Green Development of Titanium Dioxide Using Astragalus boeticus for the Degradation of Cationic and Anionic Dyes in an Aqueous Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Astragalus boeticus Extract
2.3. Synthesis of TiO2 NPs by the Extract of Astragalus boeticus
2.4. Characterization Techniques
2.5. Photodegradation Test of RY161 and CV Dyes by Elaborated Green TiO2 NPs
2.6. Reusability Test
3. Results and Discussion
3.1. Sample Characterization
3.1.1. XRD Analysis
3.1.2. FTIR Analysis
3.1.3. SEM with EDX Analysis
3.1.4. UV-Vis Analysis
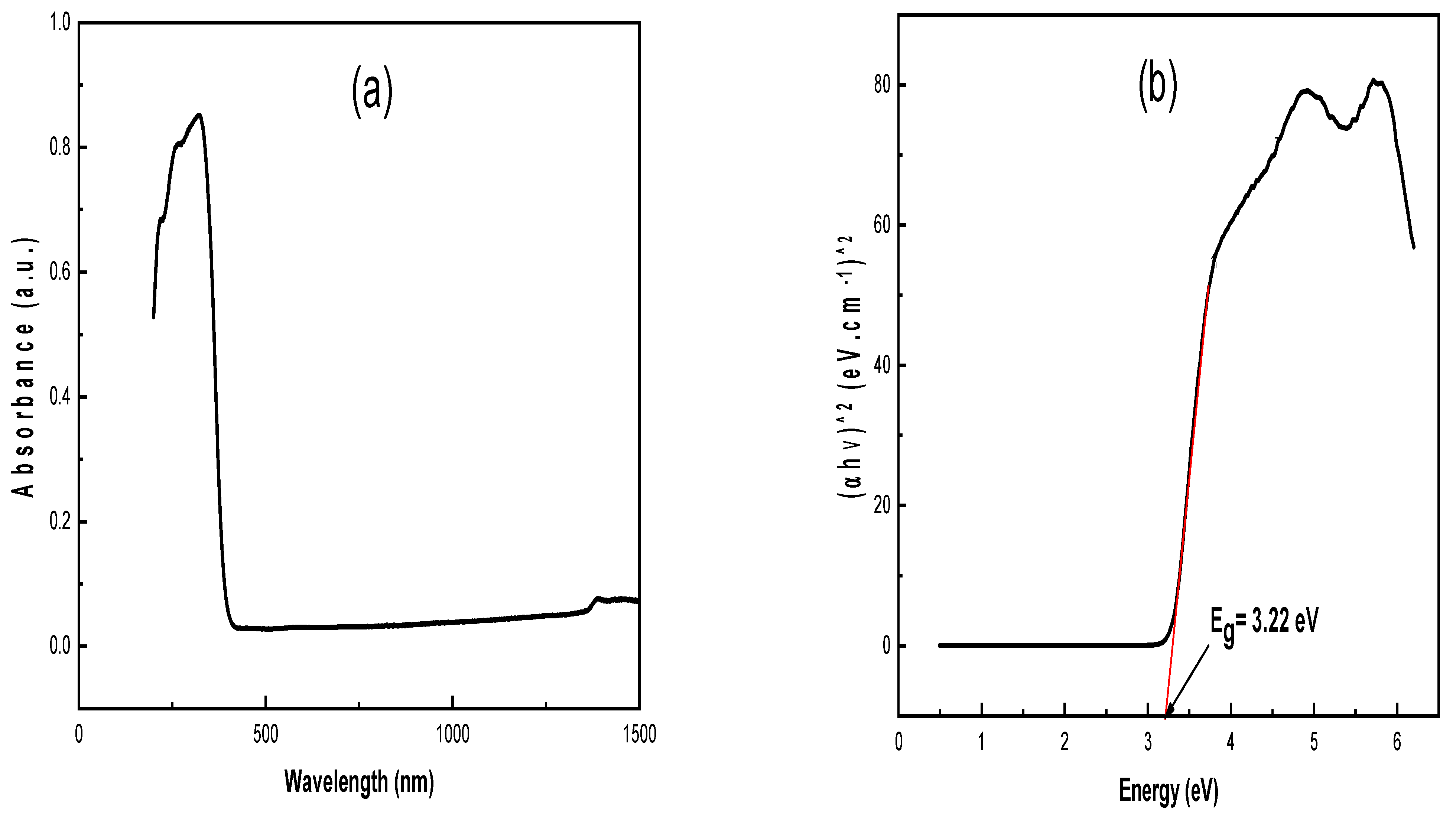
3.2. Photocatalytic Activity of TiO2 NPs for Degradation of Dyes
3.2.1. Effect of Photolysis and Adsorption
3.2.2. Effect of TiO2 NPs Mass and Kinetic Study
3.2.3. Effect of Concentration and Kinetic Study
3.2.4. Effect of Solution pH and Kinetic Study
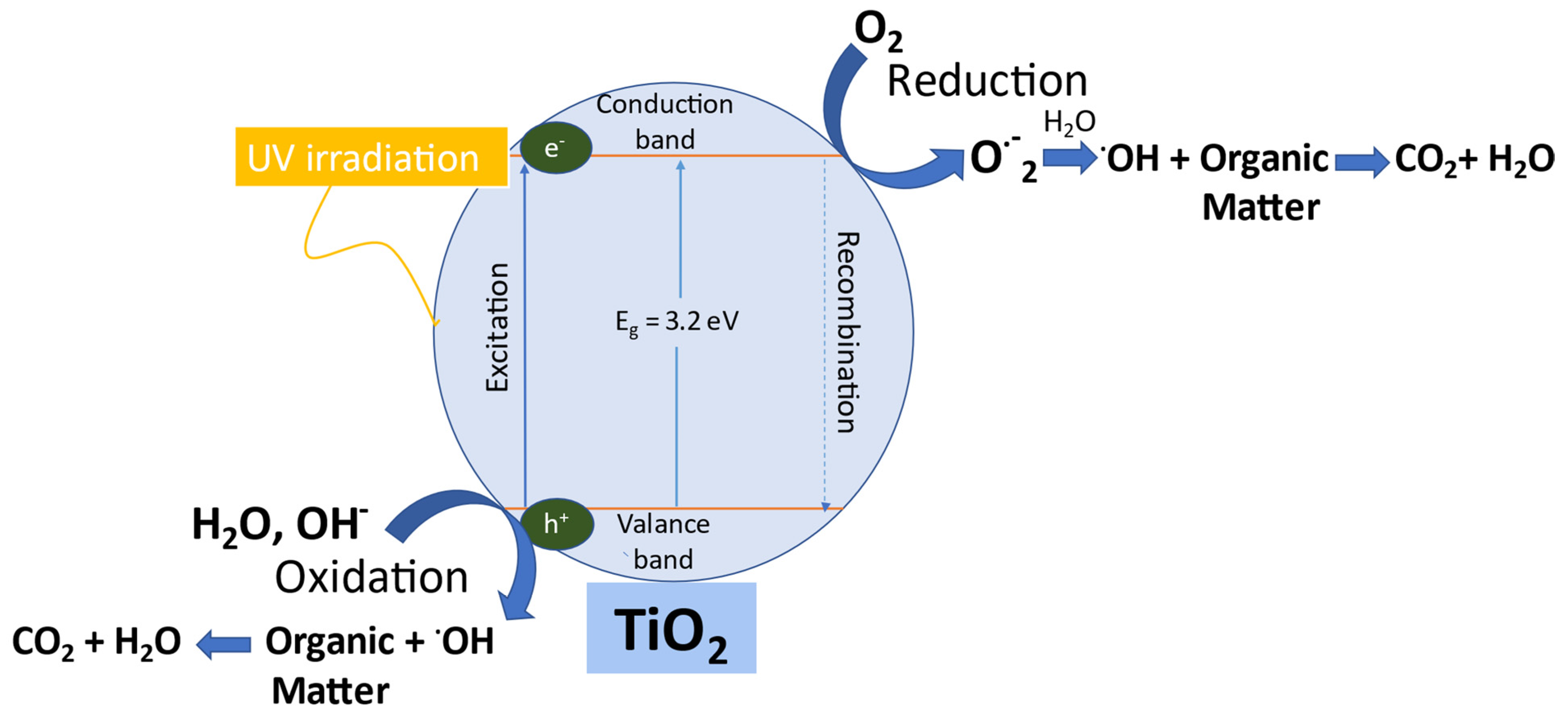
3.2.5. Mechanism Proposed for the Photocatalytic Activity of TiO2 NPs
3.3. Reusability of the catalyst TiO2 NPs
3.4. Comparative Study of the Photocatalytic Degradation of Organic Pollutants by Nanomaterials Synthesized Using a Green Way
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TiO2 | Titanium Dioxide |
| TiO2-NP | Titanium Dioxide Nanoparticle |
| TTIP | Titanium (IV) Isopropoxide |
| A.B | Astragalus boeticus |
| RY161 | Reactive Yellow 161 |
| CV | Crystal Violet |
| XRD | X-Ray Diffraction |
| FTIR | Fourier-Transmission Infrared |
| SEM | Scanning Electron Microscopy |
| EDX | Energy-Dispersive Analysis |
| UV-Vis | Ultraviolet Visible |
| eV | Electron Volt |
| pH | Hydrogen Potential |
| NaOH | Sodium Hydroxide |
| HCl | Hydrochloric Acid |
