Analysis of the Recharge Area of the Perrot Spring (Aosta Valley) Using a Hydrochemical and Isotopic Approach
Abstract
1. Introduction
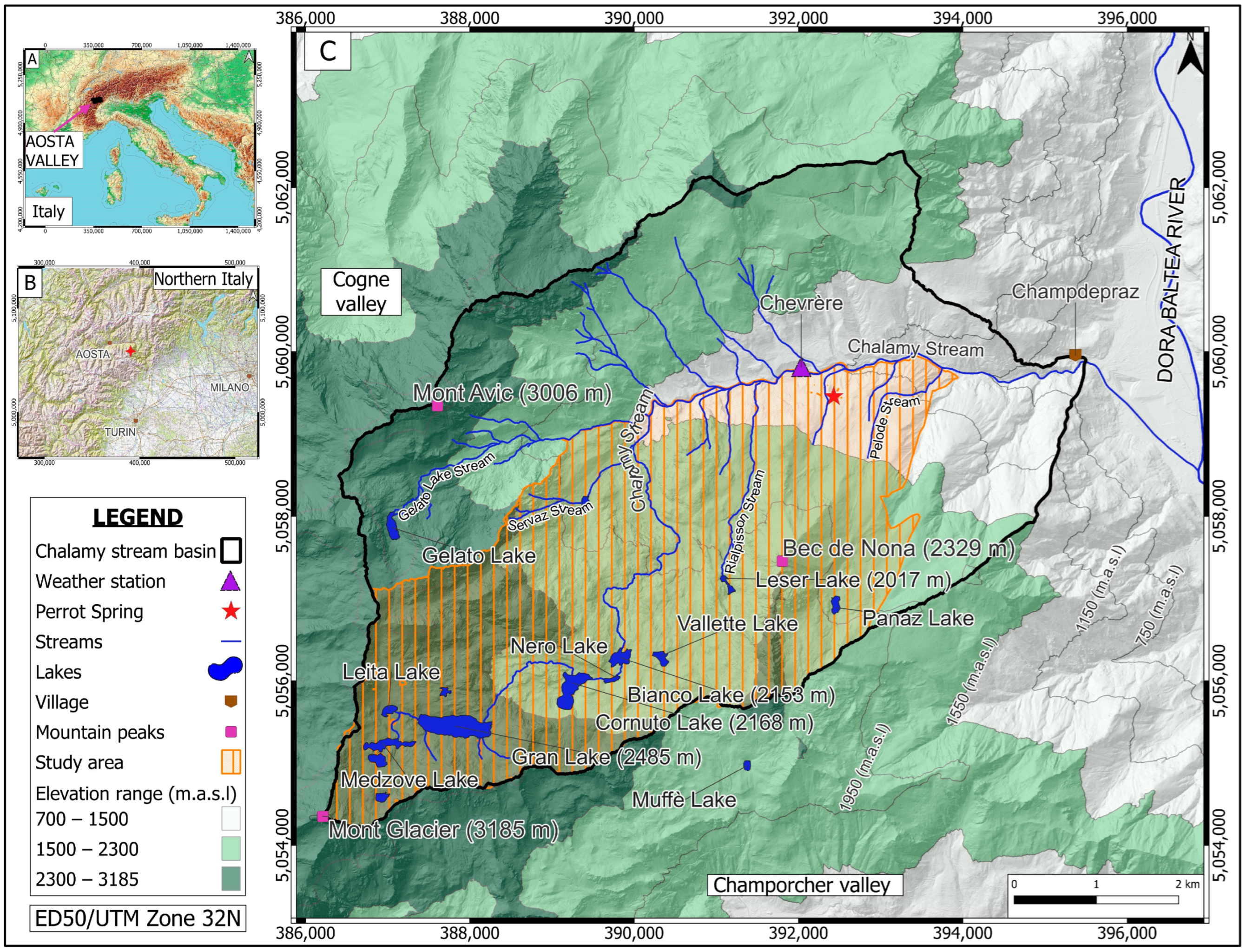
2. Study Area Description
2.1. Study Area
2.2. Geological Setting
2.3. Hydrogeological Setting
- Metamorphic bedrock.
- 2.
- Quaternary succession.
- Subglacial deposits: over consolidated deposits characterized by abundant sandy–silty matrix containing subordinate clasts. Low permeability;
- Ice-marginal deposits: deposits with a very heterogeneous texture, characterised by the presence of clasts of different sizes and shapes, mixed in a greyish-brown sandy–silty matrix with carbonate cementation. Variable permeability;
- Glaciolacustrine deposits: deposits formed by a sandy–silty matrix in which some centimetric to decimetric clasts are embedded, showing carbonate cementation. Low permeability;
- Debris and landslide deposits: deposits formed by angular decimetric clasts without matrix. Very high permeability;
- Lake and marsh deposits: silty and peat deposits characterised by a very localised distribution in depressed areas. Low permeability.
