Single-Step Modification of Brewer’s Spent Grains Using Phosphoric Acid and Application in Cheese Whey Remediation via Liquid-Phase Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbent
2.3. Adsorbent Characterization
2.4. Cheese Whey Adsorption Assays
2.4.1. Kinetic Modeling
2.4.2. Equilibrium Modeling
2.4.3. Thermodynamic Modeling
2.5. Desorption and Regeneration Experiments
2.6. Statistical Analysis
3. Results and Discussion
3.1. Adsorbents Characterization
3.2. Cheese Whey Characterization
3.3. Adsorption Experiment of Cheese Whey onto Activated Carbons
3.3.1. Acid Modification Effect
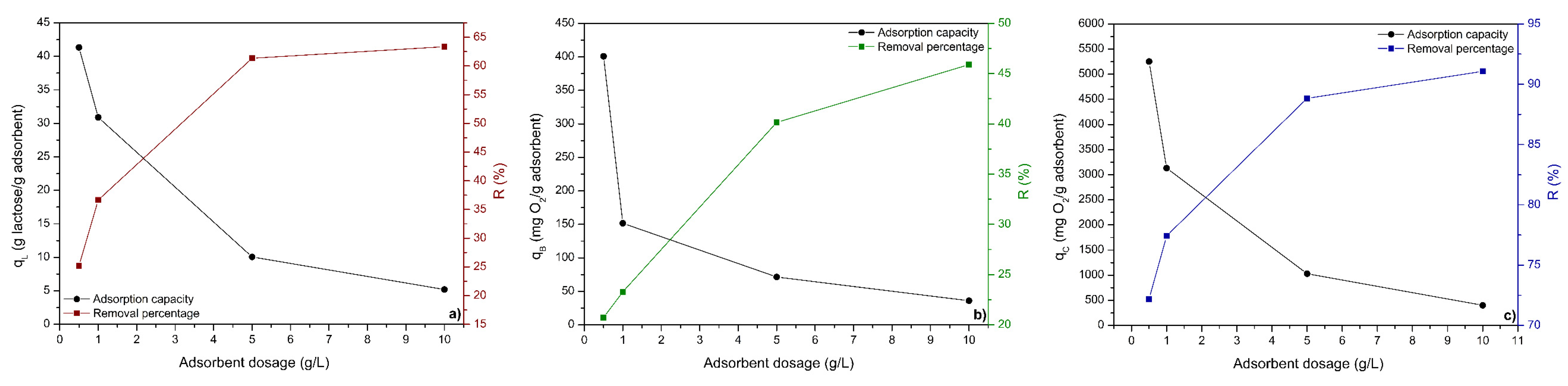
3.3.2. ACPO4 Dosage Effect
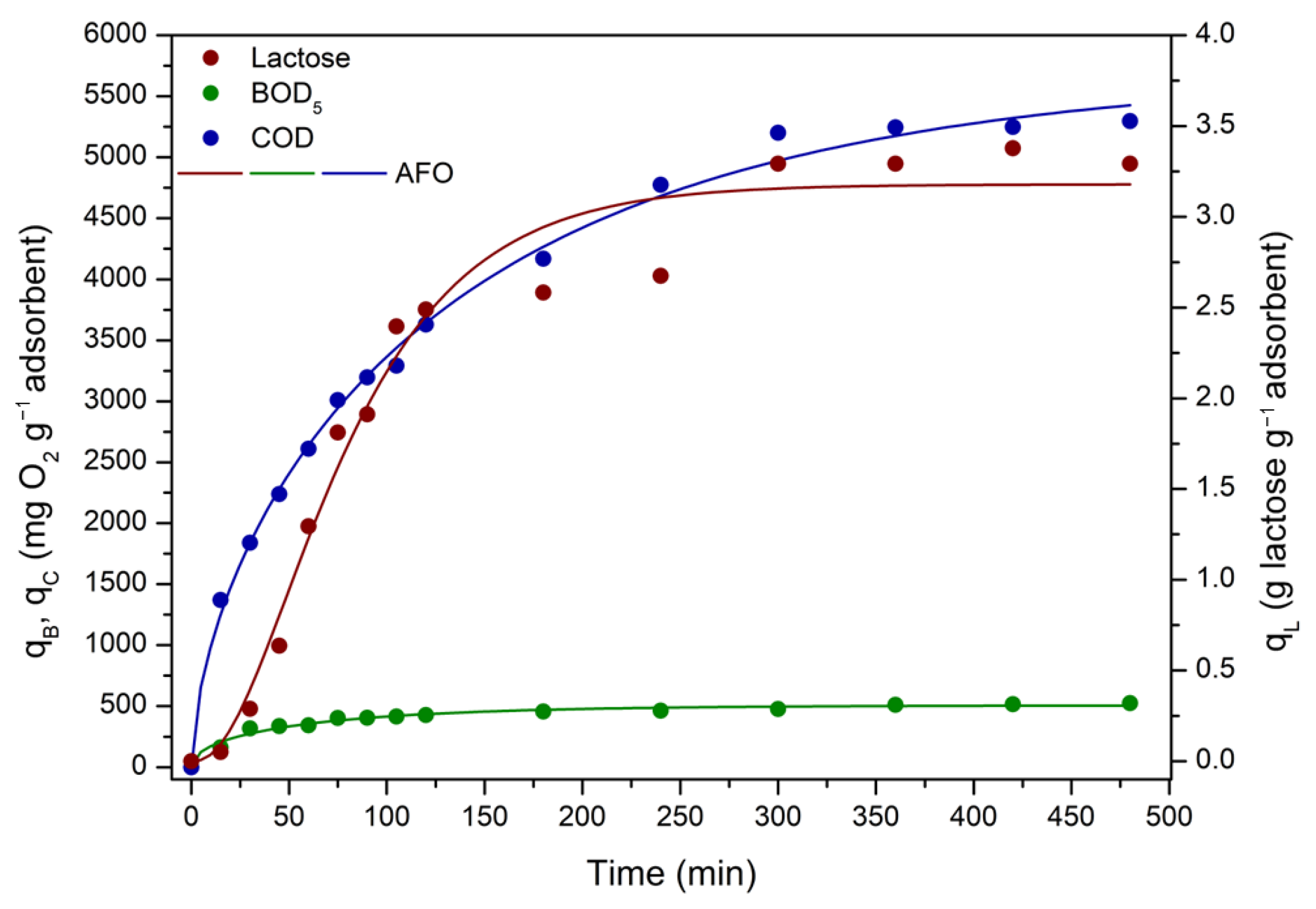
3.3.3. ACPO4 Adsorption Kinetics
3.3.4. ACPO4 Adsorption Equilibrium
3.3.5. ACPO4 Thermodynamic Parameters
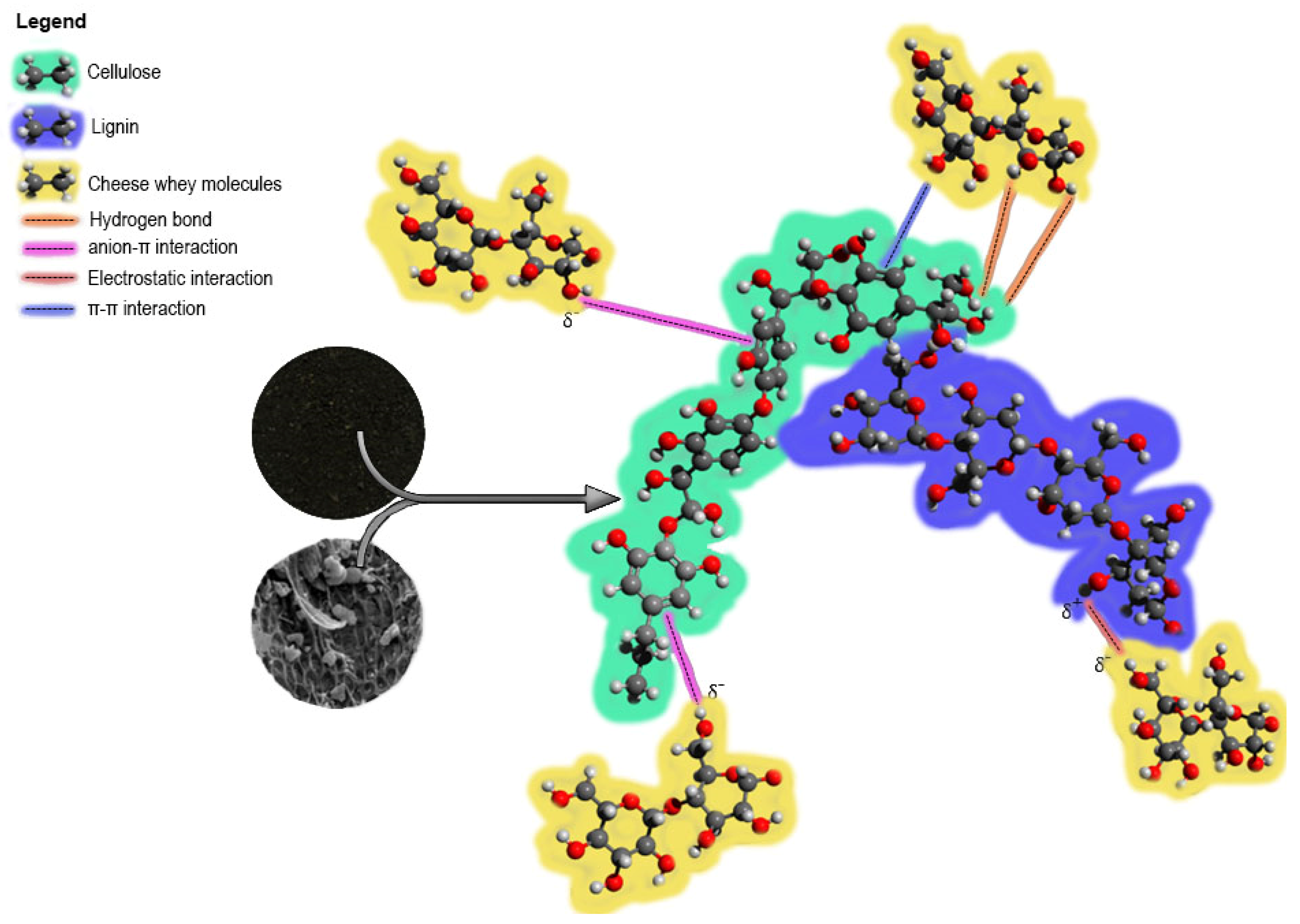
3.4. Proposal of the Adsorption Mechanism
3.5. Regeneration and Reuse of the ACPO4 Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tišma, M.; Bucic-Kojic, A.; Planinic, M. Bio-Based Products from Lignocellulosic Waste Biomass: A State of the Art. Chem. Biochem. Eng. Q. 2021, 35, 139–156. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef]
- Osman, A.I.; O’Connor, E.; McSpadden, G.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Upcycling Brewer’s Spent Grain Waste into Activated Carbon and Carbon Nanotubes for Energy and Other Applications via Two-Stage Activation. J. Chem. Technol. Biotechnol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Mahmood, A.S.N.; Brammer, J.G.; Hornung, A.; Steele, A.; Poulston, S. The Intermediate Pyrolysis and Catalytic Steam Reforming of Brewers Spent Grain. J. Anal. Appl. Pyrolysis 2013, 103, 328–342. [Google Scholar] [CrossRef]
- Vanreppelen, K.; Vanderheyden, S.; Kuppens, T.; Schreurs, S.; Yperman, J.; Carleer, R. Activated Carbon from Pyrolysis of Brewer’s Spent Grain: Production and Adsorption Properties. Waste Manag. Res. 2014, 32, 634–645. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ampese, L.C.; Mussatto, S.I.; Forster-Carneiro, T. A Bibliometric Analysis on Potential Uses of Brewer’s Spent Grains in a Biorefinery for the Circular Economy Transition of the Beer Industry. Biofuels Bioprod. Biorefining 2021, 15, 1965–1988. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-Economic Assessment of Bioenergy and Fertilizer Production by Anaerobic Digestion of Brewer’s Spent Grains in a Biorefinery Concept. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Mançano, R.R.; Battocchio, D.A.J.; Colpini, L.M.S. Adsorção de Alimento Corante Usando Carvão Ativado de Cervejarias’ Grãos Gastos. Acta Scientiarum. Technol. 2022, 45, e60443. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Viganó, J.; Castro, L.E.N.; Maciel-Silva, F.W.; Rostagno, M.A.; Mussatto, S.I.; Forster-Carneiro, T. Recovery of Sugars and Amino Acids from Brewers’ Spent Grains Using Subcritical Water Hydrolysis in a Single and Two Sequential Semi-Continuous Flow-through Reactors. Food Res. Int. 2022, 157, 111470. [Google Scholar] [CrossRef]
- Dudek, M.; Świechowski, K.; Manczarski, P.; Koziel, J.A.; Białowiec, A. The Effect of Biochar Addition on the Biogas Production Kinetics from the Anaerobic Digestion of Brewers’ Spent Grain. Energies 2019, 12, 1518. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Cara, C.; Romero, I.; Ruiz, E.; Romero-García, J.M.; Mussatto, S.I.; Castro, E. Ethanol Production from Brewers’ Spent Grain Pretreated by Dilute Phosphoric Acid. Energy Fuels 2018, 32, 5226–5233. [Google Scholar] [CrossRef]
