Occurrence of Antibiotic Resistance Genes, Antibiotics-Resistant and Multi-Resistant Bacteria and Their Correlations in One River in Central-Western Brazil
Abstract
:1. Introduction
2. Materials and Methods
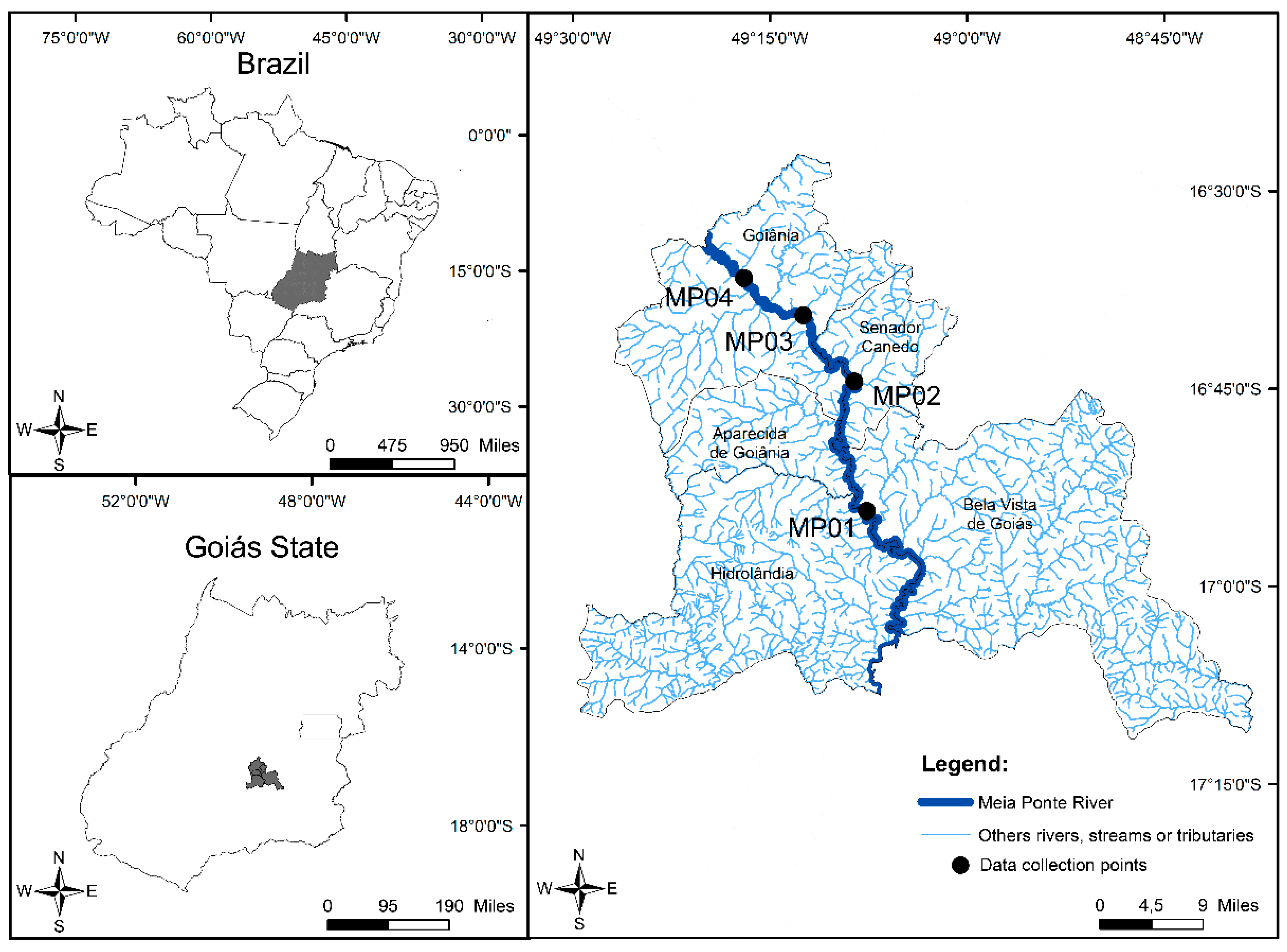
2.1. Study Area
2.2. Sample Collection
2.3. Physicochemical Analysis of Water
2.4. Culture-Dependent Method
2.4.1. Thermotolerant Coliforms and Escherichia Coli Count
2.4.2. Standard Plate Count
2.4.3. Bacterial Isolation
2.4.4. Bacterial Identification
2.4.5. Antibiogram
2.5. Culture-Independent Method
2.5.1. DNA Extraction from Environmental Samples
2.5.2. Real-Time PCR (qPCR)
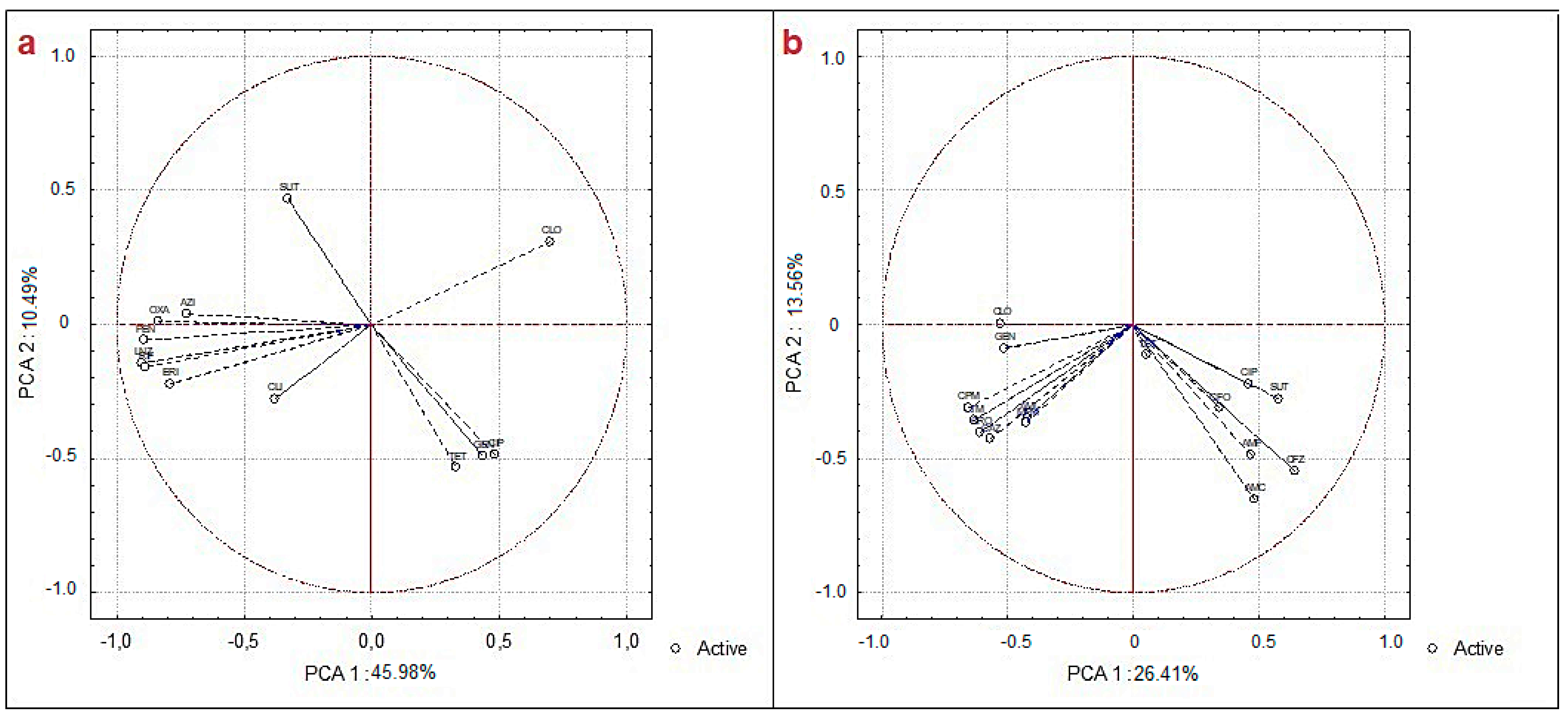
2.6. Statistical Analysis of Data
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, M.G.; Nash, S.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Indic. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Tiyasha; Tung, T.M.; Yaseen, Z.M. A survey on river water quality modelling using artificial intelligence models: 2000–2020. J. Hydrol. 2020, 585, 124670. [Google Scholar] [CrossRef]
- Kachroud, M.; Trolard, F.; Kefi, M.; Jebari, S.; Bourrié, G. Water quality indices: Challenges and application limits in the literature. Water 2019, 11, 361. [Google Scholar] [CrossRef]
- Giri, S. Water quality prospective in Twenty First Century: Status of water quality in major river basins, contemporary strategies and impediments: A review. Environ. Pollut. 2021, 271, 116332. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. The Global Risks Report 2021, 16th ed.; World Economic Forum: Geneva, Switzerland, 2021; ISBN 9782940631247. [Google Scholar]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Kumar, M.; Borah, P.; Devi, P. Priority and emerging pollutants in water. In Inorganic Pollutants in Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–49. [Google Scholar] [CrossRef]
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Li, F.; Hua, T.; Zhou, Q.; Ho, S.H. Technologies towards antibiotic resistance genes (ARGs) removal from aquatic environment: A critical review. J. Hazard. Mater. 2021, 411, 125148. [Google Scholar] [CrossRef]