3. Materials and Methods
3.1. Climatic Conditions
3.2. Water Sampling
3.3. Laboratory Analyses
3.4. Chemical and Isotopic Data Interpretation
4. Results
4.1. Climatic Setting of the Study Area
4.2. Chemical Analysis
4.3. Isotopic Analyses
5. Discussions
- -
- fractured rocky bedrock with low to very low permeability, essentially represented by the serpentinite.
- -
- very thick incoherent deposits of very high to high permeability, including ice-marginal, gravitational, and fluvial deposits that lie on incoherent deposits of low permeability, including subglacial and glaciolacustrine deposits.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The United Nations World Water Development. Groundwater: Making the Invisible Visible; The United Nations World Water Development: Paris, France, 2022. [Google Scholar]
- Bowen, G.J.; Ehleringer, J.R.; Chesson, L.A.; Stange, E.; Cerling, T.E. Stable Isotope Ratios of Tap Water in the Contiguous United States. Water Resour. Res. 2007, 43, W03419. [Google Scholar] [CrossRef]
- De Luca, D.A.; Masciocco, L.; Caviglia, C.; Destefanis, E.; Forno, M.G.; Fratianni, S.; Gattiglio, M.; Gianotti, F.; Lasagna, M.; Latagliata, V.; et al. Distribution, Discharge, Geological and Physical–Chemical Features of the Springs in the Turin Province (Piedmont, NW Italy). In Engineering Geology for Society and Territory-Volume 3; Springer International Publishing: Cham, Switzerland, 2015; pp. 253–256. [Google Scholar] [CrossRef]
- Fiorillo, F.; Pagnozzi, M.; Stevanović, Z.; Ventafridda, G. Main hydrological features and recharge analysis of the Caposele Spring Catchment, Southern Italy. Acta Carsologica 2019, 48, 6738. [Google Scholar] [CrossRef]
- Cusano, D.; Allocca, V.; Coda, S.; Lepore, D.; Vassallo, M.; De Vita, P. The Survey of Italian Springs by the National Hydrographic Service, a Forgotten Database. Structuring and Analysis of a Dataset of Campania Springs (Southern Italy). Acque Sotter. Ital. J. Groundw. 2022, 11, 31–41. [Google Scholar] [CrossRef]
- Vigna, B.; Banzato, C. The Hydrogeology of High-Mountain Carbonate Areas: An Example of Some Alpine Systems in Southern Piedmont (Italy). Environ. Earth Sci. 2015, 74, 267–280. [Google Scholar] [CrossRef]
- Howell, B.A.; Fryar, A.E.; Benaabidate, L.; Bouchaou, L.; Farhaoui, M. Variable Responses of Karst Springs to Recharge in the Middle Atlas Region of Morocco. Hydrogeol. J. 2019, 27, 1693–1710. [Google Scholar] [CrossRef]
- Bastiancich, L.; Lasagna, M.; Mancini, S.; Falco, M.; De Luca, D.A. Temperature and discharge variations in natural mineral water springs due to climate variability: A case study in the Piedmont Alps (NW Italy). Environ. Geochem. Health 2022, 44, 1971–1994. [Google Scholar] [CrossRef]
- Fensham, R.J.; Doyle, T.; Habermehl, M.A.; Laffineur, B.; Silcock, J.L. Hydrogeological assessment of Springs in the South-Central Great Artesian Basin of Australia. Hydrogeol. J. 2021, 29, 1501–1515. [Google Scholar] [CrossRef]
- Galleani, L.; Vigna, B.; Banzato, C.; Russo, S.L. Validation of a Vulnerability Estimator for Spring Protection Areas: The VESPA Index. J. Hydrol. 2011, 396, 233–245. [Google Scholar] [CrossRef]
- Stevens, L.E.; Schenk, E.R.; Springer, A.E. Springs Ecosystem Classification. Ecol. Appl. 2021, 31, 2218. [Google Scholar] [CrossRef] [PubMed]
- Petitta, M.; Banzato, F.; Lorenzi, V.; Matani, E.; Sbarbati, C. Determining Recharge Distribution in Fractured Carbonate Aquifers in Central Italy Using Environmental Isotopes: Snowpack Cover as an Indicator for Future Availability of Groundwater Resources. Hydrogeol. J. 2022, 30, 1619–1636. [Google Scholar] [CrossRef]
