Ecology Health Evaluation System Based on Fish Movement Behavior Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
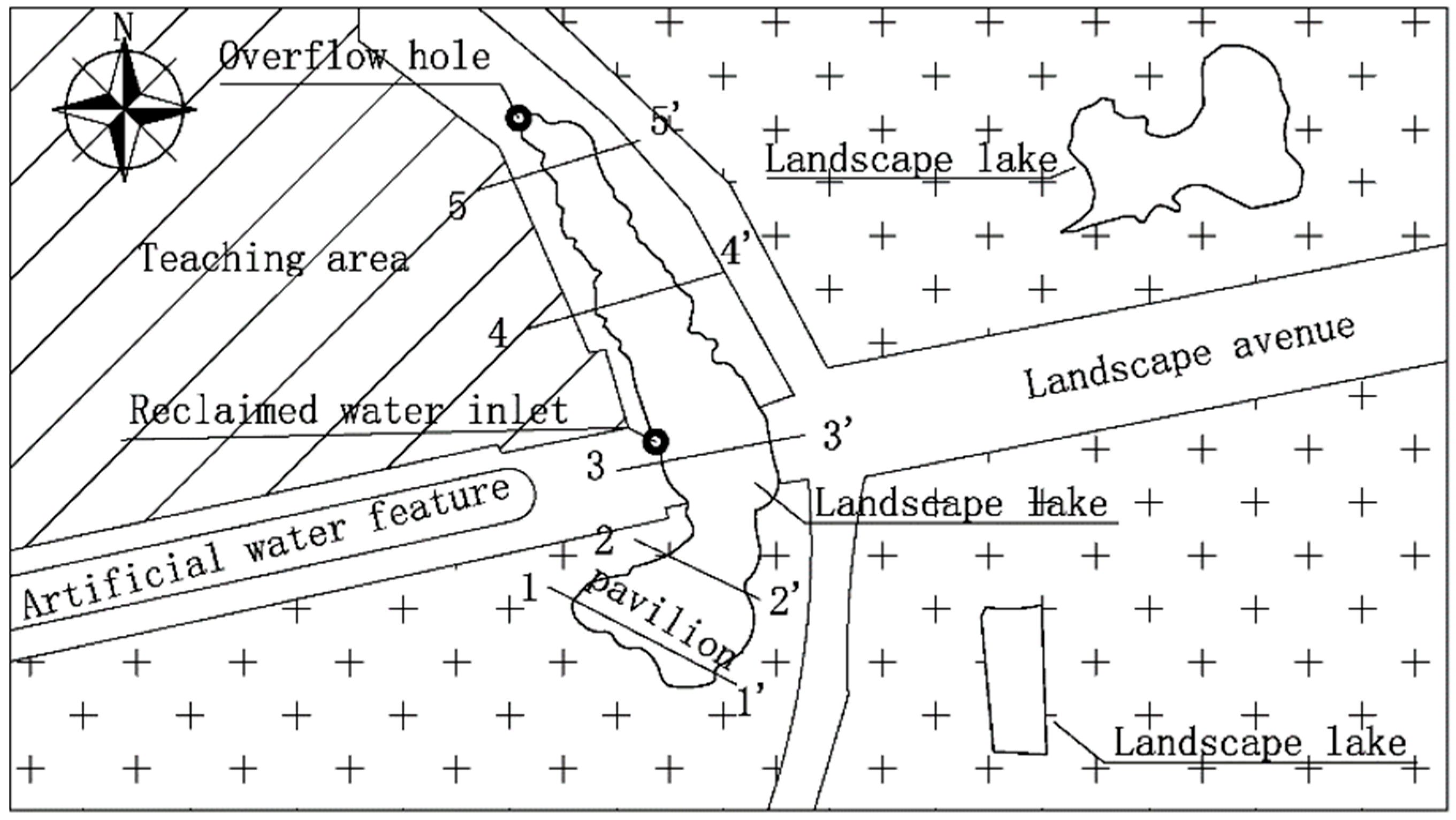
2.1.1. Experimental Device
2.1.2. Biological Indicator
2.2. Monitoring Technology and Methods
2.2.1. Water Quality Monitoring Methods
2.2.2. Fish Trajectory Monitoring Technology
2.3. Fish Swimming Speed
2.4. Evaluation of the Water Area
2.5. Data Processing Methods
3. Results and Discussion
3.1. Changes in Fish Movement Trajectories on the Time Scale
3.2. Changes in Fish Movement Trajectories on the Spatial Scale
3.3. Changes in Fish Swimming Speed
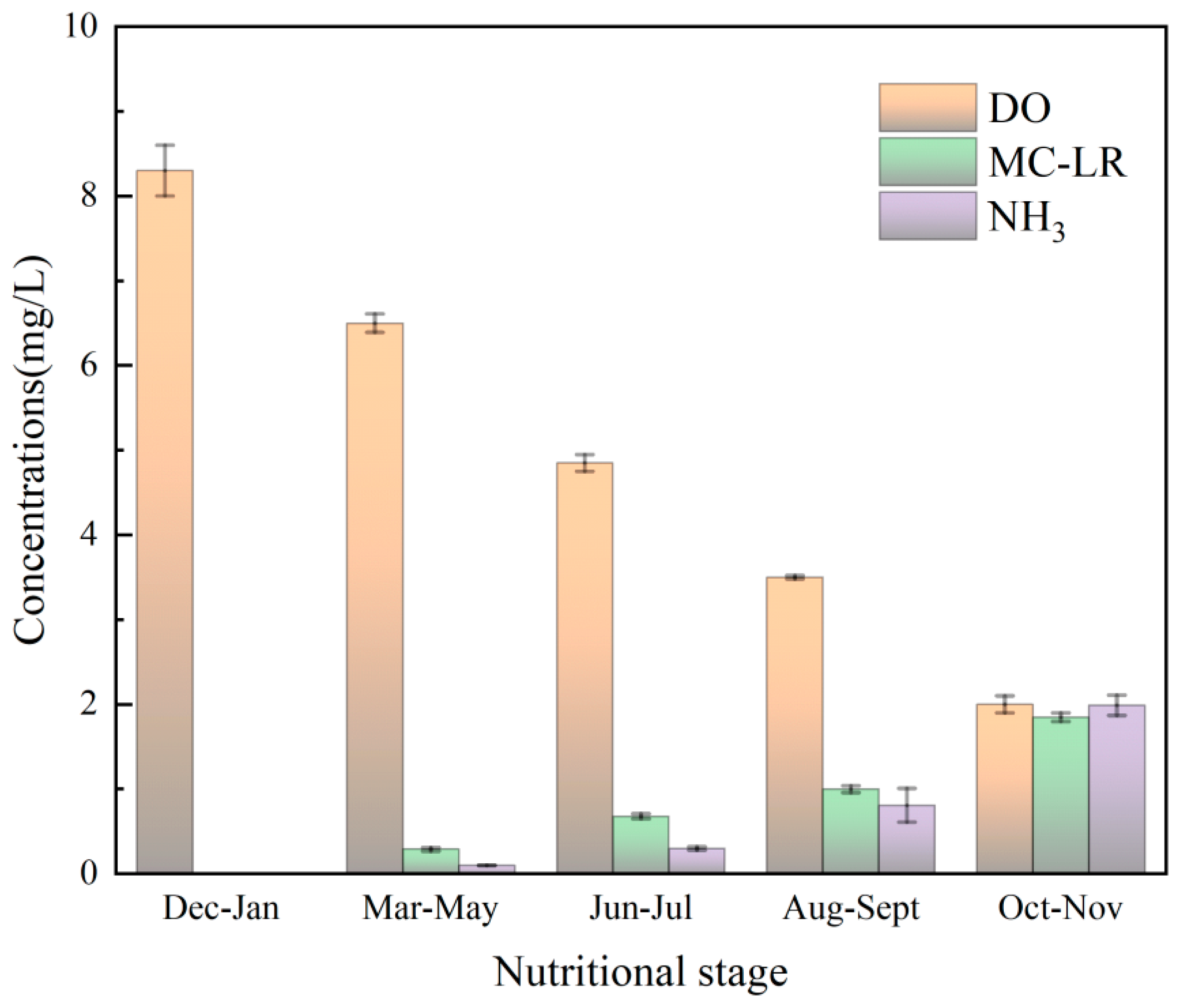
3.4. Determination of Water Quality Index Using Fish Sensitivity
3.5. Correlation Analysis
3.6. Prediction Model of the Indices of Fish Movement Behavior Response
3.7. Construction of the Water Ecological Health Evaluation System
3.7.1. Construction of the Evaluation Index System
3.7.2. Classification of Evaluation Index Levels
3.8. Application of the Aquatic Ecological Health Evaluation System
3.8.1. Analysis of Monitoring Results
3.8.2. Evaluation Result Verification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Besch, W.K.; Kemball, A.; Meyer-Waarden, K.; Scharf, B. A Biological Monitoring System Employing Rheotaxis of Fish; ASTM Special Technical Publication: West Conshohocken, PA, USA, 1976. [Google Scholar]
- Williams, K.; Goddard, P.; Wilborn, R.; Bryan, D.; Rooper, C. Fish behavior in response to an approaching underwater camera. Fish. Res. 2023, 268, 106823. [Google Scholar] [CrossRef]
