Study on the Impact of Emulsion on Mine Water Quality and Health Risk Assessment
Abstract
:1. Introduction
2. Experiments and Health Impact Assessment
2.1. Experimental Device
2.2. Experimental Steps
2.2.1. Characteristics of Water Quality Indicators
2.2.2. Measurement of Water Quality Indicators
- (1)
- Determination of pH value.
- (2)
- Determination of total dissolved solids (TDS) content.
- (3)
- Determination of the content of chloride and sulfate.
- (4)
- Determination of the content of hexavalent chromium (Cr6+).
- (5)
- Total hardness measurement.
2.3. Health Risk Assessment
3. Results Analysis
3.1. Analysis of the Influence of Emulsion on Mine Water Quality
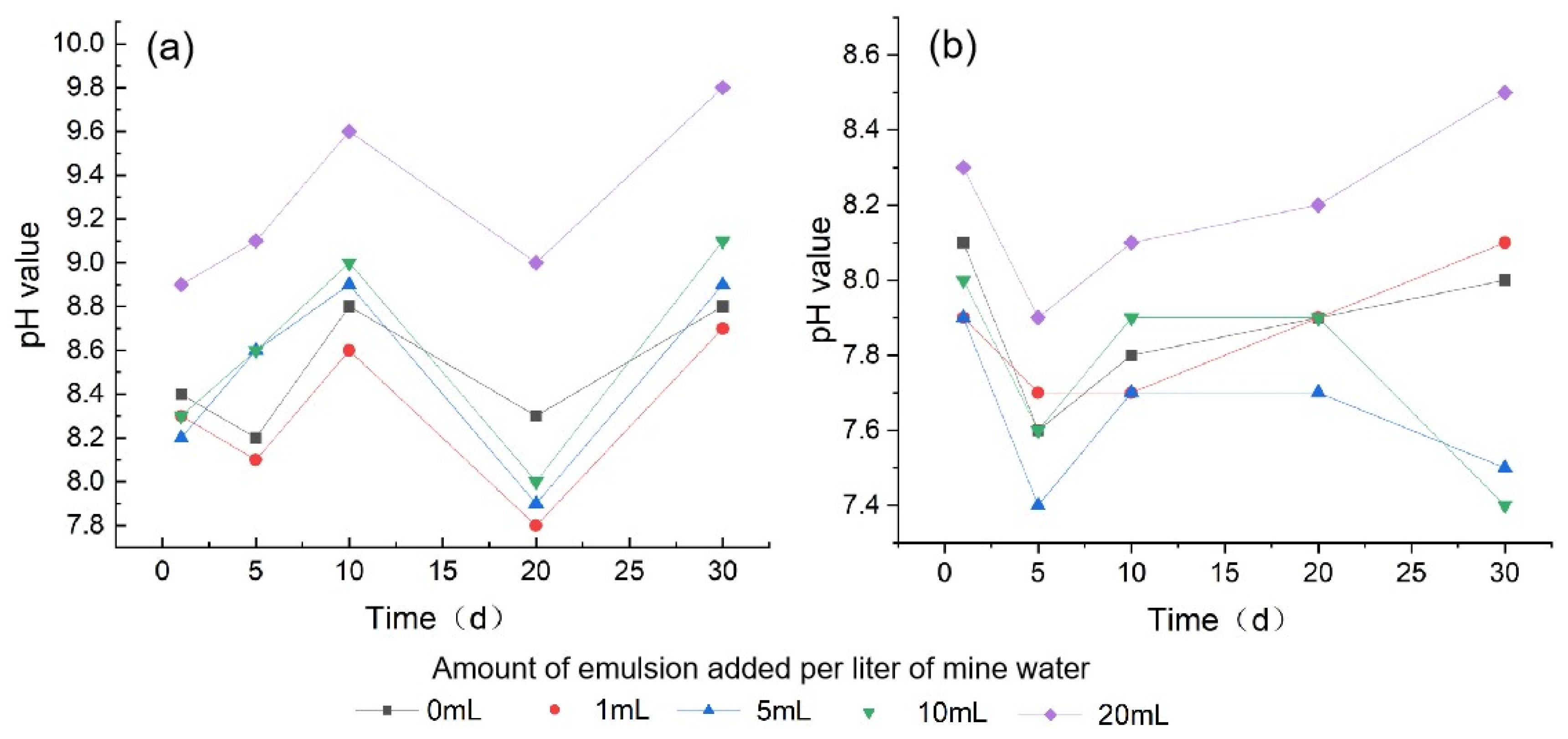
3.1.1. pH Value
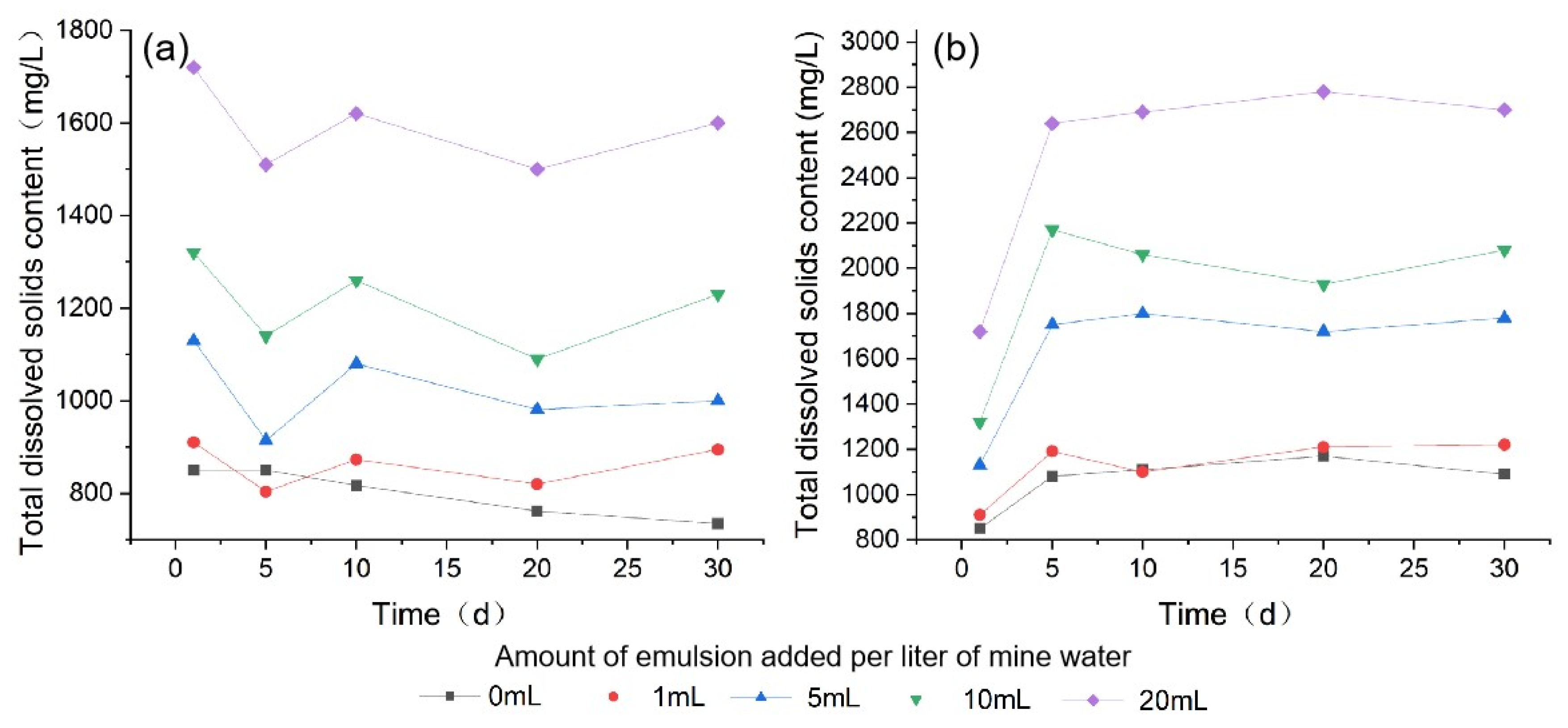
3.1.2. Total Dissolved Solids (TDS)
3.1.3. Chloride
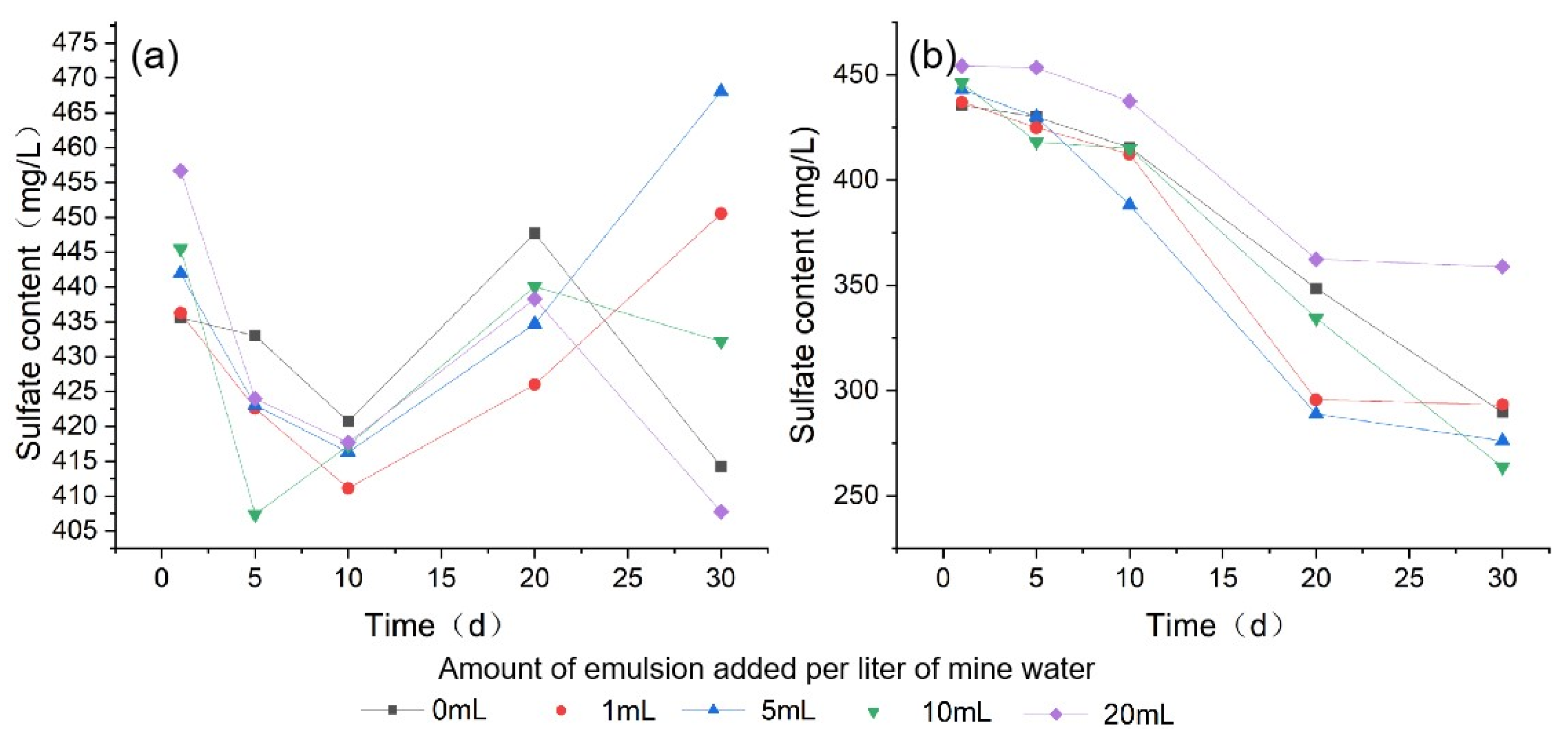
3.1.4. Sulfate
3.1.5. Hexavalent Chromium (Cr6+)
3.1.6. Total Hardness
3.2. Health Risk Assessment
3.2.1. Reference Concentration
3.2.2. Health Risk Assessment Results and Analysis
4. Discussions and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, D.; Gao, C.; Liu, L.; Tingting Yu, T.; Yihua Li, Y.H.; Wang, H.B. Customized copper/cobalt-rich ferrite spinel-based construction ceramic membrane incorporating gold tailings for enhanced treatment of industrial oily emulsion wastewater. Sep. Purif. Technol. 2023, 320, 124131. [Google Scholar] [CrossRef]
- Wei, L.H.; Li, M.; Gao, F.; Zhang, Y.B.; Li, C.W.; Zhang, Q. A novel integrated sequential air flotation in cold-rolling emulsion wastewater (CREW) treatment: Satisfying oil droplets growth laws at various stages. J. Water Process Eng. 2022, 48, 102852. [Google Scholar] [CrossRef]
- Sun, D.W.; Wang, Y.J.; Gao, J.; Liu, S.J.; Liu, X.L. Insights into the relation of crude oil components and surfactants to the stability of oily wastewater emulsions: Influence of asphaltenes, colloids, and nonionic surfactants. Sep. Purif. Technol. 2023, 307, 122804. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Luengo, A.; Zazo, J.A.; Casas, J.A. Cutting oil-water emulsion wastewater treatment by microwave assisted catalytic wet peroxide oxidation. Sep. Purif. Technol. 2021, 257, 117940. [Google Scholar] [CrossRef]