- Wierzba, S.; Rajfur, M.; Nabrdalik, M.; Kłos, A. Assessment of the Influence of Counter Ions on Biosorption of Copper Cations in Brewer’s Spent Grain—Waste Product Generated during Beer Brewing Process. Microchem. J. 2019, 145, 196–203. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Wierzba, S.; Kłos, A. Heavy Metal Sorption in Biosorbents—Using Spent Grain from the Brewing Industry. J. Clean. Prod. 2019, 225, 112–120. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Tavares, F.d.O.; Vareschini, D.T.; Barros, M.A.S.D. Biosorption Mechanisms of Cationic and Anionic Dyes in a Low-Cost Residue from Brewer’s Spent Grain. Environ. Technol. 2020, 42, 2925–2940. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-around Characterization of Brewers’ Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Molina, A.; Garcia, P.; Ruiz-Colorado, A.A.; Linares-Solano, A.; Salinas-Martínez de Lecea, C. Phosphoric Acid Activation of Recalcitrant Biomass Originated in Ethanol Production from Banana Plants. Biomass Bioenergy 2011, 35, 1196–1204. [Google Scholar] [CrossRef]
- de Castro, L.E.N.; Battocchio, D.A.J.; Ribeiro, L.F.; Colpini, L.M.S. Development of Adsorbent Materials Using Residue from Coffee Industry and Application in Food Dye Adsorption Processes. Braz. Arch. Biol. Technol. 2022, 66, e23210125. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a Dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Hasan Johir, M.A.; Zhou, J.L.; Ngo, H.H.; Nghiem, L.D.; Richardson, C.; Moni, M.A.; Bryant, M.R. Activated Carbon Preparation from Biomass Feedstock: Clean Production and Carbon Dioxide Adsorption. J. Clean. Prod. 2019, 225, 405–413. [Google Scholar] [CrossRef]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using Cheese Whey for Hydrogen and Methane Generation in a Two-Stage Continuous Process with Alternative PH Controlling Approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Aghili, F.; Ghoreyshi, A.A.; Rahimpour, A.; Rahimnejad, M. Dynamic Behavior of the Adsorption, Activated Sludge and Combined Activated Sludge-Adsorption Process for Treatment of Cheese Whey Wastewater. Desalination Water Treat. 2015, 57, 16404–16414. [Google Scholar] [CrossRef]
- Martins, R.C.; Quinta-Ferreira, R.M. Final Remediation of Post-Biological Treated Milk Whey Wastewater by Ozone. Int. J. Chem. React. Eng. 2010, 8, 142. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, T.I.; Tekerlekopoulou, A.G.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Aerobic Biological Treatment of Second Cheese Whey in Suspended and Attached Growth Reactors. J. Chem. Technol. Biotechnol. 2015, 90, 2040–2049. [Google Scholar] [CrossRef]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of Trace Organic Contaminants by a Membrane Bioreactor–Granular Activated Carbon (MBR–GAC) System. Bioresour. Technol. 2012, 113, 169–173. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. The Advantages of Clay Mineral Modification Methods for Enhancing Adsorption Efficiency in Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2020, 28, 2572–2599. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Santos, J.V.F.; Fagnani, K.C.; Alves, H.J.; Colpini, L.M.S. Evaluation of the Effect of Different Treatment Methods on Sugarcane Vinasse Remediation. J. Environ. Sci. Health 2019, 54, 791–800. [Google Scholar] [CrossRef]
- Azmi, N.S.; Yunos, K.F.M. Wastewater Treatment of Palm Oil Mill Effluent (POME) by Ultrafiltration Membrane Separation Technique Coupled with Adsorption Treatment as Pre-Treatment. Agric. Agric. Sci. Procedia 2014, 2, 257–264. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, H.E.; Mohammadi, A.; Hejna, A.; Majtacz, J.; Esmaeili, A.; Habibzadeh, S.; Saeb, M.R.; Badawi, M.; Lima, E.C.; Mąkinia, J. Wastewater Reuse in Agriculture: Prospects and Challenges. Environ. Res. 2023, 236, 116711. [Google Scholar] [CrossRef]
- da Silva, L.C.A.; Lafetá Junior, J.A.Q.; Leite, M.O.; Fontes, E.A.F.; Coimbra, J.S.R. Comparative Appraisal of HPLC, Chloramine-T and Lane–Eynon Methods for Quantification of Carbohydrates in Concentrated Dairy Products. Int. J. Dairy. Technol. 2020, 73, 795–800. [Google Scholar] [CrossRef]