- Lasagna, M.; De Luca, D.A.; Clemente, P.; Dino, G.A.; Forno, M.G.; Gattiglio, M.; Gianotti, F. Study on the Water Supply of the Montellina Spring by the Renanchio Stream (Quincinetto, Turin). Acque Sotter. Ital. J. Groundw. 2013, 2, 75–85. [Google Scholar] [CrossRef][Green Version]
- De Luca, D.A.; Cerino Abdin, E.; Forno, M.G.; Gattiglio, M.; Gianotti, F.; Lasagna, M. The Montellina Spring as an Example of Water Circulation in an Alpine DSGSD Context (NW Italy). Water 2019, 11, 700. [Google Scholar] [CrossRef]
- Yu, S.; Chae, G.; Oh, J.; Kim, S.-H.; Kim, D.-I.; Yun, S.-T. Hydrochemical and Isotopic Difference of Spring Water Depending on Flow Type in a Stratigraphically Complex Karst Area of South Korea. Front. Earth Sci. 2021, 9, 712865. [Google Scholar] [CrossRef]
- Cocca, D.; Lasagna, M.; Marchina, C.; Brombin, V.; Santillan-Quiroga, L.M.; De Luca, D.A. Assessment of the Groundwater Recharge Processes of a Shallow and Deep Aquifer System (Maggiore Valley, Northwest Italy): A Hydrogeochemical and Isotopic Approach. Hydrogeol. J. 2023, 1–22. [Google Scholar] [CrossRef]
- Bian, J.; Sun, W.; Li, J.; Li, Y.; Ma, Y.; Li, Y. Hydrochemical Formation Mechanism of Mineral Springs in Changbai Mountain (China). Environ. Earth Sci. 2023, 82, 145. [Google Scholar] [CrossRef]
- Barbeta, A.; Ogée, J.; Peñuelas, J. Stable-Isotope Techniques to Investigate Sources of Plant Water. In Advances in Plant Ecophysiology Techniques; Springer International Publishing: Cham, Switzerland, 2018; pp. 439–456. [Google Scholar] [CrossRef]
- Sprenger, M.; Weiler, M.; Volkmann, T.H. A Global Review on Isotopic and Hydrochemical Studies of the Unsaturated Zone. Earth-Sci. Rev. 2018, 185, 150–171. [Google Scholar]
- Gat, J.R. Oxygen and Hydrogen Isotopes in the Hydrologic Cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- West, J.B.; Bowen, G.J.; Cerling, T.E.; Ehleringer, J.R. Stable Isotopes as One of Nature’s Ecological Recorders. Trends Ecol. Evol. 2006, 21, 408–414. [Google Scholar] [CrossRef]
- Chen, K.; Tetzlaff, D.; Goldhammer, T.; Freymueller, J.; Wu, S.; Andrew Smith, A.; Schmidt, A.; Liu, G.; Venohr, M.; Soulsby, C. Synoptic Water Isotope Surveys to Understand the Hydrology of Large Intensively Managed Catchments. J. Hydrol. 2023, 623, 129817. [Google Scholar] [CrossRef]
- Windhorst, D.; Waltz, T.; Timbe, E.; Frede, H.-G.; Breuer, L. Impact of Elevation and Weather Patterns on the Isotopic Composition of Precipitation in a Tropical Montane Rainforest. Hydrol. Earth Syst. Sci. 2013, 17, 409–419. [Google Scholar] [CrossRef]
- Novel, J.P.; Ravello, M.; Dray, M.; Pollicini, F.; Zuppi, G.M. Isotopic Contribution (18O, 2H, 3H) in the Understanding of the Flow Pattern in Surface Water and Groundwater in the Aosta Valley (Italy). Geogr. Fis. Din. Quat. 1995, 18, 315–319. [Google Scholar]
- Calligaris, C.; Mezga, K.; Slejko, F.; Urbanc, J.; Zini, L. Groundwater Characterization by Means of Conservative (δ18O and δ2H) and non-conservative (87Sr/86Sr) Isotopic Values: The Classical Karst Region Aquifer Case (Italy–Slovenia). Geosciences 2018, 8, 321. [Google Scholar] [CrossRef]
- Capecchiacci, F.; Tassi, F.; Vaselli, O.; Bicocchi, G.; Cabassi, J.; Giannini, L.; Nisi, B.; Chiocciora, G. A Combined Geochemical and Isotopic Study of the Fluids Discharged from the Montecatini Thermal System (NW Tuscany, Italy). Appl. Geochem. 2015, 59, 33–46. [Google Scholar] [CrossRef]
