Species Diversity of Zooplankton of Small Steppe Lakes of the Northern Part of Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Bie, T.; Declerck, S.; Martens, K.; De Meester, L.; Brendonck, L. A Comparative Analysis of Cladoceran Communities from Different Water Body Types: Patterns in Community Composition and Diversity. Hydrobiologia 2007, 597, 19–27. [Google Scholar] [CrossRef]
- Davies, B.R.; Biggs, J.; Williams, P.J.; Lee, J.T.; Thompson, S.A. Comparison of the Catchment Sizes of Rivers, Streams, Ponds, Ditches and Lakes: Implications for Protecting Aquatic Biodiversity in an Agricultural Landscape. Hydrobiologia 2007, 597, 7–17. [Google Scholar] [CrossRef]
- Ermolaeva, N.I. Some Results of Zooplankton Research in Lakes of Northern Kazakhstan. Arid. Ecosyst. 2013, 12, 91–103. (In Russian) [Google Scholar]
- Krupa, E.; Barinova, S.; Romanova, S.; Aubakirova, M.; Ainabaeva, N. Planktonic invertebrates in the assessment of long-term change in water quality of the Sorbulak wastewater disposal system (Kazakhstan). Water 2020, 12, 3409. [Google Scholar] [CrossRef]
- Krupa, E.; Romanova, S.; Berkinbaev, G.; Yakovleva, N.; Sadvakasov, E. Zooplankton as indicator of the ecological state of protected aquatic ecosystems (Lake Borovoe, Burabay national nature park, Northern Kazakhstan). Water 2020, 12, 2580. [Google Scholar] [CrossRef]
- Aubakirova, M.; Krupa, E.; Mazhibayeva, Z.; Isbekov, K.; Assylbekova, S. The role of external factors in the variability of the structure of the zooplankton community of small lakes (South-East Kazakhstan). Water 2021, 13, 962. [Google Scholar] [CrossRef]
- Samarkhanov, T.N.; Myrzagaliyeva, A.B.; Chlachula, J.; Kushnikova, L.B.; Czerniawska, J.; Nigmetzhanov, S.B. Geoenvironmental Implications and Biocenosis of Freshwater Lakes in the Arid Zone of East Kazakhstan. Sustainability 2021, 13, 5756. [Google Scholar] [CrossRef]
- Dukravets, G.M.; Sidorova, A.F. On the History of Hydrobiological and Ichthyological Research at the Al-Farabi Kazakh National University. Bull. Al-Farabi Kazakh Natl. Univ. Biol. Ser. 2011, 5, 5–18. (In Russian) [Google Scholar]
- Matmuratov, S.A.; Stuge, T.S.; Troshina, T.T.; Lopatin, O.E. Zooplankton of the Korgalzhin Lake System and Its Indicator Value. Proc. Inst. Zool. 2009, 50, 198–211. (In Russian) [Google Scholar]
- Zsuga, K.; Inelova, Z.; Boros, E. Zooplankton Community Structure in Shallow Saline Steppe Inland Waters. Water 2021, 13, 1164. [Google Scholar] [CrossRef]
- Rogozin, A.G. Materials on the fauna and ecology of rotifers in the Urals, family Brachionidae (Rotifera, Eurotatoria, Ploima), genera Kellicottia, Plationus, and Platyias. Biol. Bull. 2021, 48, 950–958. [Google Scholar] [CrossRef]
- Merrix-Jones, F.L.; Thackeray, S.J.; Ormerod, S.J. A global analysis of zooplankton in natural and artificial fresh waters. J. Limnol. 2013, 72, 66–91. [Google Scholar] [CrossRef]
- Tsalolikhin, S.Y. Determinant of Freshwater Invertebrates of Russia and Adjacent Territories; Lower Invertebrates: Saint Petersburg, Russia, 1994; Volume 1. (In Russian) [Google Scholar]
- Krupa, E.G.; Dobrokhotova, O.V.; Stuge, T.S. Fauna of Calanoida (Crustacea: Copepoda) Kazakhstan and Adjacent Territories: Monograph; Etalon Print: Almaty, Kazakhstan, 2016; p. 395. (In Russian) [Google Scholar]
- Suthers, I.; Bowling, L.; Kobayashi, T.; Rissik, D. Sampling Methods for Plankton. In Plankton: A Guide to Their Ecology and Monitoring for Water Quality; CSIRO Publishing: Collingwood, Australia, 2009; pp. 73–114. [Google Scholar]
- Hart, R.C.; Bychek, E.A. Body size in freshwater planktonic crustaceans: An overview of extrinsic determinants and modifying influences of biotic interactions. Hydrobiologia 2011, 668, 61–108. [Google Scholar] [CrossRef]
- Keskitalo, J.; Salonen, K. Manual for Integrated Monitoring. Subprogramme Hydrobiology of Lakes; National Board of Waters and the Environment: Helsinki, Finland, 1994; p. 46.
