Hydrogeology and Hydrogeochemistry of Saline Groundwater Seepage Zones in Wadi Bani Malik Basin, Jeddah, Saudi Arabia: Impacts on Soil and Water Resources
Abstract
:1. Introduction
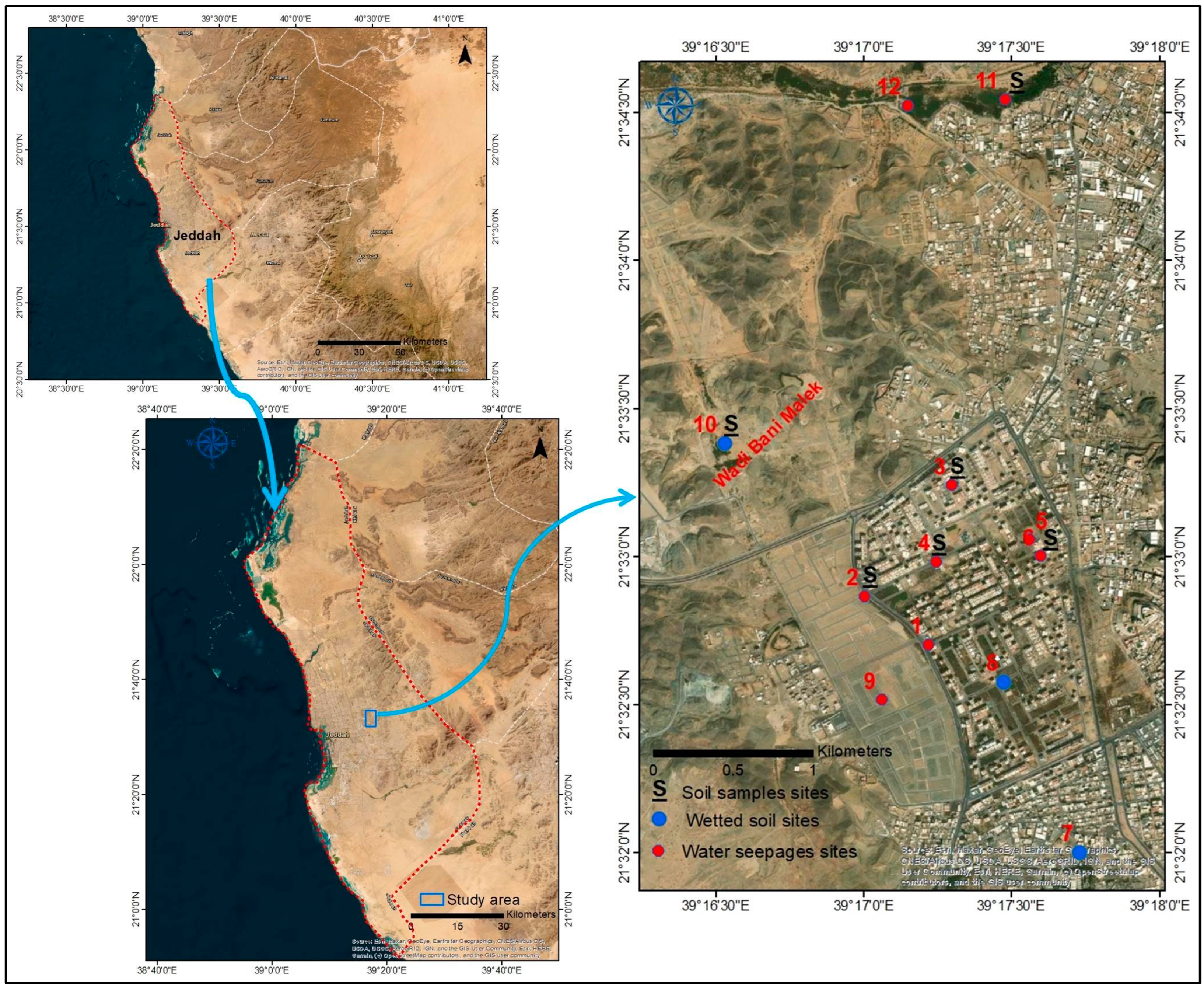
2. Study Area
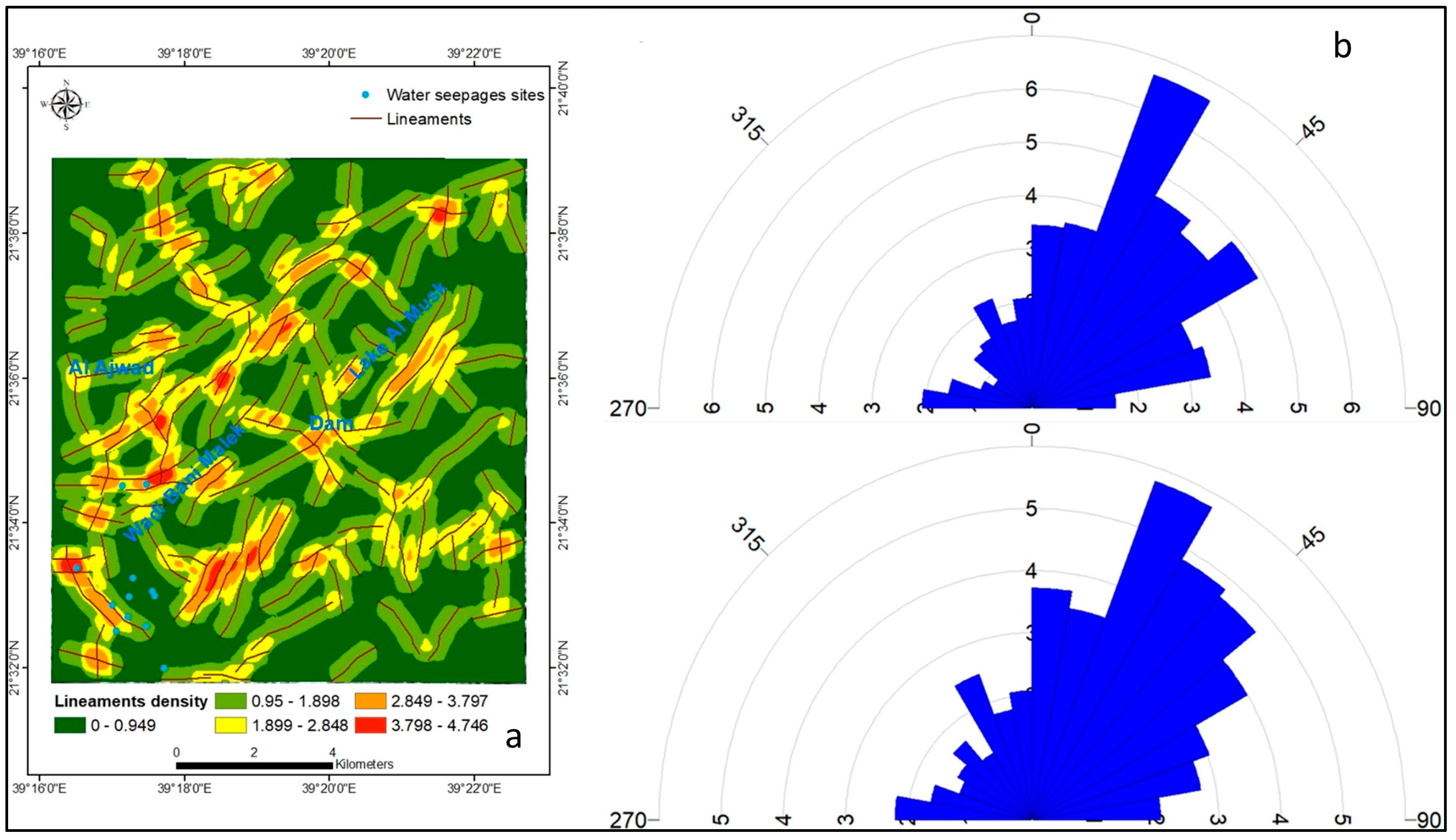
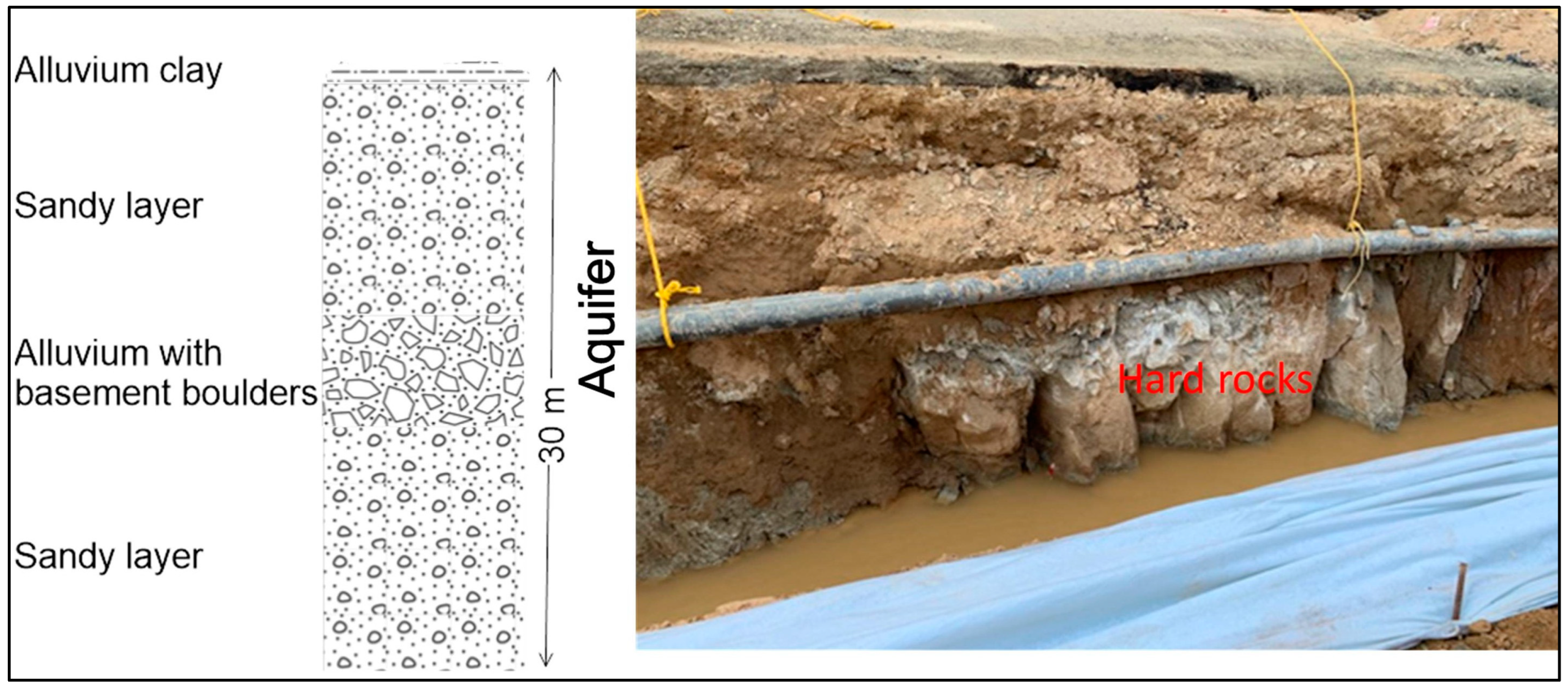
Geology and Hydrogeology
3. Materials and Methods
4. Results and Discussion
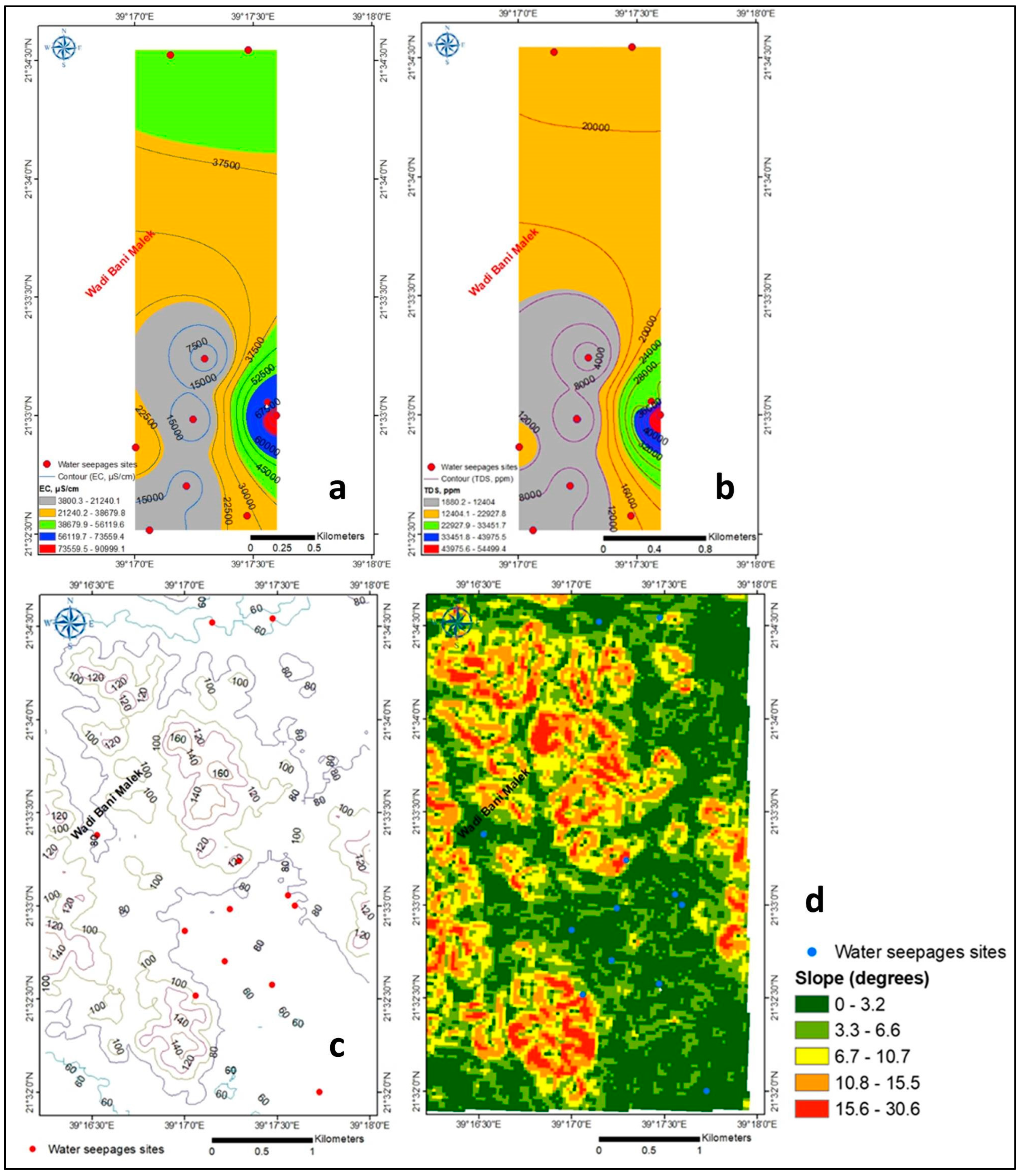
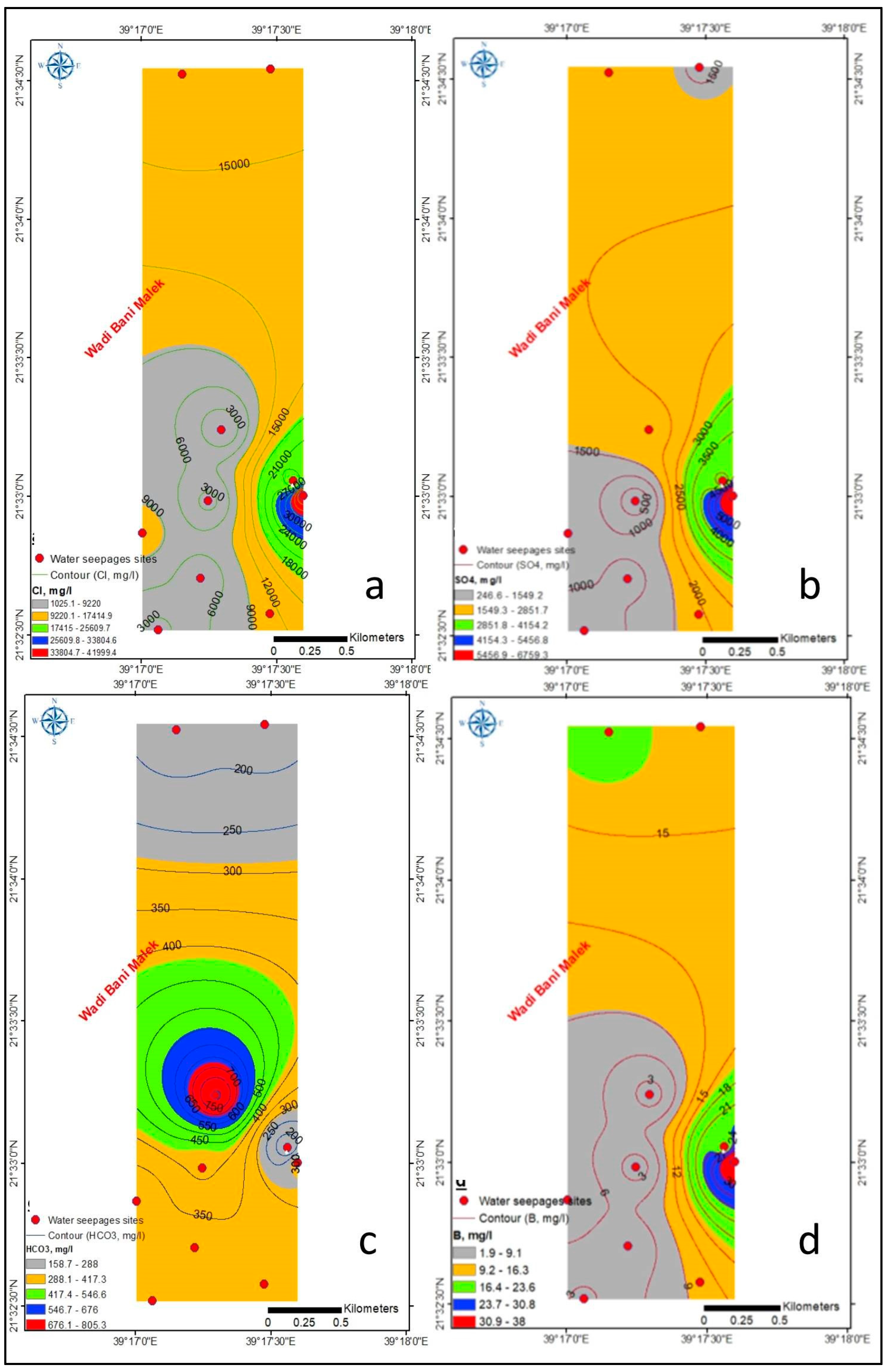
4.1. Hydrogeochemistry
4.1.1. Major Ions
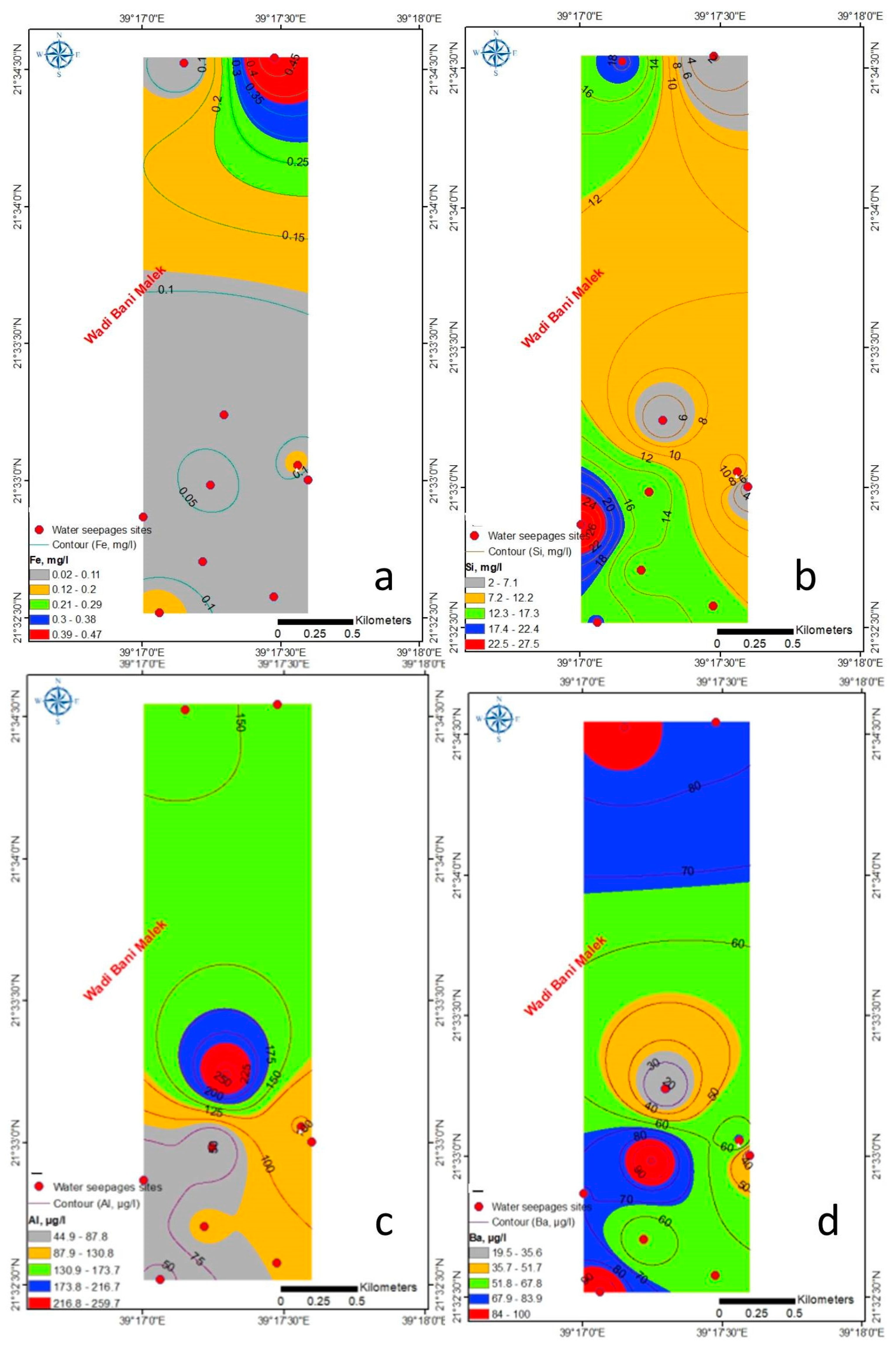
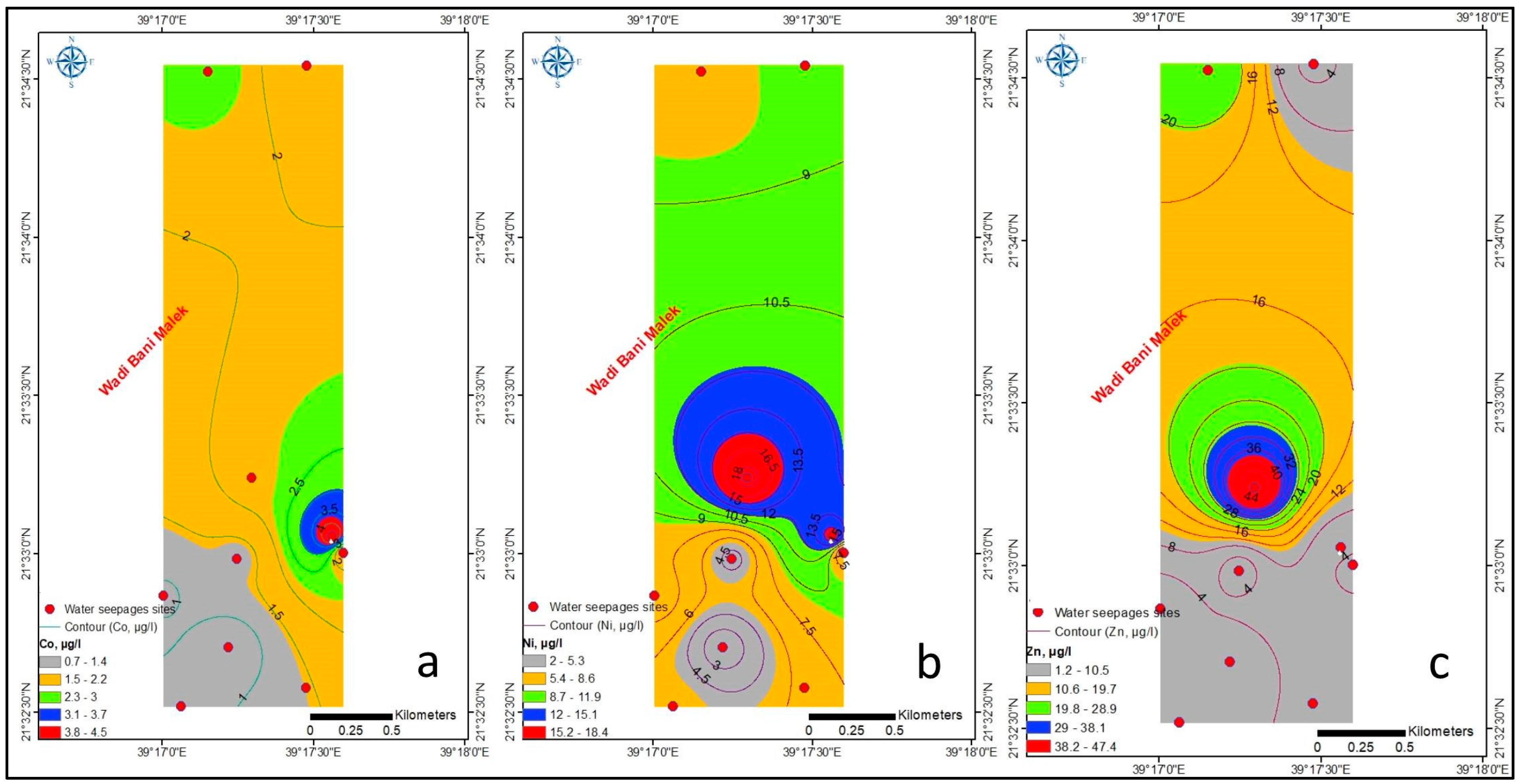
4.1.2. Trace Elements
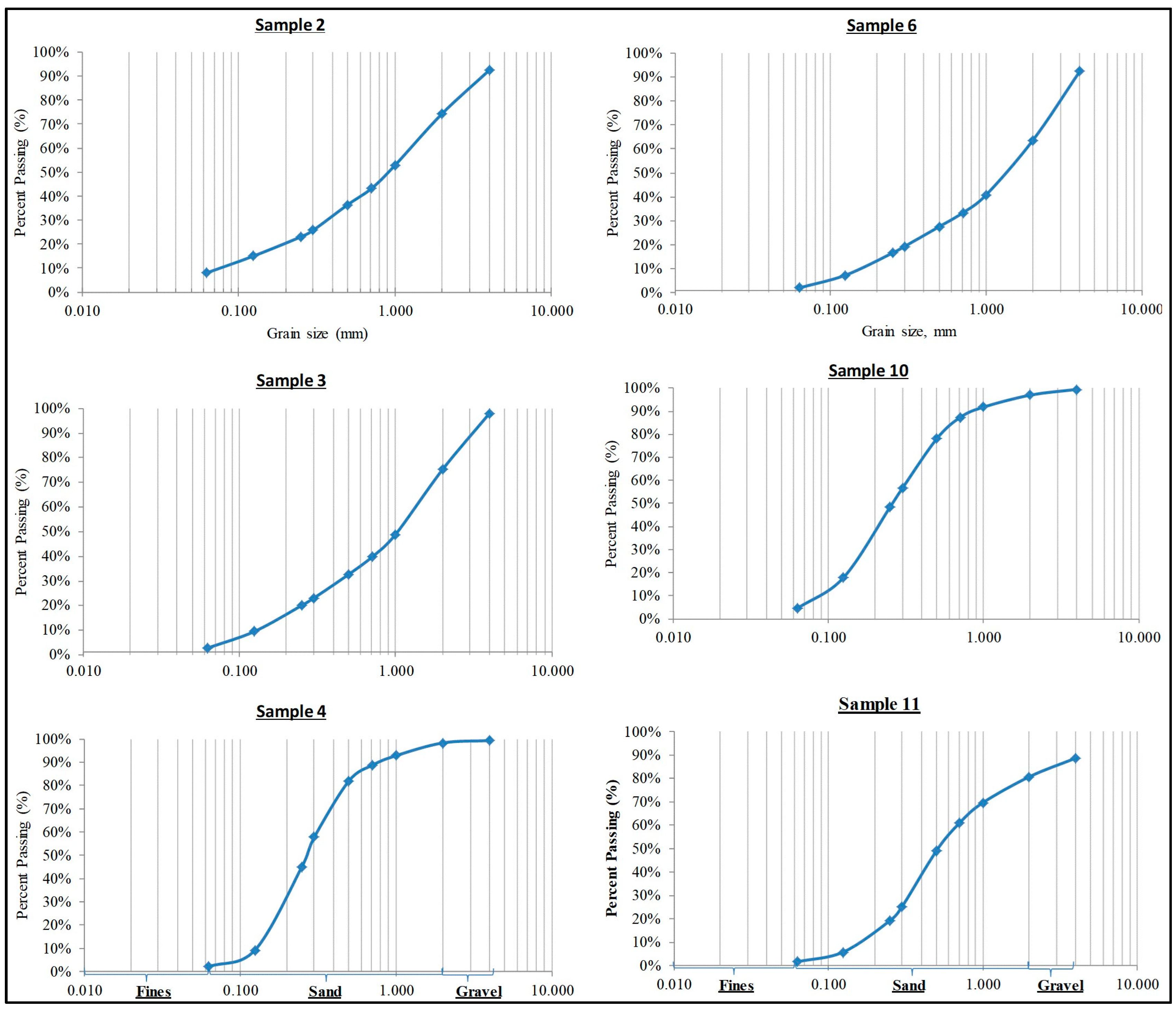
4.2. Grain Size Analysis
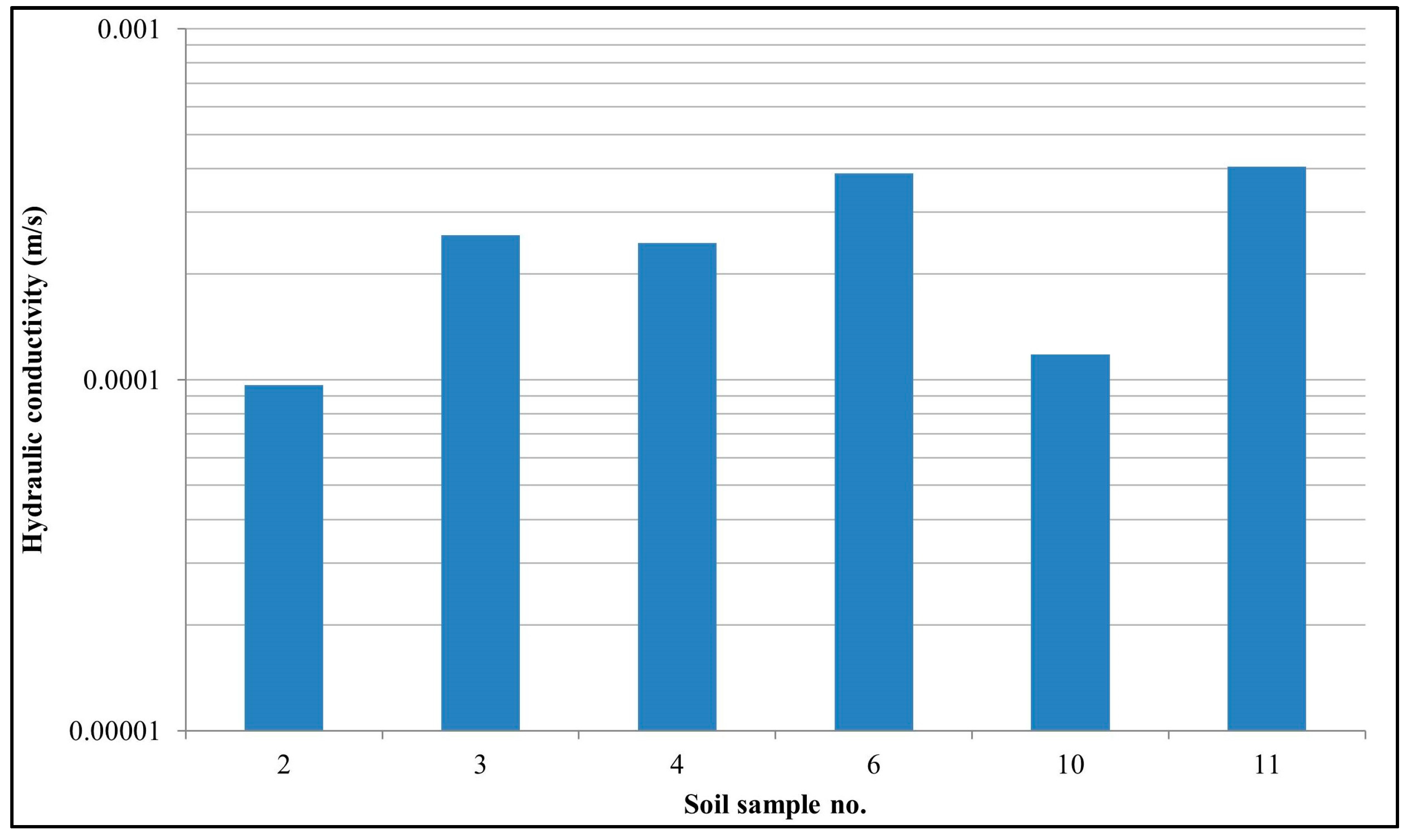
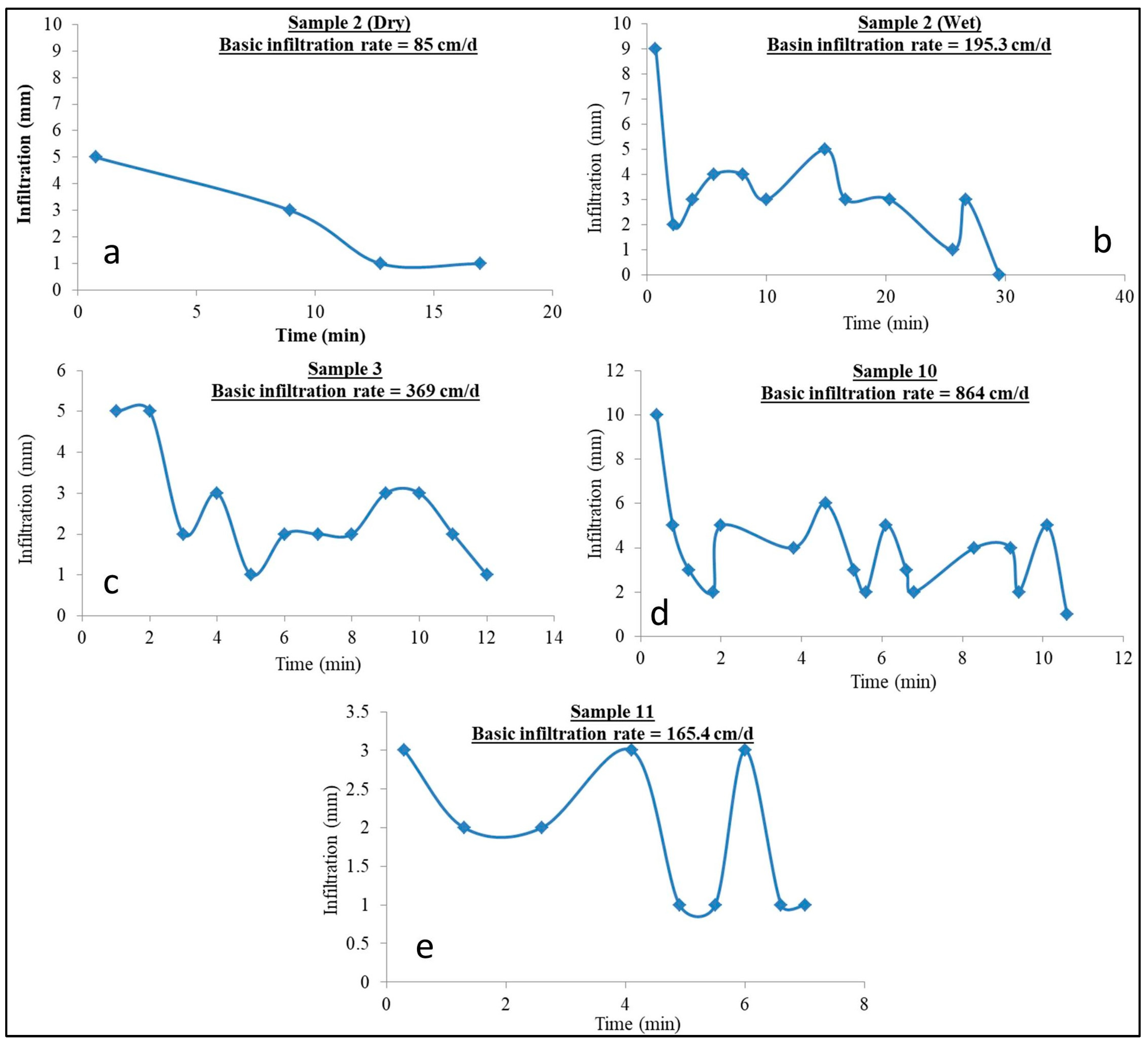
4.3. Infiltration Test
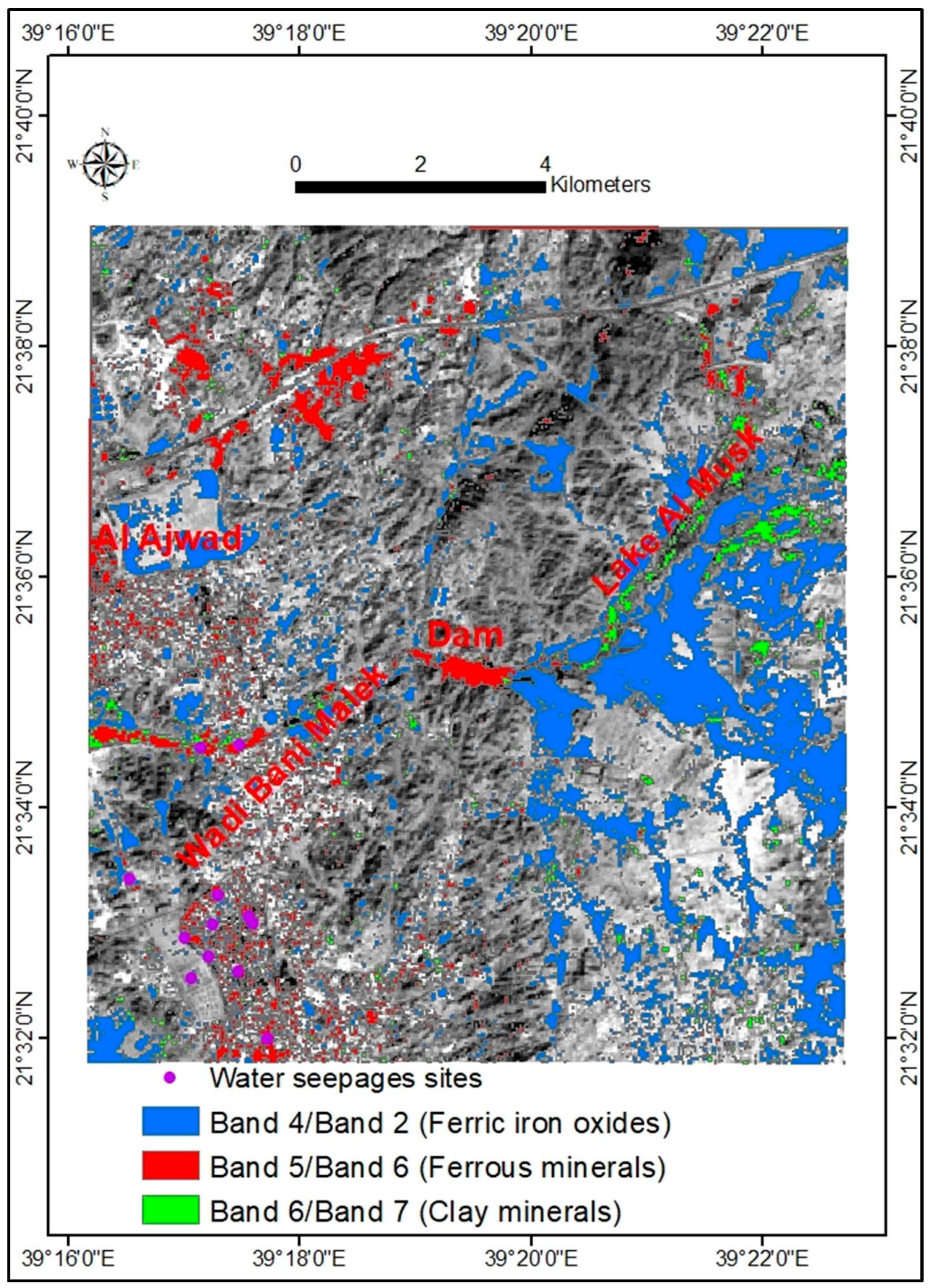
4.4. Landsat Oli 8 Band Ratio (BR) and Color Composite Images
5. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehbandi, R.; Abbasnejad, A.; Karimi, Z.; Herath, I.; Bundschuh, J. Hydrogeochemical controls on arsenic mobility in an arid inland basin, southeast of Iran: The role of alkaline conditions and salt water intrusion. Environ. Pollut. 2019, 249, 910–922. [Google Scholar] [CrossRef]
- Morris, B.L.; Lawrence, A.R.L.; Chilton, P.J.C.; Adams, B.; Calow, R.C.; Klinck, B.A. Groundwater and Its Susceptibility to Degradation: A Global Assessment of the Problemand Options for Management: Early Warning and Assessment Report Series; United Nations Environment Programme: Nairobi, Kenya, 2003. [Google Scholar]
- van Loon, G.W.; Duffy, S.J. Environmental Chemistry: A Global Perspective, 3rd ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Khan, M.Y.A.; ElKashouty, M.; Bob, M. Impact of rapid urbanization and tourism on the groundwater quality in Al Madinah city, Saudi Arabia: A monitoring and modeling approach. Arab. J. Geosci. 2020, 13, 922. [Google Scholar] [CrossRef]
- Bovolo, C.I.; Parkin, G.; Sophocleous, M. Groundwater resources, climate and vulnerability. Environ. Res. Lett. 2009, 4, 035001. [Google Scholar] [CrossRef]
- Gleick, P.H. China and Water. In The World’s Water 2008–2009: The Biennial Report on Freshwater Resources; Gelick, P.H., Morikawa, C.M., Palaniappan, M., Morrison, J., Cohen, M.J., Eds.; Island Press: Washington, DC, USA, 2009. [Google Scholar]
- Jago-on, K.A.B.; Siringan, F.P.; Balangue-Tarriela, R.; Taniguchi, M.; Reyes, Y.K.; Lloren, R.; Peña, M.A.; Bagalihog, E. Hot spring resort development in Laguna Province, Phillppines: Challenges in water use regulation. J. Hydrol. 2017, 11, 96–106. [Google Scholar]
