Potential Analysis of Atmospheric Water Harvesting Technologies from the Perspective of “Trading-in Energy for Water”
Abstract
1. Introduction
2. Atmospheric Water Harvesting by Condensation
2.1. Fundamentals of Technology & Equipment
2.2. Theoretical Energy Consumption
2.2.1. Total Energy Consumption Per Unit Water Uptake
2.2.2. Proportion of Energy Consumption in Improving Humidity
2.2.3. Energy Consumption of Single-Stage and Multi-Stage Condensation
2.3. Energy Consumption of Existing Technologies
2.3.1. Single-Stage Condensation
2.3.2. Multi-Stage Condensation
2.4. Potential Analysis of Water Uptake by Condensation
2.4.1. Influence Factors on the Harvesting Efficiency of Condensation
2.4.2. Application Prospect
3. Atmospheric Water Harvesting by Adsorption and Desorption
3.1. Fundamentals of Technology & Equipment
3.2. Theoretical Energy Consumption
3.3. Energy Consumption of Existing Technologies
3.4. Potential Analysis of Atmospheric Water Harvesting by Adsorption and Desorption
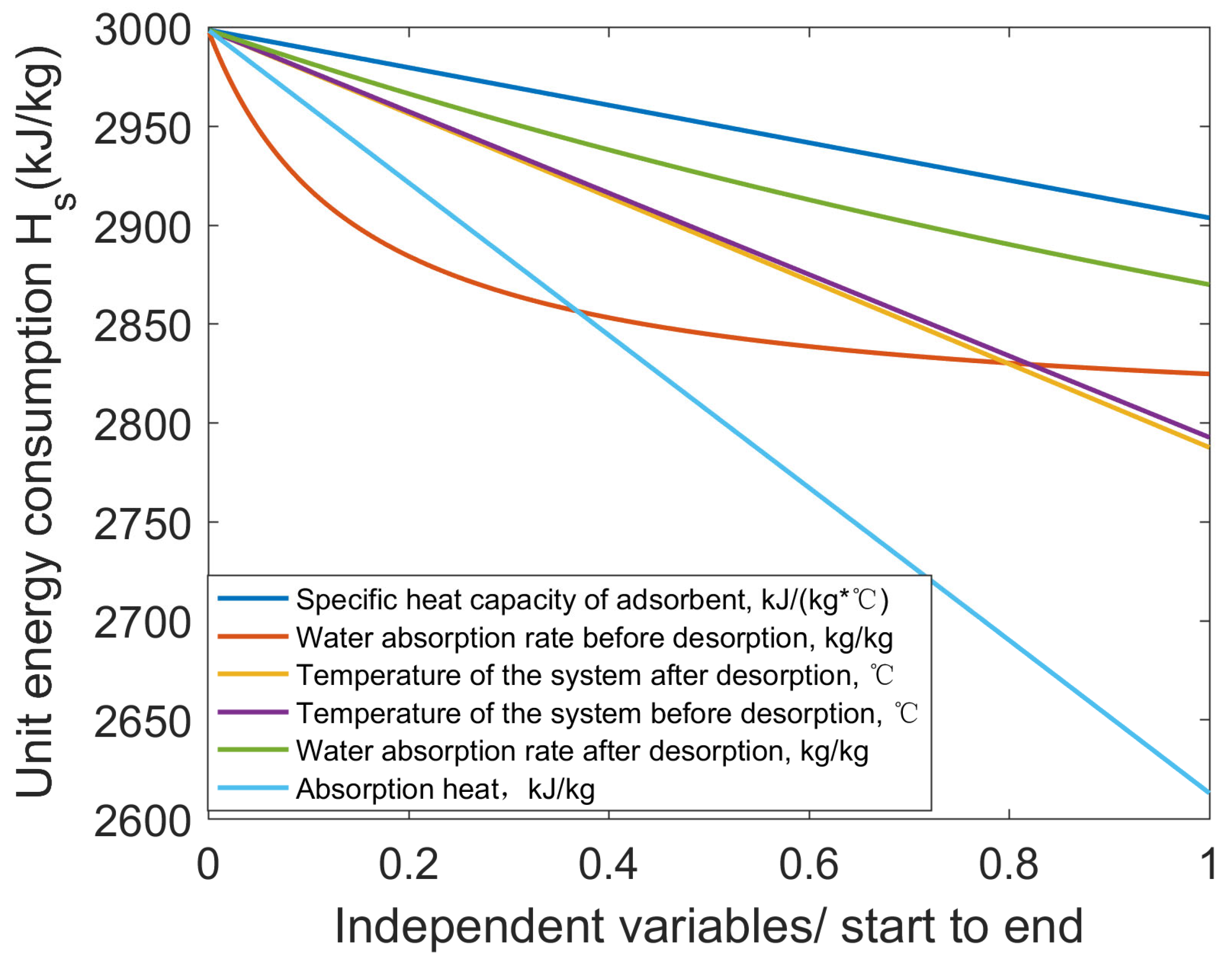
3.4.1. Influence Factors on the Harvesting Efficiency of Condensation
3.4.2. Application Prospect
4. Active Collection of Fog and Dew
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, A.Y.; Mekonnen, M.M.; Chapagain, A.K.; Mathews, R.E.; Richter, B.D. Global monthly water scarcity: Blue water footprints versus blue water availability. PLoS ONE 2012, 7, e32688. [Google Scholar] [CrossRef] [PubMed]
- Huan, S.; Liu, X. Development status of global seawater desalination industry and dynamically comparative analysis of its production cost. IOP Conf. Ser. Earth Environ. Sci. 2021, 772, 12085. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Delene, D.; Shamrukh, M. Investing in Rainfall Enhancement: An Innovative Plan for Arid Regions. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha, Qatar, 22–23 March 2016; Volume 2016. Issue 1. [Google Scholar]
- Junde, W.; Yanzhao, J.; Xiaojuan, T. Study on Analysis of Risk for Rainwater Collection and Utilization for Rural Safe Drinking Water Project. IOP Conf. Ser. Mater. Sci. Eng. 2020, 780, 72040. [Google Scholar] [CrossRef]
- Khanal, G.; Thapa, A.; Devkota, N.; Paudel, U.R. A review on harvesting and harnessing rainwater: An alternative strategy to cope with drinking water scarcity. Water Supply 2020, 20, 2951–2963. [Google Scholar] [CrossRef]
- Bass, D.A.; McFadden, B.R.; Costanigro, M.; Messer, K.D. Implicit and Explicit Biases for Recycled Water and Tap Water. Water Resour. Res. 2022, 58, e2021WR030712. [Google Scholar] [CrossRef]
- Yang, G.; Gong, M.; Zheng, X.; Lin, L.; Fan, J.; Liu, F.; Meng, J. A review of microbial corrosion in reclaimed water pipelines: Challenges and mitigation strategies. Water Pract. Technol. 2022, 17, 731–748. [Google Scholar] [CrossRef]
- Person, M.; Sazeed, N. Continental Brackish Groundwater Resources. In Unconventional Water Resources; Springer: Cham, Switzerland, 2022; pp. 111–128. [Google Scholar]
- Zhang, D.; Xie, X.; Wang, T.; Wang, B.; Pei, S. Research on Water Resources Allocation System Based on Rational Utilization of Brackish Water. Water 2022, 14, 948. [Google Scholar] [CrossRef]
