The Algicidal Potential of a Floating-Bed System against Microcystis aeruginosa in Laboratory Conditions
Abstract
:1. Introduction
2. Experimental Methods
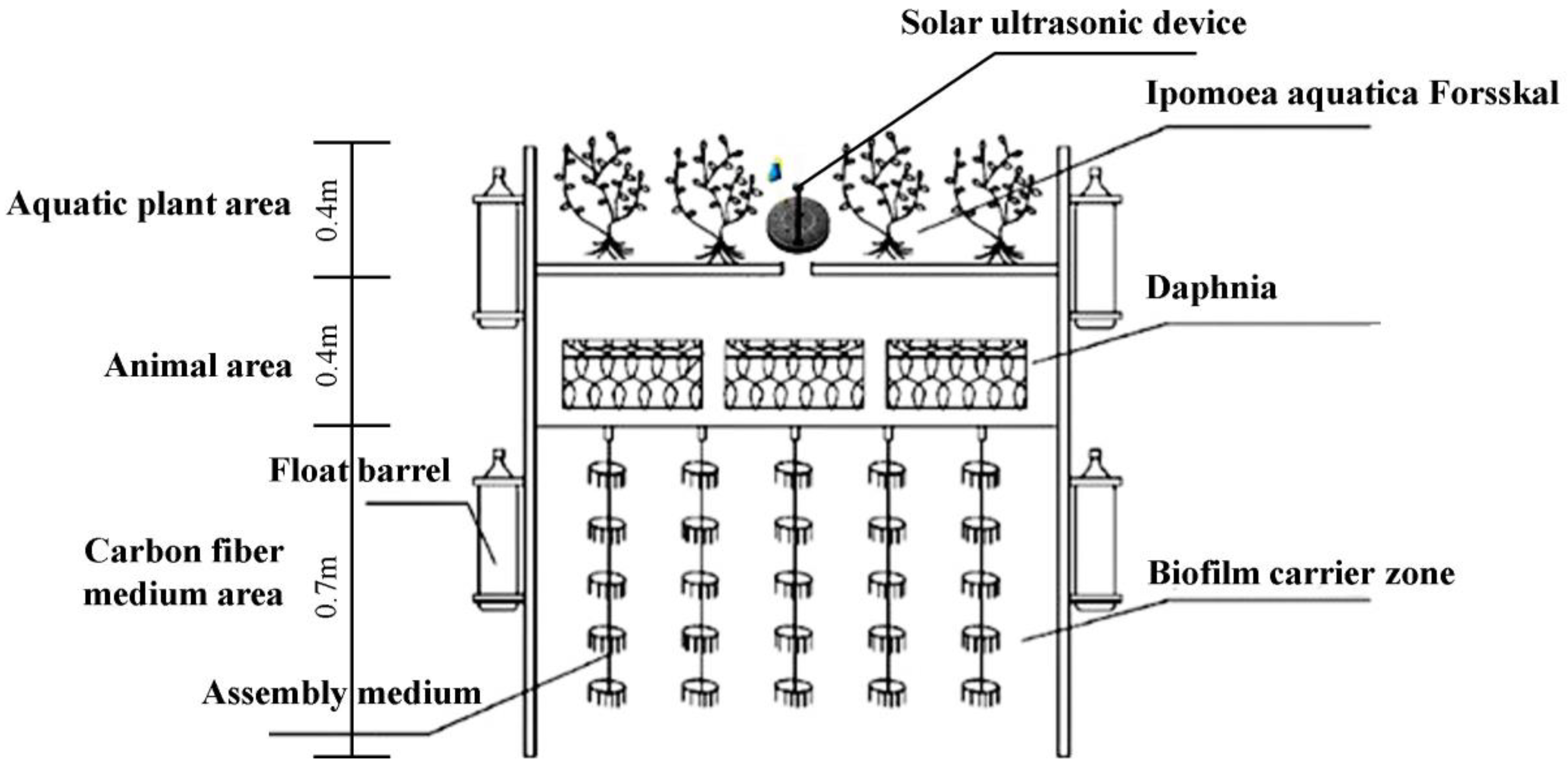
2.1. Design of Ecological Floating Bed
2.2. Experimental Water Samples
2.3. Algae Strains and Culture Conditions
2.4. Experimental Schemes
2.5. Experimental Instruments
2.6. Analysis Indicator and Method
2.6.1. Method for Extracting Crude Enzyme Solution of Algae
2.6.2. Measurement of Dehydrogenase Activity (DHA) in Algae Solution
2.6.3. Determination of Soluble Protein in Algae Solution
2.6.4. Determination of Malondialdehyde Content (MDA) in Algae Solution
- (1)
- Draw 2 mL of crude enzyme solution, and 2 mL of distilled water for the control group, add 2 mL of 0.5% (w/v) trichloroacetic acid solution, and then, add 2 mL of 0.67% (w/v) thiobarbituric acid solution. Shake well until uniform.