- Sanderson, H.; Fricker, C.; Brown, R.S.; Majury, A.; Liss, S.N. Antibiotic Resistance Genes as an Emerging Environmental Contaminant Haley. Environ. Rev. 2016, 24, 205–218. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment. Crit. Rev. Biotechnol. 2018, 38, 1195–1208. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jay, J.A.; Stenstrom, M.K. Fate of antibiotic resistance genes and antibiotic-resistant bacteria in water resource recovery facilities. Water Environ. Res. 2019, 91, 5–20. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Suzuki, S.; Pruden, A.; Virta, M.; Zhang, T. Antibiotic resistance in aquatic systems. Front. Microbiol. 2017, 8, 14. [Google Scholar] [CrossRef]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef]
- Bürgmann, H.; Frigon, D.; Gaze, W.H.; Manaia, C.M.; Pruden, A.; Singer, A.C.; Smets, B.F.; Zhang, T. Water and sanitation: An essential battlefront in the war on antimicrobial resistance. FEMS Microbiol. Ecol. 2018, 94, fiy101. [Google Scholar] [CrossRef]
- Böger, B.; Surek, M.; Vilhena, R.D.O.; Fachi, M.M.; Junkert, A.M.; Santos, J.M.; Domingos, E.L.; Cobre, A.d.F.; Momade, D.R.; Pontarolo, R. Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil. J. Hazard. Mater. 2021, 402, 123448. [Google Scholar] [CrossRef]
- Amaral, A.K.N.; Formiga, K.T.M. Análise da distribuição granulométrica ao longo da Bacia Hidrográfica do Rio Meia Ponte—Goiás. Rev. Gestão Água América Lat. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Costa, H.S.; Tejerina-Garro, F.L.; Rocha, C. Trace elements: Water-sediment interactions in tropical rivers. Environ. Sci. Pollut. Res. 2017, 24, 22018–22025. [Google Scholar] [CrossRef] [PubMed]
- De Paula, L.G.F.; Zeringóta, V.; Sampaio, A.L.N.; Bezerra, G.P.; Barreto, A.L.G.; dos Santos, A.A.; Miranda, V.C.; Paula, W.V.D.F.; Neves, L.C.; Secchis, M.V.; et al. Seasonal dynamics of Amblyomma sculptum in two areas of the Cerrado biome midwestern Brazil, where human cases of rickettsiosis have been reported. Exp. Appl. Acarol. 2021, 84, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.P.; de Paula Silva, J.A.; Carvalho Junior, M.C.; Alburquerque, W.C.A.; Scalize, P.S.; Galvão Filho, A.R.; de Jesus Pires, D.; Vieira, J.D.G.; Carneiro, L.C. Evaluation of the raw water quality: Physicochemical and toxicological approaches. Environ. Geochem. Health. 2019, 41, 2425–2442. [Google Scholar] [CrossRef]