- Liu, C.; Liang, L.; Wang, L.; Zheng, S. Allocation and Utilization of Coal Mine Water for Ecological Protection of Lakes in Semi-Arid Area of China. Sustainability 2022, 14, 9042. [Google Scholar] [CrossRef]
- Zhang, N.; He, H.; Guo, X.; Li, S.; Ni, S.; Peng, Y. Research on the development and utilization mode of mine water resources. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 12004. [Google Scholar] [CrossRef]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, R.J.; Tuinenburg, O.A. The residence time of water in the atmosphere revisited. Hydrol. Earth Syst. Sci. 2017, 21, 779–790. [Google Scholar] [CrossRef]
- Kaseke, K.F.; Wang, L. Fog and Dew as Potable Water Resources: Maximizing Harvesting Potential and Water Quality Concerns. Geohealth 2018, 2, 327–332. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). International Meteorological Vocabulary (WMO-No. 182); Secretariat of the World Meteorological Organization: Geneva, Switzerland, 1992; pp. 182, 784. [Google Scholar]
- Wu, H.T. A simple algorithm for calculating water content and enthalpy in air. Chem. Eng. Des. 1990, 2, 28–30. (In Chinese) [Google Scholar]
- Maleki, M.; Eslamian, S.; Hamouda, B. Principles and Applications of Atmospheric Water Harvesting; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 243–259. [Google Scholar]
- Li, Q.; Hao, X.Y. A review on extracting water from air. Shanxi Archit. 2016, 42, 124–126. (In Chinese) [Google Scholar] [CrossRef]
- Kim, H.; Rao, S.R.; Kapustin, E.A.; Zhao, L.; Yang, S.; Yaghi, O.M.; Wang, E.N. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, R.; Li, Y. Diversifying Water Sources with Atmospheric Water Harvesting to Enhance Water Supply Resilience. Sustainability 2022, 14, 7783. [Google Scholar] [CrossRef]
- Humphrey, J.H.; Brown, J.; Cumming, O.; Evans, B.; Howard, G.; Kulabako, R.N.; Lamontagne, J.; Pickering, A.J.; Wang, E.N. The potential for atmospheric water harvesting to accelerate household access to safe water. Lancet Planet Health 2020, 4, e91–e92. [Google Scholar] [CrossRef]
- Rao, A.K.; Fix, A.J.; Yang, Y.C.; Warsinger, D.M. Thermodynamic limits of atmospheric water harvesting. Energy Environ. Sci. 2022, 15, 4025–4037. [Google Scholar] [CrossRef]
- Gido, B.; Friedler, E.; Broday, D.M. Assessment of atmospheric moisture harvesting by direct cooling. Atmos. Res. 2016, 182, 156–162. [Google Scholar] [CrossRef]
- Magnus Formula. Available online: https://baike.baidu.com/item/%E9%A9%AC%E6%A0%BC%E7%BA%B3%E6%96%AF%E5%85%AC%E5%BC%8F/144125?fr=aladdin (accessed on 19 December 2022). (In Chinese).
- Dew Point. Available online: https://www.chemeurope.com/en/encyclopedia/Dew_point.html (accessed on 19 December 2022).
- Jin, Y.; Soukane, S.; Ghaffour, N. Salt-solution-infused thin-film condenser for simultaneous anti-frost and solar-assisted atmospheric water harvesting. Cell Rep. Phys. Sci. 2021, 2, 100568. [Google Scholar] [CrossRef]
- Yuan, S. Research of Water Harvesting Performance of Water Harvesting Device Based on Condensation Surface with Microstructures. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2018. (In Chinese). [Google Scholar]
- Yu, P.K. Design and Optimization of Semiconductor Atmospheric Water Generator. Master’s Thesis, Hefei University of Technology, Hefei, China, 2020. (In Chinese). [Google Scholar]