- Lapworth, D.J.; Krishan, G.; MacDonald, A.M.; Rao, M.S. Groundwater quality in the alluvial aquifer system of northwest India: New evidence of the extent of anthropogenic and geogenic contamination. Sci. Total Environ. 2017, 599–600, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Vengosh, A.; Rosenthal, E. Saline groundwater in Israel: Its bearing on the water crisis in the country. J. Hydrol. 1994, 156, 389–430. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, J.J. Origin of groundwater salinity and hydrogeochemical processes in the confined Quaternary aquifer of the Pearl River Delta, China. J. Hydrol. 2012, 438–439, 112–124. [Google Scholar] [CrossRef]
- Khan, M.Y.A.; ElKashouty, M.; Abdellattif, A.; Egbueri, J.C.; Taha, A.I.; Al Deep, M.; Shaaban, F. Influence of natural and anthropogenic factors on the hydrogeology and hydrogeochemistry of Wadi Itwad Aquifer, Saudi Arabia: Assessment using multivariate statistics and PMWIN simulation. Ecol. Indic. 2023, 151, 110287. [Google Scholar] [CrossRef]
- Khan, M.Y.A.; ElKashouty, M.; Khan, N.; Subyani, A.M.; Tian, F. Spatio-temporal evaluation of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater, Khulais, Saudi Arabia. Appl. Water Sci. 2023, 13, 123. [Google Scholar] [CrossRef]
- Guo, H.M.; Wang, Y.X. Geochemical characteristics of shallow groundwater in Datong Basin, northwestern China. J. Geochem. Explor. 2005, 87, 109–120. [Google Scholar] [CrossRef]
- Li, D.N.; Gao, X.B.; Wang, Y.X.; Luo, W.T. Diverse mechanisms drive fluoride enrichment in groundwater in two neighboring sites in northern China. Environ. Pollut. 2018, 237, 430–441. [Google Scholar] [CrossRef]
- Dong, C.; Wu, X.; Gao, Z.; Yang, P.; Khan, M.Y.A. A novel and efficient metal oxide fluoride absorbent for drinking water safety and sustainable development. Sustainability 2021, 13, 883. [Google Scholar] [CrossRef]
- Elumalai, V.; Nwabisa, D.P.; Rajmohan, N. Evaluation of high fluoride contaminated fractured rock aquifer in South Africageochemical and chemometric approaches. Chemosphere 2019, 235, 1–11. [Google Scholar] [CrossRef]
- Gizaw, B. The origin of high bicarbonate and fluoride concentrations in waters of the Main Ethiopian Rift Valley, east African rift system. J. Afr. Earth Sci. 1996, 22, 391–402. [Google Scholar] [CrossRef]