| PZC | Point of Zero Charge |
| OH. | Hydroxyl Radical |
| AOPs | Advanced Oxidation Processes |
References
- Hasan, I.; Rana, A. A review on in SITU green synthesis of titanium dioxide nanoparticles and their photocatalytic activities. Mater. Today Proc. 2023, 81, 916–918. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for removal of environmental pollutants and fuel production: A review. Environ. Chem. Lett. 2021, 19, 441–463. [Google Scholar] [CrossRef]
- Al-Shahrani, S. Phenomena of Removal of Crystal Violet from Wastewater Using Khulays Natural Bentonite. J. Chem. 2020, 2020, 4607657. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, M.; Liu, D.; Lei, W.; Sun, L.; Wang, X. Reactive yellow 161 decolorization by TiO2/porous boron nitridenanosheet composites in cotton dyeing effluent. ACS Sustain. Chem. Eng. 2017, 5, 1392–1399. [Google Scholar] [CrossRef]
- Ammari, Y.; Elatmani, K.; Qourzal, S.; Bakas, I.; Ejakouk, E.; Ait-Ichou, Y. Etude cinétique de la dégradation photocatalytique du colorant bleu de méthylène en présence de dioxyde de titane (TiO2), en suspension aqueuse. J. Mater. Environ. Sci. 2016, 7, 671–678. [Google Scholar]
- Srinivasan, M.; Venkatesan, M.; Arumugam, V.; Natesan, G. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019, 80, 197–202. [Google Scholar] [CrossRef]
- El Yadini, A.; Marouane, B.; Ahmido, A.; Dunlop, P.; Byrne, J.A.; El Azzouzi, M.; El Hajjaji, S. Photolysis and photodegradation of fenamiphos insecticide by using slurry and supported TiO2. J. Mater. Environ. Sci. 2013, 4, 973–980. [Google Scholar]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2017, 336, 386–396. [Google Scholar] [CrossRef]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Arshad Kamran, M.; Al-Habardi, M. Green synthesis of spherical TiO2 nanoparticles using Citrus Limetta extract: Excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar] [CrossRef]