- Baird, R.; Rice, E.; Eaton, A. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Association, W.A.P.H., Ed.; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Lagergreen, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Lopes, E.C.N.; Da Silva, R.G. Kinetics and Equilibrium Parameters of Hg(II) Adsorption on Silica–Dithizone. J. Colloid. Interface Sci. 2004, 272, 271–276. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich Equation and the Underlying Microscopic Adsorption Description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Textile Wastewater Treatment by Agro-Industrial Waste: Equilibrium Modelling, Thermodynamics and Mass Transfer Mechanisms of Cationic Dyes Adsorption onto Low-Cost Lignocellulosic Adsorbent. J. Taiwan. Inst. Chem. Eng. 2019, 96, 439–452. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A.; Aziz, N. Isotherms, Kinetics and Thermodynamics of Acid Dye Adsorption on Activated Palm Ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A Critical Review of the Estimation of the Thermodynamic Parameters on Adsorption Equilibria. Wrong Use of Equilibrium Constant in the Van’t Hoof Equation for Calculation of Thermodynamic Parameters of Adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Li, C.; Song, X.; Hein, S.; Wang, K. The Separation of GMP from Milk Whey Using the Modified Chitosan Beads. Adsorption 2010, 16, 85–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Campbell, R.; Drake, M.A.; Zhong, Q. Decolorization of Cheddar Cheese Whey by Activated Carbon. J. Dairy Sci. 2015, 98, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Guliyev, R.; Akgün, M.; Sayın Börekçi, B.; Sadak, O.; Esen, Y. Modelling and Process Optimization of Cheese Whey Wastewater Treatment Using Magnetic Nanoparticles. Biomass Convers. Biorefin 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Alrowais, R.; Said, N.; Bashir, M.T.; Ghazy, A.; Alwushayh, B.; Daiem, M.M.A. Adsorption of Diphenolic Acid from Contaminated Water onto Commercial and Prepared Activated Carbons from Wheat Straw. Water 2023, 15, 555. [Google Scholar] [CrossRef]
- Peixoto, B.S.; Mota, L.S.D.O.; Oliveira, P.C.O.D.; Veloso, M.C.D.C.; Romeiro, G.A.; Moraes, M.C.D. Highly Functionalized Microporous Activated Biochar from Syagrus Coronata Waste: Production, Characterization, and Application in Adsorption Studies. Water 2022, 14, 3525. [Google Scholar] [CrossRef]
- Ameen, M.M.; Moustafa, A.A.; Mofeed, J.; Hasnaoui, M.; Olanrewaju, O.S.; Lazzaro, U.; Guerriero, G. Factors Affecting Efficiency of Biosorption of Fe (III) and Zn (II) by Ulva Lactuca and Corallina Officinalis and Their Activated Carbons. Water 2021, 13, 3421. [Google Scholar] [CrossRef]
- Kifetew, M.; Alemayehu, E.; Fito, J.; Worku, Z.; Prabhu, S.V.; Lennartz, B. Adsorptive Removal of Reactive Yellow 145 Dye from Textile Industry Effluent Using Teff Straw Activated Carbon: Optimization Using Central Composite Design. Water 2023, 15, 1281. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Liu, H. Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha Orientalis. Water 2018, 10, 351. [Google Scholar] [CrossRef]
- Bevilacqua, R.C.; Preigschadt, I.A.; Netto, M.S.; Georgin, J.; Franco, D.S.P.; Mallmann, E.S.; Silva, L.F.O.; Pinto, D.; Foletto, E.L.; Dotto, G.L. One Step Acid Modification of the Residual Bark from Campomanesia Guazumifolia Using H2SO4 and Application in the Removal of 2,4-Dichlorophenoxyacetic from Aqueous Solution. J. Environ. Sci. Health Part B 2021, 56, 995–1006. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wu, C.; Xu, G.; Feng, D.; Zhang, Y.; Gao, N. Valorization of Solid Digestate into Activated Carbon and Its Potential for CO2 Capture. J. Anal. Appl. Pyrolysis 2023, 169, 105874. [Google Scholar] [CrossRef]
- Gil, A.; Pallarés, J.; Arauzo, I.; Cortés, C. Pyrolysis and CO2 Gasification of Barley Straw: Effect of Particle Size Distribution and Chemical Composition. Powder Technol. 2023, 424, 118539. [Google Scholar] [CrossRef]