- Soloveva, O.; Solovev, S.; Ilyin, V.; Talipova, A.; Sagdieva, T. Study of the influence of porous structure on the efficiency of emulsion separation in wastewater purification on transport. Transp. Res. Procedia 2022, 61, 402–409. [Google Scholar] [CrossRef]
- Ma, W.J.; Cao, W.X.; Lu, T.; Xiong, R.H.; Huang, C.B. Multifunctional nanofibrous membrane fabrication by a sacrifice template strategy for efficient emulsion oily wastewater separation and water purification. J. Environ. Chem. Eng. 2022, 10, 108908. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, B.; Li, B.; Shi, K.; Liu, B.; Zhang, S. Dual-functional superwetting CuCo2O4 coated stainless steel mesh for wastewater treatment: Highly efficient oil/water emulsion separation and photocatalytic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130730. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, P.; Wang, D.S. Central treatment of different emulsion wastewaters by an integrated process of physicochemically enhanced ultrafiltration and anaerobic–aerobic biofilm reactor. Bioresour. Technol. 2014, 159, 150–156. [Google Scholar] [CrossRef]
- Qu, R.; Zhao, S.; Liu, N.; Li, X.; Zhai, H.; Liu, Y.; Wei, Y.; Feng, L. 3D inner-outer asymmetric sponge for enormous-volume emulsion wastewater treatment based on a new “demulsification-transport” mechanism. Green Energy Environ. 2023, 8, 1398–1408. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Wang, Y.; Song, G.; Zhang, L. Industrial application of ceramic ultrafiltration membrane in cold-rolling emulsion wastewater treatment. Sep. Purif. Technol. 2022, 289, 120724. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Dai, J.; Liu, W.; Yang, H.; Bai, Z. Efficient extraction of phenol from wastewater by ionic micro-emulsion method: Anionic and cationic. Chin. J. Chem. Eng. 2023, 58, 137–145. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Heng, L.; Jiang, L. Nano/submicrometer-emulsion oily wastewater treatment inspired by plant transpiration. Matter 2021, 4, 1274–1286. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Zhang, G.; Yang, F.; Lin, S.; Dong, Y. Robust zirconia ceramic membrane with exceptional performance for purifying nano-emulsion oily wastewater. Water Res. 2022, 208, 117859. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, F.; Li, M.; Li, J.; Li, S.Q.; Wu, S.H. New integrated processes for treating cold-rolling mill emulsion wastewater. J. Iron Steel Res. Int. 2010, 17, 32–35. [Google Scholar] [CrossRef]
- Zhong, L.X.; Sun, C.Y.; Yang, F.L.; Dong, Y.C. Superhydrophilic spinel ceramic membranes for oily emulsion wastewater treatment. J. Water Process Eng. 2021, 42, 102161. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, M.; Zhang, Q.; Wang, Z.; Lei, X.; Deng, X.; Gao, R.; Cai, W.; Chen, G.; Fu, C.; et al. Effect of pH value on multiferroic properties of barium ferrite ceramics prepared by sol–gel method. J. Magn. Magn. Mater. 2022, 563, 169904. [Google Scholar] [CrossRef]
- Banadkooki, F.B.; Ehteram, M.; Panahi, F.; Sammen, S.S.; El-Shafie, A. Estimation of total dissolved solids (TDS) using new hybrid machine learning models. J. Hydrol. 2020, 587, 124989. [Google Scholar] [CrossRef]
- Castillo-Meza, L.E.; Cravotta, C.A.; Tasker, T.L.; Warner, N.R.; Burgos, W.D. Batch extraction method to estimate total dissolved solids (TDS) release from coal refuse and overburden. Appl. Geochem. 2020, 115, 104540. [Google Scholar] [CrossRef]
- Burgos-Castillo, R.C.; Sirés, I.; Sillanpää, M.; Brillas, E. Application of electrochemical advanced oxidation to bisphenol A degradation in water. Effect of sulfate and chloride ions. Chemosphere 2018, 194, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S.; Zheng, J.; Chang, X.; Liao, Y.; Chen, D. Degradation process of reinforced concrete under chloride and sulfate attack with and without electric field. J. Build. Eng. 2023, 78, 107588. [Google Scholar] [CrossRef]
