.; Pain-Devin, S.; Geffard, A.; et al. Seasonal monitoring of cellular energy metabolism in a sentinel species, Dreissena polymorpha (bivalve): Effect of global change? Sci. Total Environ. 2020, 725, 138450. [Google Scholar] [CrossRef]
- Verschraegen, K.; Herman, P.M.J.; Van Gansbeke, D.; Braeckman, A. Measurement of the adenylate energy charge in Nereis diversicolor and Nephtys sp. (Polychaeta: Annelida): Evaluation of the usefulness of AEC in pollution monitoring. Mar. Biol. 1985, 86, 233–240. [Google Scholar] [CrossRef]
- Guida, L.; Walker, T.I.; Reina, R.D. The adenylate energy charge as a new and useful indicator of capture stress in chondrichtyans. J. Comp. Physiol. B 2016, 186, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Findlay, S.; Marmonier, P.; Stanley, E.H.; Valett, H.M. The functional significance of the hyporheic zone in streams and rivers. Annu. Rev. Ecol. Syst. 1998, 29, 59–81. [Google Scholar] [CrossRef]
- Mueller, B.M.; Schulz, H.; Lewandowski, J. Hyporheic zone and processes. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 301–311. [Google Scholar] [CrossRef]
- Yu, K.; Duan, Y.; Liao, P.; Xie, L.; Li, Q.; Ning, Z.; Liu, C. Watershed-scale distributions of heavy metals in the hyporheic zones of a heavily polluted Maozhou River watershed, southern China. Chemoshpere 2020, 239, 124773. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Kloep, F.; Wilzcek, S.; Pusch, M.T. A river’s liver—Microbial processes within the hyporheic zone of a large lowland river. Biogeochemistry 2005, 76, 349–371. [Google Scholar] [CrossRef]
- Pacioglu, O. Ecology of the hyporheic zone: A review. Cave Karst Sci. 2009, 36, 69–76. [Google Scholar]
- Kapetanović, D.; Vardić Smrzlić, I.; Valić, D.; Teskeredžić, E.; Kolarević, S.; Sunjog, K.; Tomović, J.; Kračun-Kolarević, M.; Knežević-Vukčević, J.; Paunović, M.; et al. Microbial characterization of the Sava River. In The Sava River; Series The Handbook of Environmental Chemistry; Milačić, R., Ščančar, J., Paunović, M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2015; Volume 31, pp. 201–228. [Google Scholar] [CrossRef]
- Milaković, M.; Vestergaard, G.; González-Plaza, J.J.; Petrić, I.; Šimatović, A.; Senta, I.; Kublik, S.; Schloter, M.; Smalla, K.; Udiković-Kolić, N. Pollution from azithromycin manufacturing promotes macrolide-resistance gene propagation and induces spatial and seasonal bacterial community shifts in receiving river sediments. Environ. Int. 2019, 123, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Vdović, N.; Lučić, M.; Mikac, N.; Bačić, N. Partitioning of metal contaminants between bulk and fine-grained fraction in freshwater sediments: A critical appraisal. Minerals 2021, 11, 603. [Google Scholar] [CrossRef]
- Bakke, T.; Källqvist, T.; Ruus, A.; Breedveld, G.D.; Hylland, K. Development of sediment quality criteria in Norway. J. Soils Sediments 2010, 10, 172–178. [Google Scholar] [CrossRef]
- Gottstein, S.; Redžović, Z.; Erk, M.; Cindrić, M. The hyporheic composition of the Sava River in the city of Zagreb after more than 60 years of research. In Book of Abstracts of the International Conference City Windows in Nature, Zagreb, Croatia, 9–10/09/2021; Javna Ustanova Maksimir za Upravljanje Zaštićenim Područjima Grada Zagreba: Zagreb, Hrvatska, 2021; Available online: https://park-maksimir.hr/wp-content/uploads/2021/09/Knjiga-sazetaka.pdf (accessed on 15 May 2023).
- Žutinić, P.; Petrić, I.; Gottstein, S.; Gligora Udovič, M.; Kralj Borojević, K.; Kamberović, J.; Kolda, A.; Plenković-Moraj, A.; Trenjej, I. Microbial mats as shelter microhabitat for amphipods in an intermittent karstic spring. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 7. [Google Scholar] [CrossRef]
- Gottstein, S.; Mihaljević, Z.; Perović, G.; Kerovec, M. The distribution of amphipods (Crustacea) in different habitats along the Mura and Drava river system in Croatia. In Limnological Reports; Horvatić, J., Ed.; Faculty of Education, J.J. Strossmayer University of Osijek: Osijek, Croatia; Croatian Ecological Society: Zagreb, Croatia, 2000; Volume 33, pp. 231–236. [Google Scholar]
- Gottstein Matočec, S.; Bakran-Petricioli, T.; Bedek, J.; Bukovec, D.; Buzjak, S.; Franičević, M.; Jalžić, B.; Kerovec, M.; Kletečki, E.; Kralj, J.; et al. An overview of the cave and interstitial biota of Croatia. Nat. Croat. 2002, 11 (Suppl. 1), 1–112. Available online: https://hrcak.srce.hr/file/52154 (accessed on 22 March 2023).