- Liu, H.; Lin, J.Q.; Wang, D.S.; Huang, J.; Jiang, H.; Zhang, D.; Peng, Q.D.; Yang, J. Experimental study of the behavioral response of fish to changes in hydrodynamic indicators in A near-natural environment. Ecol. Indic. 2023, 154, 110813. [Google Scholar] [CrossRef]
- Burnett, M.J.; Süssle, V.; Saayman, T.; Jewitt, G.; O’Brien, G.C.; Downs, C.T. Detecting changes in fish behaviour in real time to alert managers to thresholds of potential concern. River Res. Appl. 2023, 1–19. [Google Scholar] [CrossRef]
- Du, J.J. Experimental Study on Behavior and Toxicological Effects of Phenol on Zebrafish. Ph.D. Thesis, Shandong Jianzhu University, Jinan, China, 2016. [Google Scholar]
- Georgieva, E.; Yancheva, V.; Velcheva, L.; Lliev, L.; Stoyanova, S. Histological and biochemical changes in liver of common carp (Cyprinus carpio L.) under metal exposure. North-West J. Zool. 2016, 12, 261–270. [Google Scholar]
- Hao, L.X.; Sun, R.H.; Chen, L.D. Health Assessment of River Ecosystem in Haihe River Basin, China. Environ. Sci. 2014, 1, 3692–3701. [Google Scholar]
- Huang, K.; Yao, Y.; Wang, X.N.; Zhang, Y.; Qian, C.; Ding, S. The Ecosystem Health Assessment based on the Fish Index of Biological Integrity in the Luanhe River Basin. Res. Environ. Sci. 2018, 31, 901–910. [Google Scholar]
- Huang, Y.Q.; Cai, D.S.; Li, M.Q.; Wu, T.H.; Li, L. Influence of changes in dissolved oxygen content on fish behavioral trajectories during water eutrophication. Appl. Ecol. Environ. Res. 2018, 17, 653–666. [Google Scholar] [CrossRef]