- Soares, A.K.; Salgado, A.D.A.; Scaratti, D.; Milograna, J.; Hora, K.E.R.; Formiga, K.T.M.; Pinho, L.; Mauro, R.A.; Duarte, L.V.; Casaroli, L.; et al. Plano de Ação da Upgrh do Rio Meia Ponte 2020, pp. 1–199. Available online: http://pbapgo.meioambiente.go.gov.br/wp-content/uploads/2020/12/RT-04-Plano-de-Acoes-UPGRH-Meia-Ponte-V01.pdf (accessed on 9 February 2023).
- Veiga, A.M.; Santos, C.C.P.; Cardoso, M.R.D.; Lino, N.C. Caracterização hidromorfológica da bacia do rio Meia Ponte. Caminhoas Geogr. 2013, 14, 126–138. [Google Scholar]
- CONAMA. Brasil Resolução CONAMA N° 357, de 17 de Março de 2005; Retificada; CONAMA: Brasilia, Brazil, 2005. Available online: https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Resolucao/2005/res_conama_357_2005_classificacao_corpos_agua_rtfcda_altrd_res_393_2007_397_2008_410_2009_430_2011.pdf (accessed on 9 February 2023).
- Bailão, E.F.L.C.; Zago, L.D.M.S.; Silva, N.C.; Machado, K.B.; D’Abadia, P.L.; de Oliveira, P.H.F.; Nabout, J.C.; de Almeida, L.M. Urban occupation increases water toxicity of an important river in central Brazil. Fronteiras 2020, 9, 73–86. [Google Scholar] [CrossRef]
- Cetesb Guia Nacional de Coleta e Preservação de Amostras. Água, Sedimento, Comunidades Aquáticas e Efluentes Líquidos; Copmhania Ambiental do Estado do São Paulo: Sao Paulo, Brazil, 2011; p. 326. Available online: https://cetesb.sp.gov.br/wp-content/uploads/2021/10/Guia-nacional-de-coleta-e-preservacao-de-amostras-2012.pdf (accessed on 9 February 2023).
- American Public Health Association (APHA). Standard Methodds for The Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2018; Volume 23, ISBN 9788578110796. Available online: https://www.wef.org/resources/publications/books/StandardMethods/ (accessed on 9 February 2023).
- Associação Brasileira de Normas Técnicas (ABNT). Água—Determinação de Oxigênio Consumido—Método do Permanganato de Potássio; SET 1989; Associação Brasileira de Normas Técnicas (ABNT): Sao Paulo, Brazil, 1989; Available online: https://www.target.com.br/produtos/normas-tecnicas/39198/nbr10739-agua-determinacao-de-oxigenio-consumido-metodo-do-permanganato-de-potassio-metodo-de-ensaio (accessed on 9 February 2023).