- Lin, Y.C.; Pei, W.L.; Sun, R.X.; Gao, C.L.; Chen, J.P.; Zheng, Y.M. Droplet Condensation on Surfaces with Special Wettability. Chem. J. Chin. Univ. 2019, 40, 1236–1241. (In Chinese) [Google Scholar]
- Zamuruyev, K.O.; Bardaweel, H.K.; Carron, C.J.; Kenyon, N.J.; Brand, O.; Delplanque, J.-P.; Davis, C.E. Continuous Droplet Removal upon Dropwise Condensation of Humid Air on a Hydrophobic Micropatterned Surface. Langmuir 2014, 30, 10133–10142. [Google Scholar] [CrossRef]
- Hand, C.T.; Peuker, S. An experimental study of the influence of orientation on water condensation of a thermoelectric cooling heatsink. Heliyon 2019, 5, e02752. [Google Scholar] [CrossRef]
- Tsutomu, H.; Kotaro, T.; Kenji, T.; Yasunari, a.M. Development of Novel Water-extraction System with Thermoelectric Module Using Solar and Wind Power in Arid Land. J. Northeast. Agric. Univ. 2010, 17, 37–42. [Google Scholar]
- Tu, R.; Hwang, Y. Performance analyses of a new system for water harvesting from moist air that combines multi-stage desiccant wheels and vapor compression cycles. Energy Convers. Manag. 2019, 198, 111811. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.-Y.; Chung, T.-S. Enhanced membrane systems to harvest water and provide comfortable air via dehumidification & moisture condensation. Sep. Purif. Technol. 2019, 220, 136–144. [Google Scholar] [CrossRef]
- Eslami, M.; Tajeddini, F.; Etaati, N. Thermal analysis and optimization of a system for water harvesting from humid air using thermoelectric coolers. Energy Convers. Manag. 2018, 174, 417–429. [Google Scholar] [CrossRef]
- Liu, J.; Zang, R.; Zhao, D.; Ji, W.; Zhang, Z. Study on properties of water extraction from air system. Refrigeration 2015, 43, 71–75. [Google Scholar] [CrossRef]
- Peeters, R.; Vanderschaeghe, H.; Rongé, J.; Martens, J.A. Energy performance and climate dependency of technologies for fresh water production from atmospheric water vapour. Environ. Sci. Water Res. Technol. 2020, 6, 2016–2034. [Google Scholar] [CrossRef]
- Zhang, R.; Zang, R.; Liu, J. Study on properties of water extraction from cooled air system under all operating conditions. Refrigeration 2016, 44, 51–55. [Google Scholar] [CrossRef]
- Chaitanya, B.; Bahadur, V.; Thakur, A.D.; Raj, R. Biomass-gasification-based atmospheric water harvesting in India. Energy 2018, 165, 610–621. [Google Scholar] [CrossRef]
- Ozkan, O.; Wikramanayake, E.D.; Bahadur, V. Modeling humid air condensation in waste natural gas-powered atmospheric water harvesting systems. Appl. Therm. Eng. 2017, 118, 224–232. [Google Scholar] [CrossRef]
- Salehi, A.A.; Ghannadi-Maragheh, M.; Torab-Mostaedi, M.; Torkaman, R.; Asadollahzadeh, M. A review on the water-energy nexus for drinking water production from humid air. Renew. Sustain. Energy Rev. 2020, 120, 109627. [Google Scholar] [CrossRef]
- Wikramanayake, E.D.; Bahadur, V. Flared natural gas-based onsite atmospheric water harvesting (AWH) for oilfield operations. Environ. Res. Lett. 2016, 11, 34024. [Google Scholar] [CrossRef]
- Wikramanayake, E.D.; Ozkan, O.; Bahadur, V. Landfill gas-powered atmospheric water harvesting for oilfield operations in the United States. Energy 2017, 138, 647–658. [Google Scholar] [CrossRef]
