Features of the Formation of Strontium Pollution of Drinking Groundwater and Associated Health Risks in the North-West of Russia
Abstract
:1. Introduction
2. Geological Conditions
3. Materials and Methods
4. Results
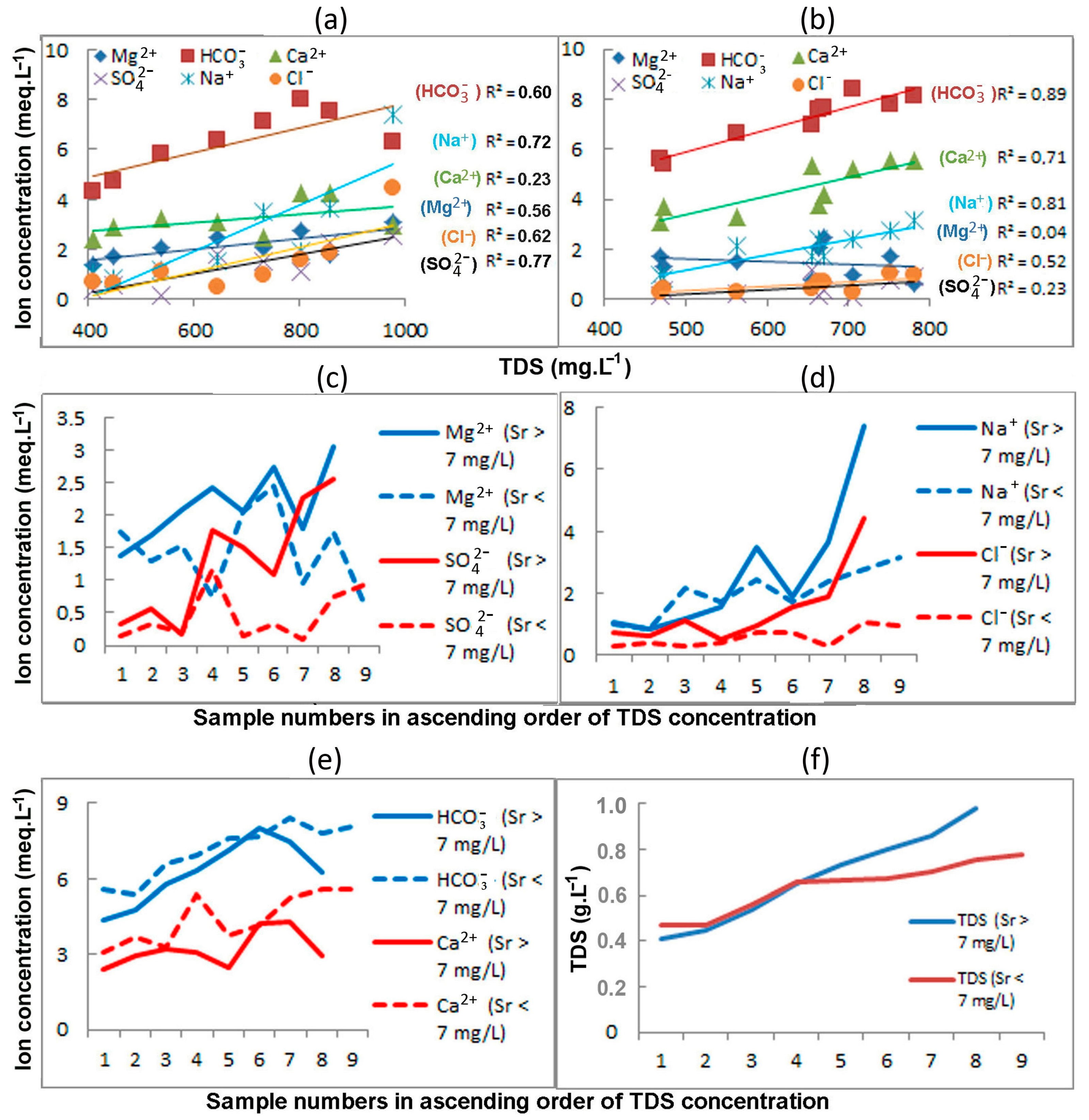
4.1. The Physicochemical Parameters of Groundwater
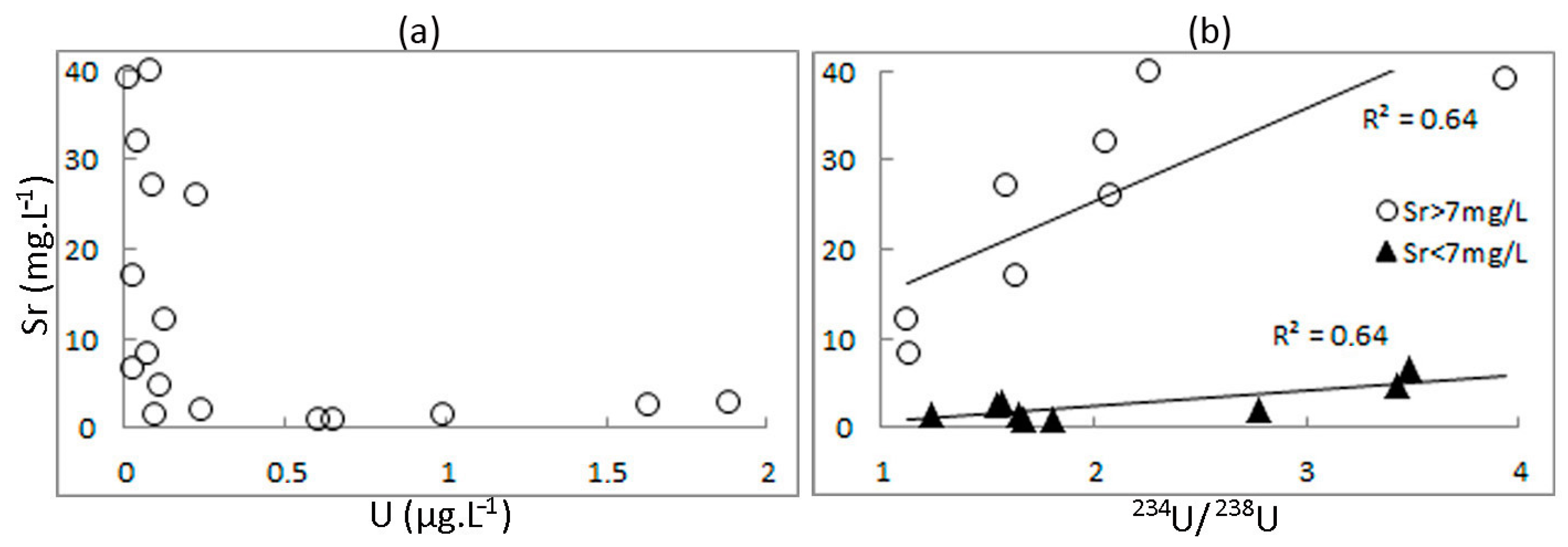
4.2. Isotopic Parameters of Groundwater