- (2)
- Put it in a boiling water bath and boil for 15 min to fully react.
- (3)
- After the time is over, immediately remove the test tube and put it in cold water for cooling. After cooling, centrifuged it for 20 min at 4000 r/min. Take the liquid at wavelengths of 600 nm, 532 nm, and 450 nm, respectively. Calculate according to Formulas (1) and (2).
2.7. Determination of Algae Chlorophyll-a
2.8. Algae Cell Characterization
2.9. Ecotoxicological Analysis of Water Samples after Treatment with an Ecological Floating-Bed System
3. Results and Discussion
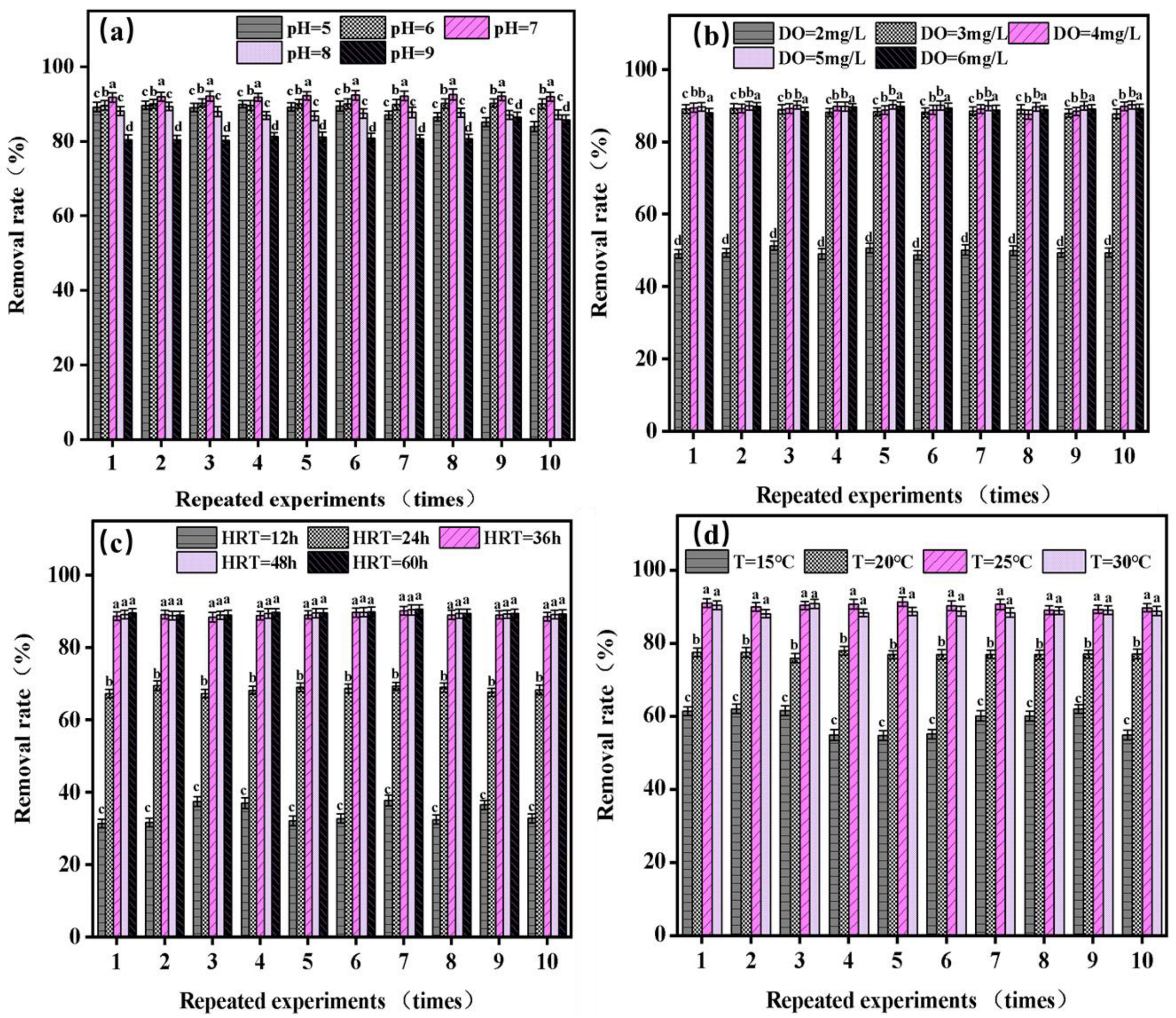
3.1. The Influencing Factors of Solar Ultrasonic Carbon Fiber Ecological Floating-Bed System Algae-Lysing
3.1.1. The Effect of Influent pH on the Variation in Chlorophyll-a Absorbance
3.1.2. The Impact of Dissolved Oxygen (DO) on the Variation in Chlorophyll-a Absorbance
3.1.3. The Influence of Hydraulic Retention Time (HRT) on the Variation in Chlorophyll-a Absorbance
3.1.4. The Effect of Temperature on the Variation in Chlorophyll-a Absorbance
3.2. Removal Mechanism of Solar Ultrasonic Carbon Fiber Ecological Floating Bed
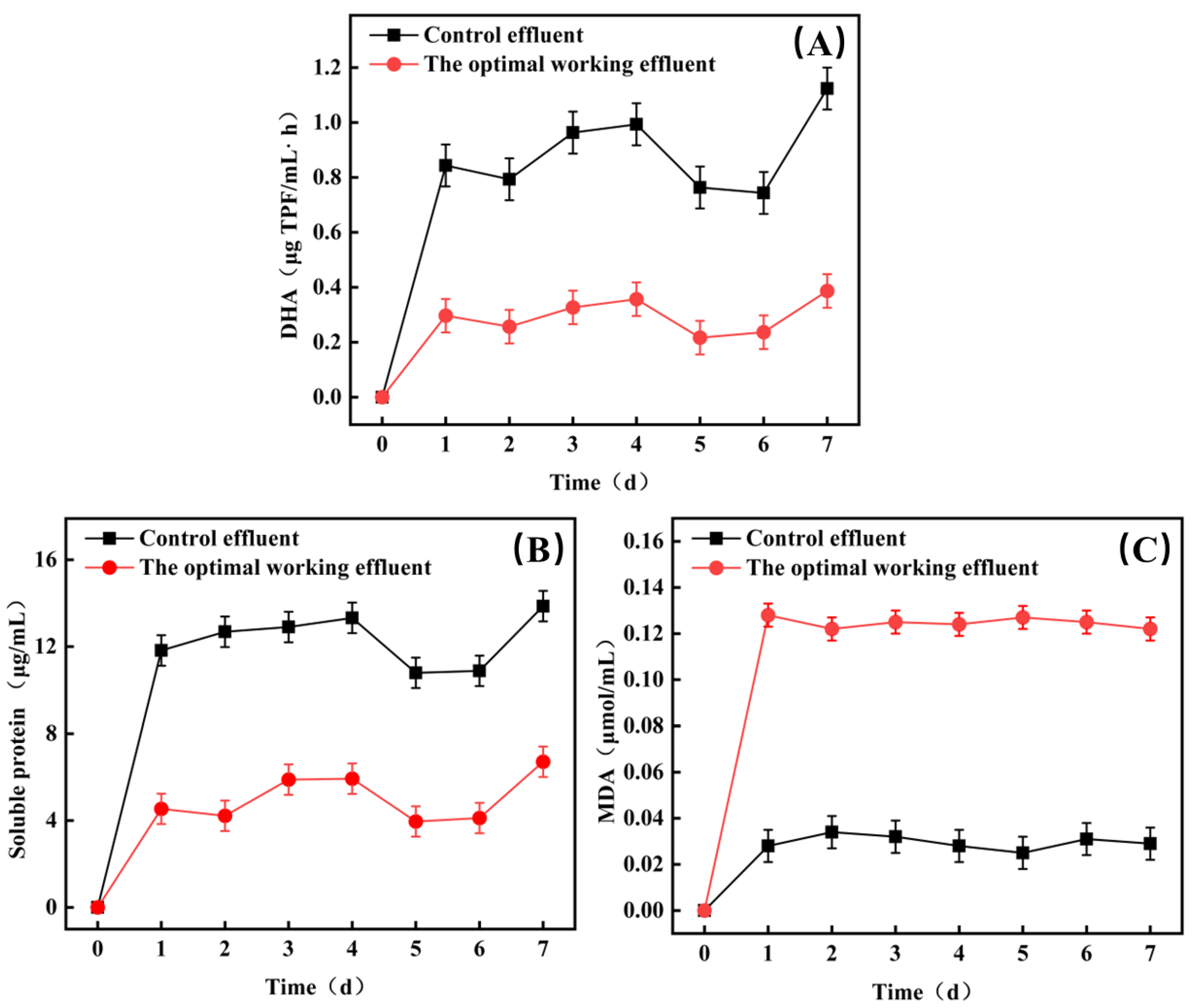
3.2.1. The Effect of Optimal Conditions on Microcystis aeruginosa Treatment
- (1)
- Variation in DHA during operation of Microcystis aeruginosa under optimal conditions
- (2)
- Changes in Soluble Protein During Operation of Microcystis aeruginosa under Optimal Conditions
- (3)
- Variation in MDA during operation of Microcystis aeruginosa under optimal conditions
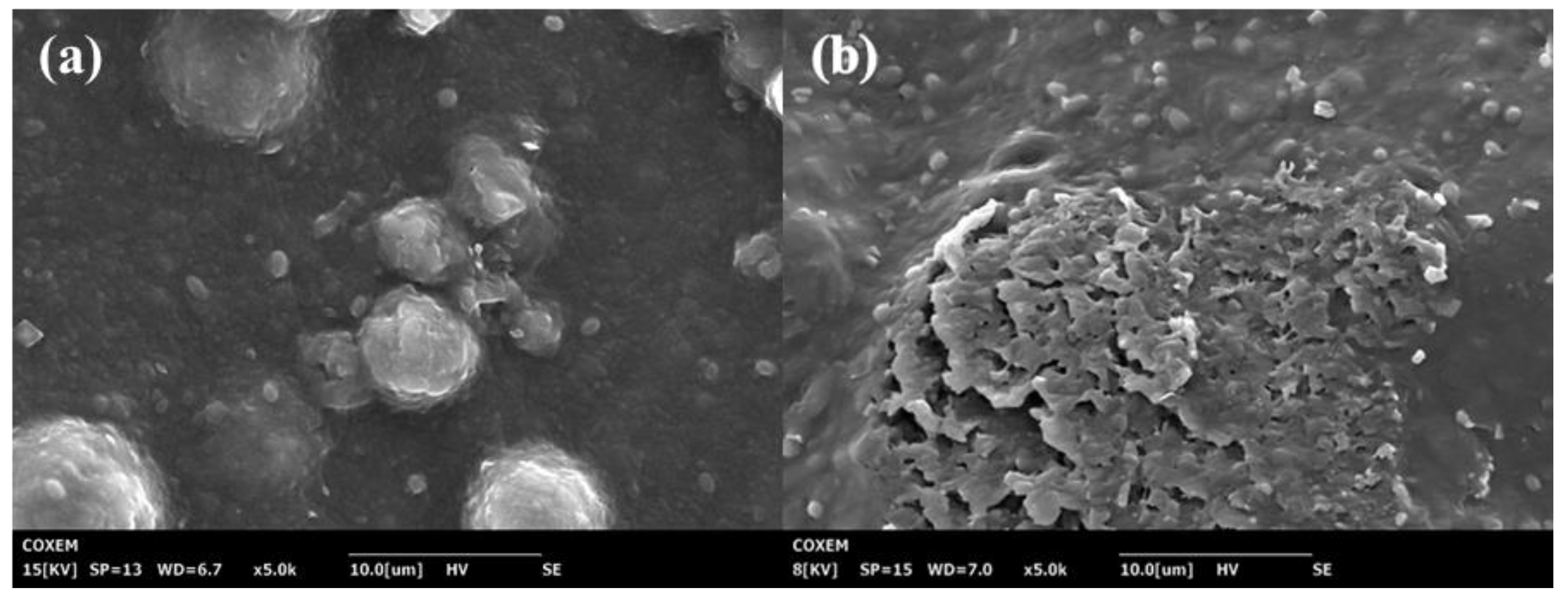