- Rango, T.; Bianchini, G.; Beccaluva, L.; Tassinari, R. Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J. Afr. Earth Sci. 2010, 57, 479–491. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Agbasi, J.C.; Ayejoto, D.A.; Khan, M.I.; Khan, M.Y.A. Extent of anthropogenic influence on groundwater quality and human health-related risks: An integrated assessment based on selected physicochemical characteristics. Geocarto Int. 2023, 38, 2210100. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Agbasi, J.C.; Ikwuka, C.F.; Chiaghanam, O.I.; Khan, M.I.; Khan, M.Y.A.; Khan, N.; Uwajingba, H.C. Nitrate health risk and geochemical characteristics of water in a semi-urban: Implications from graphical plots and statistical computing. Int. J. Environ. Anal. Chem. 2023, 1–21. [Google Scholar] [CrossRef]
- Agbasi, J.C.; Chukwu, C.N.; Nweke, N.D.; Uwajingba, H.C.; Khan MY, A.; Egbueri, J.C. Water pollution indexing and health risk assessment due to PTE ingestion and dermal absorption for nine human populations in Southeast Nigeria. Groundw. Sustain. Dev. 2023, 21, 100921. [Google Scholar] [CrossRef]
- Jacks, G.; Bhattacharya, P.; Chaudhary, V.; Singh, K.P. Controls on the genesis of some high-F groundwaters in India. Appl. Geochem. 2005, 20, 221–228. [Google Scholar] [CrossRef]
- Vikas, C.; Kushwaha, R.; Ahmad, W.; Prasannakumar, V.; Reghunath, R. Genesis and geochemistry of high fluorine-bearing groundwater from a semi-arid terrain ofNWIndia. Environ. Earth Sci. 2013, 68, 289–305. [Google Scholar] [CrossRef]
- Krishan, G.; Rao, M.S.; Vashisht, R.; Chaudhary, A.; Singh, J.; Kumar, A. Isotopic assessment of groundwater salinity: A case study of the Southwest (SW) Region of Punjab, India. Water 2022, 14, 133. [Google Scholar] [CrossRef]
- Krishan, G.; Kumar, B.; Sudarsan, N.; Rao, M.S.; Ghosh, N.C.; Taloor, A.K.; Bhattacharya, P.; Singh, S.; Kumar, C.P.; Sharma, A.; et al. Isotopes (δ18O, δD and 3H) variations in groundwater with emphasis on salinization in the state of Punjab, India. Sci. Total Environ. 2021, 789, 148051. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G.; Macdonald, D.M.J.; Nicolli, H.B.; Barros, A.J.; Tullio, J.O.; Pearce, J.M.; Alonso, M.S. Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Appl. Geochem. 2005, 20, 989–1016. [Google Scholar] [CrossRef]
- Zabala, M.E.; Manzano, M.; Vives, L. Assessment of processes controlling the regional distribution of fluoride and arsenic in groundwater of the Pampeano aquifer in the Del Azul Creek basin (Argentina). J. Hydrol. 2016, 541, 1067–1087. [Google Scholar] [CrossRef]
- Avrahamov, N.; Antler, G.; Yechieli, Y.; Gavrieli, I.; Joye, S.B.; Saxton, M.; Turchyn, A.V.; Sivan, O. Anaerobic oxidation of methane by sulfate in hypersaline groundwater of the Dead Sea aquifer. Geobiology 2014, 12, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.M.; Wang, Y.X.; Ma, T. Isotope and minor element geochemistry of high arsenic groundwater from Hangjinhouqi, the Hetao Plain, Inner Mongolia. Appl. Geochem. 2009, 24, 587–599. [Google Scholar] [CrossRef]