4.3. Dependence of Strontium on the Acid–Base and Redox Properties of Fresh Water in the Upper Permian Kazan Carbonate Aquifer (P2kz)
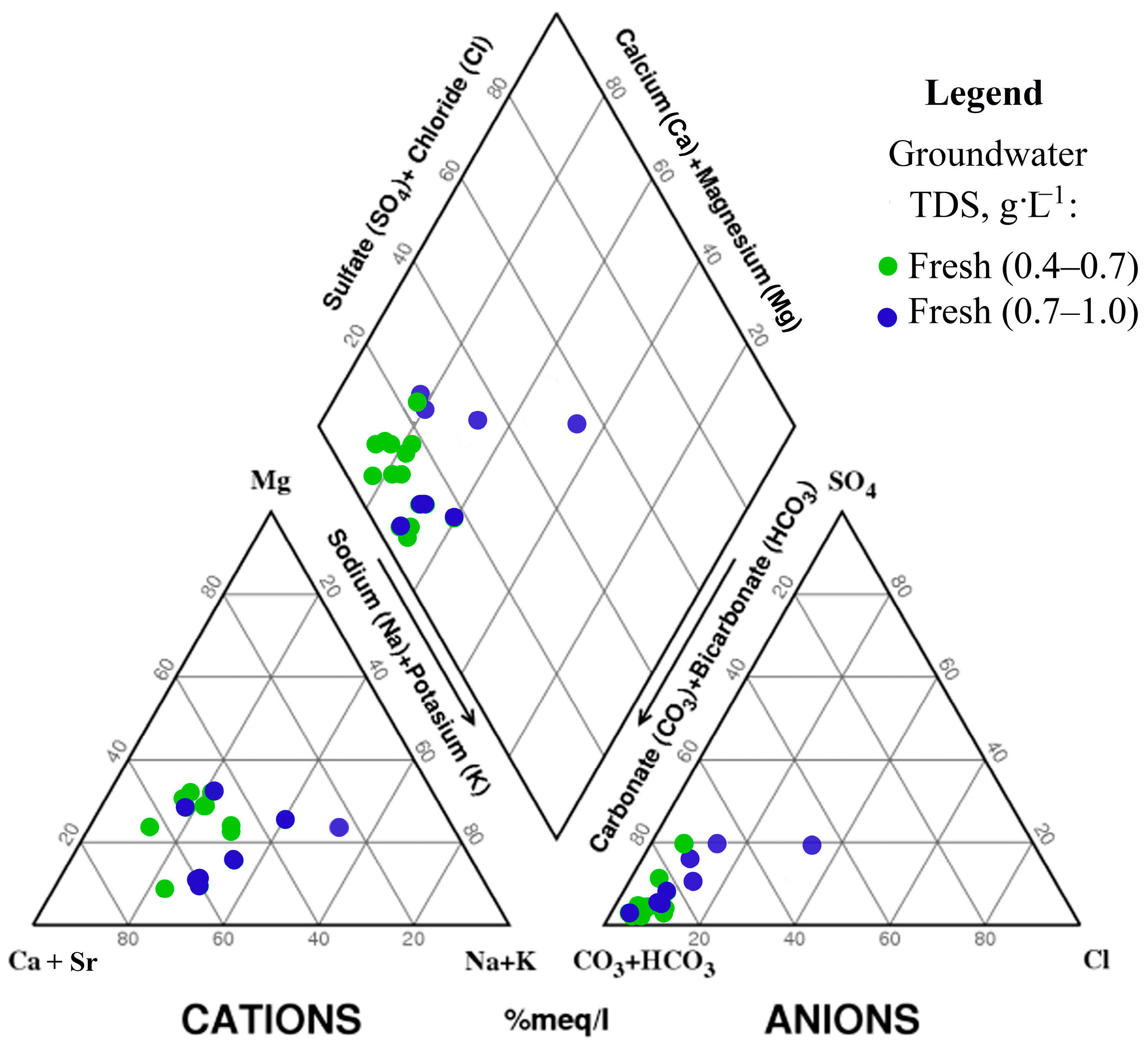
4.4. Strontium vs. Chemical Composition of the Fresh Groundwater in the Upper Permian Kazan Carbonate Aquifer
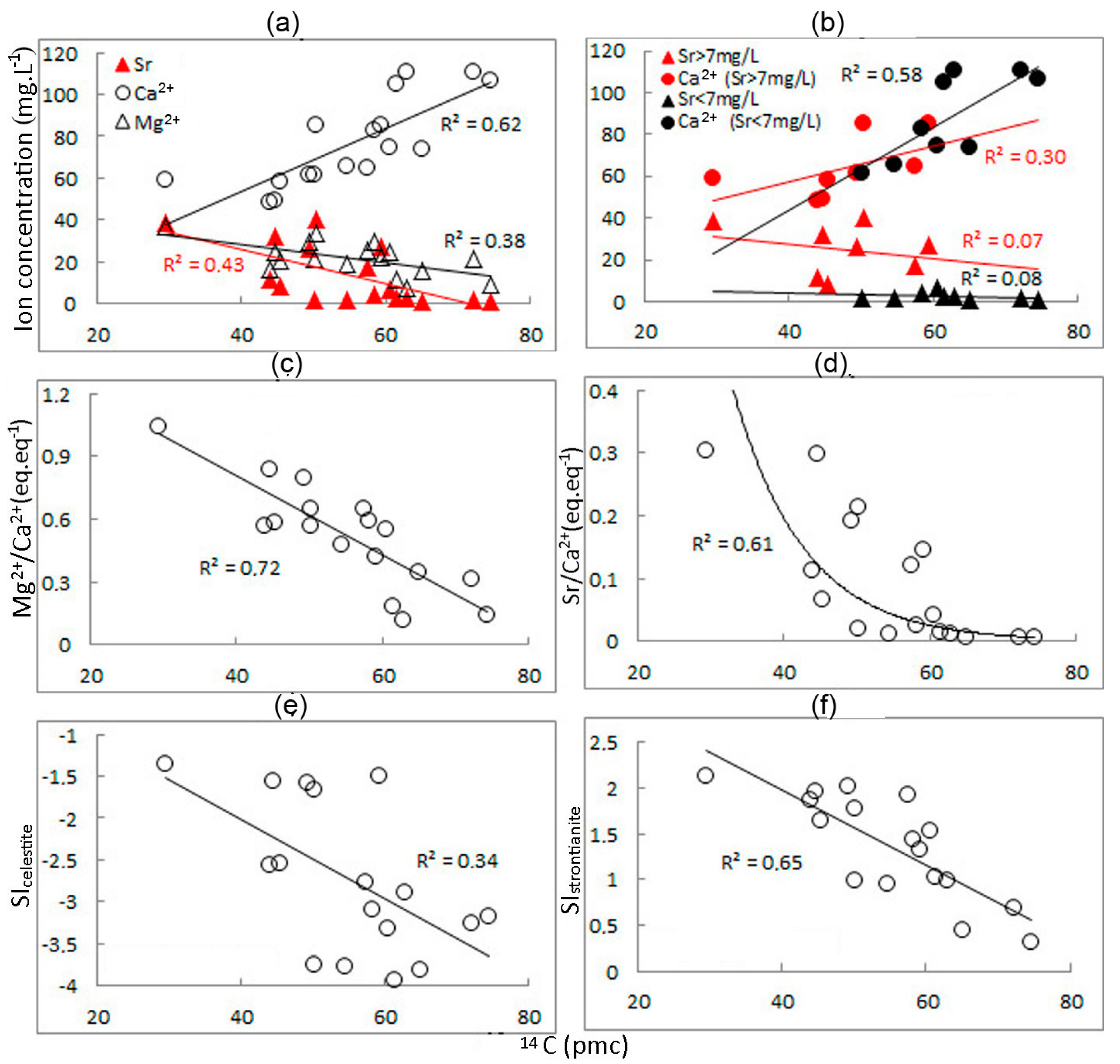
4.5. Dependence of Strontium Content on Water Age
5. Discussion
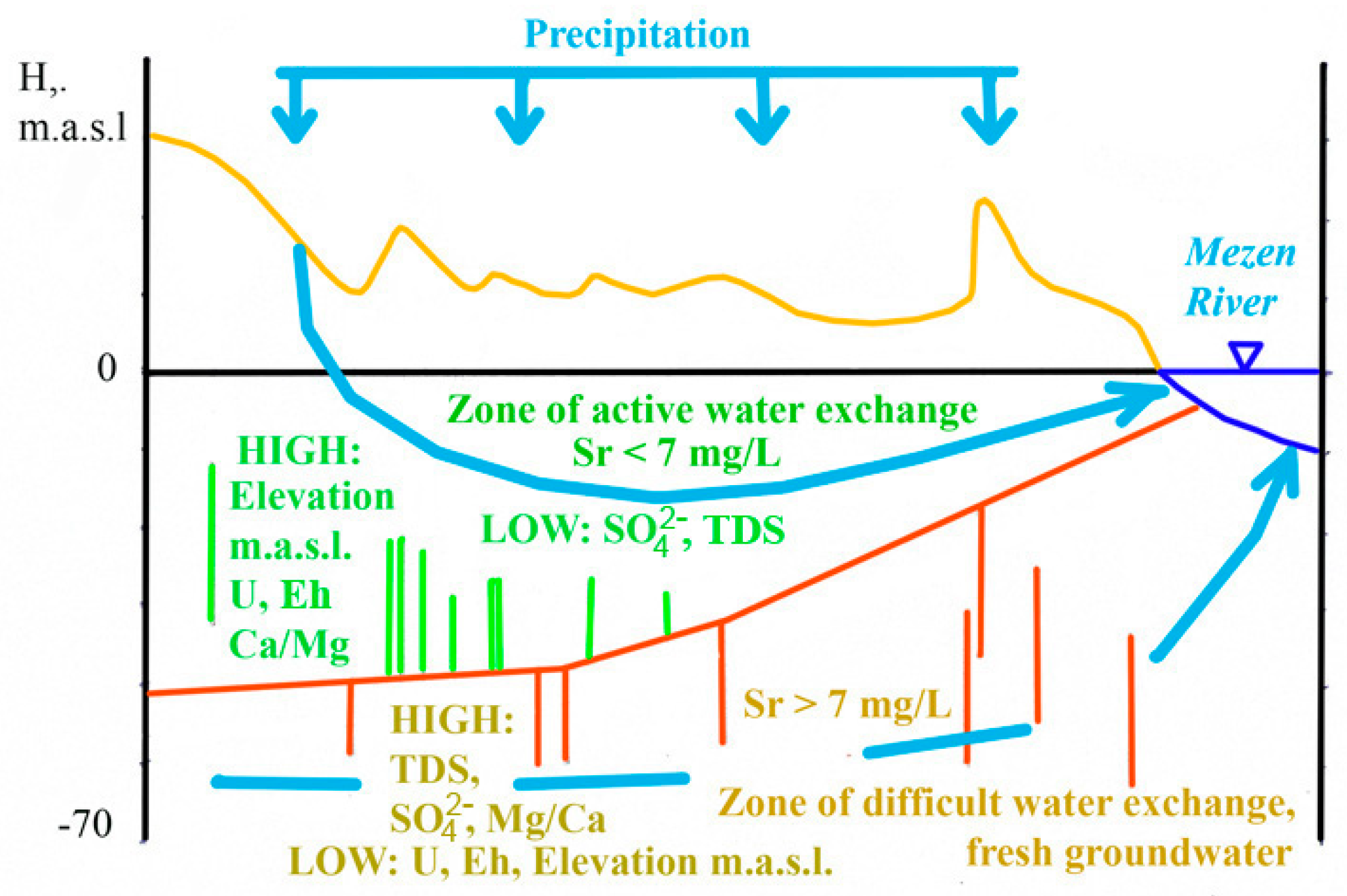
5.1. Groundwater with Strontium Concentrations above the MPC