- Frondini, F.; Zucchini, A.; Comodi, P. Water-Rock Interactions and Trace Elements Distribution in Dolomite Aquifers: The Sassolungo and Sella Systems (Northern Italy). Geochem. J. 2014, 48, 231–246. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Smedley, P.L. Residence time indicators in groundwater: The East Midlands Triassic sandstone aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Bouchaou, L.; Michelot, J.L.; Vengosh, A.; Hsissou, Y.; Qurtobi, M.; Gaye, C.B.; Bullen, T.D.; Zuppi, G.M. Application of Multiple Isotopic and Geochemical Tracers for Investigation of Recharge, Salinization, and Residence Time of Water in the Souss–Massa Aquifer, Southwest of Morocco. J. Hydrol. 2008, 352, 267–287. [Google Scholar] [CrossRef]
- Joshi, S.K.; Rai, S.P.; Sinha, R.; Gupta, S.; Densmore, A.L.; Rawat, Y.S.; Shekhar, S. Tracing Groundwater Recharge Sources in the Northwestern Indian Alluvial Aquifer Using Water Isotopes (δ18O, δ2H and 3H). J. Hydrol. 2018, 559, 835–847. [Google Scholar] [CrossRef]
- Terzer, S.; Wassenaar, L.I.; Araguás-Araguás, L.J.; Aggarwal, P.K. Global Isoscapes for δ18O and δ2H in Precipitation: Improved Using Regionalized Climatic Regression Models. Hydrol. Earth Syst. Sci. 2013, 17, 4713–4728. [Google Scholar] [CrossRef]
- Gaj, M.; Beyer, M.; Koeniger, P.; Wanke, H.; Hamutoko, J.; Himmelsbach, T. In Situ Unsaturated Zone Water Stable Isotope (δ2H and δ18O) Measurements in Semi-Arid Environments: A Soil Water Balance. Hydrol. Earth Syst. Sci. 2016, 20, 715–731. [Google Scholar] [CrossRef]
- von Freyberg, J.; Studer, B.; Kirchner, J.W. A Lab in the Field: High-Frequency Analysis of Water Quality and Stable Isotopes in Stream Water and Precipitation. Hydrol. Earth Syst. Sci. 2017, 21, 1721–1739. [Google Scholar] [CrossRef]
- Sánchez-Murillo, R.; Durán-Quesada, A.M.; Esquivel-Hernández, G.; Rojas-Cantillano, D.; Birkel, C.; Welsh, K.; Sánchez-Llull, M.; Alonso-Hernández, C.M.; Tetzlaff, D.; Soulsby, C.; et al. Deciphering Key Processes Controlling Rainfall Isotopic Variability during Extreme Tropical Cyclones. Nat. Commun. 2019, 10, 4321. [Google Scholar] [CrossRef] [PubMed]
- Stichler, W.; Schotterer, U.; Fröhlich, K.; Ginot, P.; Kull, C.; Gäggeler, H.; Pouyaud, B. Influence of Sublimation on Stable Isotope Records Recovered from High-altitude Glaciers in the Tropical Andes. J. Geophys. Res. Atmos. 2001, 106, 22613–22620. [Google Scholar] [CrossRef]
- Manciati, C.; Taupin, J.D.; Patris, N.; Leduc, C.; Casiot, C. Diverging Water Ages Inferred from Hydrodynamics, Hydrochemical and Isotopic Tracers in a Tropical Andean Volcano-Sedimentary Confined Aquifer System. Front. Water 2021, 3, 597641. [Google Scholar] [CrossRef]
- Yang, K.; Han, G.; Liu, M.; Li, X.; Liu, J.; Zhang, Q. Spatial and Seasonal Variation of O and H Isotopes in the Jiulong River, Southeast China. Water 2018, 10, 1677. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, C.; Wu, D.; Chen, G.; Lu, X.; Liu, X. Estimation of Evaporation Losses Based on Stable Isotopes of Stream Water in a Mountain Watershed. Acta Geochim. 2021, 40, 176–183. [Google Scholar] [CrossRef]
- Darling, W.G.; Bath, A.H.; Talbot, J.C. The O and H Stable Isotopic Composition of Fresh Waters in the British Isles. 2. Surface Waters and Groundwater. Hydrol. Earth Syst. Sci. 2003, 7, 183–195. [Google Scholar] [CrossRef]
- Marchina, C.; Zuecco, G.; Chiogna, G.; Bianchini, G.; Carturan, L.; Comiti, F.; Engel, M.; Natali, C.; Borga, M.; Penna, D. Alternative Methods to Determine the δ2H-δ18O Relationship: An Application to Different Water Types. J. Hydrol. 2020, 587, 124951. [Google Scholar] [CrossRef]
- Bearth, P. Die Ophiolithe Der Zone Von Zermatt-Saas Fee; Kümmerly & Frey: Bern, Switzerland, 1967; 130P. [Google Scholar]
- Bucher, K. Blueschists, Eclogites, and Decompression Assemblages of the Zermatt-Saas Ophiolite: High-Pressure Metamorphism of Subducted Tethys Lithosphere. Am. Mineral. 2005, 90, 821–835. [Google Scholar] [CrossRef]