- Shariq, M.; Singh, S.; Farooq, U.; Dhariyal, K.K.; Singh, K.; Kaur, N. Presumptive Coliform Count in Water Sample Collected from Different Sites of. Int. J. Sci. Study 2016, 3, 12. [Google Scholar] [CrossRef]
- Da Cunha, F.V.; Batista, S.B. Análise fenotípica e genotípica de bactérias heterotróficas e fixadoras de nitrogênio em sedimento na bacia do Rio Cuiabá-MT. Rev. Ambient. Agua. 2014, 9, 68–80. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Módulo 6: Detecção e identificação e bactérias de importância médica. In Manual de Microbiologia Clínica Para o Controle Assistência à Saúde; Agência Nacional de Vigilância Sanitária: Brasilia, Brazil, 2013; Volume 9. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/publicacoes/modulo-10_manual-de-microbiologia.pdf (accessed on 9 February 2023).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 29th informational supplement; CLSI document M100-S29; 29th suppl.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; ISBN 978-1-68440-034. Available online: https://clsi.org/media/2663/m100ed29_sample.pdf (accessed on 9 February 2023).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Santos, A.L.; Dos Santos, A.P.; Ito, C.R.M.; de Queiroz, P.H.P.; de Almeida, J.A.; Júnior, M.A.B.D.C.; de Oliveira, C.Z.; Avelino, M.A.G.; Wastowski, I.J.; Gomes, G.P.L.A.; et al. Profile of enterobacteria resistant to beta-lactams. Antibiotics 2020, 9, 410. [Google Scholar] [CrossRef]
- Knapp, C.W.; Zhang, W.; Sturm, B.S.M.; Graham, D.W. Differential fate of erythromycin and beta-lactam resistance genes from swine lagoon waste under different aquatic conditions. Environ. Pollut. 2010, 158, 1506–1512. [Google Scholar] [CrossRef]
- Robicsek, A.; Strahilevitz, J.; Sahm, D.F.; Jacoby, G.A.; Hooper, D.C. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 2006, 50, 2872–2874. [Google Scholar] [CrossRef]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef]
- Waldron, L.S.; Gillings, M.R. Screening foodstuffs for class 1 integrons and gene cassettes. J. Vis. Exp. 2015, 2015, e52889. [Google Scholar] [CrossRef]
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes 2001, 15, 209–215. [Google Scholar] [CrossRef]
- Heuer, H.; Smalla, K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 2007, 9, 657–666. [Google Scholar] [CrossRef]
- Lu, Z.; Na, G.; Gao, H.; Wang, L.; Bao, C.; Yao, Z. Fate of sulfonamide resistance genes in estuary environment and effect of anthropogenic activities. Sci. Total Environ. 2015, 527–528, 429–438. [Google Scholar] [CrossRef]
- Li, J.; Shao, B.; Shen, J.; Wang, S.; Wu, Y. Occurrence of chloramphenicol-resistance genes as environmental pollutants from swine feedlots. Environ. Sci. Technol. 2013, 47, 2892–2897. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 304: Cluster analysis. Singapore Med. J. 2003, 44, 614–619. [Google Scholar]
- Sória, M.; Tavares, V.E.Q.; Pinto, M.A.B.; Stumpf, L.; Zarnott5, D.; Bubolz, J.; Nörenberg, B.G. Evaluation of physicochemical water parameters in watersheds of southern southern Brazil. Rev. Ambient. Agua 2020, 15, e2596. [Google Scholar] [CrossRef]
- Mustapha, A.; Aris, A.Z.; Juahir, H.; Ramli, M.F.; Kura, N.U. River water quality assessment using environmentric techniques: Case study of Jakara River Basin. Environ. Sci. Pollut. Res. 2013, 20, 5630–5644. [Google Scholar] [CrossRef]
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Wu, H.; Yang, W.; Yao, R.; Zhao, Y.; Zhao, Y.; Zhang, Y.; Yuan, Q.; Lin, A. Evaluating surface water quality using water quality index in Beiyun River, China. Environ. Sci. Pollut. Res. 2020, 27, 35449–35458. [Google Scholar] [CrossRef]
- Ahmed, A.A.M. Prediction of dissolved oxygen in Surma River by biochemical oxygen demand and chemical oxygen demand using the artificial neural networks (ANNs). J. King Saud Univ. Eng. Sci. 2017, 29, 151–158. [Google Scholar] [CrossRef]
- Bayram, A.; Uzlu, E.; Kankal, M.; Dede, T. Modeling stream dissolved oxygen concentration using teaching–learning based optimization algorithm. Environ. Earth Sci. 2015, 73, 6565–6576. [Google Scholar] [CrossRef]
- Harvey, R.; Lye, L.; Khan, A.; Paterson, R. The influence of air temperature on water temperature and the concentration of dissolved oxygen in Newfoundland Rivers. Can. Water Resour. J. 2011, 36, 171–192. [Google Scholar] [CrossRef]