5.2. Groundwater with Strontium Content below the MPC
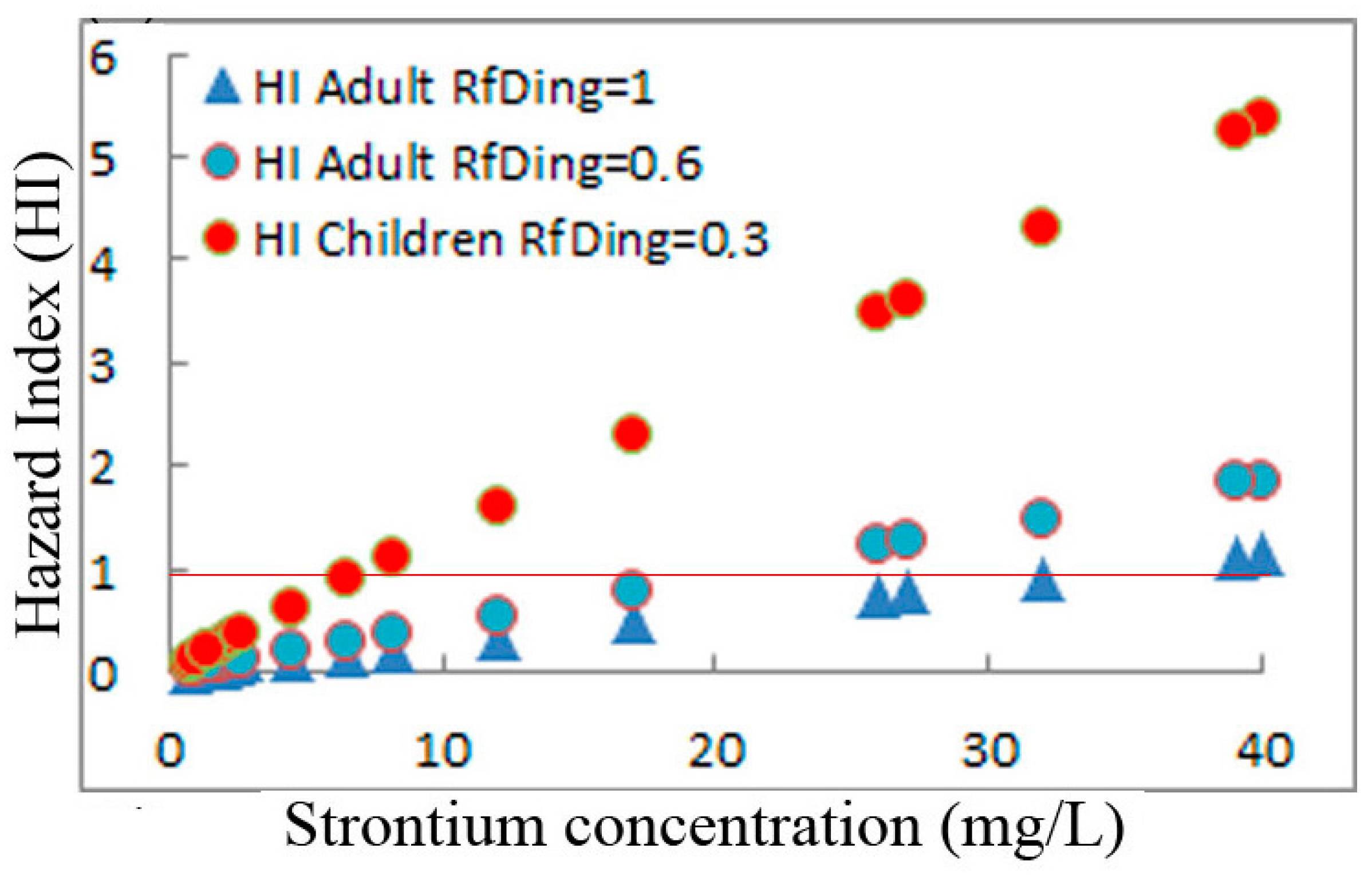
5.3. Estimation of Exposure and Human Health Risk
6. Conclusions
- (1)
- For waters with strontium concentrations above the MPC, the following features are characteristic:
- Correlations of strontium with TDS and saturation indices for celestite and gypsum, indicating the formation of these waters in sediments with high contents of celestite and gypsum.