- Li, C.C.; Gao, X.B.; Liu, Y.S.; Wang, Y.X. Impact pf anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Naftz, D.L.; Bullen, T.D.; Stolp, B.J.; Wilkowske, C.D. Utilizing geochemical, hydrologic, and boron isotopic data to assess the success of a salinity and selenium remediation project, Upper Colorado River Basin, Utah. Sci. Total Environ. 2008, 392, 1–11. [Google Scholar] [CrossRef]
- Santucci, L.; Carol, E.; Kruse, E. identification of palaeo-seawater intrusion in groundwater using minor ions in a semi-confined aquifer of the Rio de la Plata littoral (Argentina). Sci. Total Environ. 2016, 566–567, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Vengosh, A.; Dwyer, G.; Bianchini, G. Mobilization of arsenic and other naturally occurring contaminants in groundwater of the Main Ethiopian Rift aquifers. Water Res. 2013, 47, 5801–5818. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, B.R.; Nicot, J.P.; Reedy, R.C.; Kurtzman, D.; Mukherjee, A.; Nordstrom, D.K. Elevated naturally occurring arsenic in a semi-arid oxidizing system, southern high plain aquifer, Texas, USA. Appl. Geochem. 2009, 24, 2061–2071. [Google Scholar] [CrossRef]
- Singh, R.K.; Sengupta, B.; Bal, R.; Shukla, B.P.; Gurunadharao, V.S.; Srivatstava, R. Identification and mapping of chromium (VI) plume in groundwater for remediation: A case study at Kanpur Uttar Pradesh. J. Geol. Soc. India 2009, 74, 49–57. [Google Scholar] [CrossRef]
- Brindha, K.; Rajib, P.; Julien, W.; Mou, L.T.; Mahesh, K.S. Trace metals contamination in groundwater and implications on human health: Comprehensive assessment using hydrogeochemical and geostatistical methods. Environ. Geochem. Health 2020, 42, 3819–3839. [Google Scholar] [CrossRef] [PubMed]
- Vasanthavigar, M.; Srinivasamoorthy, K.; Rajiv Ganthi, R.; Vijayaraghavan, K.; Sarma, V. Characterisation and quality assessment of groundwater with a special emphasis on irrigation utility: Thirumanimuttar sub-basin, Tamil Nadu, India. Arab. J. Geosci. 2012, 5, 245–258. [Google Scholar] [CrossRef]
- Belkhiri, L.; Tiri, A.; Mouni, L. Assessment of heavy metals contamination in groundwater: A case study of the south of setif area, East Algeria. In Achievements and Challenges of Integrated River Basin Management; IntechOpen: London, UK, 2018; pp. 17–31. [Google Scholar]