- CONAMA. Brasil Resolução CONAMA no 274, de 29 de Novembro de 2000; CONAMA: Brasilia, Brazil, 2000. Available online: http://pnqa.ana.gov.br/Publicacao/Resolu%C3%A7%C3%A3o_Conama_274_Balneabilidade.pdf (accessed on 9 February 2023).
- Garbossa, L.H.P.; Souza, R.V.; Campos, C.J.A.; Vanz, A.; Vianna, L.F.N.; Rupp, G.S. Thermotolerant coliform loadings to coastal areas of Santa Catarina (Brazil) evidence the effect of growing urbanisation and insufficient provision of sewerage infrastructure. Environ. Monit. Assess. 2017, 189, 27. [Google Scholar] [CrossRef]
- Poma, V.; Mamani, N.; Iñiguez, V. Impact of urban contamination of the La Paz River basin on thermotolerant coliform density and occurrence of multiple antibiotic resistant enteric pathogens in river water, irrigated soil and fresh vegetables. Springerplus 2016, 5, 499. [Google Scholar] [CrossRef]
- Marques, L.O.D.A.; Taffarello, D.; Calijuri, M.D.C.; Mendiondo, E.M.; Ferreira, M.D.S.; Cunha, D.G.F. Phosphorus and thermotolerant coliforms’ loads in brazilian watersheds with limited data: Considerations on the integrated analysis of water quality and quantity. Rev. Bras. Recur. Hidricos 2019, 24, 1–13. [Google Scholar] [CrossRef]
- Da Cunha, D.F.; da Costa, N.M.; Barreira, C.C.M.A. Integração E Cooperação Territorial Na Região Metropolitana De Goiânia / Territorial Integration and Cooperation in the Goiania Metropolitan Region. Geo UERJ 2017, 30, 76–98. [Google Scholar] [CrossRef]
- Sylvestre, É.; Burnet, J.B.; Smeets, P.; Medema, G.; Prévost, M.; Dorner, S. Can routine monitoring of E. coli fully account for peak event concentrations at drinking water intakes in agricultural and urban rivers? Water Res. 2020, 170, 115369. [Google Scholar] [CrossRef]
- Mena-Rivera, L.; Vásquez-Bolaños, O.; Gómez-Castro, C.; Fonseca-Sánchez, A.; Rodríguez-Rodríguez, A.; Sánchez-Gutiérrez, R. Ecosystemic assessment of surface water quality in the Virilla River: Towards sanitation processes in Costa Rica. Water 2018, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, A.J.F.C.; Hollnagel, H.C.; Lima Mesquita, H.D.S.; Fontes, R.F.C. Physical, chemical and microbiological characterization of the intertidal sediments of Pereque Beach, Guarujá (SP), Brazil. Mar. Pollut. Bull. 2007, 54, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Lee, H.; Kim, Y. Relationship between Coliform bacteria and water quality factors at weir stations in the Nakdong River, South Korea. Water 2019, 11, 1171. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Chowdhary, P.; Singh, A.; Chaurasia, D.; Pandey, A.; Chandra, R.; Gupta, P. Dissemination of antibiotic resistance genes, mobile genetic elements, and efflux genes in anthropogenically impacted riverine environments. Chemosphere 2021, 273, 129693. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.R.; Gomes, R.P.; Rodrigues, A.B.; Ferreira, L.M.; Gama, A.R.; Vieira, J.D.G.; Fernandes, M.R.; Carneiro, L.C. Identification and resistance profile of gram positive bacteria from aquatic environment. Res. Soc. Dev. 2021, 10, e226101321182. [Google Scholar] [CrossRef]
- Freitas, D.Y.; Araújo, S.; Folador, A.R.C.; Ramos, R.T.J.; Azevedo, J.S.N.; Tacão, M.; Silva, A.; Henriques, I.; Baraúna, R.A. Extended spectrum beta-lactamase-producing gram-negative bacteria recovered from an amazonian lake near the city of Belém, Brazil. Front. Microbiol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Rebello, R.C.D.L.; Regua-Mangia, A.H. Potential enterovirulence and antimicrobial resistance in Escherichia coli isolates from aquatic environments in Rio de Janeiro, Brazil. Sci. Total Environ. 2014, 490, 19–27. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.; Chowdhary, P.; Pandey, A.; Gupta, P. Occurrence of emerging sulfonamide resistance (sul1 and sul2) associated with mobile integrons-integrase (intI1 and intI2) in riverine systems. Sci. Total Environ. 2021, 751, 142217. [Google Scholar] [CrossRef]
- Heck, K.; de Marco, É.G.; Duarte, M.W.; Salamoni, S.P.; van der Sand, S. Pattern of multiresistant to antimicrobials and heavy metal tolerance in bacteria isolated from sewage sludge samples from a composting process at a recycling plant in southern Brazil. Environ. Monit. Assess. 2015, 187, 328. [Google Scholar] [CrossRef]