- Kitaev, S.P. Fundamentals of Limnology for Hydrobiologists and Ichthyologists; KarSC RAS: Petrozavodsk, Russia, 2007; p. 395. (In Russian) [Google Scholar]
- Leal, T.L.; Freitas, M.F.; Alencar, N.R.O.; Lisboa, G.D.S.; Stracieri, J. Statistical analysis of solid waste generation in the state of Bahia, Brazil. Rev. Bras. Gestão Desenvolv. Reg. 2023, 19, 3–25. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS 3.22.1. Geographic Information System. Open-Source Geospatial Foundation Project. 2023. Available online: http://Qgis.osgeo.org (accessed on 2 February 2023).
- GIS Mapping Software. 2022. Available online: www.esri.com (accessed on 8 January 2023).
- Krupa, E.G. Zooplankton Structure of Ecologically Diverse Reservoirs and Watercourses of Kazakhstan. Doctoral Dissertation, Institut Zoologi, Almaty, Kazakhstan, 2010. (In Russian). [Google Scholar]
- Choi, Y.; Oh, H.-J.; Lee, D.-H.; Jang, M.-H.; Lee, K.-L.; Chang, K.-H.; Kim, H.-W. Current Utilization and Further Application of Zooplankton Indices for Ecosystem Health Assessment of Lake Ecosystems. Sustainability 2023, 15, 10950. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Chen, G.; Dalton, C.M.; Taylor, D. Cladocera as Indicators of Trophic State in Irish Lakes. J. Paleolimnol. 2010, 44, 465–481. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Grazing Potential—A Functional Plankton Food Web Metric for Ecological Water Quality Assessment in Mediterranean Lakes. Water 2019, 11, 1274. [Google Scholar] [CrossRef]
- Stamou, G.; Mazaris, A.D.; Moustaka-Gouni, M.; Špoljar, M.; Ternjej, I.; Dražina, T.; Dorak, Z.; Michaloudi, E. Introducing a Zooplanktonic Index for Assessing Water Quality of Natural Lakes in the Mediterranean Region. Ecol. Inform. 2022, 69, 101616. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J.; Karabin, A. The Suitability of Zooplankton as Lake Ecosystem Indicators: Crustacean Trophic State Index. Pol. J. Ecol. 2013, 61, 561–573. [Google Scholar]
- Mao, M.; Zhu, Y.; Zhu, X.; Jiang, Z.; Xuan, J.; Gu, J.; Du, P.; Zeng, J. Response of zooplankton to warming in a low-salinity, eutrophic bay. Ecol. Indic. 2023, 153, 110459. [Google Scholar] [CrossRef]
- Aubakirova, G.; Adilbekov, Z.; Narbayev, S. Influence of water mineralization on zooplankton productivity in reservoirs of Akmola region. Period. Tchê Química 2020, 17, 520–527. [Google Scholar] [CrossRef]