- An increase in strontium–calcium and magnesium–calcium ratios during the period when groundwater is in the aquifer, associated with the process of dedolomitization.
- Correlation of strontium concentrations with the concentrations of the main ions, except for calcium.
- Reducing conditions in the aquifer, indicating difficult water exchange in the aquifer, promoting the preservation of strontium-containing minerals.
- (2)
- For waters with strontium concentrations below the MPC, the following features are characteristic:
- Strontium concentrations do not correlate with TDS, SIcelestite, and SIgypsum, indicating the formation of water in sediments with discretely located small inclusions of celestite and gypsum.
- The processes of dedolomitization practically do not affect the growth of strontium concentrations.
- Oxidizing conditions and active water exchange in the aquifer are favorable for the formation of water with standard quality.
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Zektser, I.S. Groundwater as a Component of the Environment. In Geology and Ecosystems; Zektser, I.S., Marker, B., Ridgway, J., Rogachevskaya, L., Vartanyan, G., Eds.; Springer: Boston, MA, USA, 2006; pp. 91–105. [Google Scholar] [CrossRef]
- Krainov, S.R.; Ryzhenko, B.N.; Shvets, V.M. Geochemistry of Groundwater. Fundamental, Applied and Environmental Aspects; CenterLitNefteGaz: Moskow, Russia, 2012; p. 672. (In Russian) [Google Scholar]
- Shen, J.; Schäfer, A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikasb, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- He, X.; Li, P.; Shi, H.; Xiao, Y.; Guo, Y.; Zhao, H. Identifying strontium sources of flowback fluid and groundwater pollution using 87Sr/86Sr and geochemical model in Sulige gasfield, China. Chemosphere 2022, 306, 135594. [Google Scholar] [CrossRef] [PubMed]
- SanRaR 2.1.4.1074-01; Drinking Water. Hygienic Requirements for Water Quality of Centralized Drinking Water Supply Systems. Quality Control. Sanitary and Epidemiological Rules and Regulations Approved by the Chief State Sanitary Doctor of the Russian Federation on 26 September 2001. Russian Federation: Moscow, Russia, 2001. (In Russian)
- Amata, R.; Diamond, G.L.; Dorsey, A.; Fransen, M.E. Toxicological Profile for Strontium; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004.
- Bartley, J.C.; Reber, E.F. Toxic effects of stable strontium in young pigs. J. Nutr. 1961, 75, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Colvin, L.B.; Creger, C.R. Stable strontium and experimental bone anomalies. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1967, 26, 416. [Google Scholar]
- Colvin, L.B.; Creger, C.R.; Ferguson, T.M.; Crookshank, H.R. Experimental epiphyseal cartilage anomalies by dietary strontium. Poult. Sci. 1972, 51, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Keesari, T.; Sabarathinam, C.; Sinha, U.K.; Pethaperumal; Thilagavathi, R.; Kamaraj, P. Fate and transport of strontium in groundwater from a layered sedimentary aquifer system. Chemosphere 2022, 307, 136015. [Google Scholar] [CrossRef] [PubMed]
- Khandare, A.L.; Validandi, V.; Rajendran, A.; Singh, T.G.; Thingnganing, L.; Kurella, S.; Nagaraju, R.; Dheeravath, S.; Vaddi, N.; Kommu, S.; et al. Health risk assessment of heavy metals and strontium in groundwater used for drinking and cooking in 58 villages of Prakasam district, Andhra Pradesh, India. Env. Geochem. Health 2020, 42, 3675–3701. [Google Scholar] [CrossRef]
- Zamana, L.V.; Rikhvanov, L.P.; Soktoev, B.R.; Baranovskaya, N.V.; Epova, E.S.; Solodukhina, M.A.; Mikhailova, L.A.; Kopylova, Y.G.; Khvashchevskaya, A.A. New data on chemical composition of natural waters in the area of distribution of Urov (Kaschin–Beck) disease (Transbaikal region). Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2019, 330, 121–133. (In Russian) [Google Scholar]
- Höllriegl, V.; München, H.Z. Strontium in the environment and possible human health effects. Encycl. Environl. Health 2011, 10, 268–275. [Google Scholar] [CrossRef]
- Wang, Z. A historic overview of research and control on Kashin-Beck Disease in China. Chin. J. Endem. 1999, 18, 161–163. [Google Scholar]
- Levander, O.A. Selenium. In Trace Elements in Human and Animal Nutrition; Mertz, W., Ed.; Academic Press Inc.: Orlando, FL, USA, 1986; Volume 2, pp. 209–279. [Google Scholar]
- Malaisse, F.; Mathieu, F. (Eds.) Big Bone Disease, a Multidisciplinary Approach of Kashin–Beck Disease in Tibet Autonomous Region (PR China); Les Presses Universitaires de Gembloux: Gembloux, Belgium, 2008; p. 148. [Google Scholar]
- Vinogradov, A.P. Geochemical investigations in the area of Urov endemia. Dokl. Akad. Nauk 1939, 23, 64–67. (In Russian) [Google Scholar]
- Ermakov, V.V.; Gulyaeva, U.A.; Tyutikov, S.F.; Kuzmina, T.G.; Safonov, V.A. Biogeochemistry of calcium and strontium in the landscapes of Eastern Transbaikalia. Geochem. Int. 2017, 55, 1105–1117. [Google Scholar] [CrossRef]
- Ermakov, V.; Bech, J.; Gulyaeva, U.; Tyutikov, S.; Safonov, V.; Danilova, V.; Roca, N. Relationship of the mobile forms of calcium and strontium in soils with their accumulation in meadow plants in the area of Kashin–Beck endemia. Env. Geochem. Health 2020, 42, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Rikhvanov, L.P.; Soktoev, B.R.; Baranovskaya, N.V.; Ageeva, E.V.; Belyanovskaya, A.I.; Deriglazova, M.A.; Yusupov, D.V.; Epova, E.S.; Solodukhina, M.A.; Zamana, L.V.; et al. Integrated geochemical studies of the components of the natural environment in the endemic regions of Transbaikalia. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2021, 332, 7–25. (In Russian) [Google Scholar]
- Vsevolozhsky, V.A. Fundamentals of Hydrogeology; Publishing House of Moscow State University: Moscow, Russia, 2007; p. 448. (In Russian) [Google Scholar]