- Lee, J.; Beck, K.; Bürgmann, H. Wastewater bypass is a major temporary point-source of antibiotic resistance genes and multi-resistance risk factors in a Swiss river. Water Res. 2021, 208, 117827. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, D.; He, S.; Ye, H.; Zhang, L.; Wen, Y.; Zhang, W.; Shu, L.; Chen, S. Prevalence of antibiotic-resistant Escherichia coli in drinking water sources in Hangzhou City. Front. Microbiol. 2017, 8, 1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, G.; Hilgert, S.; Fuchs, S.; Azevedo, J.C.R. Emerging contaminants and antibiotic resistance in the different environmental matrices of Latin America. Environ. Pollut. 2019, 255, 113140. [Google Scholar] [CrossRef] [PubMed]
- Arsand, J.B.; Hoff, R.B.; Jank, L.; Bussamara, R.; Dallegrave, A.; Bento, F.M.; Kmetzsch, L.; Falção, D.A.; do Carmo Ruaro Peralba, M.; de Araujo Gomes, A.; et al. Presence of antibiotic resistance genes and its association with antibiotic occurrence in Dilúvio River in southern Brazil. Sci. Total Environ. 2020, 738, 139781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Z.; Wei, Y.; Chen, T.; Feng, W.; Chen, H. High-throughput profiling of seasonal variations of antibiotic resistance gene transport in a peri-urban river. Environ. Int. 2018, 114, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, C.; Luo, Y.; Lv, J.; Zhang, Y.; Lin, H.; Wang, L.; Xu, J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016, 213, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.; Roberts, D.J.; Du, H.N.; Yu, X.F.; Zhu, N.Z.; Meng, X.Z. Persistence of antibiotic resistance genes from river water to tap water in the Yangtze River Delta. Sci. Total Environ. 2020, 742, 140592. [Google Scholar] [CrossRef]
- Olsen, R.L.; Chappell, R.W.; Loftis, J.C. Water quality sample collection, data treatment and results presentation for principal components analysis—Literature review and Illinois River watershed case study. Water Res. 2012, 46, 3110–3122. [Google Scholar] [CrossRef]
- Menció, A.; Mas-Pla, J. Assessment by multivariate analysis of groundwater-surface water interactions in urbanized Mediterranean streams. J. Hydrol. 2008, 352, 355–366. [Google Scholar] [CrossRef]


| Parameter | Chloride (mg/L) | Electrical Conductivity (µS/cm) | Apparent Color (mg Pt/L) | Hardness (mg/L) | Nitrate (mg/L) | Oxygen Dissolved (mg/L) | pH | Water Temperature (°C) | Turbidity (NTU) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rainy period | MP01 | 1.77 | 180.00 | 150.00 * | 60.00 | 1.72 | 6.88 | 7.37 | 22.50 | 218.00 * |
| MP02 | 1.76 | 202.00 | 251.00 * | 106.71 | 1.34 | 5.79 | 7.27 | 23.00 | 97.70 | |
| MP03 | 1.40 | 179.70 | 321.00 * | 38.70 | 1.38 | 5.89 | 7.22 | 25.00 | 137.00 * | |
| MP04 | 0.53 | 131.50 | 314.00 * | 25.30 | 1.40 | 5.86 | 7.42 | 25.00 | 100.00 * | |
| average ± SD | 1.365 ± 0.58 | 173.30 ± 29.76 | 259.00 * ± 79.19 | 57.68 ± 35.67 | 1.46 ± 0.018 | 6.10 ± 0.52 | 7.32 ± 0.09 | 23.87 ± 1.31 | 138.18 * ± 56.18 | |
| Dry period | MP01 | 2.51 | 383.00 | 104.00 * | 14.67 | 0.30 | 6.59 | 7.30 | 23.50 | 10.30 |
| MP02 | 2.82 | 431.00 | 123.00 * | 17.34 | 0.00 | 10.12 | 7.11 | 24.50 | 16.30 | |
| MP03 | 1.78 | 291.00 | 66.90 | 0.00 | 0.20 | 7.02 | 7.04 | 26.00 | 13.00 | |
| MP04 | 1.09 | 187.00 | 53.20 | 0.00 | 1.30 | 0.73 * | 7.60 | 26.00 | 5.81 | |
| average ± SD | 2.05 ±0.77 | 323.00 ± 107.68 | 86.78 ± 32.31 | 8.00 ± 9.30 | 0.45 ± 0.58 | 6.12 ± 3.92 | 7.26 ± 0.25 | 25.00 ± 1.22 | 11.35 ± 4.44 | |
| Maximum recommended values for class 2 of CONAMA Regulation No. 357 * | 250.00 | NR | 75.00 | NR | 10.00 | Not inferior 5.00 | 6.0–9.0 | NR | 100.00 | |
| media ± DP total | 1.71 ± 0.73 | 248.15 ± 108.40 | 172.89 ± 107.75 | 32.84 ± 35.88 | 0.95 ± 0.67 | 6.11 ± 2.59 | 7.29 ± 0.18 | 24.44 ± 1.32 | 74.76 ± 77.18 | |
| Sample | Period | Sample Point | Culture Media | |||
|---|---|---|---|---|---|---|
| R2A | MacConkey | Salt Manitol | Violet Red | |||
| Water (CFUs/mL) | Rainy | MP01 | >2500 | 455 | 105 | 1360 |
| MP02 | >2500 | >2500 | 240 | >2500 | ||
| MP03 | >2500 | >2500 | 395 | 940 | ||
| MP04 | 1565 | 555 | 135 | 220 | ||
| Sediment (CFUs/g) | Rainy | MP01 | >25,000 | >25,000 | 15,250 | 10,800 |
| MP02 | >25,000 | >25,000 | >25,000 | >25,000 | ||
| MP03 | >25,000 | >25,000 | >25,000 | >25,000 | ||
| MP04 | >25,000 | >25,000 | >25,000 | >25,000 | ||
| Water (CFUs/mL) | Dry | MP01 | >2500 | 1005 | 35 | 95 |
| MP02 | >2500 | >2500 | 2390 | >2500 | ||
| MP03 | >2500 | >2500 | 2325 | >2500 | ||
| MP04 | >2500 | 1540 | 5 | 45 | ||
| Sediment (CFUs/g) | Dry | MP01 | >25,000 | 6100 | 6850 | 1000 |