- Lawtae, P.; Phothong, K.; Tangsathitkulchai, C.; Wongkoblap, A. Analysis of Gas Adsorption and Pore Development of Microporous-Mesoporous Activated Carbon Based on GCMC Simulation and a Surface Defect Model. J. Porous Mater. 2023, 1, 1–17. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Yu, G.; Jiang, B.; Ren, B.; Wang, S. Characteristics of in Situ Synthesized Activated Carbon and Metal-Organic Framework Composites for CH4/N2 Gas Mixture Separation. Greenh. Gases Sci. Technol. 2023, 13, 67–80. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Characterization of Char from Biomass Gasification and Its Similarities with Activated Carbon in Adsorption Applications. Appl. Energy 2018, 227, 92–99. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Cruz Junior, O.F.; Sreńscek-Nazzal, J. Design of Highly Microporous Activated Carbons Based on Walnut Shell Biomass for H2 and CO2 Storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Cruz, O.F.; Campello-Gómez, I.; Casco, M.E.; Serafin, J.; Silvestre-Albero, J.; Martínez-Escandell, M.; Hotza, D.; Rambo, C.R. Enhanced CO2 Capture by Cupuassu Shell-Derived Activated Carbon with High Microporous Volume. Carbon. Lett. 2023, 33, 727–735. [Google Scholar] [CrossRef]
- Cruz, O.F.; Gómez, I.C.; Rodríguez-Reinoso, F.; Silvestre-Albero, J.; Rambo, C.R.; Martínez-Escandell, M. Activated Carbons with High Micropore Volume Obtained from Polyurethane Foams for Enhanced CO2 Adsorption. Chem. Eng. Sci. 2023, 273, 118671. [Google Scholar] [CrossRef]
- Zięzio, M.; Charmas, B.; Jedynak, K.; Hawryluk, M.; Kucio, K. Preparation and Characterization of Activated Carbons Obtained from the Waste Materials Impregnated with Phosphoric Acid(V). Appl. Nanosci. 2020, 10, 4703–4716. [Google Scholar] [CrossRef]
- Wu, H.; Chen, R.; Du, H.; Zhang, J.; Shi, L.; Qin, Y.; Yue, L.; Wang, J. Synthesis of Activated Carbon from Peanut Shell as Dye Adsorbents for Wastewater Treatment. Adsorpt. Sci. Technol. 2019, 37, 34–48. [Google Scholar] [CrossRef]
- McLellan, T.M.; Aber, J.D.; Martin, M.E.; Melillo, J.M.; Nadelhoffer, K.J. Determination of Nitrogen, Lignin, and Cellulose Content of Decomposing Leaf Material by near Infrared Reflectance Spectroscopy. Can. J. For. Res. 2011, 21, 1684–1688. [Google Scholar] [CrossRef]
- Li, X.; Sun, C.; Zhou, B.; He, Y. Determination of Hemicellulose, Cellulose and Lignin in Moso Bamboo by Near Infrared Spectroscopy. Sci. Rep. 2015, 5, 17210. [Google Scholar] [CrossRef] [PubMed]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.W.; Holmes, M.; Hanpin, M. Variables Selection Methods in Near-Infrared Spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shim, H.E.; Yang, J.E.; Choi, Y.J.; Jeon, J. Continuous Flow Removal of Anionic Dyes in Water by Chitosan-Functionalized Iron Oxide Nanoparticles Incorporated in a Dextran Gel Column. Nanomaterials 2019, 9, 1164. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Matheus, L.R.; Albuquerque, L.J.C.; Gasparini, L.J.; Fagnani, K.C.; Alves, H.J.; Colpini, L.M.S. Production of Nanostructured Crystalline Composite Using Residual Ashes from Flocculated Sludge Burning Process in a Poultry Slaughterhouse Wastewater Treatment System. Cerâmica 2022, 68, 427–440. [Google Scholar] [CrossRef]
- de Castro, L.E.N.; Meurer, E.C.; Alves, H.J.; dos Santos, M.A.R.; Vasques, E.d.C.; Colpini, L.M.S. Photocatalytic Degradation of Textile Dye Orange-122 Via Electrospray Mass Spectrometry. Braz. Arch. Biol. Technol. 2020, 63, e20180573. [Google Scholar] [CrossRef]
- Chen, G.Q.; Qu, Y.; Gras, S.L.; Kentish, S.E. Separation Technologies for Whey Protein Fractionation. Food Eng. Rev. 2023, 15, 438–465. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Gangasalam, A.; Ismail, A.F.; Arumugam, S.; Narayanan, A. Concentration of Whey Protein from Cheese Whey Effluent Using Ultrafiltration by Combination of Hydrophilic Metal Oxides and Hydrophobic Polymer. J. Chem. Technol. Biotechnol. 2018, 93, 2576–2591. [Google Scholar] [CrossRef]
- Arunkumar, A.; Etzel, M.R. Milk Protein Concentration Using Negatively Charged Ultrafiltration Membranes. Foods 2018, 7, 134. [Google Scholar] [CrossRef]
- Von Sperling, M. Introdução à Qualidade Das Águas e Ao Tratamento de Esgotos, 2nd ed.; Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 1996; ISBN 9788570411143. [Google Scholar]
- Hu, R.; Liu, Y.; Zhu, G.; Chen, C.; Hantoko, D.; Yan, M. COD Removal of Wastewater from Hydrothermal Carbonization of Food Waste: Using Coagulation Combined Activated Carbon Adsorption. J. Water Process Eng. 2022, 45, 102462. [Google Scholar] [CrossRef]