- Bui, D.T.; Khosravi, K.; Karimi, M.; Busico, G.; Khozani, Z.S.; Nguyen, H.; Mastrocicco, M.; Tedesco, D.; Cuoco, E.; Kazakis, N. Enhancing nitrate and strontium concentration prediction in groundwater by using new data mining algorithm. Sci. Total Environ. 2020, 715, 136836. [Google Scholar] [CrossRef]
- Limantseva, O.A.; Ryzhenko, B.N. Model for Sr accumulation in the Carboniferous deposits of the Moscow artesian basin. Geochem. Int. 2008, 46, 935–944. [Google Scholar] [CrossRef]
- Limantseva, O.A.; Ryzhenko, B.N.; Cherkasova, E.V. Model for the formation of fluorine-bearing rocks in the Carboniferous deposits of the Moscow artesian basin. Geochem. Int. 2007, 45, 900–917. [Google Scholar] [CrossRef]
- Ivanova, N.I. Strontium distridution patterns in groundwater and aquifer host rocks in the southeastern part of the Severnaya Dvina artesian basin. Mosc. Univ. Geol. Bull. 2014, 69, 258–266. [Google Scholar] [CrossRef]
- Ivanova, I.S.; Shvartsev, S.L.; Pokrovsky, O.S. Distribution of strontium in groundwater of the upper hydrodynamic zone of the Sredneobsky artesian basin (Tomsk region). In Geological Evolution of the Interaction of Water with Rocks; Zamana, L.V., Shvartsev, S.L., Eds.; Publishing House of BSC SB RAS: Ulan-Ude, Russia, 2018; pp. 110–114. [Google Scholar]
- Kaleem, M.; Naseem, S.; Bashir, E.; Shahab, B.; Rafiq, T. Discrete geochemical behavior of Sr and Ba in the groundwater of Southern Mor Range, Balochistan, a tracer for igneous and sedimentary rocks weathering and related environmental issues. Appl. Geochem. 2021, 130, 104996. [Google Scholar] [CrossRef]
- Musgrove, M. The occurrence and distribution of strontium in U.S. groundwater. Appl. Geochem. 2021, 126, 104867. [Google Scholar] [CrossRef]
- Plechacek, A.; Scott, S.R.; Gotkowitz, M.B.; Ginder-Vogel, M. Strontium and radium occurrence at the boundary of a confined aquifer system. Appl. Geochem. 2022, 142, 105332. [Google Scholar] [CrossRef]
- Malov, A.I. Groundwater of the South-East White Sea: Formation, Role in Geological Processes; UB RAS: Yekaterinburg, Russia, 2003; p. 234. Available online: https://www.elibrary.ru/item.asp?id=1947446429 (accessed on 10 September 2023). (In Russian)
- Salminen, R.; Chekushin, V.; Bogatyrev, I.; Fedotova, E.; Tomilina, O.; Zhdanova, L.; Tenhola, M.; Glavatskikh, S.; Gregorauskiene, V.; Kashulina, G.; et al. Geochemical Atlas of the Eastern Barents Region; Elsevier: Amsterdam, The Netherlands, 2004; p. 548. [Google Scholar]
- USEPA. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); Final. EPA/540/R/99/005; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- Stankovsky, A.F.; Verichev, E.M.; Grib, V.P.; Dobeiko, I.P. Vendian of the southeastern White Sea. Izv. Acad. Sci. USSR. Ser. Geol. 1981, 2, 78–87. (In Russian) [Google Scholar]
- Stankovsky, A.F.; Verichev, E.M.; Dobeiko, I.P. Vendian of the southeastern White Sea. In Vendian System. Historical-Geological and Paleontological Substantiation. Vol. 2. Stratigraphy and Geological Processes; Sokolov, B.S., Fedonkin, M.A., Eds.; Nauka: Moscow, Russia, 1985; pp. 67–76. (In Russian) [Google Scholar]
- Turekian, K.K. Chemistry of the Earth; Holt, Rinehart and Winston Inc.: New York, NY, USA, 1972; p. 131. [Google Scholar]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Malov, A.I.; Sidkina, E.S.; Ershova, D.D.; Cherkasova, E.V.; Druzhinin, S.V. Time regularities of strontium concentration in drinking groundwater distant from the sea coast. Env. Geochem. Health 2023, 45, 8097–8118. [Google Scholar] [CrossRef] [PubMed]
- Malov, A.I.; Sidkina, E.S.; Ryzhenko, B.N. Model of the Lomonosov diamond deposit as a water–rock system: Migration Species, Groundwater Saturation with Rock-Forming and Ore Minerals, and Ecological Assessment of Water Quality. Geochem. Int. 2017, 55, 1118–1130. [Google Scholar] [CrossRef]
- Münnich, K.O. Messungen des 14C-Gehaltes von hartem Grundwasser. Naturwissenschaften 1957, 44, 32–34. [Google Scholar] [CrossRef]
- Münnich, K.O. Isotopendatierung von Grundwasser. Naturwissenschaften 1968, 55, 3–11. [Google Scholar] [CrossRef]
- Ingerson, E.; Pearson, F.J. Estimation of age and rate of motion of groundwater by the 14C method. In Recent Researches in the Fields of Atmosphere, Hydrosphere and Nuclear Geochemistry; Marusen: Tokyo, Japan, 1964; pp. 263–283. [Google Scholar]
- Mook, W.G. On the reconstruction of the initial 14C content of groundwater from the chemical and isotopic composition. In Proceedings of the Eighth International Conference on Radiocarbon Dating 1, Lower Hutt, New Zealand, 18–25 October 1972; Royal Society of New Zealand: Wellington, New Zealand, 1972; pp. 342–352. [Google Scholar]
- Mook, W.G. The dissolution-exchange model for dating groundwater with 14C. In Interpretation of Environmental Isotope and Hydrochemical Data in Groundwater Hydrology; IAEA: Vienna, Austria, 1976; pp. 213–225. [Google Scholar]
- Han, L.-F.; Plummer, N. A review of single-sample-based models and other approaches for radiocarbon dating of dissolved inorganic carbon in groundwater. Earth Sci. Rev. 2016, 152, 119–142. [Google Scholar] [CrossRef]
- Ferronsky, V.I.; Polyakov, V.A. Isotopes of the Earth’s Hydrosphere. Springer: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Vogel, J.C. Carbon-14 dating of groundwater. In Proceedings of the Symposium on Use of Isotopes in Hydrology, Vienna, Austria, 9–13 March 1970; IAEA: Vienna, Austria, 1970; pp. 225–237. [Google Scholar]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 ka cal BP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Stuiver, M.; Reimer, P.J.; Reimer, R.W. CALIB 8.2 [WWW Program]. Available online: http://calib.org (accessed on 16 February 2021).