- Saurina, J.; Ester López-Aviles, E.; Le Moal, A.; Hernández-Cassou, S. Determination of calcium and total hardness in natural waters using a potentiometric sensor array. Anal. Chim. Acta 2002, 464, 89–98. [Google Scholar] [CrossRef]
- Roberts, S.D.; Powell, M.D. Reduced total hardness of fresh water enhances the efficacy of bathing as a treatment for amoebic gill disease in Atlantic salmon, Salmo salar L. J. Fish Dis. 2010, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Divahar, R.; Raj, P.S.A.; Sangeetha, S.P.; Mohanakavitha, T.; Meenambal, T. Dataset on the assessment of water quality of ground water in Kalingarayan Canal, Erode district, Tamil Nadu, India. Data Brief 2020, 32, 106112. [Google Scholar] [CrossRef] [PubMed]
- Simi, R.; Subin, M.P. Hexavalent chromium (CrVI) uptake induced physiological effects and growth in Amaranthus dubius Mart. ex Thell. Appl. Ecol. Environ. Sci. 2022, 10, 1–10. [Google Scholar]
- Chen, L.; Wu, Y.; Shen, Q.; Zheng, X.; Chen, Y. Enhancement of hexavalent chromium reduction by Shewanella oneidensis MR-1 in presence of copper nanoparticles via stimulating bacterial extracellular electron transfer and environmental adaptability. Bioresour. Technol. 2022, 361, 127686. [Google Scholar] [CrossRef] [PubMed]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium Cr(VI): A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Khoshakhlagh, A.; Mohammadzadeh, M.; Manafi, S.; Yousefian, F.; Gruszecka-Kosowska, A. Inhalational exposure to formaldehyde, carcinogenic, and non-carcinogenic risk assessment: A systematic review. Environ. Pollut. 2023, 331, 121854. [Google Scholar] [CrossRef]
- Jaiswal, M.; Hussain, J.; Gupta, S.K.; Nasr, M.; Nema, A.K. Comprehensive evaluation of water quality status for entire stretch of Yamuna River, India. Environ. Monit. Assess. 2019, 191, 208. [Google Scholar] [CrossRef]