- Krylov, А.; Gerasimov, Y.; Gabrialiyan, V.; Borisenko, E.; Akopyan, A.; Nikoghosyan, A.; Malin, M.; Hovsepyan, А. Zooplankton of Lake Sevan during the Period of Continued Increase in Water Level and Decrease in Fish Density. Biol. Inland Waters 2013, 2013, 37–45. [Google Scholar] [CrossRef]
- Ochocka, A. ZIPLAS: Zooplankton Index for Polish Lakes’ Assessment: A New Method to Assess the Ecological Status of Stratified Lakes. Environ. Monit. Assess. 2021, 193, 664. [Google Scholar] [CrossRef]
- Sendacz, S.; Caleffi, S.; Santos-Soares, J. Zooplankton Biomass of Reservoirs in Different Trophic Conditions in the State of São Paulo, Brazil. Braz. J. Biol. 2006, 66, 337–350. [Google Scholar] [CrossRef]
- Van Egeren, S.J.; Dodson, S.I.; Torke, B.; Maxted, J.T. The Relative Significance of Environmental and Anthropogenic Factors Affecting Zooplankton Community Structure in Southeast Wisconsin till Plain Lakes. Hydrobiologia 2011, 668, 137–146. [Google Scholar] [CrossRef]
- Mamilov, N.; Sharakhmetov, S.; Amirbekova, F.; Bekkozhayeva, D.; Sapargaliyeva, N.; Kegenova, G.; Tanybayeva, A.; Abilkasimov, K. Past, Current and Future of Fish Diversity in the Alakol Lakes (Central Asia: Kazakhstan). Diversity 2021, 14, 11. [Google Scholar] [CrossRef]
- Zaghloul, A.; Saber, M.; Gadow, S.; Awad, F. Biological Indicators for Pollution Detection in Terrestrial and Aquatic Ecosystems. Bull. Natl. Res. Cent. 2020, 44, 127. [Google Scholar] [CrossRef]
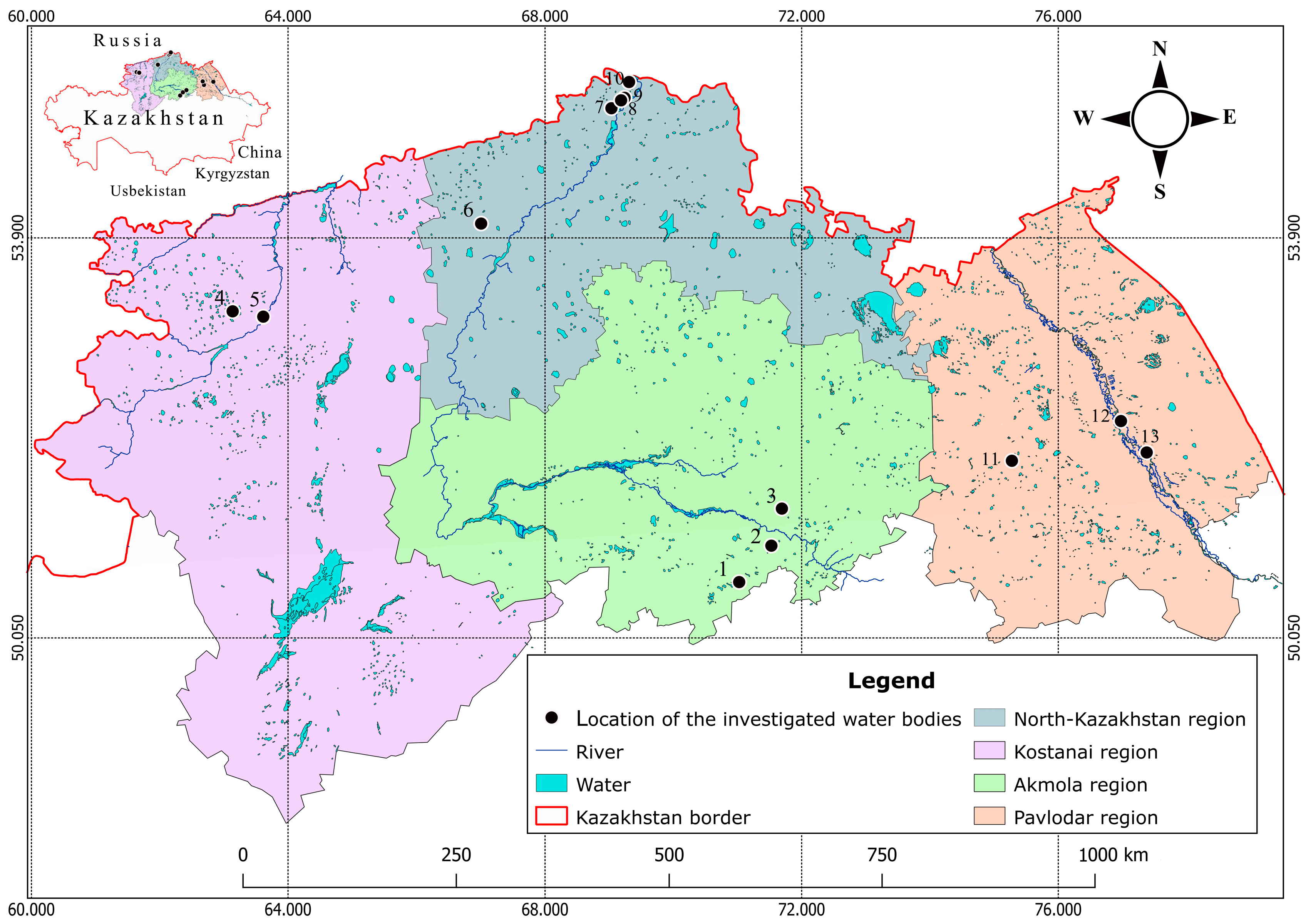


| № | Name of Water Body | WGS (lat) | WGS (lon) | Area (km2) | Depth (cm) | O2 (mg L−1) | рН | Salinity (gL−1) | Color of Water (Pt Co) | NO3− (mg L−1) | NO2− (mg L−1) | ZOO SUM (ind./m3) | TAX NUM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akmola region | |||||||||||||
| 1. | Zharlykol Lake | 50.607 | 71.026 | 14.80 | 70 | 5.38 | 6.52 | 0.51 | 30 | 2.9 | 0.02 | 36,089 | 16 |
| 2. | Maibalyk Lake | 50.967 | 71.528 | 14.60 | 60 | 3.90 | 7.60 | 3.26 | 35 | 2.3 | 0.01 | 120,149 | 36 |
| 3. | Koyandy reservoir | 51.330 | 71.691 | 1.78 | 60 | 5.42 | 7.26 | 0.44 | 40 | 3.2 | 0.02 | 103,586 | 24 |
| Kostanai region | |||||||||||||
| 4. | Zharkol Lake | 53.219 | 63.236 | 19 | 50 | 5.34 | 7.74 | 0.55 | 35 | 0 | 0 | 14,067 | 10 |
| 5. | Kostomar Lake | 53.218 | 63.136 | 12.4 | 120 | 6.37 | 8.15 | more 20 | 45 | 2.3 | 0.01 | 55,244 | 15 |