- Redžović, Z.; Erk, M.; Gottstein, S.; Sertić Perić, M.; Dautović, J.; Fiket, Ž.; Brkić, A.L.; Cindrić, M. Metal bioaccumulation in stygophilous amphipod Synurella ambulans in the hyporheic zone: The influence of environmental factors. Sci. Total Environ. 2023, 866, 161350. [Google Scholar] [CrossRef]
- GRC (Government of the Republic of Croatia). Directive on Water Quality Standard. Off. Gaz. 2019, 96. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_10_96_1879.html (accessed on 2 February 2023).
- EEA (European Environment Agency). Ecoregions for Rivers and Lakes. 2009. Available online: https://www.eea.europa.eu/data-and-maps/figures/ecoregions-for-rivers-and-lakes (accessed on 2 February 2023).
- Tomas, D.; Čurlin, M.; Marić, A.S. Assessing the surface water status in Pannonian ecoregion by the water quality index model. Ecol. Indic. 2017, 79, 182–190. [Google Scholar] [CrossRef]
- Bou, C.; Rouch, R. Un nouveau champ de recherche sur la faune aquatique souterraine. Acad. Sci. Paris 1967, 265, 369–370. [Google Scholar]
- Karaman, G.S. Crustacea—Amphipoda of freshwaters. In Fauna d’Italia; Edizioni Calderini: Bologna, Italy, 1993; Volume 31, pp. 1–337. [Google Scholar]
- Redžović, Z.; Erk, M.; Svetličić, E.; Dončević, L.; Gottstein, S.; Hozić, A.; Cindrić, M. Determination of adenylate nucleotides in amphipod Gammarus fossarum by ion-pair reverse phase liquid chromatography: Possibilities of positive pressure micro-solid phase extraction. Separations 2021, 8, 20. [Google Scholar] [CrossRef]
- Díaz Enrich, M.J.; Villamarín, J.A.; Ramos Martínez, J.I.; Ibarguren, I. Measurement of adenosine 3′,5′-cyclic monophosphate and guanosine 3′, 5′-cyclic monophosphate in mussel (Mytilus galloprovincialis Lmk.) by high-performance liquid chromatography with diode array detection. Anal. Biochem. 2000, 285, 105–112. [Google Scholar] [CrossRef]
- Czarnecka, J.; Cieślak, M.; Michał, K. Application of solid phase extraction and high-performance liquid chromatography to qualitative and quantitative analysis of nucleotides and nucleosides in human cerebrospinal fluid. J. Chromatogr. B 2005, 822, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Thébault, M.T.; Raffin, J.P.; Picado, A.M.; Mendonça, E.; Skorkowski, E.F.; Le Gal, Y. Coordinated changes of adenylate energy charge and ATP/ADP: Use in ecotoxicological studes. Ecotoxicol. Environ. Saf. 2000, 46, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagina, K.P.; Lubyaga, Y.A.; Shatilina, Z.; Bedulina, D.; Gurkov, A.; Axenov-Gribanov, D.V.; Baduev, B.; Kondrateva, E.S.; Gubanov, M.; Zadereev, E.; et al. Salinity modulates thermotolerance, energy metabolism and stress response in amphipods Gammarus lacustris. PeerJ 2016, 4, e2657. [Google Scholar] [CrossRef] [PubMed]
- Robles-Romo, A.; Arjona, O.; Racotta, I.S. Influence of sampling, storage, processing and optimal experimental conditions on adenylate energy charge in penaeid shrimp. Arch. Biol. Sci. 2014, 66, 651–666. [Google Scholar] [CrossRef]
- Hervant, F.; Mathieu, J.; Garin, D.; Fréminet, A. Behavioral, ventilatory, and metabolic responses to severe hypoxia and subsequent recovery of the hypogean Niphargus rhenorhodanensis and the epigean Gammarus fossarum (Crustacea: Amphipoda). Physiol. Zool. 1995, 68, 223–244. [Google Scholar] [CrossRef]
- Hervant, F. The activities of enzymes associated with the intermediary and energy metabolism in hypogean and epigean crustaceans. Comptes Rendus Acad. Sci.-Ser. III 1996, 319, 1071–1077. [Google Scholar]
- Redžović, Z.; Sokolova, I.; Erk, M.; Sokolov, E.; Gottstein, S. Anaerobic potential of Synurella ambulans (Müller, 1846) (Crustacea, Amphipoda) from hyporheic zone of Sava River and Stalnjak Spring, Croatia. In Book of Abstracts of 3rd Faculty of Science PhD Student Symposium, Zagreb, Croatia, 22/02/2019; Prirodoslovno-Matematički Fakultet Sveučilišta u Zagrebu: Zagreb, Croatia, 2019; Available online: https://www.pmf.unizg.hr/_download/repository/Knjiga_sazetaka_SSDS_PMF-a_2019.pdf (accessed on 3 February 2023).