| MP02 | >25,000 | >25,000 | 11,850 | >25,000 | ||
| MP03 | >25,000 | >25,000 | 1150 | 11,400 | ||
| MP04 | >25,000 | >25,000 | >25,000 | 5900 | ||
| Antibiotic | Rainy Period | Dry Period | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | R | I | S | |
| AMI | 13.04% (6/46) | 6.52% (3/46) | 80.43% (37/46) | 8.33% (4/48) | 6.25% (3/48) | 85.42% (41/48) | 10.64% (10/94) | 6.38% (6/94) | 82.98% (78/94) |
| AMC | 91.30% (42/46) | 0% (0/46) | 8.7% (4/46) | 84.21% (32/38) | 7.89% (3/38) | 7.89% (3/38) | 88.10% (74/84) | 3.57% (3/84) | 8.33% (7/84) |
| AMP | 61.84% (47/76) | 1.32% (1/76) | 36.84% (28/76) | 70.83% (51/72) | 1.39% (1/72) | 27.78% (20/72) | 66.22% (98/148) | 1.35% (2/148) | 32.43% (48/148) |
| ATM | 36.96% (17/46) | 2.17% (1/46) | 60.87% (28/46) | 29.55% (13/44) | 0% (0/44) | 70.45% (31/44) | 33.33% (30/90) | 1.11% (1/90) | 65.56% (59/90) |
| CFZ | 95.65% (44/46) | 0% (0/46) | 4.35% (2/46) | 78.95% (30/38) | 0% (0/38) | 21.05% (8/38) | 88.10% (74/84) | 0% (0/84) | 11.90% (10/84) |
| CPM | 32.61% (15/46) | 0% (0/46) | 67.39% (31/46) | 2.08% (1/48) | 16.67% (8/48) | 81.25% (39/48) | 17.02% (16/94) | 8.51% (8/94) | 74.47% (70/94) |
| CFO | 57.89% (44/76) | 2.63% (2/76) | 39.47% (30/76) | 60.53% (46/76) | 1.32% (1/76) | 38.16% (29/76) | 59.21% (90/152) | 1.97% (3/152) | 38.82% (59/152) |
| CAZ | 30.43% (14/46) | 4.35% (2/46) | 65.22% (30/46) | 6.25% (3/48) | 22.92% (11/48) | 70.83% (34/48) | 18.09% (17/94) | 13.83% (13/94) | 68.09% (64/94) |
| CRO | 26.09% (12/46) | 6.52% (3/46) | 67.39% (31/46) | 25.00% (12/48) | 12.5% (6/48) | 62.50% (30/48) | 25.53% (24/94) | 9.57% (9/94) | 64.89% (61/94) |
| CIP | 35.35% (35/99) | 5.05% (5/99) | 59.60% (59/99) | 21.36% (22/103) | 9.71% (10/103) | 68.93% (71/103) | 28.22% (57/202) | 7.43% (15/202) | 64.36% (130/202) |
| CLO | 41.41% (41/99) | 8.08% (8/99) | 50.51% (50/99) | 39.39% (39/99) | 8.08% (8/99) | 52.53% (52/99) | 40.40% (80/198) | 8.08% (16/198) | 51.52% (102/198) |
| GEN | 14.14% (14/99) | 4.04% (4/99) | 81.82% (81/99) | 21.65% (21/97) | 2.06% (2/97) | 76.29% (74/97) | 17.86% (35/196) | 3.06% (6/196) | 79.08% (155/196) |
| MPM | 17.39% (8/46) | 2.17% (1/46) | 80.43% (37/46) | 14.58% (7/48) | 4.17% (2/48) | 81.25% (39/48) | 15.96% (15/94) | 3.19% (3/94) | 80.85% (76/94) |
| SUT | 58.59% (58/99) | 1.01% (1/99) | 40.40% (40/99) | 40.21% (39/97) | 4.12% (4/97) | 55.67% (54/97) | 49.49% (97/196) | 2.55% (5/196) | 47.96% (94/196) |
| TET | 44.44% (44/99) | 5.05% (5/99) | 50.51% (50/99) | 31.07% (32/103) | 9.71% (10/103) | 59.22% (61/103) | 37.62% (76/202) | 7.43% (15/202) | 54.95% (111/202) |
| AZI | 43.40% (23/53) | 1.89% (1/53) | 54.72% (29/53) | 59.18% (29/49) | 4.08% (2/49) | 36.73% (18/49) | 50.98% (52/102) | 2.94% (3/102) | 46.08% (47/102) |
| CLI | 90.57% (48/53) | 0% (0/53) | 9.43% (5/53) | 87.27% (48/55) | 0% (0/55) | 12.73% (7/55) | 88.89% (96/108) | 0% (0/108) | 11.11% (12/108) |
| ERI | 49.06% (26/53) | 0% (0/53) | 50.94% (27/53) | 54.55% (30/55) | 5.45% (3/55) | 40.00% (22/55) | 51.85% (56/108) | 2.78% (3/108) | 45.37% (49/108) |
| OXA | 52.83% (28/53) | 0% (0/53) | 47.17% (25/53) | 69.39% (34/49) | 0% (0/49) | 30.61% (15/49) | 60.78% (62/102) | 0% (0/102) | 39.22% (40/102) |
| LNZ | 49.06% (26/53) | 0% (0/53) | 50.94% (27/53) | 56.36% (31/55) | 0% (0/55) | 43.64% (24/55) | 52.78% (57/108) | 0% (0/108) | 47.22% (51/108) |
| PEN | 49.06% (26/53) | 0% (0/53) | 50.94% (27/53) | 67.27% (37/55) | 0% (0/55) | 32.73% (18/55) | 58.33% (63/108) | 0% (0/108) | 41.67% (45/108) |
| RIF | 47.17% (25/53) | 0% (0/53) | 52.83% (28/53) | 58.18% (32/55) | 0% (0/55) | 41.82% (23/55) | 52.78% (57/108) | 0% (0/108) | 47.22% (51/108) |
| VAN | 6.67% (2/30) | 0% (0/30) | 93.33% (28/30) | 47.06% (16/34) | 0% (0/34) | 52.94% (18/34) | 28.13% (18/64) | 0% (0/64) | 71.88% (46/64) |
| Antibiotic Class | Target Gene | Water | Sediment | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainy Period | Dry Period | Rainy Period | Dry Period | ||||||||||||||||
| MP01 | MP02 | MP03 | MP04 | MP01 | MP02 | MP03 | MP04 | MP01 | MP02 | MP03 | MP04 | MP01 | MP02 | MP03 | MP04 | p [% (Amount)] | a [% (Amount)] | ||
| β-lactams | blaKPC | p | p | p | p | a | a | a | p | p | p | p | p | p | p | p | p | 81.25% (13/16) | 18.75% (3/16) |
| blaCTX-M | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | 0% (0/16) | 100% (16/16) | |