- Liang, H.; Liu, Y.; Rao, K.F.; Ma, M.; Xu, W.; Tang, L.; Ma, S.; Pan, X.F.; Chen, H.M. The Behavioral Responses of the Medaka under the Stress of Heavy Metal Copper in Water Environment. Asian J. Ecotoxicol. 2016, 11, 345–352. [Google Scholar]
- Li, L.H. Anomaly Water Quality Monitoring Based on Fish Movement. Ph.D. Thesis, Yanshan University, Qinhuangdao, China, 2017. [Google Scholar]
- Liu, S.; Fu, S.J. Effects of food availability on metabolism, behaviour, growth and their relationships in a triploid carp. J. Exp. Biol. 2017, 220, 4711–4719. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lun, F.; Yang, Z.G. Study on Acute Toxicity of Trichlorfon to Yellow River Carp and Its Safety Evaluation. Heilongjiang Anim. Sci. Vet. Med. 2018, 2, 184–186. [Google Scholar]
- Lv, Y.F.; Zhao, X.; Wang, J.F. Acute Toxicity and Response of Antioxidant Enzymes of Phenanthrene Stress on Carp. J. Donghua Univ. 2018, 44, 309–316. [Google Scholar]
- Ma, H.; Tsai, T.F.; Liu, C.C. Real-time monitoring of water quality using temporal trajectory of live fish. Expert Syst. Appl. 2010, 37, 5158–5171. [Google Scholar] [CrossRef]
- Morgan, W.S. Fish locomotor behavior patterns as a monitoring tool. Water Pollut. Control Fed. 1979, 51, 580–589. [Google Scholar]
- Newbold, L.R.; Kemp, P.S. Influence of corrugated boundary hydrodynamics on the swimming performance and behavior of juvenile common carp (Cyprinus carpio). Ecol. Eng. 2015, 82, 112–120. [Google Scholar] [CrossRef]