- Schill, R.O.; Köhler, H.-R. Does the environment or the source of the population define stress status and energy supply in the freshwater amphipod, Gammarus fossarum? Ecotoxicology 2004, 13, 683–695. [Google Scholar] [CrossRef]
- De la Fuente, I.M.; Cortés, J.M.; Valero, E.; Desroches, M.; Rodrigues, S.; Malaina, I.; Martínez, L. On the dynamics of the adenylate energy system: Homeorhesis vs homeostasis. PLoS ONE 2014, 9, e108676. [Google Scholar] [CrossRef]
- Gottstein, S.; Redžović, Z.; Erk, M.; Sertić Perić, M.; Dautović, J.; Cindrić, M. Life history traits of the stygophilous amphipod Synurella ambulans in hyporheic zone of the Sava River, Croatia. Water, 2023; submitted. [Google Scholar]
- Zapata-Restrepio, L.M.; Hauton, C.; Hudson, M.D.; Williams, I.D.; Hauton, D. Toxicity of tributyltin to the European flat oyster Ostrea edulis: Metabolomic responses indicate impacts to energy metabolism, biochemical composition and reproductive maturation. PLoS ONE 2023, 18, e0280777. [Google Scholar] [CrossRef] [PubMed]
- Dole-Olivier, M.J.; Marmonier, P. Patch distribution of interstitial communities: Prevailing factors. Freshw. Biol. 1992, 27, 177–191. [Google Scholar] [CrossRef]
- Becher, J.; Englisch, C.; Griebler, C.; Bayer, P. Groundwater fauna downtown—Drivers, impacts and implications for subsurface ecosystems in urban areas. J. Contam. Hydrol. 2022, 248, 104021. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. Hyporheic rehabilitation in rivers: Restoring vertical connectivity. Freshw. Biol. 2007, 52, 632–650. [Google Scholar] [CrossRef]
- Harvey, J.; Gooseff, M. River corridor science: Hydrologic exchange and ecological consequences from bedforms to basins. Water Resour. Res. 2015, 51, 6893–6922. [Google Scholar] [CrossRef]
- Jakob, L.; Bedulina, D.S.; Axenov-Gribanov, D.V.; Ginzburg, M.; Shatilina, Z.M.; Lubyaga, Y.A.; Madyarova, E.V.; Gurkov, A.N.; Timofeyev, M.A.; Pörtner, H.O.; et al. Uptake kinetics and subcellular compartmentalization explain lethal but not sublethal effects of cadmium in two closely related amphipod species. Environ. Sci. Technol. 2017, 51, 7208–7218. [Google Scholar] [CrossRef]
- Alonso, A.; Camargo, J.A. Toxicity of nitrite to three species of freshwater invertebrates. Environ. Toxicol. 2006, 21, 90–94. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. 2003, 135, 9–24. [Google Scholar] [CrossRef]
- Astiz, S.; Sabater, F. Anthropic effects on the meiofauna and physicochemical characteristics of the hyporheic zone in a Mediterranean stream. Eur. J. Environ. Sci. 2015, 5, 2. [Google Scholar] [CrossRef]
- Jansson, M. Phosphate uptake and utilization by bacteria and algae. Hydrobiologia 1988, 170, 177–189. [Google Scholar] [CrossRef]
- Marsden, I.D.; Rainbow, P.S. Does the accumulation of trace metals in crustaceans affect their ecology—The amphipod example? J. Exp. Mar. Biol. Ecol. 2004, 300, 343–371. [Google Scholar] [CrossRef]
- Viarengo, A.; Nott, J.A. Mechanisms of heavy metal cation homeostasis in marine invertebrates. Comp. Biochem. Physiol. 1993, 104, 355–372. [Google Scholar] [CrossRef]