- Piaz, G.V.D.; Ernst, W.G. Areal Geology and Petrology of Eclogites and Associated Metabasites of the Piemonte Ophiolite Nappe, Breuil—St. Jacques Area, Italian Western Alps. Tectonophysics 1978, 51, 99–126. [Google Scholar] [CrossRef]
- Dal Piaz, G.; Cortiana, G.; Del Moro, A.; Martin, S.; Pennacchioni, G.; Tartarotti, P. Tertiary Age and Paleostructural Inferences of the Eclogitic Imprint in the Austroalpine Outliers and Zermatt–Saas Ophiolite, Western Alps. Int. J. Earth Sci. 2001, 90, 668–684. [Google Scholar] [CrossRef]
- Reinecke, T. Prograde High- to Ultrahigh-Pressure Metamorphism and Exhumation of Oceanic Sediments at Lago Di Cignana, Zermatt-Saas Zone, Western Alps. Lithos 1998, 42, 147–189. [Google Scholar] [CrossRef]
- Fontana, E.; Panseri, M.; Tartarotti, P. Oceanic Relict Textures in the Mount Avic Serpentinites, Western Alps. Ofioliti 2008, 33, 105–118. [Google Scholar]
- Zanoni, D.; Rebay, G.; Bernardoni, J.; Spalla, M.I. Using Multiscale Structural Analysis to Infer High-/Ultrahigh-Pressure Assemblages in Subducted Rodingites of the Zermatt-Saas Zone at Valtournanche, Italy. J. Virtual Explor. 2012, 41, 290. [Google Scholar] [CrossRef]
- Martin, S.; Tartarotti, P. Polyphase HP Metamorphism in the Ophiolitic Glaucophanites of the Lower St. Marcel Valley (Aosta. Italy). Ofioliti 1989, 14, 135–156. [Google Scholar]
- Tartarotti, P.; Benciolini, L.; Monopoli, B. Brecce Serpentinitiche Nel Massiccio Ultrabasico Del Monte Avic (Falda Ofiolitica Piemontese): Possibili Evidenze Di Erosione Sottomarina. Atti Tic Sc. Terra 1998, 7, 73–86. [Google Scholar]
- Fontana, E.; Tartarotti, P.; Panseri, M.; Buscemi, S. Geological Map of the Mount Avic Massif (Western Alps Ophiolites). J. Maps 2015, 11, 126–135. [Google Scholar] [CrossRef][Green Version]
- Martin, S.; Kienast, J.R. The HP-LT Manganiferous Quartzites of Praborna. Piemonte Ophiolite Nappe. Italian Western Alps. Schweiz. Mineral. Und Petrogr. Mitteilungen 1987, 67, 339–360. [Google Scholar]
- Forno, M.G.; Gattiglio, M.; Ghignone, S.; De Luca, D.A.; Santillan Quiroga, L.M. Geological Significance of the Perrot Spring in Mont Avic Natural Park (NW Alps). Water 2023, 15, 3042. [Google Scholar] [CrossRef]
- Dal Piaz, G.V.; Gianotti, F.; Monopoli, B.; Pennacchioni, G.; Tartarotti, P.; Schiavo, A. Note Illustrative Della Carta Geologica d’Italia Alla Scala 1:50.000. Foglio 091 “Chatillon”. Regione Autonoma Valle D’Aosta, Italy, 2010. [Google Scholar]
- Centro Funzionale Regione Autonoma Vall’Aosta. Dati Osservati del Centro Funzionale RAVDA. Available online: https://presidi2.regione.vda.it/str_dataview_download (accessed on 7 July 2023).
- ISO 7888:1985; Water Quality Determination of Electrical Conductivity. International Organization for Standardization: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/14838.html (accessed on 26 May 2023).
- ISO 10523:2008; Water Quality Determination of PH. International Organization for Standardization: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/51994.html (accessed on 26 May 2023).
- U.S. EPA Method 300.1, Determination of Inorganic Anions in Drinking Water by Ion Chromatography. U.S. Cincinnati; 1997. Available online: https://www.epa.gov/esam/epa-method-3001-revision-10-determination-inorganic-anions-drinking-water-ion-chromatography (accessed on 26 May 2023).
- ISO 14911:1998; Water Quality Determination of Dissolved Li+, Na+, NH4+, K+, Mn2+, Ca2+, Mg2+, Sr2+ and Ba2+ Using Ion Chromatography Method for Water and Wastewater. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/25591.html (accessed on 26 May 2023).