| North Kazakhstan region | |||||||||||||
| 6. | Zharken Lake | 54.031 | 67.003 | 5 | 50 | 2.15 | 8.25 | 2.23 | 55 | 3.0 | 0.01 | 54,218 | 23 |
| 7. | Solenoe Lake | 55.077 | 69.034 | 3.00 | 70 | 7.8 | 7.72 | 8.96 | 35 | 0 | 0 | 581,013 | 6 |
| 8. | Lebyazhe Lake | 55.149 | 69.187 | 4.80 | 100 | 6.58 | 8.6 | 2.57 | 40 | 2.5 | 0.02 | 161,232 | 16 |
| 9. | Polkovnikovo Lake | 55.168 | 69.240 | 1.50 | 80 | 8.5 | 7.55 | 1.13 | 30 | 3.0 | 0.05 | 144,175 | 12 |
| 10. | Solontsy Lake | 55.307 | 69.327 | 4.4 | 150 | 2.44 | 8.42 | 1.32 | 40 | 2.0 | 0.02 | 154,151 | 24 |
| Pavlodar region | |||||||||||||
| 11. | Ashchikol Lake | 51.795 | 75.277 | 6.40 | 80 | 7.16 | 8.02 | more 10 | 40 | 2.5 | 0.01 | 370,786 | 13 |
| 12. | Kondratievs koe Lake | 52.178 | 76.977 | 0.1 | 120 | 4.24 | 7.84 | 1.35 | 35 | 3.0 | 0.01 | 190,464 | 25 |
| 13. | Presnoe Lake | 51.881 | 77.414 | 0.37 | 90 | 8.7 | 7.86 | 1.43 | 30 | 3.3 | 0.02 | 125,420 | 19 |
| Species | Zharlykol | Koiandy | Maibalyk | Kostomar | Zharkol | Zharken | Lebiazhe | Polkovnikovo | Solenoe | Solontsy | Ashchikol | Kondratievskoe | Presnoe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rotifera | |||||||||||||
| Asplanchna priodonta (Gosse, 1850) | 1 | 1 | 1 | ||||||||||
| Asplanchna girodi (Guerne, 1888) | 1 | 1 | 1 | ||||||||||
| Asplanchna intermedia (Hudson, 1886) | 1 | ||||||||||||
| Asplanchna priodonta (Gosse, 1850) | 1 | 1 | 1 | 1 | |||||||||
| Asplanchna sieboldi (Leydig, 1854) | 1 | ||||||||||||
| Bdelloida gen. sp. | 1 | ||||||||||||
| Bipalpus hudsoni (Imhof, 1891) | 1 | ||||||||||||
| Brachionus angularis (Gosse, 1851) | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Brachionus calyciflorus amphiceros (Ehrenberg, 1838) | 1 | 1 | 1 | 1 | |||||||||
| Brachionus diversicornis (Daday, 1883) | 1 | ||||||||||||
| Brachionus plicatilis (Muller, 1786) | 1 | 1 | 1 | ||||||||||
| Brachionus plicatilis longicornis (Fadeev, 1786) | 1 | ||||||||||||
| Brachionus quadridentatus ancylognathus (Schmarda, 1859) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Brachionus quadridentatus dorcas (Gosse, 1851) | 1 | ||||||||||||
| Brachionus quadridentatus (Hermann, 1783) | 1 | 1 | 1 | 1 | |||||||||
| Brachionus urceus (Linnaeus, 1758) | 1 | ||||||||||||
| Brachionus variabilis (Hempel, 1896) | 1 | 1 | 1 | ||||||||||