3.2.2. Effect of Optimal Working Conditions on Spatial Structure of Microcystis aeruginosa
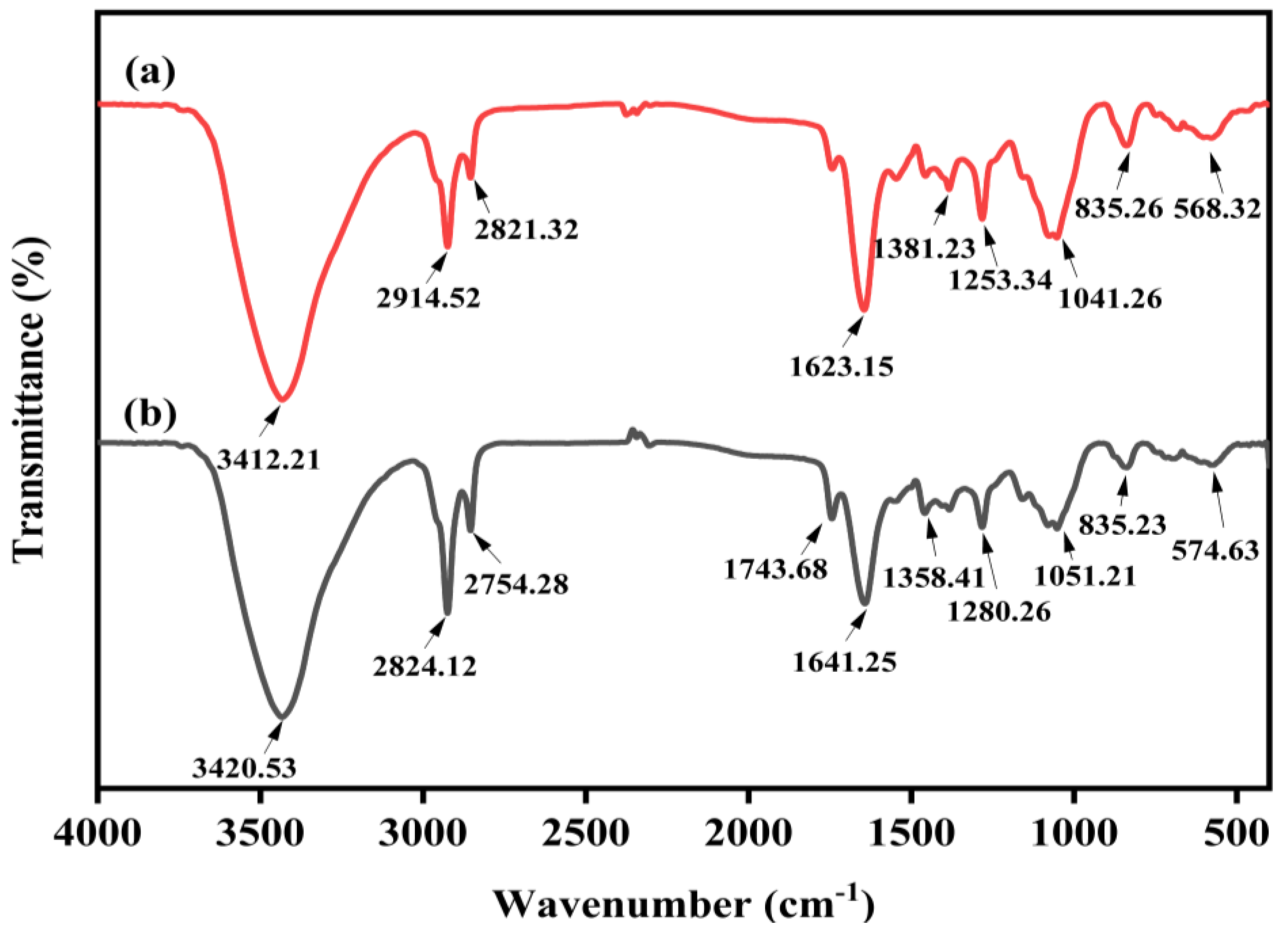
3.2.3. The Effect of Optimal Conditions on the Functional Groups of Microcystis aeruginosa
3.3. Study on Algae-Lysing Toxicity of Solar Ultrasonic Carbon Fiber Ecological Floating Bed
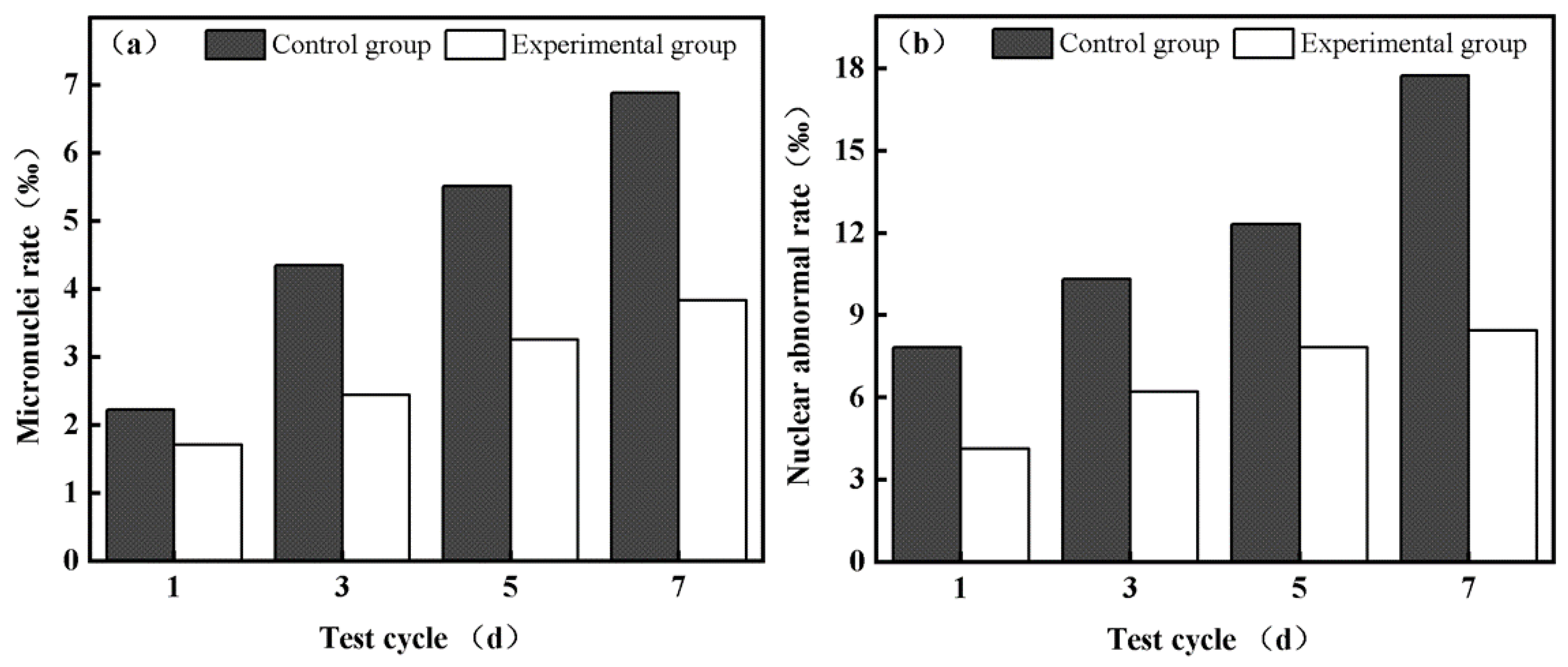
3.3.1. Analysis of Micronucleus Test Results
3.3.2. Analysis of Comet Experiment Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Cai, X.; Wang, S.; Yang, X. Analysis of the causes of cyanobacteria bloom: A review. J. Resour. Ecol. 2020, 11, 405–413. [Google Scholar]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Neves, R.A.; Nascimento, S.M.; Santos, L.N. Harmful algal blooms and shellfish in the marine environment: An overview of the main molluscan responses, toxin dynamics, and risks for human health. Environ. Sci. Pollut. Res. 2021, 28, 55846–55868. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; do Nascimento Moura, A. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Alonso-Rodrıguez, R.; Páez-Osuna, F. Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: A review with special reference to the situation in the Gulf of California. Aquaculture 2003, 219, 317–336. [Google Scholar] [CrossRef]
- Yan, T.; Li, X.-D.; Tan, Z.-J.; Yu, R.-C.; Zou, J.-Z. Toxic effects, mechanisms, and ecological impacts of harmful algal blooms in China. Harmful Algae 2022, 111, 102148. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-based methods for harmful algal blooms control: A review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, P.; Wang, Y. Algicidal efficiency and mechanism of Phanerochaete chrysosporium against harmful algal bloom species. Algal Res. 2015, 12, 182–190. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae 2021, 109, 102099. [Google Scholar] [CrossRef]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Xu, S.; Lyu, P.; Zheng, X.; Yang, H.; Xia, B.; Li, H.; Zhang, H.; Ma, S. Monitoring and control methods of harmful algal blooms in Chinese freshwater system: A review. Environ. Sci. Pollut. Res. 2022, 29, 56908–56927. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Wang, Y.; Sun, L.-Q.; Zheng, Y.-C.; Zhao, J.-C. Research and application status of ecological floating bed in eutrophic landscape water restoration. Sci. Total Environ. 2020, 704, 135434. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ao, Y.; Yang, X.; Li, T. Treating eutrophic water for nutrient reduction using an aquatic macrophyte (Ipomoea aquatica Forsskal) in a deep flow technique system. Agric. Water Manag. 2008, 95, 607–615. [Google Scholar] [CrossRef]
- Li, X.-N.; Song, H.-L.; Li, W.; Lu, X.-W.; Nishimura, O. An integrated ecological floating-bed employing plant, freshwater clam and biofilm carrier for purification of eutrophic water. Ecol. Eng. 2010, 36, 382–390. [Google Scholar] [CrossRef]
- Freese, H.M.; Schink, B. Composition and stability of the microbial community inside the digestive tract of the aquatic crustacean Daphnia magna. Microb. Ecol. 2011, 62, 882–894. [Google Scholar] [CrossRef]