- Iqbal, T.; Masood, A.; Khalid, N.R.; Tahir, M.B.; Asiri, A.M.; Alrobei, H. Green synthesis of novel lanthanum doped copper oxide nanoparticles for photocatalytic application: Correlation between experiment and COMSOL simulation. Ceram. Int. 2022, 48, 13420–13430. [Google Scholar] [CrossRef]
- Nabi, G.; Raza, W.; Tahir, M.B. Green Synthesis of TiO2 Nanoparticle Using Cinnamon Powder Extract and the Study of Optical Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1425–1429. [Google Scholar] [CrossRef]
- Iqbal, T.; Farman, S.; Afsheen, S.; Riaz, K.N. Novel study to correlate efficient photocatalytic activity of WO3 and Cr doped TiO2 leading to enhance the shelf-life of the apple. Appl. Nanosci. 2022, 12, 87–99. [Google Scholar] [CrossRef]
- Umar, A.; Chauhan, M.S.; Chauhan, S.; Kumar, R.; Kumar, G.; Al-Sayari, S.A.; Hwang, S.W.; Al-Hajry, A. Large-scale synthesis of ZnO balls made of fluffy thin nanosheets by simple solution process: Structural, optical and photocatalytic properties. J. Colloid Interface Sci. 2011, 363, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Eskandari Azar, B.; Ramazani, A.; Taghavi Fardood, S.; Morsali, A. Green synthesis and characterization of ZnAl2O4@ZnO nanocomposite and its environmental applications in rapid dye degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Shen, J.; Zeng, H.; Chen, C.; Xu, S. Applied Clay Science A facile fabrication of Ag 2 O-Ag/ZnAl-oxides with enhanced visible-light photocatalytic performance for tetracycline degradation. Appl. Clay Sci. 2020, 185, 105413. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater, Sci. Total Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef]