- Khademi, H.; Gabarrón, M.; Abbaspour, A.; Martínez-Martínez, S.; Faz, A.; Acosta, J.A. Environmental impact assessment of industrial activities on heavy metals distribution in street dust and soil. Chemosphere 2019, 217, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 330. [Google Scholar] [CrossRef]
- Shaxson, T.F.; Barber, R.G. Optimizing Soil Moisture for Plant Production: The Significance of Soil Porosity (No. 79); Food and Agriculture Organization: Rome, Italy, 2003. [Google Scholar]
- Zhao, K.; Fu, W.; Qiu, Q.; Ye, Z.; Li, Y.; Tunney, H.; Dou, C.; Zhou, K.; Qian, X. Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma 2019, 337, 453–462. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nation (FAO). Saudi Arabia irrigation in the Middle East regions in figure. Aquatat Survey 2008. In Land FAO and Water Division Report; Freken, K., Ed.; FAO: Rome, Italy, 2009; Volume 34, pp. 325–337. [Google Scholar]
- Khan, M.Y.A.; El Kashouty, M.; Gusti, W.; Kumar, A.; Subyani, A.M.; Alshehri, A. Geo-temporal signatures of physicochemical and heavy metals pollution in Groundwater of Khulais region—Makkah Province, Saudi Arabia. Front. Environ. Sci. 2022, 9, 699. [Google Scholar] [CrossRef]
- Ouda, O.K.M.; Shawesh, A.; Al-Olabi, T.; Younes, F.; Al-Waked, R. Review of domestic water conservation practices in Saudi Arabia. Appl. Water Sci. 2013, 3, 689–699. [Google Scholar] [CrossRef]
- Hakami, B.A.; Seif ES, S.A.; El-Shater, A.A. Environmental pollution assessment of Al-Musk Lake, Jeddah, Saudi Arabia. Nat. Hazards 2020, 101, 429–448. [Google Scholar] [CrossRef]
- Saleem, H.A.; Subyani, A.M.; Elfeki, A. Solute transport model for groundwater contamination in Wadi Bani Malik, Jeddah, Saudi Arabia. Arab. J. Geosci. 2019, 12, 148. [Google Scholar] [CrossRef]
- Ewea, H.A.S.A. Hydrological analysis of flooding wastewater lake in Jeddah, Saudi Arabia. JKAU Meteorol. Environ. Arid Land Agric. Sci. 2010, 21, 125–144. [Google Scholar] [CrossRef]
- Elfeki, A.; Ewea, H.; Al-Amri, N. Linking groundwater flow and transport models, GIS technology, satellite images and uncertainty quantification for decision making: Buraiman Lake case study Jeddah, Saudi Arabia. Int. J. Water Resour. Arid. Environ. 2011, 1, 295–303. [Google Scholar]
- Rehman, F.; Cheema, T. Effects of sewage waste disposal on the groundwater quality and agricultural potential of a floodplain near Jeddah, Saudi Arabia. Arab. J. Geosci. 2016, 9, 307. [Google Scholar] [CrossRef]
- Bahabri, A.A. Geo-Environmental Assessments of Polluted Water and Soil Due to Sewage Water at Wadi Uranah, Southwest of Makkah. Ph.D. Thesis, King Abdul Aziz University, Jeddah, Saudi Arabia, 2011. [Google Scholar]