| Cephalodella sp. | 1 | ||||||||||||
| Euchlanis dapidula (Parise, 1966) | 1 | ||||||||||||
| Euchlanis dilatata (Ehrenberg, 1832) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Euchlanis lyra (Hudson, 1886) | 1 | ||||||||||||
| Filinia longiseta (Ehrenberg,1834) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Filinia terminalis (Plate, 1886) | 1 | ||||||||||||
| Hexarthra fennica (Levander, 1892) | 1 | 1 | 1 | 1 | |||||||||
| Kellicottia longispina (Kellicott, 1879) | 1 | ||||||||||||
| Keratella cochlearis (Gosse, 1851) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Keratella quadrata (Müller, 1786) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Keratella tropica (Apstein,1907) | 1 | 1 | |||||||||||
| Lecane bulla (Gosse,1851) | 1 | 1 | |||||||||||
| Lecane crenata (Harring, 1913) | 1 | ||||||||||||
| Lecane (s. str.) luna (Müller, 1776) | 1 | 1 | 1 | ||||||||||
| Lepadella (s. str.) triptera (Еhrenberg, 1830) | 1 | ||||||||||||
| Lophocharis oxysternon (Gosse, 1851) | 1 | ||||||||||||
| Mytilina mucronata (Müller, 1773) | 1 | 1 | |||||||||||
| Notholca acuminata (Ehrenberg, 1832) | 1 | 1 | 1 | 1 | |||||||||
| Notommata collaris (Ehrenberg, 1832) | 1 | ||||||||||||
| Notommatidae gen. sp. | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Platyias quadricornis (Ehrenberg, 1832) | 1 | 1 | 1 | ||||||||||
| Polyarthra vulgaris (Carlin, 1943) | 1 | ||||||||||||
| Polyarthra dolichoptera (Idelson, 1925) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Polyarthra vulgaris (Carlin, 1943) | 1 | 1 | |||||||||||
| Pompholyx sulcata (Hudson, 1885) | 1 | ||||||||||||
| Synchaeta stylata (Wierzejski, 1893) | 1 | 1 | |||||||||||
| Testudinella patina (Hermann, 1783) | 1 | 1 | |||||||||||
| Trichotria pocillum (Müller, 1776) | 1 | ||||||||||||
| Trichotria truncata (Whitelegge, 1889) | 1 | ||||||||||||
| Total number of Rotifera: 46 | 11 | 16 | 20 | 4 | 3 | 5 | 8 | 7 | 4 | 10 | 5 | 14 | 11 |
| Cladocera | |||||||||||||
| Alona rectangula (G.O. Sars, 1862) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Bosmina (Bosmina) longirostris (O.F. Müller, 1785) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bythotrephes longimanus (Leydig, 1860) | 1 | ||||||||||||
| Ceriodaphnia pulchella (Sars, 1862) | 1 | ||||||||||||
| Ceriodaphnia quadrangula (O.F. Müller, 1785) | 1 | 1 | 1 | ||||||||||