- ISO 9963-1:1994; Water Quality Determination of Alkalinity Part 1: Determination of Total and Composite Alkalinity. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/17868.html (accessed on 26 May 2023).
- Zuecco, G.; Marchina, C.; Gelmini, Y.; Amin, A.; van Meerveld, H.J.; Penna, D.; Borga, M. Ressi Experimental Catchment: Ecohydrological Research in the Italian pre-Alps. Hydrol. Process. 2021, 35, 3. [Google Scholar] [CrossRef]
- Giustini, F.; Brilli, M.; Patera, A. Mapping Oxygen Stable Isotopes of Precipitation in Italy. J. Hydrol. Reg. Stud. 2016, 8, 162–181. [Google Scholar] [CrossRef]
- AINEVA. Analisi Meteo-Climatologiche e Nivo-Valangologiche su Alpi e Appennini 2021-22. Neve e Valanghe-Aineva. Available online: https://aineva.it/pubblicazioni/resoconto-inverno-2021-22/ (accessed on 25 September 2023).
- Celico, P. Prospezioni Idrogeologiche; Liguori Editore: Napoli, Italy, 1986; Volume 2, Available online: https://catalogo-unito.sebina.it/SebinaOpac/resource/prospezioni-idrogeologiche/UTO01172659?tabDoc=tabcontiene (accessed on 25 September 2023).
- Grappein, B.; Lasagna, M.; Capodaglio, P.; Caselle, C.; De Luca, D.A. Hydrochemical and Isotopic Applications in the Western Aosta Valley (Italy) for Sustainable Groundwater Management. Sustainability 2021, 13, 487. [Google Scholar] [CrossRef]
- Scandellari, F. Gli Isotopi Stabili Nell’acqua Fra Suolo, Pianta e Atmosfera. Italus Hortus 2018, 24, 51–67. [Google Scholar] [CrossRef]










| Lithology | Hydraulic Conductivity k (m/s) | Type of Permeability | |
|---|---|---|---|
| Lake and marsh deposits | Very low (10−7 > k > 10−9) | Porosity | |
| Debris and landslide deposits | Very high (k > 10−2) | Porosity | |
| Subglacial deposits | Very low (10−7 > k > 10−9) | Porosity | |
| Ice-marginal deposits | Low (10−5 > k > 10−7) | Porosity | |
| Glaciolacustrine | Low and very low (10−5 > k > 10−9) | Porosity | |
| Metamorphic bedrock (serpentinite) | Fractured | Low (10−5 > k > 10−7) | Fractures |
| Mylonitized | Medium (10−4 > k > 10−5) | ||
| Foliate and fractured | High (10−2 > k > 10−4) | ||
| Massive | Impermeable (k < 10−9) | ||
| Sampling Date | 2021 | 2022 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 May | 6 May | 3 July | 11 November | 7 July | 8 July | 20 September | ||||||
| Monitoring Point ID | Location | X UTM ED 50 | Y UTM ED 50 | Elevation (m a.s.l.) | A | B | A | A | B | A | B | A |
| C_1 | Park Office | 395,752 | 5,060,071 | 414 | x | x | x | x | ||||
| C_2 | Park Location 2 | 393,786 | 5,059,742 | 965 | x | x | x | x | ||||
| C_3 | Park Location 3 | 392,011 | 5,059,822 | 1269 | x | x | x | x | ||||
| C_4 | near Perrot Spring | 391,942 | 5,058,810 | 1561 | x | x | x | |||||
| C_5 | near Bianco Lake | 389,798 | 5,056,012 | 2160 | x | x | ||||||
| C_6 | near Gran Lake | 388,078 | 5,055,339 | 2503 | x | |||||||
| S_1 | Perrot Spring | 392,417 | 5,059,457 | 1300 | x | x | x | x | x | |||
| R_1 | Chalamy Stream | 388,314 | 5,055,804 | 2295 | x | x | ||||||
| R_2 | Chalamy Strea | 387,318 | 5,055,539 | 2532 | x | x | ||||||