- Gonzalez-Piedra, S.; Hernández-García, H.; Perez-Morales, J.M.; Acosta-Domínguez, L.; Bastidas-Oyanedel, J.R.; Hernandez-Martinez, E. A Study on the Feasibility of Anaerobic Co-Digestion of Raw Cheese Whey with Coffee Pulp Residues. Energies 2021, 14, 3611. [Google Scholar] [CrossRef]
- Bella, K.; Venkateswara Rao, P. Anaerobic Co-Digestion of Cheese Whey and Septage: Effect of Substrate and Inoculum on Biogas Production. J. Environ. Manag. 2022, 308, 114581. [Google Scholar] [CrossRef]
- Wen, J.; Liu, Z.; Xi, H.; Huang, B. Synthesis of Hierarchical Porous Carbon with High Surface Area by Chemical Activation of (NH4)2C2O4 Modified Hydrochar for Chlorobenzene Adsorption. J. Environ. Sci. 2023, 126, 123–137. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, J.; Zhang, Y.; Du, Q.; Feng, D.; Dong, H.; Peng, Y.; Zhang, T.; Xie, M. Ultra-Microporous Biochar-Based Carbon Adsorbents by a Facile Chemical Activation Strategy for High-Performance CO2 Adsorption. Fuel Process. Technol. 2023, 241, 107613. [Google Scholar] [CrossRef]
- Singh, G.; Maria Ruban, A.; Geng, X.; Vinu, A. Recognizing the Potential of K-Salts, Apart from KOH, for Generating Porous Carbons Using Chemical Activation. Chem. Eng. J. 2023, 451, 139045. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, Z.; Huang, J.; Hu, W.; Xie, D.; Qiao, Y. Efficient Adsorption of Ammonia on Activated Carbon from Hydrochar of Pomelo Peel at Room Temperature: Role of Chemical Components in Feedstock. J. Clean. Prod. 2023, 406, 137076. [Google Scholar] [CrossRef]
- Zhang, J.W.; Mariska, S.; Pap, S.; Tran, H.N.; Chao, H.P. Enhanced Separation Capacity of Carbonaceous Materials (Hydrochar, Biochar, and Activated Carbon) toward Potential Toxic Metals through Grafting Copolymerization. Sep. Purif. Technol. 2023, 320, 124229. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.R.; Encina, P.A.G.; Fdz-Polanco, F. Anaerobic Treatment of Cheese-Production Wastewater Using a UASB Reactor. Bioresour. Technol. 1991, 37, 271–276. [Google Scholar] [CrossRef]
- Rodgers, M.; Zhan, X.-M.; Dolan, B. Mixing Characteristics and Whey Wastewater Treatment of a Novel Moving Anaerobic Biofilm Reactor. J. Environ. Sci. Health Part A 2004, 39, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.; Prazeres, A.R.; Carvalho, F.; Beltrán, F. Treatment of Cheese Whey Wastewater: Combined Coagulation−Flocculation and Aerobic Biodegradation. J. Agric. Food Chem. 2010, 58, 7871–7877. [Google Scholar] [CrossRef]
- Rivas, J.; Prazeres, A.R.; Carvalho, F. Aerobic Biodegradation of Precoagulated Cheese Whey Wastewater. J. Agric. Food Chem. 2011, 59, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Franco, D.S.P.; Schadeck Netto, M.; Allasia, D.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Transforming Shrub Waste into a High-Efficiency Adsorbent: Application of Physalis Peruvian Chalice Treated with Strong Acid to Remove the 2,4-Dichlorophenoxyacetic Acid Herbicide. J. Environ. Chem. Eng. 2021, 9, 104574. [Google Scholar] [CrossRef]
- Caponi, N.; Schnorr, C.; Franco, D.S.P.; Netto, M.S.; Vedovatto, F.; Tres, M.V.; Zabot, G.L.; Abaide, E.R.; Silva, L.F.O.; Dotto, G.L. Potential of Subcritical Water Hydrolyzed Soybean Husk as an Alternative Biosorbent to Uptake Basic Red 9 Dye from Aqueous Solutions. J. Environ. Chem. Eng. 2022, 10, 108603. [Google Scholar] [CrossRef]
- de Azevedo, C.F.; Machado, F.M.; de Souza, N.F.; Silveira, L.L.; Lima, E.C.; Andreazza, R.; Bergamnn, C.P. Comprehensive Adsorption and Spectroscopic Studies on the Interaction of Carbon Nanotubes with Diclofenac Anti-Inflammatory. Chem. Eng. J. 2023, 454, 140102. [Google Scholar] [CrossRef]
- Tran, H.N. Applying Linear Forms of Pseudo-Second-Order Kinetic Model for Feasibly Identifying Errors in the Initial Periods of Time-Dependent Adsorption Datasets. Water 2023, 15, 1231. [Google Scholar] [CrossRef]
- Guo, Y.; Su, C.; Chen, H.; Wang, J.; Liu, B.; Zeng, Z.; Li, L. Hierarchical Porous Carbon with Tunable Apertures and Nitrogen/Oxygen Heteroatoms for Efficient Adsorption and Separation of VOCs. Chem. Eng. J. 2023, 471, 144558. [Google Scholar] [CrossRef]