- Jaiswal, M.; Gupta, S.K.; Chabukdhara, M.; Nasr, M.; Nema, A.K.; Hussain, J.; Malik, T. Heavy metal contamination in the complete stretch of Yamuna river: A fuzzy logic approach for comprehensive health risk assessment. PLoS ONE 2022, 17, e0272562. [Google Scholar] [CrossRef]
- Oni, A.A.; Babalola, S.O.; Adeleye, A.D.; Olagunju, T.E.; Amama, I.A.; Omole, E.O.; Adegboye, E.A.; Ohore, O.G. Non-carcinogenic and carcinogenic health risks associated with heavy metals and polycyclic aromatic hydrocarbons in well-water samples from an automobile junk market in Ibadan, SW-Nigeria. Heliyon 2022, 8, e10688. [Google Scholar] [CrossRef]
- Dehbandi, R.; Moore, F.; Keshavarzi, B. Geochemical sources, hydrogeochemical behavior, and health risk assessment of fluoride in an endemic fluorosis area, central Iran. Chemosphere 2018, 193, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, S.; Bina, B.; Tarrahi, M.J.; Amiri, F.; Ebrahimi, A. Distribution and health risk assessment of natural fluoride of drinking groundwater resources of Isfahan, Iran, using GIS. Environ. Monit. Assess. 2018, 190, 137. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Infants | Children | Youth | Mam | Woman |
|---|---|---|---|---|---|
| IR (L/d) | 0.25 | 1.50 | 1.70 | 3.00 | 2.30 |
| BW (kg) | 6 | 20 | 54 | 75 | 69 |
| Index | Statistics | Emulsion Added (mL/L, Static Test) | Emulsion Added (mL/L, Dynamic Test) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | 20 | 0 | 1 | 5 | 10 | 20 | ||
| pH | Minimum | 8.19 | 7.77 | 7.92 | 8.03 | 8.86 | 7.61 | 7.71 | 7.42 | 7.42 | 7.92 |
| Maximum | 8.81 | 8.68 | 8.89 | 9.08 | 9.84 | 8.12 | 8.12 | 7.89 | 8.02 | 8.53 | |
| Mean | 8.50 | 8.30 | 8.51 | 8.60 | 9.25 | 7.89 | 7.87 | 7.66 | 7.76 | 8.20 | |
| SD | 0.29 | 0.38 | 0.43 | 0.45 | 0.43 | 0.20 | 0.17 | 0.19 | 0.25 | 0.23 | |
| TDS | Minimum | 735 | 804 | 914 | 1090 | 1500 | 850 | 910 | 1130 | 1320 | 1720 |
| Maximum | 850 | 910 | 1130 | 1320 | 1720 | 1170 | 1220 | 1800 | 2170 | 2780 | |
| Mean | 803 | 860 | 1021 | 1208 | 1590 | 1060 | 1126 | 1636 | 1912 | 2506 | |