- Assessment and Analysis of New Energy Electricity Consumption in China. Available online: http://www.chinapower.com.cn/zx/hyfx/20220315/138719.html (accessed on 19 December 2022). (In Chinese).
- Patel, J.; Patel, K.; Mudgal, A.; Panchal, H.; Sadasivuni, K.K. Experimental investigations of atmospheric water extraction device under different climatic conditions. Sustain. Energy Technol. Assess. 2020, 38, 100677. [Google Scholar] [CrossRef]
- Kim, H.; Rao, S.R.; LaPotin, A.; Lee, S.; Wang, E.N. Thermodynamic analysis and optimization of adsorption-based atmospheric water harvesting. Int. J. Heat Mass Transf. 2020, 161, 120253. [Google Scholar] [CrossRef]
- Siegel, N.; Conser, B. A Techno-Economic Analysis of Solar-Driven Atmospheric Water Harvesting. J. Energy Resour. Technol. 2020, 143, 90907. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wang, R.Z.; Xia, Z.Z. Continuous Cycle Unit for Extracting Water from Air. J. Chem. Ind. Eng. 2004, 55, 1002–1005. (In Chinese) [Google Scholar]
- Wu, Q.; Su, W.; Li, Q.; Tao, Y.; Li, H. Enabling Continuous and Improved Solar-Driven Atmospheric Water Harvesting with Ti(3)C(2)-Incorporated Metal-Organic Framework Monoliths. ACS Appl. Mater. Interfaces 2021, 13, 38906–38915. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, T.; Yan, T.; Wu, S.; Wu, M.; Chao, J.; Huo, X.; Wang, P.; Wang, R. Ultrahigh solar-driven atmospheric water production enabled by scalable rapid-cycling water harvester with vertically aligned nanocomposite sorbent. Energy Environ. Sci. 2021, 14, 5979–5994. [Google Scholar] [CrossRef]
- Qi, H.; Wei, T.; Zhao, W.; Zhu, B.; Liu, G.; Wang, P.; Lin, Z.; Wang, X.; Li, X.; Zhang, X.; et al. An Interfacial Solar-Driven Atmospheric Water Generator Based on a Liquid Sorbent with Simultaneous Adsorption-Desorption. Adv. Mater. 2019, 31, e1903378. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, Y.; Wu, M.; Hong, S.; Wang, P. Improving atmospheric water production yield: Enabling multiple water harvesting cycles with nano sorbent. Nano Energy 2020, 67, 104255. [Google Scholar] [CrossRef]
- Mulchandani, A.; Malinda, S.; Edberg, J.; Westerhoff, P. Sunlight-driven atmospheric water capture capacity is enhanced by nano-enabled photothermal desiccants. Environ. Sci. Nano 2020, 7, 2584–2594. [Google Scholar] [CrossRef]
- Terzis, A.; Ramachandran, A.; Wang, K.; Asheghi, M.; Goodson, K.E.; Santiago, J.G. High-Frequency Water Vapor Sorption Cycling Using Fluidization of Metal-Organic Frameworks. Cell Rep. Phys. Sci. 2020, 1, 100057. [Google Scholar] [CrossRef]
- Park, H.; Haechler, I.; Schnoering, G.; Ponte, M.D.; Schutzius, T.M.; Poulikakos, D. Enhanced Atmospheric Water Harvesting with Sunlight-Activated Sorption Ratcheting. ACS Appl. Mater. Interfaces 2022, 14, 2237–2245. [Google Scholar] [CrossRef]
- Hanikel, N.; Prevot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid Cycling and Exceptional Yield in a Metal-Organic Framework Water Harvester. ACS Cent Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef]