- Mydeen, S.S.; Kumar, R.R.; Sambathkumar, S.; Kottaisamy, M.; Vasantha, V.S. Facile Synthesis of ZnO/AC Nanocomposites using Prosopis Juliflora for Enhanced Photocatalytic Degradation of Methylene Blue and Antibacterial Activity. Optik 2020, 224, 165426. [Google Scholar] [CrossRef]
- Moradnia, F.; Fardood, S.T.; Ramazani, A.; Osali, S.; Abdolmaleki, I. Green sol—gel synthesis of CoMnCrO 4 spinel nanoparticles and their photocatalytic application. Micro Nano Lett. 2020, 15, 674–677. [Google Scholar] [CrossRef]
- Moradnia, F.; Taghavi, S.; Ramazani, A.; Kumar, V. Green synthesis of recyclable MgFeCrO4 spinel nanoparticles for rapid photodegradation of direct black 122 dye. J. Photochem. Photobiol. A Chem. 2020, 392, 112433. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Mohd Kaus, N.H.; Jiang, Z.T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Metal Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Bhullar, S.; Goyal, N.; Gupta, S. Rapid green-synthesis of TiO2 nanoparticles for therapeutic applications. RSC Adv. 2021, 11, 30343–30352. [Google Scholar] [CrossRef]
- Halim, W. Synthèse Contrôlée par Additifs Latex de Nanoparticules Mésoporeuses à Base de TiO2: M (M=Pd, Ag, Cu, Ni…): Caractérisation et Applications en Photocatalyse. 01 Février 2021. Available online: https://tel.archives-ouvertes.fr/tel-03279447%0Ahttps://tel.archives-ouvertes.fr/tel-03279447/document (accessed on 27 September 2023).