- de Azevedo, C.F.; Rodrigues, D.L.C.; Silveira, L.L.; Lima, E.C.; Osorio, A.G.; Andreazza, R.; de Pereira, C.M.P.; Poletti, T.; Machado Machado, F. Comprehensive Adsorption and Spectroscopic Studies on the Interaction of Magnetic Biochar from Black Wattle Sawdust with Beta-Blocker Metoprolol. Bioresour. Technol. 2023, 388, 129708. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.A.; Thue, P.S.; Lima, É.C.; Grimm, A.; Naushad, M.; Dotto, G.L.; dos Reis, G.S. Adsorption of Omeprazole on Biobased Adsorbents Doped with Si/Mg: Kinetic, Equilibrium, and Thermodynamic Studies. Molecules 2023, 28, 4591. [Google Scholar] [CrossRef]
- Yanan, C.; Srour, Z.; Ali, J.; Guo, S.; Taamalli, S.; Fèvre-Nollet, V.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Silva, L.F.O.; et al. Adsorption of Paracetamol and Ketoprofenon Activated Charcoal Prepared from the Residue of the Fruit of Butiacapitate: Experiments and Theoretical Interpretations. Chem. Eng. J. 2023, 454, 139943. [Google Scholar] [CrossRef]
- Gabriela Elvir-Padilla, L.; Ileana Mendoza-Castillo, D.; Villanueva-Mejía, F.; Bonilla-Petriciolet, A. Molecular Aggregation Effect on the Antagonistic Adsorption of Pharmaceuticals from Aqueous Solution Using Bone Char: DFT Calculations and Multicomponent Experimental Studies. J. Mol. Liq. 2023, 369, 120957. [Google Scholar] [CrossRef]
- Lima, E.C.; Sher, F.; Guleria, A.; Saeb, M.R.; Anastopoulos, I.; Tran, H.N.; Hosseini-Bandegharaei, A. Is One Performing the Treatment Data of Adsorption Kinetics Correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
- Lima, É.C.; Dehghani, M.H.; Guleria, A.; Sher, F.; Karri, R.R.; Dotto, G.L.; Tran, H.N. Adsorption: Fundamental Aspects and Applications of Adsorption for Effluent Treatment. Green. Technol. Defluoridation Water 2021, chapter 3, 41–88. [Google Scholar] [CrossRef]
- Cantu, Y.; Remes, A.; Reyna, A.; Martinez, D.; Villarreal, J.; Ramos, H.; Trevino, S.; Tamez, C.; Martinez, A.; Eubanks, T.; et al. Thermodynamics, Kinetics, and Activation Energy Studies of the Sorption of Chromium(III) and Chromium(VI) to a Mn3O4 Nanomaterial. Chem. Eng. J. 2014, 254, 374–383. [Google Scholar] [CrossRef]
- Dotto, G.L.; Vieillard, J.; Pinto, D.; Lütke, S.F.; Silva, L.F.O.; dos Reis, G.S.; Lima, É.C.; Franco, D.S.P. Selective Adsorption of Gadolinium from Real Leachate Using a Natural Bentonite Clay. J. Environ. Chem. Eng. 2023, 11, 109748. [Google Scholar] [CrossRef]
- Khadhri, N.; El Khames Saad, M.; Ben Mosbah, M.; Moussaoui, Y. Batch and Continuous Column Adsorption of Indigo Carmine onto Activated Carbon Derived from Date Palm Petiole. J. Environ. Chem. Eng. 2019, 7, 102775. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Maschio, L.J.; da Silva, R.E.; da Silva, M.L.C.P. Adsorption of Cr(VI) from Aqueous Solution by Hydrous Zirconium Oxide. J. Hazard. Mater. 2010, 173, 630–636. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nano Zerovalent Iron Particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, S.; Fu, L.; Zhang, L. Efficient Metal-Organic Framework Adsorbents for Removal of Harmful Heavy Metal Pb(II) from Solution: Activation Energy and Interaction Mechanism. J. Environ. Chem. Eng. 2023, 11, 109335. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z.; Li, J.; You, Y.; Ma, X.; Li, J.; Cheng, X. Preparation of Silicon-Doped Ferrihydrite for Adsorption of Lead and Cadmium: Property and Mechanism. Chin. Chem. Lett. 2021, 32, 3169–3174. [Google Scholar] [CrossRef]
- Cojocaru, C.; Samoila, P.; Pascariu, P. Chitosan-Based Magnetic Adsorbent for Removal of Water-Soluble Anionic Dye: Artificial Neural Network Modeling and Molecular Docking Insights. Int. J. Biol. Macromol. 2019, 123, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.M.; Lachos-Perez, D.; Forster-Carneiro, T.; Rostagno, M.A. Sub- and Supercritical Water Hydrolysis of Agricultural and Food Industry Residues for the Production of Fermentable Sugars: A Review. Food Bioprod. Process. 2016, 98, 95–123. [Google Scholar] [CrossRef]