- Sun, J.; An, B.; Zhang, K.; Xu, M.; Wu, Z.; Ma, C.; Li, W.; Liu, S. Moisture-indicating cellulose aerogels for multiple atmospheric water harvesting cycles driven by solar energy. J. Mater. Chem. A 2021, 9, 24650–24660. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Xing, Z.; Liu, X.; Dai, Y.; Wang, R.; Ge, T. Viability of a practical multicyclic sorption-based water harvester with improved water yield. Water Res. 2021, 211, 118029. [Google Scholar] [CrossRef] [PubMed]
- Asim, N.; Badiei, M.; Alghoul, M.A.; Mohammad, M.; Samsudin, N.A.; Amin, N.; Sopian, K. Sorbent-based air water-harvesting systems: Progress, limitation, and consideration. Rev. Environ. Sci. Bio/Technol. 2020, 20, 257–279. [Google Scholar] [CrossRef]
- LaPotin, A.; Kim, H.; Rao, S.R.; Wang, E.N. Adsorption-Based Atmospheric Water Harvesting: Impact of Material and Component Properties on System-Level Performance. Acc. Chem. Res. 2019, 52, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Ge, T.S.; Dai, Y.J.; Wang, R.Z.; Xie, S.T. Status of Solar-driven Sorption-based Atmosphere Water Harvesting. Sol. Energy 2020, 1, 33–46. (In Chinese) [Google Scholar]
- Zhao, H.Z.; Lei, M.; Huang, T.H.; Liu, T.; Zhang, M. A review on the development of water extraction from atmospheric air. Appl. Chem. Ind. 2020, 49, 414–419+425. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, X.; Lu, H.; Zhao, F.; Yu, G. Atmospheric Water Harvesting: A Review of Material and Structural Designs. ACS Mater. Lett. 2020, 2, 671–684. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, L.W.; Wang, J.Y.; Wang, R.Z. Alternative of Sorbents and Heat Storage Materials for Heat Storage Sorption Air Intake. J. Refrig. 2018, 39, 74–79+98. (In Chinese) [Google Scholar]
- Lord, J.; Thomas, A.; Treat, N.; Forkin, M.; Bain, R.; Dulac, P.; Behroozi, C.H.; Mamutov, T.; Fongheiser, J.; Kobilansky, N.; et al. Global potential for harvesting drinking water from air using solar energy. Nature 2021, 598, 611–617. [Google Scholar] [CrossRef]
- Zhuang, S.; Qi, H.; Wang, X.; Li, X.; Liu, K.; Liu, J.; Zhang, H. Advances in Solar-Driven Hygroscopic Water Harvesting. Glob. Chall. 2021, 5, 2000085. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, Y.; Wen, Z.; Han, W.; Wang, S.; Cao, Y.; He, R.X.; Liu, Y.; Chen, B. Enhanced adsorption-based atmospheric water harvesting using a photothermal cotton rod for freshwater production in cold climates. RSC Adv. 2021, 11, 35695–35702. [Google Scholar] [CrossRef]
- Guan, H.; Sebben, M.; Bennett, J. Radiative- and artificial-cooling enhanced dew collection in a coastal area of South Australia. Urban Water J. 2013, 11, 175–184. [Google Scholar] [CrossRef]
- Peng, Y.; He, Y.; Yang, S.; Ben, S.; Cao, M.; Li, K.; Liu, K.; Jiang, L. Magnetically Induced Fog Harvesting via Flexible Conical Arrays. Adv. Funct. Mater. 2015, 25, 5967–5971. [Google Scholar] [CrossRef]
- Damak, M.; Varanasi, K.K. Electrostatically driven fog collection using space charge injection. Sci. Adv. 2018, 4, eaao5323. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, D.; Jerez-Hanckes, C. Electrostatic fog water collection. J. Electrost. 2018, 96, 128–133. [Google Scholar] [CrossRef]
- Sharifvaghefi, S.; Kazerooni, H. Fog harvesting: Combination and comparison of different methods to maximize the collection efficiency. SN Appl. Sci. 2021, 3, 516. [Google Scholar] [CrossRef]
- Watergen. Water from Air. Available online: https://www.watergen.com/ (accessed on 30 December 2022).