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigation and Development; UNESCO: Paris, France, 1967; Volume 33, pp. 44–52. [Google Scholar]
- Schoeller, H. Geochemistry of groundwater. In Groundwater Studies—An International Guide for Research and Practice; UNESCO: Paris, France, 1977; Volume 15, pp. 1–18. [Google Scholar]
- Steffanowski, J.; Banning, A. Uraniferous dolomite: A natural source of high groundwater uranium concentrations in northern Bavaria, Germany? Env. Earth Sci. 2017, 76, 508. [Google Scholar] [CrossRef]
- Plummer, L.N. Defining reactions and mass transfer in part of the Floridan Aquifer. Water Resour. Res. 1977, 13, 801–812. [Google Scholar] [CrossRef]
- Back, W.; Hanshaw, B.B.; Plummer, L.N.; Rahn, P.H.; Rightmere, C.T.; Rubin, M. Process and rate of dedolomitization: Mass transfer and 14C dating in a regional carbonate aquifer. Geol. Soc. Am. Bull. 1983, 94, 1415–1429. [Google Scholar] [CrossRef]
- Musgrove, M.; Banner, J.L. Controls on the spatial and temporal variability of vadose dripwater geochemistry: Edwards aquifer, central Texas. Geochem. Cosmochim. Acta 2004, 68, 1007–1020. [Google Scholar] [CrossRef]
- Malov, A.I. Features of the formation of high strontium concentrations in drinking groundwater near sea coast. Dokl. Earth Sci. 2023, 512, 898–901. [Google Scholar] [CrossRef]
- USEPA. Health Effects Support Document for Strontium; EPA 820-P-14-0012014; U.S. Environmental Protection Agency: Washington, DC, USA, 2014. Available online: www.epa.gov/safewater/ccl/pdf/Strontium.pdf (accessed on 10 September 2023).
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; OSWER 9355.4-24; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Gerba, C.P. Risk Assessment. Environmental and Pollution Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 541–563. [Google Scholar] [CrossRef]
- Means, B. Risk-Assessment Guidance for Superfund, Volume 1. In Human Health Evaluation Manual, Part A; Interim Report Final No. PB-90–155581/XAB; EPA-540/1–89/002; Environmental Protection Agency; Office of Solid Waste and Emergency Response: Washington, DC, USA, 1989. Available online: https://www.osti.gov/biblio/7037757 (accessed on 18 January 2022).
- Ondayo, M.A.; Watts, M.J.; Hamilton, E.M.; Mitchell, C.; Mankelow, J.; Osano, O. Artisanal gold mining in Kakamega and Vihiga counties, Kenya: Potential human exposure and health risk. Env. Geochem. Health 2023, 45, 6543–6565. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Regional Screening Levels (RSLs). Calculator. Available online: https://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search (accessed on 3 August 2023).
- Zhang, H.; Zhou, X.; Wang, L.; Wang, W.; Xu, J. Concentrations and potential health risks of strontium in drinking water from Xi’an, Northwest China. Ecotoxicol. Environ. Saf. 2018, 164, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zimoch, I.; Łobos, E. Evaluation of health risk caused by chloroform in drinking water. Desalin. Water Treat. 2015, 57, 1027–1033. [Google Scholar] [CrossRef]
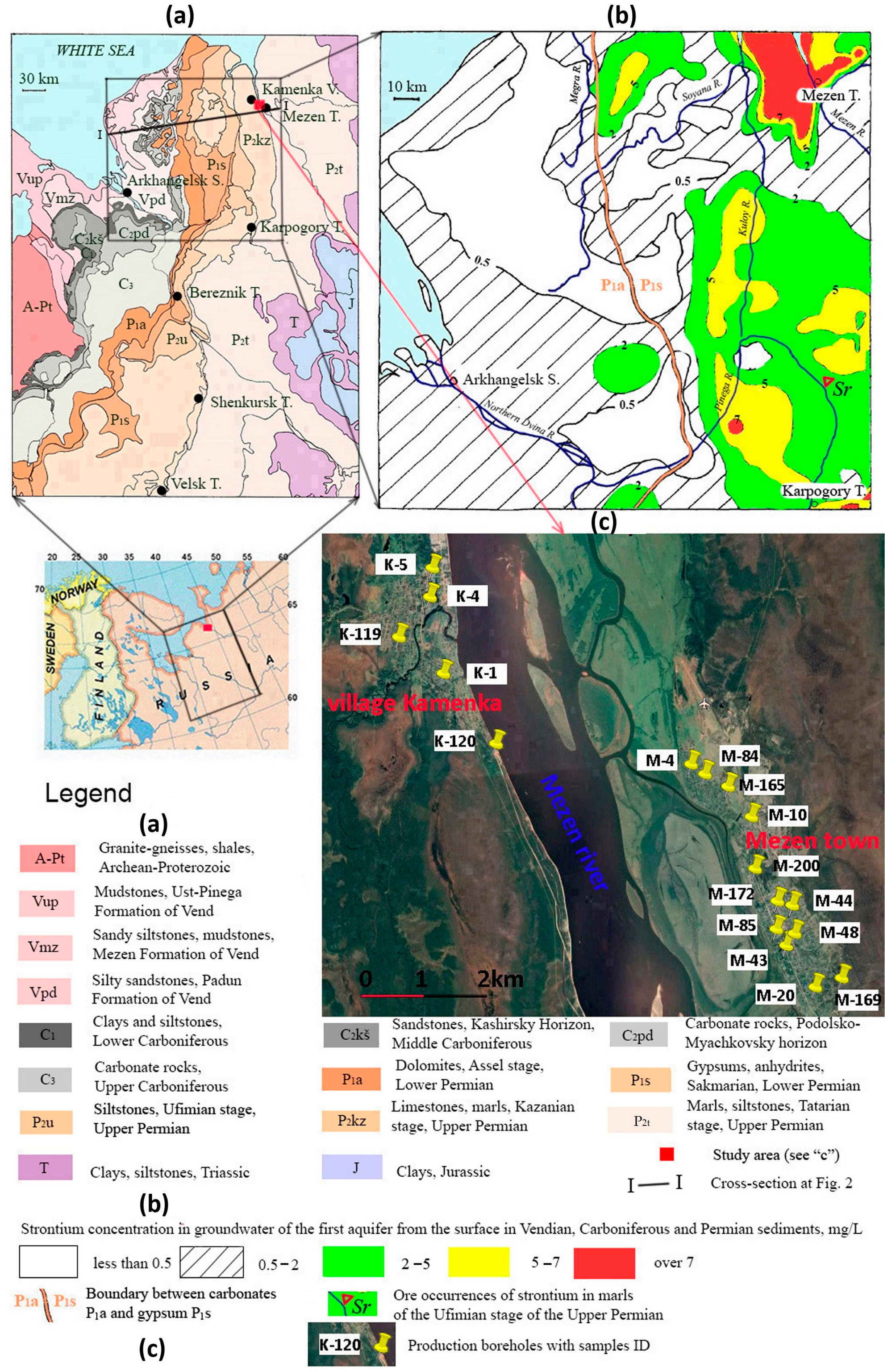
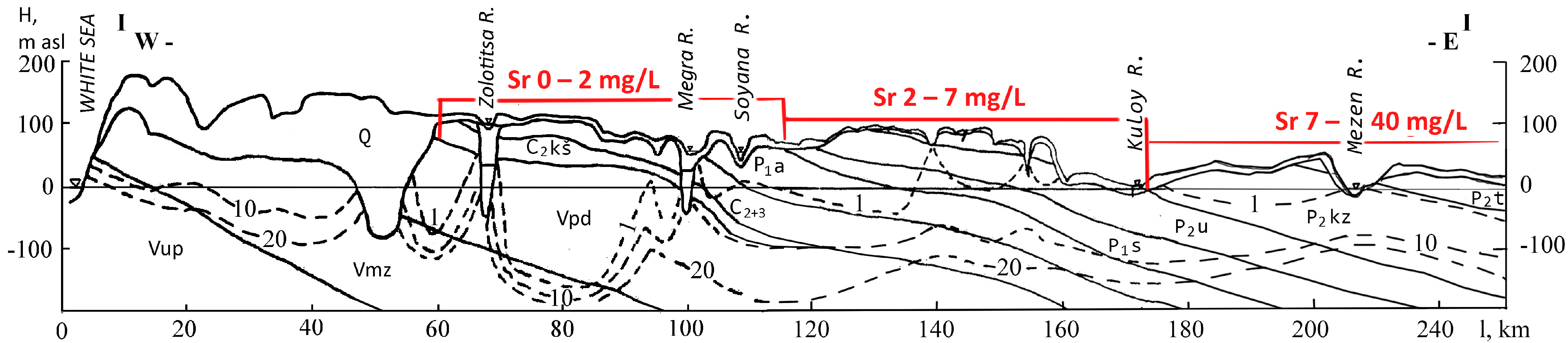




| Sample ID | Location |
H,
m.a.s.l. |
Sample Date//
Depth (m) |
T
(°C) |
pH
(Unit) |
Eh
(mV) |
O2 (mg·L−1) |
TDS
(mg·L−1) |
Sr
(mg·L−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K-120 | N 65.87238 E 44.13921 | 6 | 18 June//40–60 | 5.3 | 8.03 | 151 | 3.0 | 410 | 12.09 | ||
| K-1 | N 65.88087 E 44.12091 | 22 | 18 June//40–60 | 7.1 | 7.99 | 155 | 8.0 | 446 | 8.23 | ||
| M-200 | N 65.85261 E 44.23201 | 11 | 21 June//40–60 | 4.8 | 7.90 | −60 | 2.8 | 469 | 1.91 | ||
| M-84 | N 65.86652 E 44.21601 | 13 | 20 June//40–60 | 5.3 | 7.77 | 73 | 3.2 | 473 | 0.76 | ||