- Wang, L.T.; Ge, C.X.; Wang, J.C.; Dai, J.; Zhang, P.J.; Li, Y.H. Effects of different combinations of Bacillus on immunity and antioxidant activities in common carp. Aquac. Int. 2017, 25, 2091–2099. [Google Scholar] [CrossRef]
- Xu, Z.X.; Jiang, Y.P. Lake Eutrophication Assessment: Comprehensive Water Quality Identification Index. J. Tong Ji Univ. 2009, 37, 1044–1048. [Google Scholar]
- Yu, J.; Zhao, M.; Hu, Q.W.; Chen, P.M. Ecosystem health assessment of Egong Bay fisheries area in Shenzhen based on index of biological integrity of fish. J. South. Agric. 2017, 48, 524–531. [Google Scholar]
- Zhang, J.S.; Huang, Y.; Han, X.B.; Huang, Y.L. Application of changes of the fish behavior in the water quality monitoring. Water Wastewater Eng. 2013, 7, 166–170. [Google Scholar]
- Stanton, T. 30 years of advances in active bioacoustics: A personal perspective. Methods Oceanogr. 2012, 1–2, 49–77. [Google Scholar] [CrossRef]
- Song, C.; Yan, R.X.; Jiang, M.W.; Peng, R.B.; Han, Q.X. Testing the applicability of marine biotic indices for ecological quality in the northern East China Sea. J. Sea Res. 2023, 195, 102441. [Google Scholar] [CrossRef]
- GB11913-89; Water Quality—Determination of Dissolved Oxygen—Electrochemical Probe Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1989.
- GB/T 20466-2006; Determination of Microcystins in Water. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2006.
- GB7481-87; Water Quality―Determination of Ammonia Nitrogen―Salicylic Acid Spectrophotometry. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1987.