- Hiltunen, M.; Honkanen, M.; Taipale, S.; Strandberg, U.; Kankaala, P. Trophic upgrading via the microbial food web may link terrestrial dissolved organic matter to Daphnia. J. Plankton Res. 2017, 39, 861–869. [Google Scholar] [CrossRef]
- Zhang, R.; Jin, R.; Liu, G.; Zhou, J.; Li, C.L. Study on nitrogen removal performance of sequencing batch reactor enhanced by low intensity ultrasound. Bioresour. Technol. 2011, 102, 5717–5721. [Google Scholar] [CrossRef]
- Yang, M.; Shi, D.; Wang, Y.; Ebadi, A.G.; Toughani, M. Study on Interaction of Coomassie Brilliant Blue G-250 with Bovine Serum Albumin by Multispectroscopic. Int. J. Pept. Res. Ther. 2021, 27, 421–431. [Google Scholar] [CrossRef]
- Draper, H.; Squires, E.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; Gauthier, L.; Baudrimont, M.; Gonzalez, P.; Mailhes, C.; Ferrier, V.; Devaux, A. Comparative evaluation of the toxicity and genotoxicity of cadmium in amphibian larvae (Xenopus laevis and Pleurodeles waltl) using the comet assay and the micronucleus test. Environ. Toxicol. Int. J. 2007, 22, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Poletta, G.; Larriera, A.; Kleinsorge, E.; Mudry, M. Genotoxicity of the herbicide formulation Roundup ®(glyphosate) in broad-snouted caiman (Caiman latirostris) evidenced by the Comet assay and the Micronucleus test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 672, 95–102. [Google Scholar] [CrossRef]