| L_1 | Leser Lake | 390,992 | 5,057,071 | 2017 | x | x | ||||||
| L_2 | Vallette Lake | 390,305 | 5,056,100 | 2182 | x | x | ||||||
| L_3 | Bianco Lake | 389,818 | 5,056,055 | 2159 | x | x | x | |||||
| L_4 | Nero Lake | 389,718 | 5,055,861 | 2169 | x | x | x | |||||
| L_5 | Gran Lake | 388,055 | 5,055,272 | 2494 | x | x | x | |||||
| L_6 | Leita Lake | 386,974 | 5,055,430 | 2532 | x | x | ||||||
| L_8 | Leita Superiore Lake | 387,008 | 5,055,058 | 2563 | x | x | ||||||
| L_9 | North of the Gran Lake | 387,567 | 5,055,658 | 2527 | x | x | ||||||
| L_10 | Cornuto Lake | 389,378 | 5,055,834 | 2173 | x | x | x | |||||
| L_11 | Muffé Lake | 391,295 | 5,054,715 | 2078 | x | x | x | |||||
| ID | EC | pH | T | Na+ | K+ | Ca2+ | Mg2+ | Li+ | NH4+ | Cl− | NO2− | F− | Br− | HCO3− | SO42− | NO3− | Error |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S_1 (6 May 2021) Perrot Spring | 81 | 8.20 | 4.8 | N/A | N/A | 10.33 | 4.30 | N/A | N/A | 1.50 | N/A | N/A | N/A | 51.90 | 2.10 | 2.80 | N/A |
| S_1 (7 February 2022) Perrot Spring | 88 | 9.40 | 5.0 | N/A | N/A | 3.70 | 9.90 | N/A | N/A | 0.55 | N/A | N/A | N/A | 57.83 | 2.10 | 2.50 | N/A |
| S_1 (8 July 2022) Perrot Spring | 87 | 8.89 | 6.5 | 0.34 | 0.39 | 4.82 | 11.21 | 0.05 | 0.05 | 0.25 | <0.005 | <0.010 | <0.010 | 61.49 | 1.85 | 2.65 | 4.37 |
| R_1 (8 July 2022) Chalamy Stream | 29 | 7.64 | 10.7 | 0.18 | 0.08 | 3.18 | 3.13 | <0.010 | 0.06 | 0.06 | <0.005 | <0.010 | <0.010 | 20.98 | 2.82 | 0.40 | 1.8 |
| R_2 (8 July 2022) Chalamy Stream | 35 | 7.73 | 15.0 | 0.18 | 0.13 | 5.36 | 2.24 | <0.010 | 0.08 | 0.15 | <0.005 | <0.010 | <0.010 | 29.28 | 0.10 | 0.03 | 1.97 |
| L_1 (8 July 2022) Leser Lake | 118 | 9.26 | 18.3 | 0.22 | 0.28 | 3.04 | 17.35 | <0.010 | 0.43 | 0.26 | <0.005 | <0.010 | <0.010 | 82.96 | 4.19 | 1.66 | 4.57 |
| L_2 (8 July 2022) Vallette Lake | 68 | 8.81 | 22.1 | 0.24 | 0.15 | 6.97 | 5.78 | <0.010 | 0.25 | 0.18 | <0.005 | <0.010 | <0.010 | 26.84 | 19.36 | 0.07 | 0.25 |
| L_3 (8 July 2022) Bianco Lake | 67 | 8.93 | 17.3 | 0.32 | 0.53 | 7.23 | 4.10 | <0.010 | 0.11 | 0.37 | <0.005 | 0.15 | <0.010 | 25.62 | 15.50 | 0.86 | 2.89 |
| L_4 (8 July 2022) Nero Lake | 65 | 7.89 | 13.5 | 0.25 | 0.26 | 8.55 | 4.85 | <0.010 | 0.08 | 0.14 | <0.005 | <0.010 | <0.010 | 27.82 | 17.19 | 1.13 | 0.7 |
| L_5 (8 July 2022) Gran Lake | 31 | 7.85 | 15.1 | 0.35 | 0.62 | 9.20 | 3.02 | <0.010 | 0.09 | 0.12 | <0.005 | 0.03 | <0.010 | 20.25 | 22.30 | 0.37 | 4.09 |
| L_6 (8 July 2022) Leita Lake | 31 | 8.04 | 16.0 | 0.34 | 0.30 | 6.52 | 1.14 | <0.010 | 0.13 | 0.13 | <0.005 | <0.010 | <0.010 | 20.01 | 4.13 | 0.05 | 3.8 |
| L_8 (8 July 2022) Leita Superiore Lake | 74 | 7.73 | 15.5 | 3.50 | 0.40 | 6.60 | 0.40 | <0.010 | 0.07 | 0.12 | <0.005 | 0.03 | <0.010 | 21.72 | 7.40 | 0.98 | 0.17 |
| L_9 (8 July 2022) North of the Gran Lake | 9 | 7.67 | 18.8 | 0.14 | 0.25 | 1.40 | 0.92 | <0.010 | 0.18 | 0.04 | <0.005 | 0.02 | <0.010 | 9.76 | 0.88 | 0.03 | 3.40 |
| L_10 (8 July 2022) Cornuto Lake | 58 | 8.19 | 19.5 | 0.60 | 1.10 | 12.00 | 2.80 | <0.010 | 0.11 | 0.36 | <0.005 | 0.03 | <0.010 | 36.60 | 13.39 | 0.91 | 0.89 |