| blaSHV | a | p | p | a | p | a | a | a | a | a | a | a | a | p | p | p | 37.50% (6/16) | 62.50% (10/16) | |
| blaOXA | p | p | p | p | a | a | a | p | p | p | p | p | p | p | p | a | 75.00% (12/16) | 25.00% (4/16) | |
| blaCMY | p | p | p | p | a | a | a | p | p | p | p | p | p | p | p | p | 81.25% (13/16) | 18.75% (3/16) | |
| blaTEM | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | 0% (0/16) | 100% (16/16) | |
| Quinolones | qnrA | p | p | p | p | p | a | a | p | p | p | p | p | p | p | p | p | 87.50% (14/16) | 12.50% (3/16) |
| qnrB | p | p | p | p | p | p | p | p | p | p | p | a | p | p | p | p | 93.75% (15/16) | 6.25% (1/16) | |
| qnrS | p | p | p | p | a | a | a | a | p | p | p | p | p | p | p | p | 75.00% (12/16) | 25.00% (4/16) | |
| Fluoroquinolone | aac(‘6)-ib | p | p | p | p | a | p | p | p | p | p | p | p | p | p | p | p | 93.75% (15/16) | 6.25% (1/16) |
| Sulfonamides | sul1 | p | p | p | p | a | p | p | p | p | p | p | p | p | p | p | p | 93.75% (15/16) | 6.25% (1/16) |
| sul2 | p | p | p | p | p | p | p | p | p | p | p | p | p | p | p | p | 100% (16/16) | 0% (0/16) | |
| sul3 | p | p | p | p | p | a | a | p | p | p | p | p | p | p | p | a | 81.25% (13/16) | 18.75% (3/16) | |
| Tetracyclines | tet(A) | p | p | p | p | p | p | a | a | p | p | p | p | p | p | p | p | 87.50% (14/16) | 12.50% (2/16) |
| tet(B) | p | p | p | p | a | a | a | a | p | p | p | p | p | p | p | p | 75.00% (12/16) | 25.00% (4/16) | |
| tet(M) | p | p | p | p | p | a | a | a | p | p | p | p | p | p | p | p | 81.25% (13/16) | 18.75% (3/16) | |
| tet(O) | p | p | p | p | a | p | p | a | p | p | p | p | p | p | p | a | 81.25% (13/16) | 18.75% (3/16) | |
| Macrolides | ermB | p | p | p | p | a | a | a | p | p | p | p | p | p | p | p | p | 81.25% (13/16) | 18.75% (3/16) |
| ermC | p | p | p | p | p | p | p | p | p | p | p | p | p | p | p | p | 100% (16/16) | 0% (0/16) | |
| Integron integrase class 1 | IntI1 | p | a | p | p | a | a | a | a | p | p | p | p | p | p | p | p | 68.75% (11/16) | 31.25% (5/16) |
| Amphenicols | floR | p | p | p | p | a | p | p | a | p | p | p | p | p | p | p | p | 87.50% (14/16) | 1.50% (3/16) |
| cfr | p | p | p | p | a | p | a | a | p | p | p | p | p | p | p | p | 81.25% (13/16) | 18.755% (3/16) | |
| cmlA | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | 0% (0/16) | 100% (16/16) | |
| fexB | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | 0% (0/16) | 100% (16/16) | |
| Total | p [% (amount)] | 79.17% (19/24) | 79.17% (19/24) | 83.33% (20/24) | 79.17% (19/24) | 33.33% (8/24) | 37.50% (9/24) | 29.17% (7/24) | 45.83% (11/24) | 79.17% (19/24) | 79.17% (19/24) | 79.17% (19/24) | 75.00% (18/24) | 79.17% (19/24) | 83.33% (20/24) | 83.33% (20/24) | 70.83% (17/24) | 68.49% (263/384) | - |
| a [% (amount)] | 20.83% (5/24) | 20.83% (5/24) | 16.67% (4/24) | 20.83% (5/24) | 66.67% (16/24) | 62.50% (15/24) | 70.83% (17/24) | 54.17% (13/24) | 20.83% (5/24) | 20.83% (5/24) | 20.83% (5/24) | 25.00% (6/24) | 20.83% (5/24) | 16.67% (4/24) | 16.67% (4/24) | 29.17% (7/24) | - | 31.51% (121/384) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, R.P.; Oliveira, T.R.; Rodrigues, A.B.; Ferreira, L.M.; Vieira, J.D.G.; Carneiro, L.C. Occurrence of Antibiotic Resistance Genes, Antibiotics-Resistant and Multi-Resistant Bacteria and Their Correlations in One River in Central-Western Brazil. Water 2023, 15, 747. https://doi.org/10.3390/w15040747
Gomes RP, Oliveira TR, Rodrigues AB, Ferreira LM, Vieira JDG, Carneiro LC. Occurrence of Antibiotic Resistance Genes, Antibiotics-Resistant and Multi-Resistant Bacteria and Their Correlations in One River in Central-Western Brazil. Water. 2023; 15(4):747. https://doi.org/10.3390/w15040747
Chicago/Turabian StyleGomes, Raylane Pereira, Thais Reis Oliveira, Ariadne Bernardes Rodrigues, Leandro Martins Ferreira, José Daniel Gonçalves Vieira, and Lilian Carla Carneiro. 2023. "Occurrence of Antibiotic Resistance Genes, Antibiotics-Resistant and Multi-Resistant Bacteria and Their Correlations in One River in Central-Western Brazil" Water 15, no. 4: 747. https://doi.org/10.3390/w15040747
APA StyleGomes, R. P., Oliveira, T. R., Rodrigues, A. B., Ferreira, L. M., Vieira, J. D. G., & Carneiro, L. C. (2023). Occurrence of Antibiotic Resistance Genes, Antibiotics-Resistant and Multi-Resistant Bacteria and Their Correlations in One River in Central-Western Brazil. Water, 15(4), 747. https://doi.org/10.3390/w15040747