| Ceriodaphnia reticulata (Jurine, 1820) | 1 | 1 | 1 | 1 | |||||||||
| Ceriodaphnia sp. | 1 | 1 | 1 | ||||||||||
| Chydorus sphaericus (O.F. Müller, 1776) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Daphnia (Ctenodaphnia) magna (Straus 1826) | 1 | ||||||||||||
| Daphnia (Daphnia) galeata (G.O. Sars, 1864) | 1 | 1 | 1 | 1 | |||||||||
| Daphnia (Daphnia) hyalina (Leydig, 1860) | 1 | ||||||||||||
| Daphnia (Daphnia) longispina (O. F. Müller, 1785) | 1 | ||||||||||||
| Daphnia (Daphnia) pulex (De Geer, 1778) | 1 | ||||||||||||
| Daphnia (Daphnia) cucullata (Sars, 1862) | 1 | 1 | 1 | ||||||||||
| Diaphanosoma brachyurum (Liévin, 1848) | 1 | 1 | |||||||||||
| Moina mongolica (Daday, 1901) | 1 | 1 | |||||||||||
| Pleuroxus aduncus (Jurine, 1820) | 1 | ||||||||||||
| Polyphemus pediculus (Linnaeus, 1761) | 1 | 1 | 1 | 1 | |||||||||
| Scapholeberis mucronata (O.F.Müller, 1776) | 1 | 1 | 1 | ||||||||||
| Simocephalus mixtus (Sars, 1903) | 1 | ||||||||||||
| Simocephalus serrulatus (Koch, 1841) | 1 | ||||||||||||
| Total number of Cladocera: 21 | 2 | 4 | 10 | 5 | 3 | 9 | 4 | 2 | 0 | 7 | 5 | 7 | 4 |
| Copepoda | |||||||||||||
| Acanthodiaptomus denticornis (Wierzejski, 1887) | 1 | 1 | |||||||||||
| Arctodiaptomus salinus (Daday 1885) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Cletocamptus retrogressus (Schmankevitsch, 1875) | 1 | 1 | |||||||||||
| Cryptocyclops bicolor (Sars G.O., 1863) | 1 | ||||||||||||
| Cyclopoida gen.sp. | 1 | 1 | 1 | 1 | |||||||||
| Cyclops vicinus (Uljanin, 1875) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Diacyclops languidoides (Lilljeborg, 1901) | 1 | ||||||||||||
| Diaptomidae gen.sp. (Sars) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Eudiaptomus transylvanicus (Daday, 1890) | 1 | ||||||||||||
| Ergasilidae gen.sp. | 1 | ||||||||||||
| Eucyclops denticulatus (Graeter, 1903) | 1 | ||||||||||||
| Eucyclops macrurus (Sars G.O., 1863) | 1 | ||||||||||||
| Eucyclops serrulatus (Lilljeborg, 1901) | 1 | 1 | |||||||||||
| Eucyclops speratus (Lilljeborg, 1901) | 1 | ||||||||||||
| Eudiaptomus graciloides (Lilljeborg, 1888) | 1 | 1 | |||||||||||
| Eurytemora affinis (Poppe, 1880) | 1 | 1 | |||||||||||
| Harpacticoida gen.sp. | 1 | 1 | 1 | ||||||||||
| Megacyclops viridis (Jurine, 1820) | 1 | 1 | |||||||||||