- Rashed, M.; Niyazi, B. Environmental impact assessment of the former Al-Musk lake wastewater dumpsite using electromagnetic induction technique. Earth Syst. Environ. 2017, 1, 10. [Google Scholar] [CrossRef]
- Daoudi, M.; Niang, A.J. Flood Risk and Vulnerability of Jeddah City, Saudi Arabia. In Recent Advances in Flood Risk Management; IntechOpen: London, UK, 2019; pp. 634–654. [Google Scholar]
- Moore, T.A.; Al-Rehaili, M.H. Geologic Map of the Makkah Quadrangle, Sheet 21D, Kingdom of Saudi Arabia; Ministry of Petroleum and Mineral Resources, Deputy Ministry For Mineral Resources Publication: Jeddah, Saudi Arabia, 1989. [Google Scholar]
- Lecia Geosystems & GIS Mapping Division. Erdas Field Guide, 7th ed.; Erdas: Atlanta, GA, USA, 2003; 698p. [Google Scholar]
- APHA. Standard Methods of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; pp. 2–61. ISBN 0875530478. [Google Scholar]
- ASTM Standards. Annual Book of ASTM Standards 11.01; American Society for Testing and Materials: Easton, MD, USA, 1980; p. 83. [Google Scholar]
- Rehman, F.; Cheema, T. Boron contamination in groundwater at a sewage waste disposal facility near Jeddah, Saudi Arabia. Environ. Earth Sci. 2017, 76, 218. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Partani, S.; Araghi, A.; Barati, R.; Farnoush, H.; Torabi Haghighi, A. Iran’s groundwater hydrochemistry. Earth Space Sci. 2021, 8, e2021EA001793. [Google Scholar] [CrossRef]
- Mirani Moghadam, H.; Karami, G.H.; Bagheri, R.; Barati, R. Death time estimation of water heritages in Gonabad Plain, Iran. Environ. Earth Sci. 2021, 80, 127. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Sadegh, M.; Sarvarzadeh, F.; Akbarzadeh, A.E.; Karandish, F.; Barati, R.; Taherpour, H. Anthropogenic decline of ancient, sustainable water systems: Qanats. Groundwater 2023, 61, 139–146. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; pp. xx, 3, 47, 161–162, 164, 177, 183, 223–224, 311, 323–324, 343, 387, 389–390, 394, 398–399, 413–415, 430. [Google Scholar]
- WHO. Boron in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; Report WHO/HSC/WSH/09.01/2; WHO: Geneva, Switzerland, 2009; p. 28. [Google Scholar]
- Weast, R.C. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1985; pp. D165–D166. [Google Scholar]
- EPA. Regulatory Determinations Support Document for Selected Contaminants for the Second Drinking Water Contaminant Candidate List (CCL2); Office of Water (4607M), EPA 815-R-08-012; EPA: Washington, DC, USA, 2008. [Google Scholar]
- ISO 9390; Water Quality—Determination of Borate—Spectrometric Method Using Azomethine-H. International Organization for Standardization: Geneva, Switzerland, 1990.