- Abaide, E.R.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Reasons for Processing of Rice Coproducts: Reality and Expectations. Biomass Bioenergy 2019, 120, 240–256. [Google Scholar] [CrossRef]
- Li, S.; Qi, B.; Luo, J.; Zhuang, Y.; Wan, Y. Ultrafast Selective Adsorption of Pretreatment Inhibitors from Lignocellulosic Hydrolysate with Metal-Organic Frameworks: Performance and Adsorption Mechanisms. Sep. Purif. Technol. 2021, 275, 119183. [Google Scholar] [CrossRef]

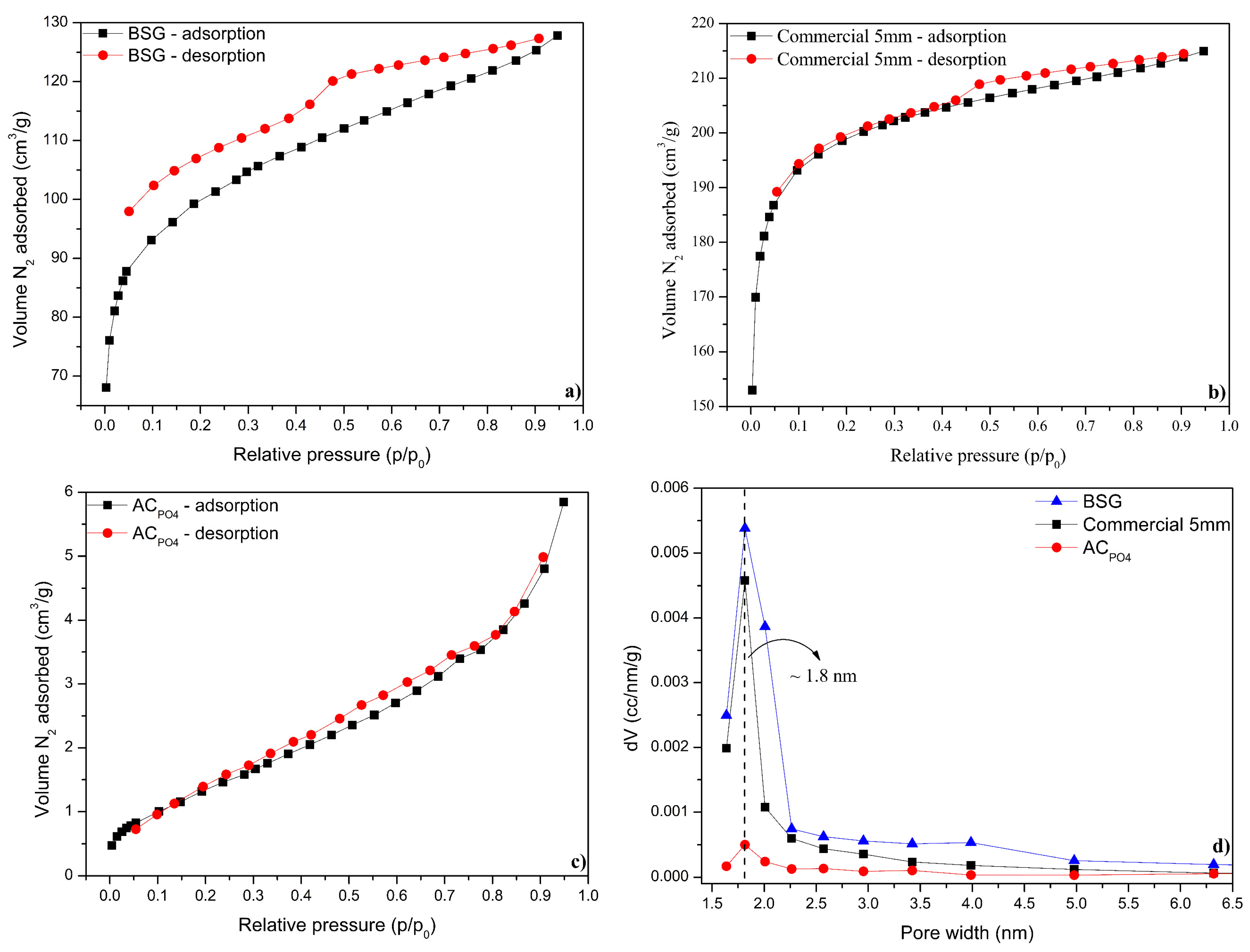
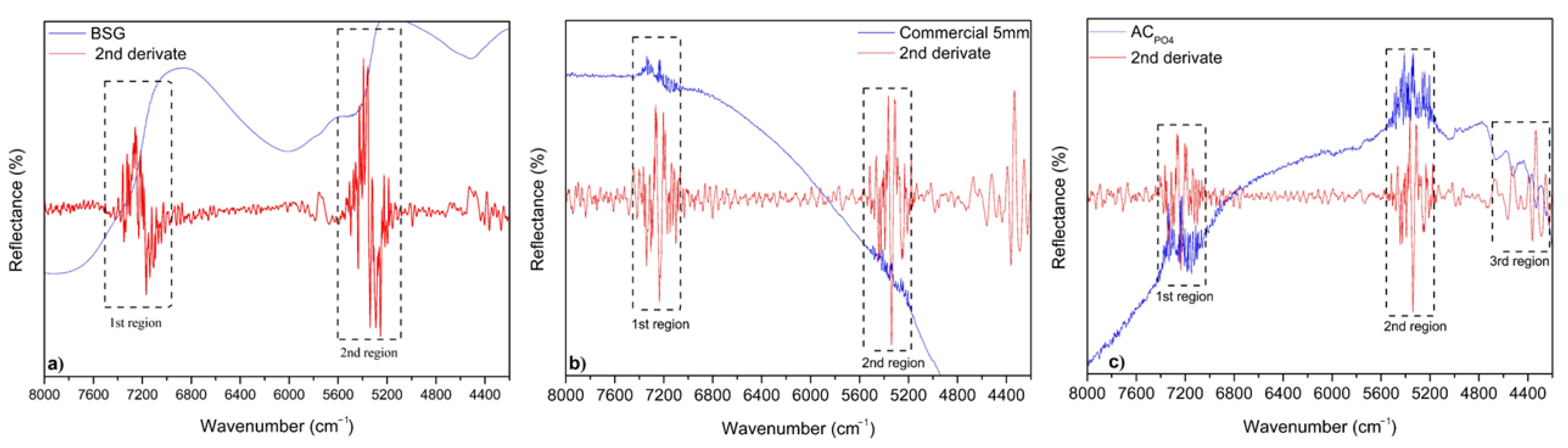
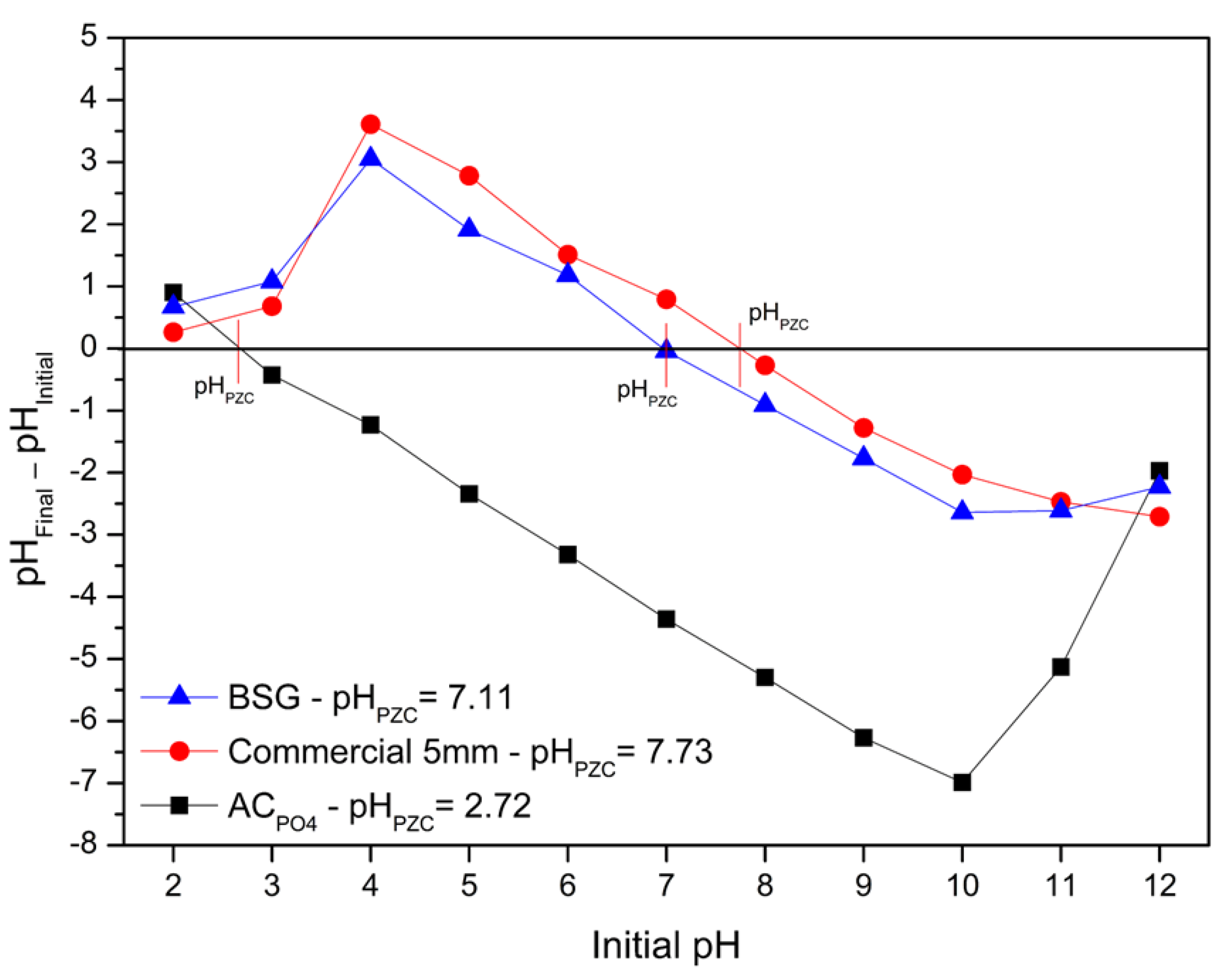
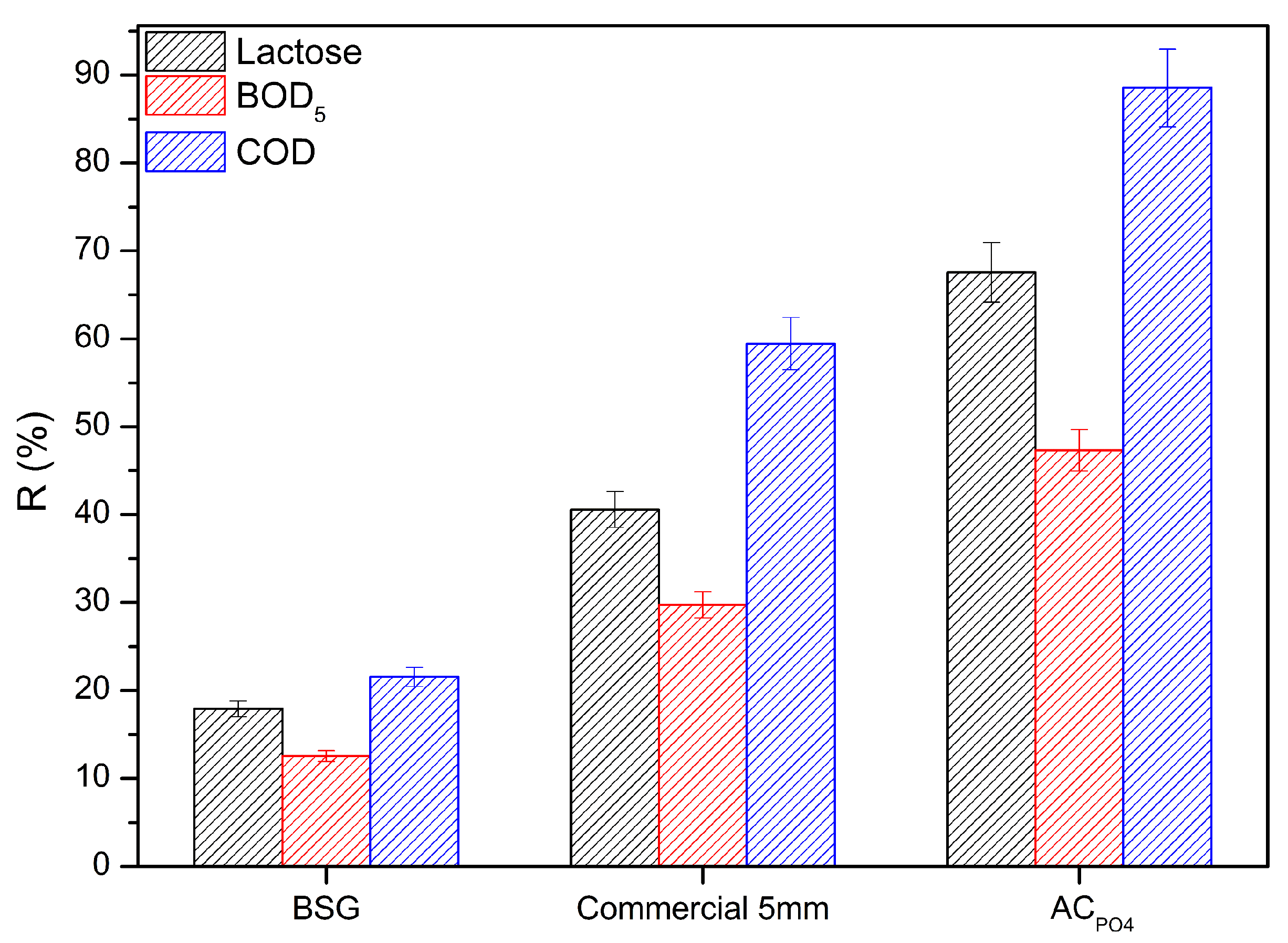


| Adsorbent | Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Si | Mg | K | Ca | P | |
| BSG | 86.35 | 13.23 | 0.42 | 0.26 | 0.11 | 0.74 | - |
| Commercial 5 mm | 86.35 | 13.23 | 0.42 | - | |||
| PO4 | 54.47 | 30.72 | 1.74 | 13.07 | |||
| Adsorbent | So (m2 g−1) | Vp (cm3 g−1) | dp (nm) |
|---|---|---|---|
| BSG | 104.3 | 0.11 | 2.03 |
| Commercial 5 mm | 377.5 | 0.33 | 1.10 |
| ACPO4 | 605.1 | 0.41 | 2.01 |
| Precursor | BET Surface Area (m2 g−1) | Activation Temperature (°C) | Reference |
|---|---|---|---|
| BSG | 605.1 | 400 | This study |
| Spent coffee grounds | 614.8 | 800 | [62] |
| Peanut shells | 590.7 | 400 | [63] |
| BSG | 768.4 | 500 | [8] |
| Rice husk residue | 585.0 | 400 | [63] |
| Technique | Removal (%) | Reference | ||
|---|---|---|---|---|
| Lactose | BOD5 | COD | ||
| Adsorption with activated carbon | 63 | 46 | 91 | This study |
| Upflow anaerobic sludge blanket | – | – | 90 | [82] |
| Vertically moving biofilm system | – | – | 89 | [83] |
| Activated sludge | – | – | 90 | [23] |
| Coagulation–flocculation with FeCl3 | 54 | 23 | 32 | [84] |
| Coagulation–flocculation with Al2(SO4)3 | 49 | 35 | 36 | |
| Ozone | 40 | 43 | 63 | [24] |
| Precipitation with lime | 56 | 45 | 55 | [85] |
| Precipitation with NaOH | 34 | 44 | 50 | |
| Model | Parameter | Absorbate | ||
|---|---|---|---|---|
| Lactose | BOD5 | COD | ||
| PFO | qe | 3.44 g lactose g−1 adsorbent | 482.24 mg O2 g−1 adsorbent | 5234.06 mg O2 g−1 adsorbent |
| k1 | 8.37 × 10−3 min−1 | 24.93 × 10−3 min−1 | 10.98 × 10−3 min−1 | |
| t1/2 | 87.10 min | 28.02 min | 62.68 min | |
| t0.95 | 359.32 min | 121.25 min | 279.52 min | |
| R2adj | 0.95 | 0.95 | 0.97 | |
| SD | 0.27 g lactose g−1 adsorbent | 31.15 mg O2 g−1 adsorbent | 252.03 mg O2 g−1 adsorbent | |
| BIC | −28.54 | 94.54 | 148.90 | |
| PSO | qe | 3.52 g lactose g−1 adsorbent | 518.40 mg O2 g−1 adsorbent | 5315.40 mg O2 g−1 adsorbent |
| k2 | 1.16 × 10−3 g lactose g−1 adsorbent min−1 | 0.06 × 10−3 mg O2 g−1 adsorbent min−1 | 0.02 × 10−3 mg O2 g−1 adsorbent min−1 | |
| t1/2 | 136.93 min | 27.09 min | 61.93 min | |
| t0.95 | 382.95 min | 308.90 min | 333.04 min | |
| R2adj | 0.94 | 0.96 | 0.98 | |
| SD | 0.30 g lactose g−1 adsorbent | 18.43 mg O2 g−1 adsorbent | 171.17 mg O2 g−1 adsorbent | |
| BIC | −25.97 | 70.89 | 168.74 | |
| AFO | qe | 3.18 g lactose g−1 adsorbent | 506.44 mg O2 g−1 adsorbent | 5303.37 mg O2 g−1 adsorbent |
| kAV | 19.56 × 10−3 min−1 | 10.69 × 10−3 min−1 | 5.22 × 10−3 min−1 | |
| nAV | 2.57 | 0.47 | 0.59 | |
| t1/2 | 73.88 min | 24.86 min | 61.18 min | |
| t0.95 | 200.34 min | 217.30 min | 315.99 min | |
| R2adj | 0.97 | 0.97 | 0.99 | |
| SD | 0.21 g lactose g−1 adsorbent | 23.41 mg O2 g−1 adsorbent | 107.93 mg O2 g−1 adsorbent | |
| BIC | −40.38 | 83.13 | 119.81 | |
| Model | Parameter | T (K) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 288 | 298 | 308 | 318 | ||||||||||
| Adsorbate | |||||||||||||
| Lactose | BOD5 | COD | Lactose | BOD5 | COD | Lactose | BOD5 | COD | Lactose | BOD5 | COD | ||
| Langmuir | qmL * | 4.55 | 713.50 | 7378.63 | 5.41 | 988.04 | 9050.27 | 6.86 | 1115.78 | 10,657.12 | 7.44 | 1349.31 | 12,220.00 |
| kL (L mg−1) | 0.0001 | 0.0004 | 0.00002 | 0.0005 | 0.0004 | 0.00004 | 0.40 | 0.77 | 0.11 | 0.54 | 1.02 | 0.16 | |
| R2adj | 0.995 | 0.999 | 0.995 | 0.998 | 0.998 | 0.981 | 0.996 | 0.997 | 0.973 | 0.998 | 0.993 | 0.998 | |