- de Oliveira, E.M.; Suzuki, M.F.; do Nascimento, P.A.; da Silva, M.A.; Okazaki, K. Evaluation of the effect of 90Sr β-radiation on human blood cells by chromosome aberration and single cell gel electrophoresis (comet assay) analysis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2001, 476, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Cavas, T. In vivo genotoxicity evaluation of atrazine and atrazine–based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem. Toxicol. 2011, 49, 1431–1435. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Sun, Y.; Sun, W.; Xu, Y.; Zheng, H. Synthesis and characterization of ampholytic flocculant CPCTS-gP (CTA-DMDAAC) and its flocculation properties for microcystis aeruginosa removal. Processes 2018, 6, 54. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsuzuki, K.; Nishijima, N.; Takagi, T. Algae-removal performance of a fluidized-bed biofilm reactor system for lake water treatment. Water Sci. Technol. 2001, 43, 277–283. [Google Scholar] [CrossRef]
- Li, J.; Wu, L.; Dai, Y.; Wang, Q.; Wang, S.; Zhang, L. Effects of different nitrogen–phosphorus ratio on the freshwater phytoplankton growth and the variations of environmental factors. Ecol. Environ. 2007, 16, 342–346. [Google Scholar]
- You, L.; Cui, L.; Liu, Z.; Yang, B.; Huang, Z. Correlation analysis of parameters in algal growth. Environ. Sci. Technol. 2007, 30, 42–44. [Google Scholar]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part II-mechanical and biological control methods. Harmful Algae 2021, 109, 102119. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Shi, X.; Zhao, Z.; Ni, L.; Wu, Y.; Jiao, T. Correlation between chlorophyll a concentration and environmental factors in shallow lakes in plain river network areas of Suzhou. J. Lake Sci. 2008, 20, 556–562. [Google Scholar]