| Mesocyclops leuckarti (Claus, 1857) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Neutrodiaptomus incongruens (Poppe, 1888) | |||||||||||||
| Thermocyclops crassus (Fischer, 1853) | 1 | 1 | 1 | 1 | 1 | ||||||||
| Thermocyclops dybowskii (Lande, 1890) | 1 | ||||||||||||
| Thermocyclops sp. | 1 | ||||||||||||
| Thermocyclops taihokuensis (Harada, 1931) | 1 | ||||||||||||
| Thermocyclops vermifer (Lindberg, 1935) | 1 | ||||||||||||
| Total number of Copepoda: 25 | 3 | 4 | 6 | 6 | 4 | 9 | 4 | 3 | 2 | 7 | 3 | 4 | 4 |
| In total: 92 | 16 | 24 | 36 | 15 | 10 | 23 | 16 | 12 | 6 | 24 | 13 | 25 | 16 |
| Lake | Population, thousand ind./m3 | Biomass, g/m3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Rotifera | Cladocera | Copepoda | Total | Rotifera | Cladocera | Copepoda | Total | |
| Zharlykol Lake | 12.48 | 20.73 | 2.88 | 36.09 | 0.03 | 0.22 | 0.13 | 0.38 |
| Maibalyk Lake | 64.24 | 1.70 | 54.22 | 120.15 | 0.21 | 0.33 | 0.03 | 0.57 |
| Koyandy Reservoir | 87.46 | 0.70 | 15.14 | 103.29 | 0.06 | 0.05 | 0.12 | 0.23 |
| Zharkol Lake | 0.24 | 4.73 | 9.10 | 14.07 | 0.00 | 0.24 | 0.40 | 0.64 |
| Kostomar Lake | 0.01 | 42.54 | 12.69 | 55.23 | 0.00 | 3.50 | 0.32 | 3.83 |
| Zharken Lake | 5.66 | 31.33 | 17.23 | 54.22 | 0.00 | 2.34 | 1.12 | 3.46 |
| Solenoe Lake | 522.86 | 1.49 | 56.66 | 581.01 | 3.06 | 0.17 | 0.80 | 4.02 |
| Lebyazhe Lake | 139.16 | 3.48 | 18.59 | 161.23 | 0.07 | 0.02 | 0.05 | 0.13 |
| Polkovnikovo Lake | 124.60 | 1.34 | 18.24 | 144.18 | 1.07 | 0.08 | 0.34 | 1.50 |
| Solontsy Lake | 19.24 | 116.88 | 17.71 | 153.82 | 0.01 | 19.34 | 0.26 | 19.61 |
| Ashchikol Lake | 1.60 | 25.85 | 343.34 | 370.79 | 0.00 | 55.83 | 13.41 | 69.24 |
| Kondratievskoe Lake | 6.51 | 63.84 | 120.11 | 190.47 | 0.03 | 0.33 | 0.46 | 0.82 |
| Presnoe Lake | 6.38 | 50.45 | 68.59 | 125.42 | 0.01 | 0.79 | 1.62 | 2.42 |
| Water Bodies | Zharlykol Lake | Koyandy Reservoir | Maibalyk Lake | Kostomar Lake | Zharkol Lake | Zharken Lake | Lebyazhe Lake | Polkovnikovo Lake | Solenoe Lake | Solontsy Lake | Ashchikol Lake | Kondratievskoe Lake | Presnoe Lake |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zharlykol Lake | 1 | ||||||||||||
| Koyandy Reservoir | 0.350 | 1 | |||||||||||
| Maibalyk Lake | 0.214 | 0.281 | 1 | ||||||||||
| Kostomar Lake | 0.375 | 0.150 | 0.393 | 1 | |||||||||
| Zharkol Lake | 0.286 | 0 | 0.038 | 0.357 | 1 | ||||||||
| Zharken Lake | 0.146 | 0.245 | 0.338 | 0.293 | 0.108 | 1 | |||||||