| SD | 0.09 | 4.75 | 165.35 | 0.06 | 8.87 | 432.10 | 0.10 | 17.84 | 577.46 | 0.77 | 32.43 | 171.29 | |
| BIC | −20.60 | 18.80 | 54.30 | −24.84 | 25.05 | 63.90 | −19.93 | 32.03 | 66.80 | −22.40 | 38.00 | 54.65 | |
| Freundlich | kF + | 1.07 | 4.27 | 11.71 | 1.88 | 6.05 | 29.75 | 2.16 | 29.92 | 322.26 | 2.84 | 57.06 | 853.09 |
| nF | 1.46 | 1.80 | 1.78 | 2.29 | 1.77 | 1.99 | 2.13 | 2.47 | 3.27 | 2.47 | 2.81 | 4.29 | |
| R2adj | 0.989 | 0.991 | 0.975 | 0.988 | 0.989 | 0.973 | 0.985 | 0.988 | 0.948 | 0.997 | 0.998 | 0.978 | |
| SD | 0.15 | 16.48 | 381.15 | 0.15 | 27.05 | 511.61 | 0.20 | 36.14 | 848.75 | 0.11 | 18.59 | 601.47 | |
| BIC | −15.83 | 31.24 | 62.65 | −15.62 | 36.18 | 65.56 | −12.55 | 39.09 | 70.65 | −19.63 | 32.44 | 67.21 | |
| Dubinin–Radushkevich | qmDR * | 4.25 | 496.32 | 6219.23 | 4.27 | 911.62 | 8286.64 | 5.17 | 1016.97 | 9794.23 | 8.06 | 1726.89 | 11,237.52 |
| β (mol2 kJ−2) | 31.23 | 25.66 | 31.19 | 20.27 | 17.00 | 20.80 | 21.32 | 12.92 | 11.88 | 8.07 | 5.35 | 10.59 | |
| Es (kJ mol−1) | 0.13 | 0.14 | 0.13 | 0.16 | 0.17 | 0.16 | 0.15 | 0.20 | 0.21 | 0.25 | 0.31 | 0.22 | |
| R2adj | 0.997 | 0.996 | 0.985 | 0.998 | 0.998 | 0.928 | 0.997 | 0.996 | 0.977 | 0.999 | 0.999 | 0.999 | |
| SD | 0.06 | 10.50 | 296.43 | 0.06 | 10.52 | 833.99 | 0.09 | 18.19 | 532.19 | 0.04 | 10.35 | 127.37 | |
| BIC | −24.07 | 22.97 | 49.69 | −18.04 | 22.98 | 57.97 | −14.67 | 27.37 | 54.37 | −21.79 | 22.85 | 42.93 | |
| Hill | qmH * | 3.69 | 719.89 | 6721.23 | 4.22 | 1043.76 | 8229.11 | 4.72 | 1371.13 | 12,759.16 | 12.77 | 3940.99 | 12,857.92 |
| C1/2 (mg L−1) | 3.17 | 2697.27 | 27,607.02 | 1.77 | 2591.11 | 21,454.73 | 1.83 | 1549.59 | 11,212.78 | 3.45 | 4526.72 | 6488.04 | |
| nH | 1.36 | 0.99 | 1.19 | 1.16 | 1.03 | 1.18 | 1.25 | 0.90 | 0.89 | 0.75 | 0.58 | 0.96 | |
| R2adj | 0.998 | 0.999 | 0.996 | 0.999 | 0.999 | 0.998 | 0.998 | 0.998 | 0.999 | 0.999 | 0.999 | 0.999 | |
| SD | 0.07 | 5.31 | 146.99 | 0.06 | 9.75 | 124.25 | 0.09 | 18.57 | 95.62 | 0.04 | 9.63 | 188.63 | |
| BIC | −27.50 | 17.51 | 44.08 | −28.66 | 22.38 | 42.74 | −25.35 | 27.53 | 40.64 | −28.69 | 22.28 | 46.07 | |
| Adsorbate | T (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | Ea (kJ mol−1) |
|---|---|---|---|---|---|
| Lactose | 288 | −3.31 | 238.26 | 830.12 | 29.24 |
| 298 | −6.26 | ||||
| 308 | −23.74 | ||||
| 318 | −25.31 | ||||
| BOD5 | 288 | −5.66 | 237.16 | 829.87 | 21.71 |
| 298 | −5.86 | ||||
| 308 | −25.42 | ||||
| 318 | −26.99 | ||||
| COD | 288 | 1.51 | 265.72 | 910.37 | 39.25 |
| 298 | −0.15 | ||||
| 308 | −20.44 | ||||
| 318 | −22.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, L.E.N.; Matheus, L.R.; Mançano, R.R.; Sganzerla, W.G.; da Rosa, R.G.; Barroso, T.L.C.T.; Ferreira, V.C.; Colpini, L.M.S. Single-Step Modification of Brewer’s Spent Grains Using Phosphoric Acid and Application in Cheese Whey Remediation via Liquid-Phase Adsorption. Water 2023, 15, 3682. https://doi.org/10.3390/w15203682
Castro LEN, Matheus LR, Mançano RR, Sganzerla WG, da Rosa RG, Barroso TLCT, Ferreira VC, Colpini LMS. Single-Step Modification of Brewer’s Spent Grains Using Phosphoric Acid and Application in Cheese Whey Remediation via Liquid-Phase Adsorption. Water. 2023; 15(20):3682. https://doi.org/10.3390/w15203682
Chicago/Turabian StyleCastro, Luiz Eduardo Nochi, Larissa Resende Matheus, Rosana Rabelo Mançano, William Gustavo Sganzerla, Rafael Gabriel da Rosa, Tiago Linhares Cruz Tabosa Barroso, Vanessa Cosme Ferreira, and Leda Maria Saragiotto Colpini. 2023. "Single-Step Modification of Brewer’s Spent Grains Using Phosphoric Acid and Application in Cheese Whey Remediation via Liquid-Phase Adsorption" Water 15, no. 20: 3682. https://doi.org/10.3390/w15203682
APA StyleCastro, L. E. N., Matheus, L. R., Mançano, R. R., Sganzerla, W. G., da Rosa, R. G., Barroso, T. L. C. T., Ferreira, V. C., & Colpini, L. M. S. (2023). Single-Step Modification of Brewer’s Spent Grains Using Phosphoric Acid and Application in Cheese Whey Remediation via Liquid-Phase Adsorption. Water, 15(20), 3682. https://doi.org/10.3390/w15203682