| Nutrient Level | ρ (Chl-a)/ (mg/m3) | ρ (TP)/ (mg/L) | ρ (TN)/ (mg/L) | ρ (COD)/ (mg/L) | ρ (Transparency)/ (mg/m3) |
|---|---|---|---|---|---|
| Oligotrophication | ≤1.0 | ≤0.004 | ≤0.05 | ≤0.40 | ≥5.0 |
| Mesotrophic | ≤26.0 | ≤0.050 | ≤0.50 | ≤4.00 | ≥1.0 |
| Eutrophic | ≤160.0 | ≤0.800 | ≤6.00 | ≤25.00 | ≥0.3 |
| Model | Correlation Coefficient (R) | Coefficient of Determination () | Adjusted Coefficient of Determination (R2) | Standard Estimate Error (S) |
|---|---|---|---|---|
| 1 | 0.997 a | 0.994 | 0.994 | 0.099846 |
| 1 | 0.962 a | 0.926 | 0.925 | 0.045911 |
| 1 | 0.998 a | 0.996 | 0.996 | 0.010239 |
| Consideration Level | Evaluation Index | Unit | Index Description | Measurement and Calculation Method |
|---|---|---|---|---|
| Fish movement behavior response | m | Distance of fish from pollution source. Distance of fish from the water surface. Fish swimming speed. | Acoustic tracking and positioning system or prediction model | |
| m | ||||
| m·s−1 | ||||
| Water quality indices based on fish sensitivity | DO | mg·L−1 | An important index that reflects the state of water pollution and its self-purification ability and the main index that affects the movement of fish. | Electrochemical probe method [24] |
| MC-LR | mg·L−1 | A freshwater cyanotoxin with the strongest toxicity and the most serious damage to fish among all microcystins, a strong liver tumor promoter with the widest distribution. | High-performance liquid chromatography (HPLC) method for the determination of microcystins in water [25] | |
| NH3 | mg·L−1 | Nitrogen in the form of non-ionic ammonia NH3 is an important indicator of water pollution, and its toxicity to fish can reach tens or even hundreds of times that of ionic ammonia. | Ammonia nitrogen NH3-N conversion algorithm Spectrophotometric method [26] |
| Serial Number | Index | Symbol | Unit | Health Level | Note | ||||
|---|---|---|---|---|---|---|---|---|---|
| Health | Sub-Health | Mild Disease | Disease | Severe Disease | |||||
| 1 | Dissolved oxygen | DO | mg·L−1 | 8.30 | 6.50 | 5.00 | 3.50 | 2.00 | ≤ |
| 2 | Microcystin-LR | MC-LR | mg·L−1 | 0 | 0.30 | 0.68 | 1.00 | 1.85 | ≤ |
| 3 | Non-ionic ammonia | NH3 | mg·L−1 | 0 | 0.10 | 0.30 | 0.85 | 2.00 | ≤ |
| 4 | Distance of fish from pollution source | m | Unlimited | 15 | 30 | 70 | 200 | ≥ | |
| 5 | Distance of fish from the water surface | m | Unlimited | Unlimited | 0.4 h | 0.3 h | 0.2 h | h is the water depth | |
| 6 | Fish swimming speed | m·s−1 | 0.55 | 0.45 | 0.35 | 0.30 | 0.25 | ≤ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Pang, R.; Li, X.; Li, W.; Yang, Z.; Wang, D.; Dong, K. Ecology Health Evaluation System Based on Fish Movement Behavior Response. Water 2023, 15, 4066. https://doi.org/10.3390/w15234066
Huang Y, Pang R, Li X, Li W, Yang Z, Wang D, Dong K. Ecology Health Evaluation System Based on Fish Movement Behavior Response. Water. 2023; 15(23):4066. https://doi.org/10.3390/w15234066
Chicago/Turabian StyleHuang, Yuequn, Rongcong Pang, Xiangtong Li, Wenjing Li, Zhanpeng Yang, Dunqiu Wang, and Kun Dong. 2023. "Ecology Health Evaluation System Based on Fish Movement Behavior Response" Water 15, no. 23: 4066. https://doi.org/10.3390/w15234066
APA StyleHuang, Y., Pang, R., Li, X., Li, W., Yang, Z., Wang, D., & Dong, K. (2023). Ecology Health Evaluation System Based on Fish Movement Behavior Response. Water, 15(23), 4066. https://doi.org/10.3390/w15234066