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Kiruthika, A.R. Photocatalytic disinfection of micro-organisms: Mechanisms and applications. Environ. Technol. Innov. 2021, 24, 101909. [Google Scholar] [CrossRef]
- Liu, A.; Li, Z.; Wu, Z.; Xia, X. Talanta Study on the photocatalytic reaction kinetics in a TiO2 nanoparticles coated microreactor integrated micro fluidics device. Talanta 2018, 182, 544–548. [Google Scholar] [CrossRef]
- El Yadini, A.; Saufi, H.; Dunlop, P.S.M.; Byrne, J.A.; El Azzouzi, M.; El Hajjaji, S. Supported TiO2 on Borosilicate Glass Plates for Efficient Photocatalytic Degradation of Fenamiphos. J. Catal. 2014, 2014, 413693. [Google Scholar] [CrossRef]
- Alamelu, K.; Ja, B.M. TiO2-Pt composite photocatalyst for photodegradation and chemical reduction of recalcitrant organic pollutants. J. Environ. Chem. Eng. 2018, 6, 5720–5731. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis, characterization and catalytic activity of the Pd/TiO2 nanoparticles for the ligand-free Suzuki—Miyaura coupling reaction. J. Colloid Interface Sci. 2016, 465, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 746–750. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, I.C.; Tiwari, A.; Srivastava, P.; Bahadur, L. Green synthesis of TiO2 nanoparticles using Citrus limon juice extract as a bio-capping agent for enhanced performance of dye-sensitized solar cells. Surf. Interfaces 2022, 28, 101652. [Google Scholar] [CrossRef]
- Surya, C.; Arul, N.A.; Pandiyan, V.; Ravikumar, S.; Amutha, P.; Sobral, A.J.F.N.; Krishnakumar, B. Costus speciosus leaf extract assisted CS-Pt-TiO 2 composites: Synthesis, characterization and their bio and photocatalytic applications. J. Mol. Struct. 2019, 1195, 787–795. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Varma, K.S.; Shukla, A.D.; Tayade, R.J.; Mishra, M.K.; Nguyen, V.H.; Gandhi, V. Interaction of levofloxacin with reverse micelle sol-gel synthesized TiO2 nanoparticles: Revealing ligand-to-metal charge transfer (LMCT) mechanism enhances photodegradation of antibiotics under visible light. Mater. Lett. 2022, 309, 131304. [Google Scholar] [CrossRef]
- Graziani, V.; Esposito, A.; Scognamiglio, M.; Chambery, A.; Russo, R.; Ciardiello, F.; Troiani, T.; Potenza, N.; Fiorentino, A.; D’Abrosca, B. Spectroscopic characterization and cytotoxicity assessment towards human colon cancer cell lines of acylated cycloartane glycosides from Astragalus boeticus L. Molecules 2019, 24, 1725. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Zaid, A.N.; Abuzant, A.; Khalaf, S.; Abu-Hassan, N. Phytochemical and biological properties of four Astragalus species commonly used in traditional Palestinian medicine. Eur. J. Integr. Med. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Prohens, J.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Prohens, R.; Herraiz, F.J.; De Ron, A.M. Swedish coffee (Astragalus boeticus L.), a neglected coffee substitute with a past and a potential future. Genet. Resour. Crop Evol. 2014, 61, 287–297. [Google Scholar] [CrossRef]
- Yu, Z.C.; Zheng, X.T.; Lin, W.; He, W.; Shao, L.; Peng, C.L. Photoprotection of Arabidopsis leaves under short-term high light treatment: The antioxidant capacity is more important than the anthocyanin shielding effect. Plant Physiol. Biochem. 2021, 166, 258–269. [Google Scholar] [CrossRef]