| M-10 | N 65.86009 E 44.23067 | 10 | 19 June//40–60 | 5.5 | 7.87 | −121 | 2.1 | 538 | 17.10 | ||
| M-20 | N 65.83675 E 44.26264 | 28 | 21 June//40–60 | 4.0 | 7.89 | −103 | 2.2 | 562 | 1.50 | ||
| M-85 | N 65.84367 E 44.23960 | 10 | 19 June//40–60 | 5.6 | 7.82 | 8 | 6.0 | 646 | 26.31 | ||
| M-4 | N 65.86776 E 44.20915 | 11 | 20 June//40–60 | 4.2 | 7.45 | 66 | 0.6 | 656 | 1.02 | ||
| M-169 | N 65.83541 E 44.25338 | 19 | 21 June//40–60 | 5.0 | 7.78 | −58 | 3.4 | 663 | 6.60 | ||
| M-48 | N 65.84318 E 44.24603 | 12 | 22 June//40–60 | 5.3 | 7.86 | −78 | 2.8 | 669 | 4.62 | ||
| M-44 | N 65.84731 E 44.24515 | 17 | 22 June//40–60 | 5.3 | 7.67 | −6 | 3.0 | 705 | 2.40 | ||
| M-43 | N 65.84137 E 44.24300 | 9 | 19 June//40–60 | 6.5 | 7.61 | −109 | 0.6 | 731 | 32.00 | ||
| M-165 | N 65.86820 E 44.22311 | 17 | 20 June//40–60 | 4.6 | 7.56 | 58 | 2.0 | 752 | 1.50 | ||
| M-172 | N 65.84790 E 44.23991 | 13 | 22 June//40–60 | 8.2 | 7.64 | 38 | 5.6 | 780 | 2.70 | ||
| K-4 | N 65.89190 E 44.11613 | 10 | 17 June//40–60 | 6.0 | 7.28 | −41 | 4.5 | 803 | 40.11 | ||
| K-5 | N 65.89627 E 44.11808 | 11 | 17 June//40–60 | 5.7 | 7.06 | −116 | 0.0 | 857 | 27.14 | ||
| K-119 | N 65.88612 E 44.10291 | 12 | 17 June//40–60 | 5.0 | 7.67 | −16 | 3.1 | 979 | 39.06 | ||
| Sample ID | Na+(mg.L−1) | Ca2+ | Mg2+ | K+ | Cl− | SO42− | HCO3− | Ca2+/Sr | Water Type
a (-) | ||
| K-120 | 24.0 | 48.3 | 16.8 | 3.61 | 24.8 | 16.0 | 265 | 4.0 | Ca-Mg-HCO3 | ||
| K-1 | 18.9 | 58.1 | 20.5 | 3.73 | 21.2 | 26.7 | 289 | 7.1 | Ca-Mg-HCO3 | ||
| M-200 | 23.1 | 61.3 | 21.1 | 3.49 | 10.6 | 7.1 | 341 | 32.0 | Ca-Mg-HCO3 | ||
| M-84 | 19.8 | 74.1 | 15.7 | 3.60 | 14.2 | 15.6 | 330 | 98.0 | Ca-HCO3 | ||
| M-10 | 27.4 | 64.5 | 25.2 | 3.68 | 38.9 | 8.3 | 353 | 3.8 | Ca-Mg-HCO3 | ||
| M-20 | 49.0 | 65.3 | 18.7 | 3.71 | 10.6 | 9.2 | 404 | 43.0 | Ca-Na-HCO3 | ||
| M-85 | 35.4 | 61.3 | 29.6 | 3.91 | 17.7 | 85.1 | 387 | 2.4 | Ca-Mg-HCO3 | ||
| M-4 | 38.9 | 107.0 | 9.1 | 4.25 | 15.9 | 56.5 | 424 | 107.0 | Ca-HCO3 | ||
| M-169 | 55.9 | 74.9 | 25.1 | 4.16 | 24.8 | 6.5 | 465 | 11.0 | Ca-Na-Mg-HCO3 | ||
| M-48 | 39.8 | 83.0 | 29.8 | 3.78 | 24.8 | 15.7 | 467 | 18.0 | Ca-Mg-HCO3 | ||
| M-44 | 54.5 | 105.0 | 11.8 | 4.22 | 10.6 | 4.4 | 512 | 44.0 | Ca-Na-HCO3 | ||
| M-43 | 80.0 | 49.3 | 25.1 | 4.31 | 33.6 | 72.9 | 433 | 2.2 | Na-Ca-HCO3 | ||
| M-165 | 63.9 | 111.0 | 21.3 | 4.62 | 37.2 | 36.0 | 476 | 74.0 | Ca-Na-HCO3 | ||
| M-172 | 72.4 | 111.0 | 7.9 | 3.90 | 33.6 | 44.0 | 494 | 41 | Ca-Na-HCO3 | ||
| K-4 | 43.7 | 85.0 | 33.4 | 4.13 | 54.9 | 52.3 | 489 | 2.1 | Ca-Mg-HCO3 | ||
| K-5 | 84.0 | 85.4 | 21.9 | 6.41 | 65.8 | 108.0 | 458 | 3.2 | Ca-Na-HCO3 | ||
| K-119 | 170.0 | 58.9 | 37.2 | 11.10 | 158.0 | 123.0 | 382 | 1.5 | Na-Mg-Ca-HCO3-Cl | ||
| Sample ID | Dolomite | Calcite | Strontianite | Anhydrite | Gypsum | Celestite |
|---|---|---|---|---|---|---|
| K-120 | 0.88 | 0.42 | 1.87 | −2.98 | −2.56 | −2.57 |
| K-1 | 0.90 | 0.43 | 1.64 | −2.70 | −2.28 | −2.54 |
| M-200 | 0.91 | 0.44 | 0.98 | −3.25 | −2.84 | −3.76 |
| M-84 | 0.58 | 0.38 | 0.45 | −2.83 | −2.42 | −3.81 |
| M-10 | 0.98 | 0.44 | 1.92 | −3.19 | −2.78 | −2.76 |
| M-20 | 1.03 | 0.54 | 0.95 | −3.13 | −2.72 | −3.77 |
| M-85 | 0.84 | 0.33 | 2.02 | −2.23 | −1.82 | −1.59 |
| M-4 | −0.03 | 0.27 | 0.30 | −2.16 | −1.75 | −3.18 |
| M-169 | 1.08 | 0.53 | 1.52 | −3.26 | −2.85 | −3.31 |
| M-48 | 1.33 | 0.64 | 1.44 | −2.85 | −2.44 | −3.10 |
| M-444 | 0.75 | 0.60 | 1.02 | −3.29 | −2.88 | −3.92 |
| M-43 | 0.38 | 0.09 | 1.96 | −2.40 | −1.98 | −1.57 |
| M-165 | 0.79 | 0.50 | 0.69 | −2.39 | −1.98 | −3.25 |
| M-172 | 0.44 | 0.55 | 0.99 | −2.28 | −1.87 | −2.88 |
| K-4 | 0.16 | 0.04 | 1.77 | −2.34 | −1.93 | −1.66 |