| L_11 (8 July 2022) Muffé Lake | 176 | 8.79 | 18.2 | 0.26 | 0.21 | 7.22 | <0.010 | <0.010 | 0.12 | 0.25 | <0.005 | <0.010 | <0.010 | 12.93 | 6.54 | 1.28 | 0.14 |
| Year | 2021 | 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 May | 10 July | 10 November | 8 July | 20 September | ||||||
| Monitoring Point ID | Elevation | δ²H | δ¹⁸O | δ²H | δ¹⁸O | δ²H | δ¹⁸O | δ²H | δ¹⁸O | δ²H | δ¹⁸O |
| C_1 (Park office) | 414 | −26.4 | −4.64 | −72.2 | −10.61 | −40.3 | −7.10 | −30.1 | −6.34 | ||
| C_2 (Park Location 2) | 965 | −34.1 | −5.72 | −85.7 | −12.35 | −40.6 | −7.54 | −39.8 | −7.76 | ||
| C_3 (Park Location 3) | 1269 | −40.8 | −6.57 | −86.0 | −12.41 | −45.4 | −8.22 | −36.7 | −7.14 | ||
| C_4 (near Perrot Spring) | 1561 | −92.8 | −13.24 | −71.3 | −11.13 | −41.6 | −7.53 | ||||
| C_5 (near Bianco Lake) | 2160 | −74.8 | −11.19 | −48.7 | −8.65 | ||||||
| C_6 (near Gran Lake) | 2503 | −52.5 | −9.33 | ||||||||
| S_1 (Perrot Spring) | 1300 | −71.8 | −10.49 | −73.4 | −11.06 | ||||||
| R_1 (Chalamy Stream) | 2295 | −96.0 | −13.41 | ||||||||
| R_2 (Chalamy Stream) | 2532 | −76.1 | −11.42 | ||||||||
| L_1 (Leser Lake) | 2017 | −68.7 | −10.71 | ||||||||
| L_2 (Vallette Lake) | 2182 | −61.8 | −9.81 | ||||||||
| L_3 (Bianco Lake) | 2159 | −74.7 | −10.81 | −58.5 | −9.78 | ||||||
| L_4 (Nero Lake) | 2169 | −75.0 | −11.08 | −66.7 | −10.65 | ||||||
| L_5 (Gran Lake) | 2494 | −79.7 | −11.46 | −71.6 | −11.16 | ||||||
| L_6 (Leita Lake) | 2532 | −71.8 | −11.27 | ||||||||
| L_8 (Leita Superiore Lake) | 2563 | −73.7 | −11.16 | ||||||||
| L_9 (North of the Gran Lake) | 2527 | −78.1 | −11.49 | ||||||||
| L_10 (Cornuto Lake) | 2173 | −73.5 | −10.89 | −77.1 | −11.52 | ||||||
| L_11 (Muffé Lake) | 2078 | −73.3 | −10.88 | −72.6 | −11.13 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santillán-Quiroga, L.M.; Cocca, D.; Lasagna, M.; Marchina, C.; Destefanis, E.; Forno, M.G.; Gattiglio, M.; Vescovo, G.; De Luca, D.A. Analysis of the Recharge Area of the Perrot Spring (Aosta Valley) Using a Hydrochemical and Isotopic Approach. Water 2023, 15, 3756. https://doi.org/10.3390/w15213756
Santillán-Quiroga LM, Cocca D, Lasagna M, Marchina C, Destefanis E, Forno MG, Gattiglio M, Vescovo G, De Luca DA. Analysis of the Recharge Area of the Perrot Spring (Aosta Valley) Using a Hydrochemical and Isotopic Approach. Water. 2023; 15(21):3756. https://doi.org/10.3390/w15213756
Chicago/Turabian StyleSantillán-Quiroga, Luis Miguel, Daniele Cocca, Manuela Lasagna, Chiara Marchina, Enrico Destefanis, Maria Gabriella Forno, Marco Gattiglio, Giacomo Vescovo, and Domenico Antonio De Luca. 2023. "Analysis of the Recharge Area of the Perrot Spring (Aosta Valley) Using a Hydrochemical and Isotopic Approach" Water 15, no. 21: 3756. https://doi.org/10.3390/w15213756
APA StyleSantillán-Quiroga, L. M., Cocca, D., Lasagna, M., Marchina, C., Destefanis, E., Forno, M. G., Gattiglio, M., Vescovo, G., & De Luca, D. A. (2023). Analysis of the Recharge Area of the Perrot Spring (Aosta Valley) Using a Hydrochemical and Isotopic Approach. Water, 15(21), 3756. https://doi.org/10.3390/w15213756