- Drhimer, F.; Rahmani, M.; Regraguy, B.; El Hajjaji, S.; Mabrouki, J.; Amrane, A.; Fourcade, F.; Assadi, A.A. Treatment of a Food Industry Dye, Brilliant Blue, at Low Concentration Using a New Photocatalytic Configuration. Sustainability 2023, 15, 5788. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Vijayalakshmi, S.; Sadasivuni, K.K.; Ojiaku, A.A.; Verma, U. Facile fabrication of CuO nanoparticles via microwave-assisted method: Photocatalytic, antimicrobial and anticancer enhancing performance. Int. J. Environ. Anal. Chem. 2022, 102, 1095–1108. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Liu, X.; Yang, L.; Ding, J.; Dong, F.; Li, Y.; Irfan, M.; Zhang, P. Fast photocatalytic degradation of dyes using low-power laser-fabricated Cu2O-Cu nanocomposites. RSC Adv. 2018, 8, 20277–20286. [Google Scholar] [CrossRef] [PubMed]
- Al-taweel, S.S.; Saud, H.R. New route for synthesis of pure anatase TiO2 nanoparticles via utrasound- assisted sol-gel method. J. Chem. Pharm. Res. 2016, 8, 620–626. [Google Scholar]
- Ajmal, N.; Saraswat, K.; Bakht, M.A.; Riadi, Y.; Ahsan, M.J.; Noushad, M. Cost-effective and eco-friendly synthesis of titanium dioxide (TiO2) nanoparticles using fruit’s peel agro-waste extracts: Characterization, in vitro antibacterial, antioxidant activities. Green Chem. Lett. Rev. 2019, 12, 244–254. [Google Scholar] [CrossRef]
- Tesfaye, L.; Ramaswamy, K.; Bekele, B.; Saka, A.; Nagaprasad, N. Experimental investigation on the impacts of annealing temperatures on titanium dioxide nanoparticles structure, size and optical properties synthesized through sol-gel methods. Mater. Today Proc. 2021, 45, 5752–5758. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Ali, M.; Anjum, R.; Haleem, A.; Siddiq, M.; Sakhawat, S.; Al, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Vetrivel, V.; Rajendran, K.; Kalaiselvi, V. Synthesis and characterization of Pure Titanium dioxide nanoparticles by Sol-gel method. Int. J. ChemTech Res. 2015, 7, 1090–1097. [Google Scholar]
- Moradnia, F.; Taghavi, S.; Ramazani, A.; Min, B.; Woo, S.; Varma, R.S. Magnetic Mg0.5Zn0.5FeMnO4 nanoparticles: Green sol-gel synthesis, characterization, and photocatalytic applications. J. Clean. Prod. 2021, 288, 125632. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, D.; Jiang, D.; Wei, W.; Qian, K.; Chen, M.; Xie, J. Bamboo leaf-assisted formation of carbon/nitrogen co-doped anatase TiO2 modified with silver and graphitic carbon nitride: Novel and green synthesis and cooperative photocatalytic activity. Dalton Trans. 2014, 43, 13792–13802. [Google Scholar] [CrossRef]
- Chandra, I.; Singh, S.; Senapati, S.; Srivastava, P.; Bahadur, L. Green synthesis of TiO2 nanoparticles using Bixa orellana seed extract and its application for solar cells. Sol. Energy 2019, 194, 952–958. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Helali, S. Application de la photocatalyse pour la dégradation des polluants chimiques et bactériologiques dans l’eau en utilisant des catalyseurs irradiés par des photons de lumière naturelle ou artificielle (UV-A/UV-B). Doctoral dissertation, Université Claude Bernard-Lyon I, Villeurbanne, France, 2012. Français. NNT: 2012LYO10316.. [Google Scholar]
- Atrak, K.; Ramazani, A.; Taghavi, S. Eco-friendly synthesis of Mg0.5Ni0.5AlxFe2-xO4 magnetic nanoparticles and study of their photocatalytic activity for degradation of direct blue 129 dye. J. Photochem. Photobiol. A Chem. 2019, 382, 111942. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Jeevanantham, S.; Ramamurthy, R.; Reshma, B. Simultaneous removal of Cu(II) and reactive green 6 dye from wastewater using immobilized mixed fungal biomass and its recovery. Chemosphere 2021, 271, 129519. [Google Scholar] [CrossRef] [PubMed]