| SD | 52 | 46 | 85 | 93 | 90 | 122 | 130 | 284 | 342 | 442 | |
| Chloride | Minimum | 295.6 | 304.9 | 306.8 | 304.6 | 292.2 | 307.3 | 308.1 | 309.8 | 306.3 | 311.0 |
| Maximum | 315.7 | 321.5 | 318.2 | 325.7 | 321.9 | 341.1 | 398.1 | 409.2 | 414.4 | 488.0 | |
| Mean | 307.8 | 310.9 | 311.7 | 313.4 | 306.7 | 319.0 | 334.5 | 356.4 | 332.9 | 405.6 | |
| SD | 8.0 | 7.0 | 4.2 | 8.8 | 11.1 | 13.5 | 39.5 | 46.4 | 45.8 | 84.7 | |
| Sulfate | Minimum | 414.3 | 411.1 | 416.3 | 407.4 | 407.8 | 289.7 | 293.3 | 276.1 | 263.7 | 358.9 |
| Maximum | 447.7 | 450.5 | 468.1 | 445.5 | 456.7 | 435.6 | 437.1 | 443.1 | 446.2 | 454.3 | |
| Mean | 430.3 | 429.3 | 436.8 | 428.5 | 428.9 | 383.9 | 372.6 | 365.3 | 375.5 | 413.3 | |
| SD | 13.1 | 14.9 | 20.1 | 15.9 | 19.1 | 63.1 | 71.9 | 78.4 | 75.1 | 48.5 | |
| Cr6+ | Minimum | 0.00 | 0.01 | 0.06 | 0.47 | 1.30 | 0.00 | 0.01 | 0.03 | 0.12 | 0.60 |
| Maximum | 0.00 | 0.03 | 0.10 | 0.73 | 1.82 | 0.00 | 0.02 | 0.09 | 0.47 | 1.51 | |
| Mean | 0.00 | 0.02 | 0.08 | 0.61 | 1.60 | 0.00 | 0.02 | 0.06 | 0.25 | 0.93 | |
| SD | 0.00 | 0.01 | 0.02 | 0.12 | 0.20 | 0.00 | 0.01 | 0.02 | 0.13 | 0.38 | |
| Total hardness | Minimum | 142.1 | 150.1 | 130.1 | 112.1 | 98.2 | 178.1 | 188.2 | 170.1 | 168.3 | 175.2 |
| Maximum | 206.2 | 188.2 | 180.1 | 122.1 | 105.3 | 222.2 | 238.2 | 252.2 | 266.2 | 304.2 | |
| Mean | 165.3 | 171.3 | 151.3 | 117.3 | 101.0 | 201.8 | 208.6 | 221.8 | 219.2 | 230.4 | |
| SD | 26.7 | 18.0 | 22.7 | 4.9 | 2.6 | 16.5 | 20.8 | 32.8 | 35.6 | 48.2 | |
| Amount of Emulsion (mL) Added to 1 L Mine Water | Maximum Value | Minimum Value | Absolute Value of Difference in Amplitude of Change |
|---|---|---|---|
| 0 | 8.80 | 8.19 | 0.61 |
| 1 | 8.68 | 7.77 | 0.91 |
| 5 | 8.89 | 7.92 | 0.97 |
| 10 | 9.08 | 8.03 | 1.05 |
| 20 | 9.84 | 8.86 | 0.98 |
| Amount of Emulsion (mL) Added to 1 L Mine Water | Initial Value Measured on Day l | Fluctuation Amplitude (Compared to Last Measurement) | |||
|---|---|---|---|---|---|
| Day 5 | Day 10 | Day 20 | Day 30 | ||