- Solmaz, A.; Işık, M. Optimization of membrane photobioreactor; the effect of hydraulic retention time on biomass production and nutrient removal by mixed microalgae culture. Biomass Bioenergy 2020, 142, 105809. [Google Scholar] [CrossRef]
- Fernandez-Marchante, C.; Asensio, Y.; Lobato, J.; Villaseñor, J.; Cañizares, P.; Rodrigo, M. Influence of hydraulic retention time and carbon loading rate on the production of algae. J. Biotechnol. 2018, 282, 70–79. [Google Scholar] [CrossRef]
- Silveira, C.F.; de Assis, L.R.; de Sousa Oliveira, A.P.; Calijuri, M.L. Valorization of swine wastewater in a circular economy approach: Effects of hydraulic retention time on microalgae cultivation. Sci. Total Environ. 2021, 789, 147861. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Wang, M.; Shi, K. Effects of temperature on the optical properties of Microcystis aeruginosa and Scenedesmus obliquus. J. Freshw. Ecol. 2016, 31, 361–375. [Google Scholar] [CrossRef]
- Chettri, D.; Sharma, B.; Verma, A.K.; Verma, A.K. Significance of Microbial Enzyme Activities in Agriculture. Microbiol. Act. Soil Plant Health Manag. 2021, 351–373. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, L.; Zhang, W.; Yang, G.; Zhao, Z.; Wang, Y.; Li, X. Comparative toxic effects of microplastics and nanoplastics on Chlamydomonas reinhardtii: Growth inhibition, oxidative stress, and cell morphology. J. Water Process Eng. 2021, 43, 102291. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, X.; Sun, S.; Wang, L.; Liu, W.; Jiang, X.; Wang, J. Effects of mesotrione on oxidative stress, subcellular structure, and membrane integrity in Chlorella vulgaris. Chemosphere 2020, 247, 125668. [Google Scholar] [CrossRef] [PubMed]