- Huang, X.P.; O’Brien, P.J.; Templeton, D.M. Mitochondrial involvement in genetically determined transition metal toxicity: I. Iron toxicity. Chem.-Biol. Interact. 2006, 163, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]



| HPLC Conditions | Composition, Concentration and Values |
|---|---|
| Mobile phase | 150 mM KH2PO4/K2HPO4 and 100 mM KCl; pH = 6 |
| Ion-pairing agent | 10 mM tetrabutylammonium (TBA) hydroxide |
| Flow rate | 1 mL min−1 |
| Detection/band width | 260 nm/80 nm |
| Injection volume | 3 µL |
| Elution time | 12 min |
| 1-UP | 2-DOWN | |||||
|---|---|---|---|---|---|---|
| N | AEC | ATP/ADP | N | AEC | ATP/ADP | |
| Winter 2018 | 10 | 0.41 ± 0.04 * | 0.91 ± 0.18 A,B | 4 | 0.34 ± 0.04 a,b | 0.73 ± 0.12 |
| Spring 2019 | 6 | 0.46 ± 0.06 * | 1.38 ± 0.37 A,* | 6 | 0.31 ± 0.05 b | 0.69 ± 0.22 |
| Summer 2019 | 6 | 0.42 ± 0.01 | 0.93 ± 0.07 A,B | 6 | 0.41 ± 0.04 a | 0.87 ± 0.14 |
| Autumn 2019 | 6 | 0.40 ± 0.02 * | 0.85 ± 0.11 B | 6 | 0.37 ± 0.02 a,b | 0.79 ± 0.06 |
| 1-UP | 2-DOWN | |
|---|---|---|
| Keq | Keq | |
| Winter 2018 | 1.395 ± 0.186 A (10) | 1.313 ± 0.178 (4) |
| Spring 2019 | 2.345 ± 0.463 B,* (6) | 1.454 ± 0.340 (6) |
| Summer 2019 | 1.369 ± 0.221 A (6) | 1.243 ± 0.159 (6) |
| Autumn 2019 | 1.322 ± 0.222 A (6) | 1.332 ± 0.166 (6) |
| Model | Coefficients | ||||||
|---|---|---|---|---|---|---|---|
| Independent Variables | R2 | F | p * | β | t | p ** | |
| (I) group 1 | 0.527 | 12.511 | 1 × 10−6 | ||||
| Conductivity | −1.395 | −4.451 | 5.6 × 10−5 | ||||
| Dissolved O2 | −0.971 | −3.681 | 6.2 × 10−4 | ||||
| Total water hardness | 0.893 | 3.976 | 2.5 × 10−4 | ||||
| Alkalinity | −0.491 | −2.476 | 1.7 × 10−2 | ||||
| (II) group 2 | 0.415 | 16.665 | 3 × 10−6 | ||||
| Nitrites | −0.463 | −3.857 | 3.5 × 10−4 | ||||
| Phosphates | −0.309 | −2.579 | 1.3 × 10−2 | ||||
| (III) group 3 | 0.420 | 9.045 | 1.1 × 10−3 | ||||
| Fe in water | 0.611 | 3.848 | 7.3 × 10−4 | ||||
| Na in water | −0.447 | −2.817 | 9.3 × 10−3 | ||||
| (IV) group 4 | 0.268 | 5.121 | 4 × 10−2 | ||||
| Ni in sediment | −0.518 | −2.263 | 4 × 10−2 | ||||
| Metal accumulation 5 | 0.791 | 37.912 | <1 × 10−7 | ||||
| Fe in S. ambulans | −0.920 | −8.554 | <1 × 10−7 | ||||
| Zn in S. ambulans | 1.483 | 4.219 | 4.2 × 10−4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redžović, Z.; Erk, M.; Gottstein, S.; Cindrić, M. Energy Status of Stygophilous Amphipod Synurella ambulans as a Promising Biomarker of Environmental Stress in the Hyporheic Zone. Water 2023, 15, 3083. https://doi.org/10.3390/w15173083
Redžović Z, Erk M, Gottstein S, Cindrić M. Energy Status of Stygophilous Amphipod Synurella ambulans as a Promising Biomarker of Environmental Stress in the Hyporheic Zone. Water. 2023; 15(17):3083. https://doi.org/10.3390/w15173083
Chicago/Turabian StyleRedžović, Zuzana, Marijana Erk, Sanja Gottstein, and Mario Cindrić. 2023. "Energy Status of Stygophilous Amphipod Synurella ambulans as a Promising Biomarker of Environmental Stress in the Hyporheic Zone" Water 15, no. 17: 3083. https://doi.org/10.3390/w15173083
APA StyleRedžović, Z., Erk, M., Gottstein, S., & Cindrić, M. (2023). Energy Status of Stygophilous Amphipod Synurella ambulans as a Promising Biomarker of Environmental Stress in the Hyporheic Zone. Water, 15(17), 3083. https://doi.org/10.3390/w15173083