- Moss, S.A.; Nagpal, N.; Branch, W.P. Ambient Water Quality Guidelines for Boron; British Columbia Technical Report; Water Protection Branch, Ministry of Water, Land and Air Protection: Victoria, BC, Canada, 2003; p. 112. [Google Scholar]
- Vengosh, A.; Kolodny, Y.; Spivack, A. Application of isotopes techniques to investigate groundwater pollution. IAEA TECHDOC 1998, 1046, 17–38. [Google Scholar]
- Ravenscroft, P.; McArthur, J. Mechanism of regional enrichment of groundwater by boron: The examples of Bangladesh and Michigan, USA. Appl. Geochem. 2004, 19, 1413–1430. [Google Scholar] [CrossRef]
- Koda, E.; Miszkowska, A.; Sieczka, A. Levels of organic pollution indicators in groundwater at the old landfill and waste management site. Appl. Sci. 2017, 7, 638. [Google Scholar] [CrossRef]
- Ukah, B.U.; Egbueri, J.C.; Unigwe, C.O.; Ubido, O.E. Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. Int. J. Energy Water Resour. 2019, 3, 291–303. [Google Scholar] [CrossRef]
- Casagrande, A. Classification and Identification of Soils. Trans. Am. Soc. Civ. Eng. 1948, 113, 901–930. [Google Scholar] [CrossRef]
- Onur, E.M. Predicting the Permeability of Sandy Soils from Grain Size Distributions. Ph.D. Dissertation, Kent State University, Portage County, OH, USA, 2014. [Google Scholar]
- Hazen, A. Some Physical Properties of Sands and Gravels, with Special Reference to Their Use in Filtration; 24th Annual Report; Massachusetts State Board of Health, Pub. Doc.: Boston, MA, USA, 1892; Volume 34, pp. 539–556. [Google Scholar]
- Diamond, J.; Shanley, T. Infiltration rate assessment of some major soils. Ir. Geogr. 2003, 36, 32–46. [Google Scholar] [CrossRef]
- Bhave, S.; Sreeja, P. Influence of initial soil condition on infiltration characteristics determined using a disk infiltrometer. ISH J. Hydraul. Eng. 2013, 19, 291–296. [Google Scholar] [CrossRef]
- Singh, B.; Sihag, P.; Singh, D. Study of infiltration characteristics of locally soils. J. Civ. Eng. Environ. Technol. 2014, 1, 9–13. [Google Scholar]
- Drury, S.A. Image Interpretation in Geology, 2nd ed.; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Lillesand, T.M.; Kiefer, R.W. Remote Sensing and Image Interpretation, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; 724p. [Google Scholar]
- Gupta, R.P. Remote Sensing Geology; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; London, UK; Paris, France; Tokyo, Japan; Hong Kong, China, 1991; xvi + 356p, ISBN 3 540 52805 9. Price DM 198.00 (Hard Covers). [Google Scholar]

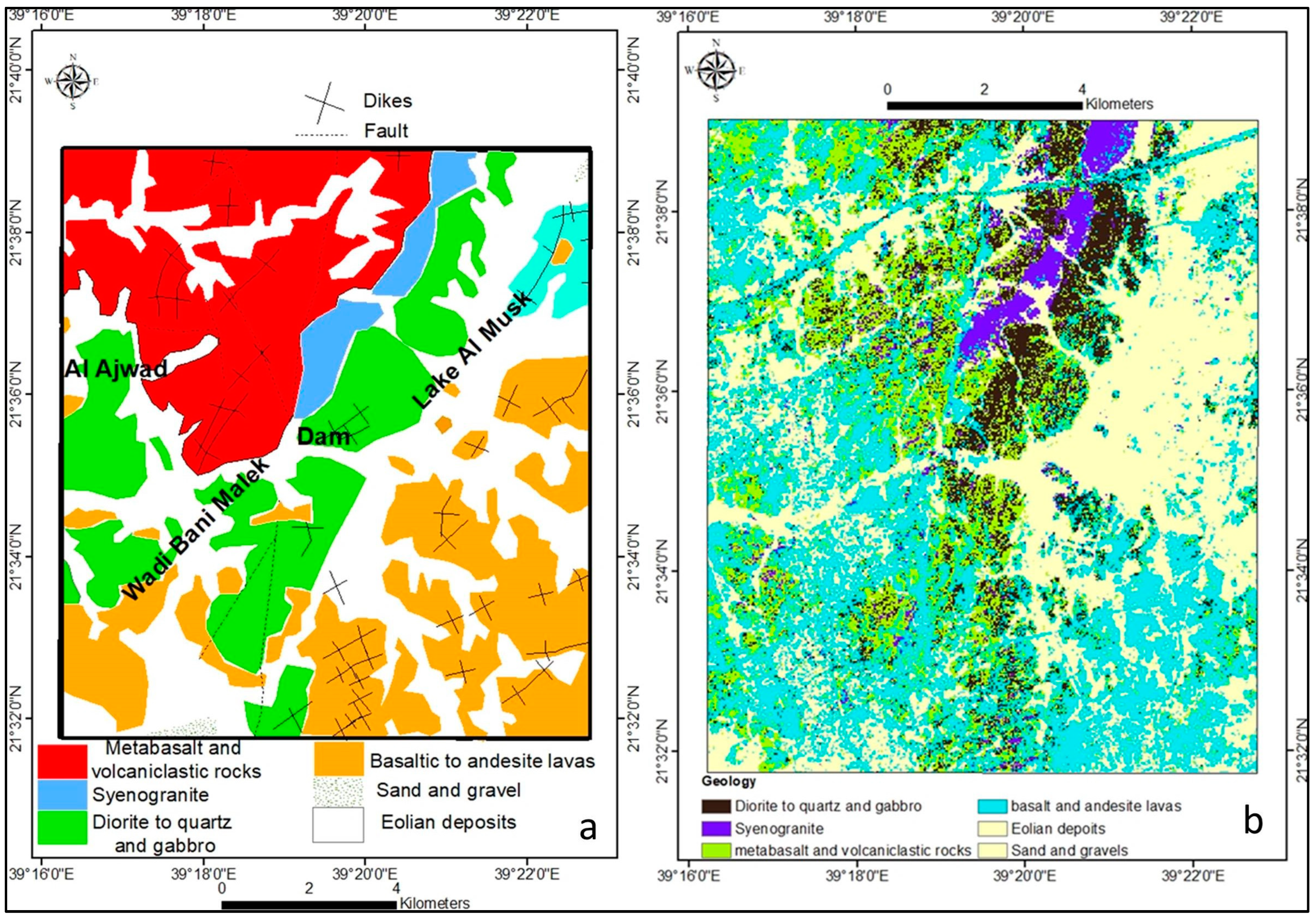


| Geology | Area (km2) | Area (%) | No. of AOI |
|---|---|---|---|
| Diorite to quartz and gabbro | 14.9 | 9.8 | 11 |
| Syenogranite | 5.3 | 3.5 | 7 |
| Metabasalt and volcaniclastic rocks | 25.1 | 16.6 | 16 |
| Basalt and andesite lavas | 48.7 | 32.2 | 28 |
| Eolian deposits and sand gravels | 57.3 | 37.9 | 25 |
| Total No. of AOI | 87 |
| EC | TDS | PH | Temp | Ca | Mg | Na | K | HCO3 | SO4 | Cl | B | Fe | Si | NO3 | Al | Ba | Co | Ni | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | 1 | |||||||||||||||||||
| TDS | 0.992 ** | |||||||||||||||||||
| PH | 0.36 | 0.384 | ||||||||||||||||||
| Temp | −0.337 | −0.254 | 0.075 | |||||||||||||||||
| Ca | 0.455 | 0.375 | −0.331 | −0.822 | ||||||||||||||||
| Mg | 0.822 ** | 0.772 * | −0.08 | −0.687 | 0.865 ** | |||||||||||||||
| Na | 0.975 ** | 0.987 ** | 0.446 | −0.212 | 0.268 | 0.702 * | ||||||||||||||
| K | 0.621 | 0.589 | −0.314 | −0.552 | 0.644 | 0.823 ** | 0.569 | |||||||||||||
| HCO3 | −0.517 | −0.448 | −0.09 | 0.452 | −0.692 | −0.63 | −0.338 | −0.229 | ||||||||||||
| SO4 | 0.894 ** | 0.930 ** | 0.469 | −0.049 | 0.055 | 0.541 | 0.969 ** | 0.503 | −0.127 | |||||||||||
| Cl | 0.986 ** | 0.997 ** | 0.352 | −0.261 | 0.385 | 0.779 * | 0.980 ** | 0.605 | −0.432 | 0.927 ** | ||||||||||
| B | 0.992 ** | 0.993 ** | 0.424 | −0.329 | 0.392 | 0.780 * | 0.987 ** | 0.597 | −0.454 | 0.925 ** | 0.989 ** | |||||||||
| Fe | 0.205 | 0.137 | −0.114 | −0.397 | 0.509 | 0.399 | 0.106 | 0.114 | −0.392 | −0.05 | 0.121 | 0.167 | ||||||||
| Si | −0.362 | −0.381 | −0.519 | 0.109 | 0.144 | −0.102 | −0.468 | 0.021 | −0.235 | −0.507 | −0.371 | −0.428 | −0.446 | |||||||
| NO3 | −0.176 | −0.157 | −0.068 | 0.224 | −0.164 | −0.276 | −0.195 | −0.473 | −0.092 | −0.174 | −0.151 | −0.195 | −0.281 | 0.255 | ||||||
| Al | 0.03 | 0.029 | −0.03 | −0.394 | −0.019 | 0.079 | 0.131 | 0.37 | 0.543 | 0.213 | 0.042 | 0.099 | 0.097 | −0.579 | −0.376 | |||||