- Alahiane, S.; Qourzal, S.; El Ouardi, M.; Belmouden, M.; Assabbane, A. Adsorption et photodégradation du colorant indigo carmine en milieu aqueux en présence de TiO2/UV/O2 (Adsorption and photocatalytic degradation of indigo carmine dye in aqueous solutions using TiO2/UV/O2). J. Mater. Environ. Sci. 2013, 4, 239–250. [Google Scholar]
- Elsellami, L.; Vocanson, F.; Dappozze, F.; Puzenat, E.; Païsse, O.; Houas, A.; Guillard, C. Kinetic of adsorption and of photocatalytic degradation of phenylalanine effect of pH and light intensity. Appl. Catal. A Gen. 2010, 380, 142–148. [Google Scholar] [CrossRef]
- Preo, T.; Kallay, N. Point of Zero Charge and Surface Charge Density of TiO 2 in Aqueous Electrolyte Solution as Obtained by Potentiometric Mass Titration. Croat. Chem. Acta 2006, 79, 95–106. [Google Scholar]
- Azeez, F.; Al-hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Bessekhouad, Y. Propriétés photocatalytiques de TiO2 nanocristallins dopés par des cations (Li+, Na+ et K+) et des hétérojonctions à base de sulfures et d’oxydes métalliques/TiO2; Autre; Université Paul Verlaine: Metz, France, 2003; Français. NNT: 2003METZ017S. tel-01749893. [Google Scholar]
- Pushpamalini, T.; Keerthana, M.; Sangavi, R.; Nagaraj, A.; Kamaraj, P. Comparative analysis of green synthesis of TiO2 nanoparticles using four different leaf extract. Mater. Today Proc. 2020, 40, S180–S184. [Google Scholar] [CrossRef]









| Plant Used for the Synthesis of TiO2 NPs | Pollutant Conc. | Catalyst Conc. | Time (min) Percentage | Morphology | Size (nm) | Ref |
|---|---|---|---|---|---|---|
| Jatropha curcas | Tannery wastewater (Cr) 6.88 mg/L | 5 g/5 L | 5 h (76.48%) | Spherical | 75 nm | [9] |
| Citrus limetta | RhB dye 10 mg/L | 0.7 g/50 mL | 80 min (90%) | Spherical | 80–100 nm | [10] |
| Syzygium cumini | Lead (Pb) 8.621 mg/L | 0.3 g/500 mL | 17 h (82.53%) | Spherical | 10 nm | [29] |
| Citrus limon juice | RG-19 6.7 Mm | 0.03 g/100 mL | 60 min (99.88%) | Spherical | 10–21 nm | [32] |
| Piper betel | Vert Malachite 100 ppm | 100 mg/50 mL | 50 min (100%) | - | 6.6 nm | [60] |
| Ocimum tenuiflorm | 50 min (100%) | 7.0 nm | ||||
| Moringa oleifera | 30 min (100%) | 6.6 nm | ||||
| Coriandrum sativum | 50 min (100%) | 6.8 nm | ||||
| Astragalus boeticus | RY161 30 mg/L CV 10 mg/L | 0.1 g/100 mL | 90 min (100%) 30 min (94.06%) | Spherical | 68 nm | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maanane, F.; El Yadini, A.; El Alouani, M.; Mabrouki, J.; Saufi, H.; Tabyaoui, M. Green Development of Titanium Dioxide Using Astragalus boeticus for the Degradation of Cationic and Anionic Dyes in an Aqueous Environment. Water 2023, 15, 3471. https://doi.org/10.3390/w15193471
Maanane F, El Yadini A, El Alouani M, Mabrouki J, Saufi H, Tabyaoui M. Green Development of Titanium Dioxide Using Astragalus boeticus for the Degradation of Cationic and Anionic Dyes in an Aqueous Environment. Water. 2023; 15(19):3471. https://doi.org/10.3390/w15193471
Chicago/Turabian StyleMaanane, Fadwa, Adil El Yadini, Marouane El Alouani, Jamal Mabrouki, Hamid Saufi, and Mohamed Tabyaoui. 2023. "Green Development of Titanium Dioxide Using Astragalus boeticus for the Degradation of Cationic and Anionic Dyes in an Aqueous Environment" Water 15, no. 19: 3471. https://doi.org/10.3390/w15193471
APA StyleMaanane, F., El Yadini, A., El Alouani, M., Mabrouki, J., Saufi, H., & Tabyaoui, M. (2023). Green Development of Titanium Dioxide Using Astragalus boeticus for the Degradation of Cationic and Anionic Dyes in an Aqueous Environment. Water, 15(19), 3471. https://doi.org/10.3390/w15193471