- Mantsch, H.; McElhaney, R. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef]
- Theerawitayaart, W.; Prodpran, T.; Benjakul, S. Enhancement of hydrophobicity of fish skin gelatin via molecular modification with oxidized linoleic acid. J. Chem. 2019, 2019, 5462471. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, G.; Wang, F.; Huang, X.; Li, Y.; Liang, D.; Zhang, M.; Sun, D. Study on the Algae Lysis Method of White Rot Fungi Algae Control System. Water 2022, 14, 903. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Liang, D.; Tang, C.; Huang, Y.; Sun, D. The Algicidal Potential of a Floating-Bed System against Microcystis aeruginosa in Laboratory Conditions. Water 2023, 15, 3607. https://doi.org/10.3390/w15203607
Zeng G, Liang D, Tang C, Huang Y, Sun D. The Algicidal Potential of a Floating-Bed System against Microcystis aeruginosa in Laboratory Conditions. Water. 2023; 15(20):3607. https://doi.org/10.3390/w15203607
Chicago/Turabian StyleZeng, Guoming, Dong Liang, Cheng Tang, Yuanyuan Huang, and Da Sun. 2023. "The Algicidal Potential of a Floating-Bed System against Microcystis aeruginosa in Laboratory Conditions" Water 15, no. 20: 3607. https://doi.org/10.3390/w15203607
APA StyleZeng, G., Liang, D., Tang, C., Huang, Y., & Sun, D. (2023). The Algicidal Potential of a Floating-Bed System against Microcystis aeruginosa in Laboratory Conditions. Water, 15(20), 3607. https://doi.org/10.3390/w15203607