| Ba | −0.212 | −0.266 | −0.009 | −0.226 | 0.389 | 0.063 | −0.374 | −0.212 | −0.595 | −0.54 | −0.274 | −0.26 | 0.192 | 0.504 | −0.096 | −0.624 | ||||
| Co | 0.376 | 0.308 | 0.469 | −0.443 | 0.157 | 0.286 | 0.385 | 0.305 | −0.189 | 0.322 | 0.259 | 0.38 | 0.052 | −0.233 | −0.319 | 0.317 | −0.062 | |||
| Ni | 0.046 | 0.008 | 0.066 | −0.163 | −0.112 | 0.023 | 0.132 | 0.366 | 0.392 | 0.177 | −0.025 | 0.059 | 0.023 | −0.288 | −0.539 | 0.652 | −0.421 | 0.730 * | ||
| Zn | −0.273 | −0.265 | −0.107 | −0.218 | −0.22 | −0.195 | −0.156 | 0.247 | 0.708 * | −0.03 | −0.25 | −0.202 | −0.233 | −0.256 | −0.379 | 0.894 ** | −0.498 | 0.277 | 0.688 * | 1 |
| Parameter | Min. | Max. | Average | WHO [62] |
|---|---|---|---|---|
| EC | 3800 | 91,000 | 32,547.78 | |
| TDS | 1880 | 54,500 | 17,184.44 | 1000 |
| PH | 7.06 | 8.88 | 8.17 | 6.5–8.5 |
| Ca | 55.94 | 1540 | 768.28 | 100 |
| Mg | 18.25 | 580 | 298.98 | 150 |
| Na | 1127.6 | 27,022 | 8406.86 | 200 |
| K | 15.2 | 106.3 | 62.57 | 200 |
| HCO3− | 158.6 | 805.3 | 330.82 | |
| SO42− | 246.5 | 6759.4 | 1999.21 | 250 |
| Cl | 1025 | 42,000 | 12,624.56 | 250 |
| B | 1.91 | 38 | 12.22 | 2.4 |
| Fe | 0.02 | 0.47 | 0.12 | 0.3 |
| Si | 1.95 | 27.5 | 12.42 | 25 |
| NO32− | 2.2 | 35 | 10.59 | 50 |
| Al | 44.86 | 259.66 | 113.17 | 1 |
| Ba | 19.5 | 100.01 | 67.39 | 0.7 |
| Co | 0.66 | 4.5 | 1.78 | 2 |
| Ni | 2.01 | 18.41 | 8.59 | 0.07 |
| Zn | 1.22 | 47.37 | 9.96 | 3 |
| BOD | <1 | 11.3 | 10 | |
| As | <0.0015 | 0.02468 | 0.01 | |
| Cd | <0.0005 | <0.0005 | 0.003 | |
| Cr | <0.00015 | 0.00461 | 0.05 | |
| Cu | <0.00015 | 0.0092 | 2 | |
| Pb | <0.00011 | 0.00083 | 0.01 |
| Sample No. | BOD | NO3 | As | Cd | Cr | Cu | Pb |
|---|---|---|---|---|---|---|---|
| mg/L | mg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| 1 | <1 | 35 | 5.25 | <0.5 | 0.73 | 5.18 | <1.1 |
| 2 | 2.8 | 13.6 | 7.35 | <0.5 | <0.15 | 0.96 | <1.1 |
| 3 | 2.8 | 2.2 | 7.82 | <0.5 | 4.61 | 2.9 | <1.1 |
| 4 | 1.7 | 15 | < 1.5 | <0.5 | <0.15 | 1.21 | 0.83 |
| 5 | 5.6 | 10 | 24.68 | <0.5 | <0.15 | 9.02 | <1.1 |
| 6 | <1 | 9 | 20.42 | <0.5 | <0.15 | 0.57 | <1.1 |
| 9 | 11.3 | 2.4 | 10.26 | <0.5 | 0.13 | <0.15 | <1.1 |
| 11 | 2.8 | 2.2 | <1.5 | <0.5 | <0.15 | <0.15 | 3.47 |
| 12 | <1 | 5.9 | 22.27 | <0.5 | <0.15 | <0.15 | <1.1 |
| S. No. | (D10) | (D25) | (D30) | (D60) | (D75) | Cu | Cc | C | K (m/s) | Fine (%) | Sand (%) | Gravel (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 0.08 | 0.34 | 0.38 | 1.34 | 1.99 | 16.67 | 1.35 | 2.42 | 9.70 × 10−5 | 8.1 | 84.2 | 7.7 |
| 3 | 0.13 | 0.29 | 0.45 | 1.42 | 2.08 | 10.85 | 1.06 | 2.69 | 2.60 × 10−4 | 2.8 | 95.3 | 1.9 |
| 4 | 0.13 | 0.18 | 0.2 | 0.32 | 0.44 | 2.5 | 0.96 | 1.57 | 2.40 × 10−4 | 2.1 | 97.3 | 0.6 |
| 6 | 0.16 | 0.44 | 0.59 | 1.84 | 2.79 | 11.49 | 1.17 | 2.53 | 3.90 × 10−4 | 2.1 | 90.2 | 7.7 |
| 10 | 0.09 | 0.15 | 0.17 | 0.33 | 0.47 | 3.74 | 1.04 | 1.75 | 1.20 × 10−4 | 4.5 | 95 | 0.5 |
| 11 | 0.16 | 0.3 | 0.34 | 0.69 | 1.48 | 4.21 | 1.02 | 2.23 | 4 × 10−4 | 1.7 | 87.1 | 11.2 |
| Ratio | Min. | Max. | Mean | SD | Threshold Value | Confidence % |
|---|---|---|---|---|---|---|
| Band 4/Band 2 | 0 | 255 | 140.6 | 60.1 | 201 | 92 |
| Band 5/Band 6 | 0 | 255 | 96.2 | 49.3 | 194 | 95 |
| Band 6/Band 7 | 0 | 255 | 106.2 | 52.7 | 212 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElKashouty, M.; Khan, M.Y.A.; Alharbi, K.; Pande, C.B.; Subyani, A.M.; Tian, F. Hydrogeology and Hydrogeochemistry of Saline Groundwater Seepage Zones in Wadi Bani Malik Basin, Jeddah, Saudi Arabia: Impacts on Soil and Water Resources. Water 2023, 15, 3464. https://doi.org/10.3390/w15193464
ElKashouty M, Khan MYA, Alharbi K, Pande CB, Subyani AM, Tian F. Hydrogeology and Hydrogeochemistry of Saline Groundwater Seepage Zones in Wadi Bani Malik Basin, Jeddah, Saudi Arabia: Impacts on Soil and Water Resources. Water. 2023; 15(19):3464. https://doi.org/10.3390/w15193464
Chicago/Turabian StyleElKashouty, Mohamed, Mohd Yawar Ali Khan, Khalid Alharbi, Chaitanya B. Pande, Ali M. Subyani, and Fuqiang Tian. 2023. "Hydrogeology and Hydrogeochemistry of Saline Groundwater Seepage Zones in Wadi Bani Malik Basin, Jeddah, Saudi Arabia: Impacts on Soil and Water Resources" Water 15, no. 19: 3464. https://doi.org/10.3390/w15193464
APA StyleElKashouty, M., Khan, M. Y. A., Alharbi, K., Pande, C. B., Subyani, A. M., & Tian, F. (2023). Hydrogeology and Hydrogeochemistry of Saline Groundwater Seepage Zones in Wadi Bani Malik Basin, Jeddah, Saudi Arabia: Impacts on Soil and Water Resources. Water, 15(19), 3464. https://doi.org/10.3390/w15193464