| K-5 | −0.59 | −0.24 | 1.32 | −2.02 | −1.61 | −1.51 |
| K-119 | 0.79 | 0.25 | 2.14 | −2.19 | −1.77 | −1.35 |
| Sample ID | 14C
(pmc) |
δ13C
(‰) | 14C a (pMC) | 14C0 b (pmc) | 14C0 c | 14C0 d | 14C0 e |
|---|---|---|---|---|---|---|---|
| K-120 | 44.04 ± 0.47 | −10.6 | 42.79 ± 0.46 | 45.00 | NC | 60.42 | 85 |
| K-1 | 45.4 ± 0.41 | −10.7 | 44.1 ± 0.4 | 45.36 | NC | 60.99 | 85 |
| M-200 | 50.15 ± 0.53 | −9.1 | 48.56 ± 0.51 | 39.64 | NC | 51.87 | 85 |
| M-84 | 65.02 ± 0.58 | −13.2 | 63.49 ± 0.57 | 54.29 | 50.47 | 76.95 | 85 |
| M-10 | 57.45 ± 0.66 | −8.7 | 55.58 ± 0.64 | 38.21 | NC | 49.59 | 85 |
| M-20 | 54.57 ± 0.54 | −13.3 | 53.29 ± 0.53 | 54.64 | 53.98 | 75.81 | 85 |
| M-85 | 49.32 ± 0.61 | −11.8 | 49.02 ± 0.59 | 49.29 | NC | 67.26 | 85 |
| M-4 | 74.47 ± 0.66 | −14.6 | 72.92 ± 0.65 | 59.29 | 63.17 | 83.22 | 85 |
| M-169 | 60.53 ± 0.65 | −15.7 | 59.4 ± 0.64 | 63.21 | 90.87 | 89.49 | 85 |
| M-48 | 58.3 ± 0.54 | −15.8 | 57.23 ± 0.5 | 63.57 | 93.62 | 90.06 | 85 |
| M-44 | 61.39 ± 0.60 | −13.5 | 59.98 ± 0.59 | 55.36 | 52.79 | 76.95 | 85 |
| M-43 | 44.64 ± 0.55 | −9.9 | 43.29 ± 0.53 | 42.50 | NC | 56.43 | 85 |
| M-165 | 72.2 ± 0.67 | −12.0 | 70.32 ± 0.65 | 50.00 | 24.61 | 68.4 | 85 |
| M-172 | 62.87 ± 0.56 | −15.2 | 61.64 ± 0.55 | 61.43 | 77.44 | 86.64 | 85 |
| K-4 | 50.24 ± 0.44 | −9.8 | 48.71 ± 0.43 | 42.14 | NC | 55.86 | 85 |
| K-5 | 59.23 ± 0.54 | −13.3 | 57.84 ± 0.53 | 54.64 | 21.96 | 75.81 | 85 |
| K-119 | 29.53 ± 0.49 | −8.6 | 28.56 ± 0.47 | 37.86 | NC | 49.02 | 85 |
| Sample ID | 14C Age b (year BP) | 14C Age c | 14C Age d | 14C Age e | U (ppb) | 234U/238U (unit) | |
| K-120 | 424 ± 104 | NC | 2675 ± 163 | 6171 ± 144 | 0.130 | 1.12 | |
| K-1 | 214 ± 213 | NC | 2529 ± 169 | 5968 ± 204 | 0.075 | 1.13 | |
| M-200 | modern | NC | 236 ± 235 | 5027 ± 187 | 0.243 | 2.78 | |
| M-84 | modern | modern | 1337 ± 187 | 2237 ± 82 | 0.649 | 1.67 | |
| M-10 | modern | NC | modern | 3472 ± 102 | 0.026 | 1.63 | |
| M-20 | 208 ± 207 | modern | 2836 ± 84 | 4014 ± 128 | 0.094 | 1.65 | |
| M-85 | modern | NC | 2628 ± 138 | 5143 ± 163 | 0.225 | 2.08 | |
| M-4 | modern | modern | 842 ± 78 | 1049 ± 121 | 0.607 | 1.81 | |
| M-169 | 570 ± 76 | 3588 ± 109 | 3468 ± 91 | 2898 ± 109 | 0.027 | 3.48 | |
| M-48 | 804 ± 99 | 4369 ± 131 | 4540 ± 240 | 3336 ± 106 | 0.108 | 3.43 | |
| M-44 | modern | modern | 1801 ± 92 | 2810 ± 65 | 1.633 | 1.54 | |
| M-43 | modern | NC | 1820 ± 84 | 6130 ± 136 | 0.042 | 2.06 | |
| M-165 | modern | modern | modern | 1259 ± 76 | 0.994 | 1.23 | |
| M-172 | modern | 1632 ± 83 | 2791 ± 61 | 2605 ± 118 | 1.885 | 1.57 | |
| K-4 | modern | NC | 817 ± 88 | 4935 ± 92 | 0.078 | 2.26 | |
| K-5 | modern | modern | 2007 ± 104 | 3186 ± 140 | 0.085 | 1.59 | |
| K-119 | 2424 ± 272 | NC | 4662 ± 206 | 9828 ± 285 | 0.014 | 3.94 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malov, A.I. Features of the Formation of Strontium Pollution of Drinking Groundwater and Associated Health Risks in the North-West of Russia. Water 2023, 15, 3846. https://doi.org/10.3390/w15213846
Malov AI. Features of the Formation of Strontium Pollution of Drinking Groundwater and Associated Health Risks in the North-West of Russia. Water. 2023; 15(21):3846. https://doi.org/10.3390/w15213846
Chicago/Turabian StyleMalov, Alexander I. 2023. "Features of the Formation of Strontium Pollution of Drinking Groundwater and Associated Health Risks in the North-West of Russia" Water 15, no. 21: 3846. https://doi.org/10.3390/w15213846
APA StyleMalov, A. I. (2023). Features of the Formation of Strontium Pollution of Drinking Groundwater and Associated Health Risks in the North-West of Russia. Water, 15(21), 3846. https://doi.org/10.3390/w15213846