| 0 | 850 | 0 | −33 | −55 | −27 |
| 1 | 910 | −106 | 69 | −53 | 75 |
| 5 | 1130 | −216 | 166 | −99 | 19 |
| 10 | 1320 | −180 | 120 | −170 | 140 |
| 20 | 1720 | −210 | 110 | −120 | 100 |
| Index | Non-Carcinogenic Risk Threshold Concentrations | ||||
|---|---|---|---|---|---|
| Infants | Children | Youth | Mam | Woman | |
| Cr6+ | 0.072 | 0.040 | 0.095 | 0.075 | 0.090 |
| Chloride content (mg/L) | 2.40 | 1.33 | 3.18 | 2.50 | 3.00 |
| Index | Amount of Emulsion Added (mL) | Risk Index of HQ | ||||
|---|---|---|---|---|---|---|
| Infants | Children | Youth | Mam | Woman | ||
| Cr6+ | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.57 | 1.02 | 0.43 | 0.55 | 0.46 | |
| 5 | 1.06 | 1.90 | 0.80 | 1.01 | 0.84 | |
| 10 | 6.51 | 11.72 | 4.92 | 6.25 | 5.21 | |
| 20 | 20.96 | 37.72 | 15.84 | 20.12 | 16.77 | |
| Chloride content | 0 | 128.54 | 231.38 | 97.12 | 123.40 | 102.83 |
| 1 | 131.54 | 236.78 | 99.39 | 126.28 | 105.23 | |
| 5 | 127.16 | 228.88 | 96.08 | 122.07 | 101.73 | |
| 10 | 130.86 | 235.56 | 98.87 | 125.63 | 104.69 | |
| 20 | 123.15 | 221.67 | 93.04 | 118.22 | 98.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Liu, Y.; Wu, M.; Li, J.; He, R.; Hao, C.; Fan, X.; Sun, C. Study on the Impact of Emulsion on Mine Water Quality and Health Risk Assessment. Water 2023, 15, 4086. https://doi.org/10.3390/w15234086
Qiu Y, Liu Y, Wu M, Li J, He R, Hao C, Fan X, Sun C. Study on the Impact of Emulsion on Mine Water Quality and Health Risk Assessment. Water. 2023; 15(23):4086. https://doi.org/10.3390/w15234086
Chicago/Turabian StyleQiu, Youli, Yu Liu, Min Wu, Jie Li, Ruimin He, Chunming Hao, Xing Fan, and Chaoxing Sun. 2023. "Study on the Impact of Emulsion on Mine Water Quality and Health Risk Assessment" Water 15, no. 23: 4086. https://doi.org/10.3390/w15234086
APA StyleQiu, Y., Liu, Y., Wu, M., Li, J., He, R., Hao, C., Fan, X., & Sun, C. (2023). Study on the Impact of Emulsion on Mine Water Quality and Health Risk Assessment. Water, 15(23), 4086. https://doi.org/10.3390/w15234086