| Lebyazhe Lake | 0.242 | 0.293 | 0.456 | 0.364 | 0 | 0.286 | 1 | ||||||
| Polkovnikovo Lake | 0.200 | 0.263 | 0.407 | 0.267 | 0 | 0.103 | 0.516 | 1 | |||||
| Solenoe Lake | 0.348 | 0.065 | 0.043 | 0.174 | 0.632 | 0.063 | 0.083 | 0.095 | 1 | ||||
| Solontsy Lake | 0.273 | 0.346 | 0.441 | 0.227 | 0.200 | 0.415 | 0.400 | 0.429 | 0.229 | 1 | |||
| Ashchikol Lake | 0.276 | 0.270 | 0.264 | 0.414 | 0.160 | 0.211 | 0.267 | 0.074 | 0.100 | 0.195 | 1 | ||
| Kondratievskoe Lake | 0.276 | 0.340 | 0.493 | 0.311 | 0.049 | 0.222 | 0.348 | 0.419 | 0.056 | 0.456 | 0.238 | 1 | |
| Presnoe Lake | 0.171 | 0.233 | 0.169 | 0.171 | 0.129 | 0.273 | 0.169 | 0.121 | 0.154 | 0.340 | 0.250 | 0.250 | 1 |
| Name of Water Body | Main Components | ||
|---|---|---|---|
| PC 1 | PC 2 | PC 3 | |
| Zharlykol Lake | −1.2995 | −0.39924 | −1.0659 |
| Koyandy Reservoir | 0.48019 | 2.2314 | −1.6759 |
| Maibalyk Lake | 3.731 | −1.8439 | 1.0181 |
| Kostomar Lake | −0.6118 | −1.9326 | 0.27934 |
| Zharkol Lake | −2.2268 | −1.1999 | 0.28282 |
| Zharken Lake | 0.031702 | 1.2497 | 3.2707 |
| Lebyazhe Lake | 1.0577 | −0.40156 | −0.40403 |
| Polkovnikovo Lake | 0.59731 | −0.26252 | −1.1374 |
| Solenoe Lake | −1.9249 | −0.60174 | −0.18336 |
| Solontsy Lake | 0.69291 | 1.8814 | 0.70725 |
| Ashchikol Lake | −0.80699 | −0.69883 | 0.009319 |
| Kondratievskoe Lake | 1.4938 | 0.4997 | −1.7531 |
| Presnoe Lake | −1.2146 | 1.4781 | 0.65205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satybaldiyeva, G.; Sapargaliyeva, N.; Sharakhmetov, S.; Inelova, Z.; Boros, E.; Krupa, E.; Utarbayeva, A.; Shupshibayev, K. Species Diversity of Zooplankton of Small Steppe Lakes of the Northern Part of Kazakhstan. Water 2023, 15, 4054. https://doi.org/10.3390/w15234054
Satybaldiyeva G, Sapargaliyeva N, Sharakhmetov S, Inelova Z, Boros E, Krupa E, Utarbayeva A, Shupshibayev K. Species Diversity of Zooplankton of Small Steppe Lakes of the Northern Part of Kazakhstan. Water. 2023; 15(23):4054. https://doi.org/10.3390/w15234054
Chicago/Turabian StyleSatybaldiyeva, Gulmira, Nazym Sapargaliyeva, Sayat Sharakhmetov, Zarina Inelova, Emil Boros, Elena Krupa, Aizhan Utarbayeva, and Kazbek Shupshibayev. 2023. "Species Diversity of Zooplankton of Small Steppe Lakes of the Northern Part of Kazakhstan" Water 15, no. 23: 4054. https://doi.org/10.3390/w15234054