- Air Water Machine. Available online: http://www.fndtech.com/ (accessed on 30 December 2022). (In Chinese).
- Renewable Drinking Water|SOURCE Water. Available online: https://www.source.co/ (accessed on 30 December 2022).
- Atlantis Solar Environmental Products. Available online: http://www.atlantissolar.com/ (accessed on 30 December 2022).
- Pure & Sustainable Water—Drinkableair Technologies. Available online: https://drinkableair.tech/ (accessed on 30 December 2022).
- SunToWater Water Generator. Making Water from Air. Available online: http://suntowater.com/ (accessed on 19 December 2022).
- WEDEW-SkySource. Available online: https://www.skysource.org/wedew (accessed on 19 December 2022).
- Rainmaker Air-to-Water Technologies. Available online: https://rainmakerww.com/technology-air-to-water/ (accessed on 19 December 2022).
- Bagheri, F. Performance investigation of atmospheric water harvesting systems. Water Resour. Ind. 2018, 20, 23–28. [Google Scholar] [CrossRef]
- Ferwati, M.S. Water harvesting cube. SN Appl. Sci. 2019, 1, 779. [Google Scholar] [CrossRef]
- Elashmawy, M.; Alshammari, F. Atmospheric water harvesting from low humid regions using tubular solar still powered by a parabolic concentrator system. J. Clean. Prod. 2020, 256, 120329. [Google Scholar] [CrossRef]
- Srivastava, S.; Yadav, A. Extraction of water particles from atmospheric air through a Scheffler reflector using different solid desiccants. Int. J. Ambient. Energy 2018, 41, 1357–1369. [Google Scholar] [CrossRef]

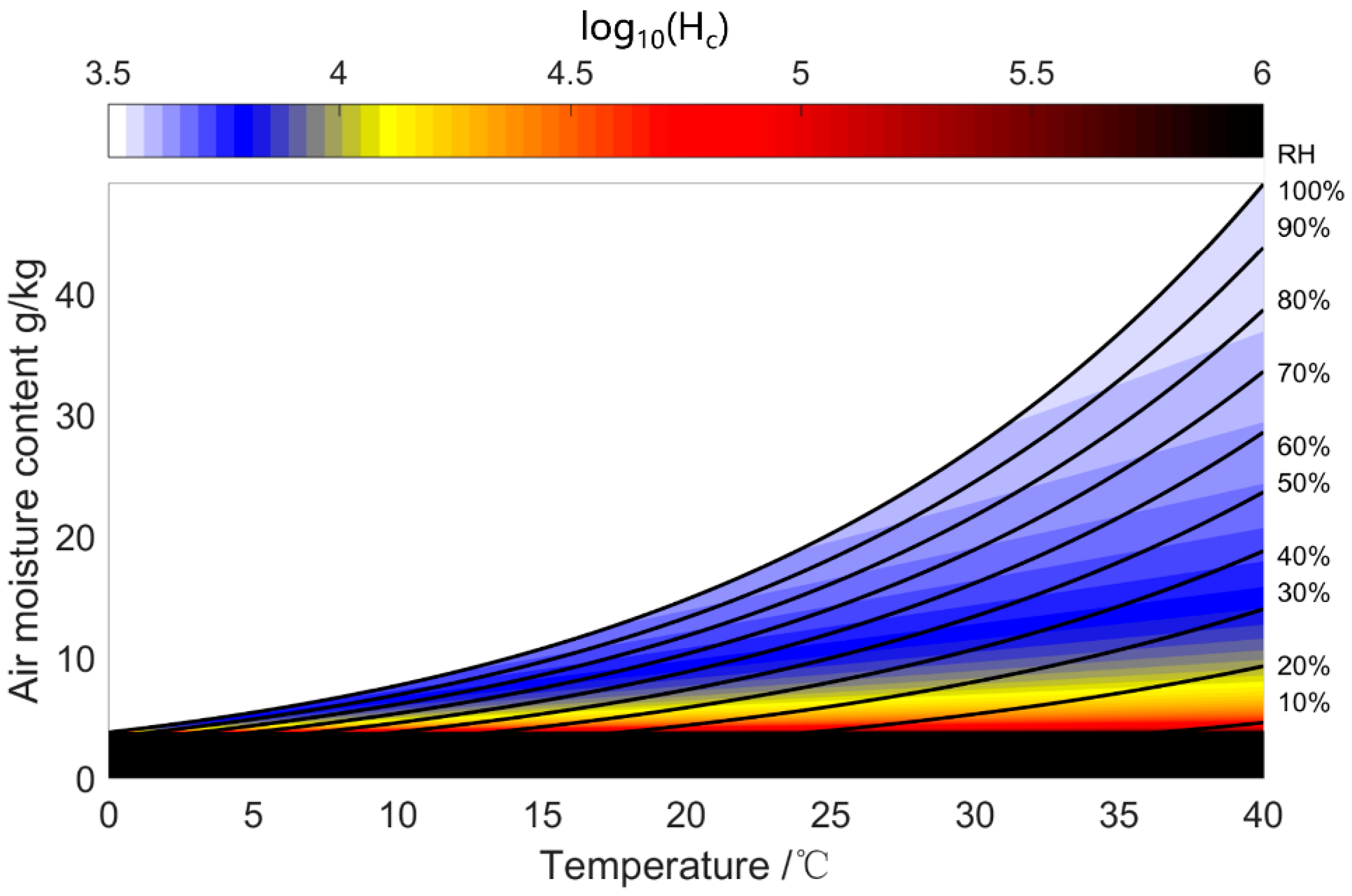
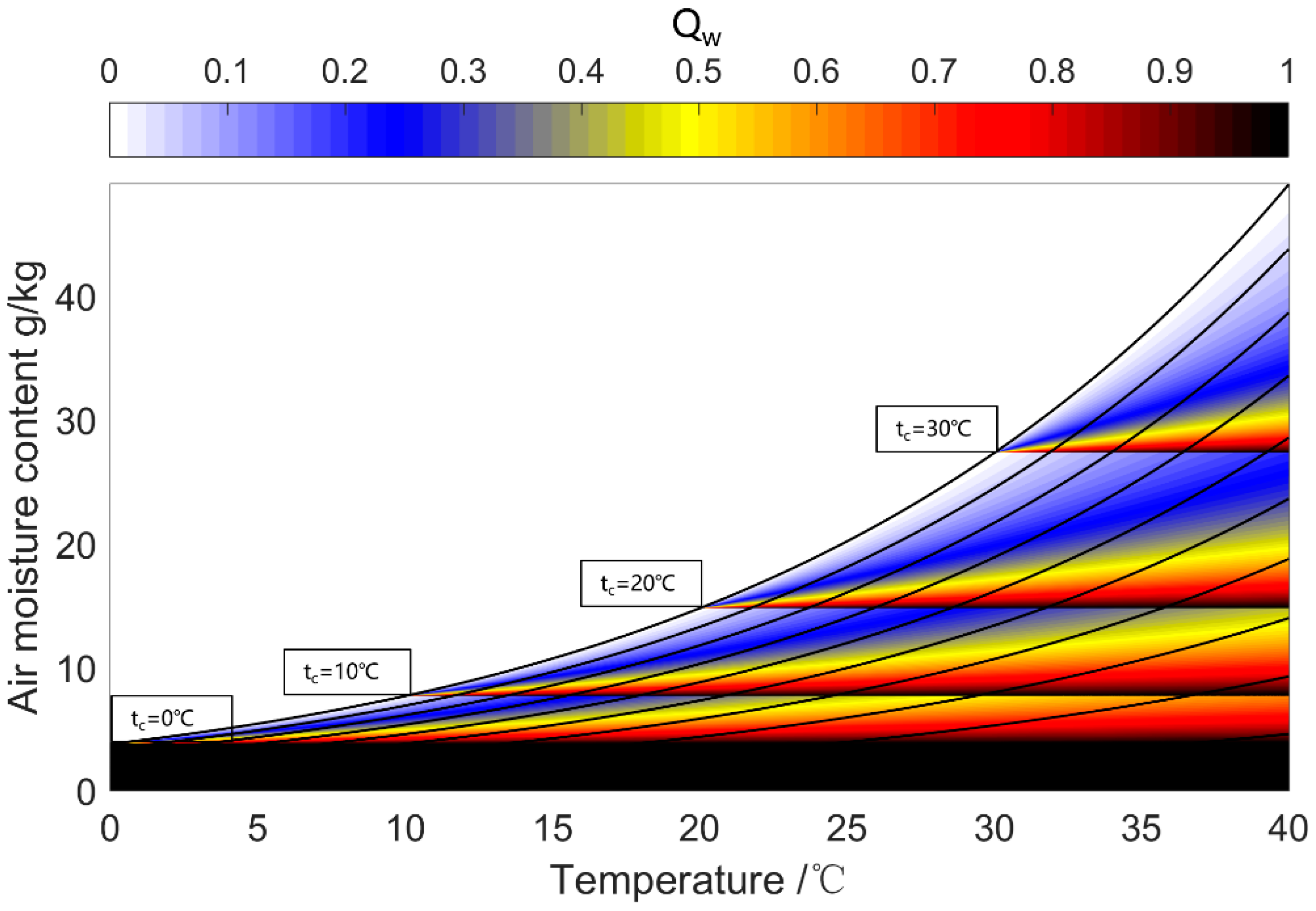
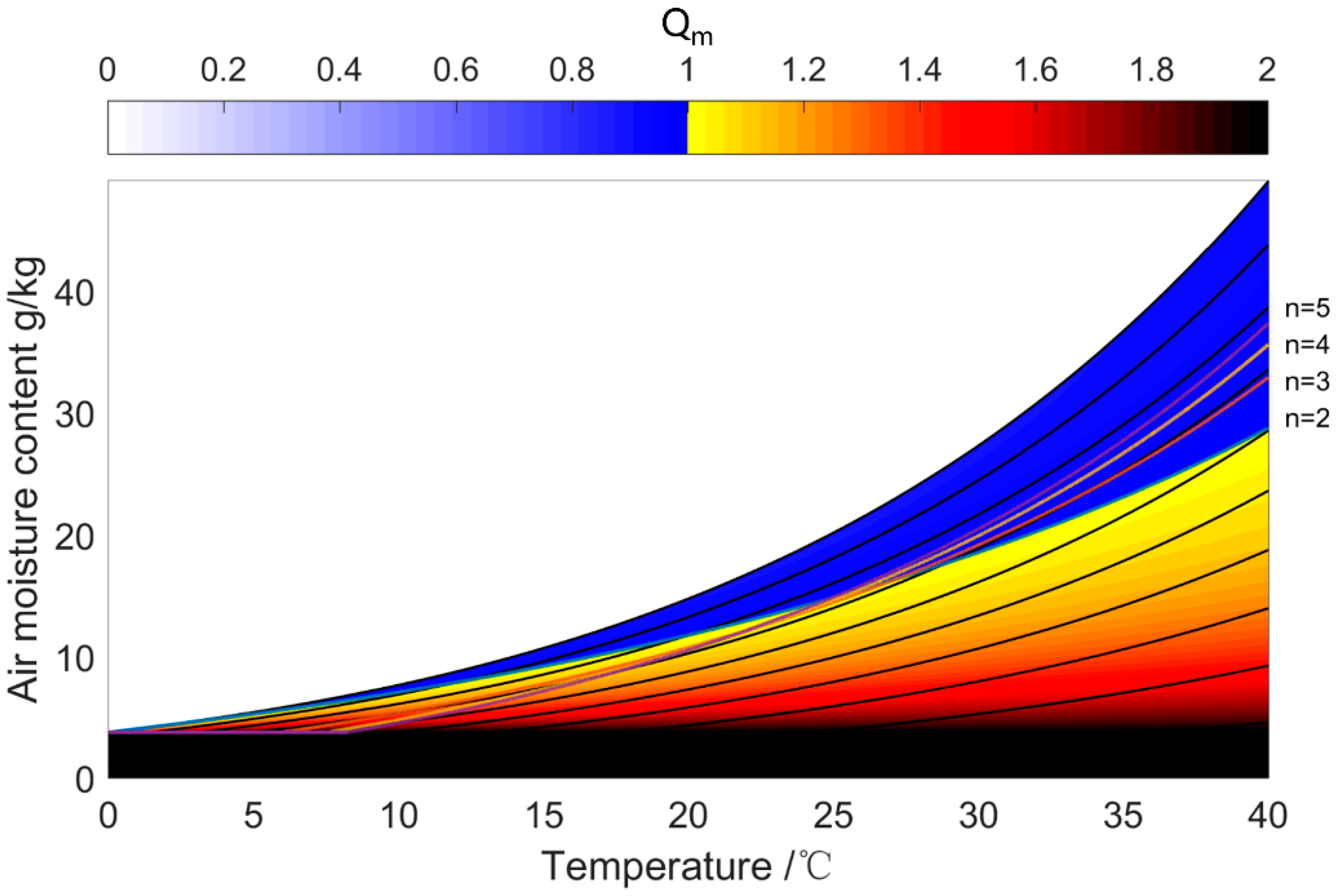
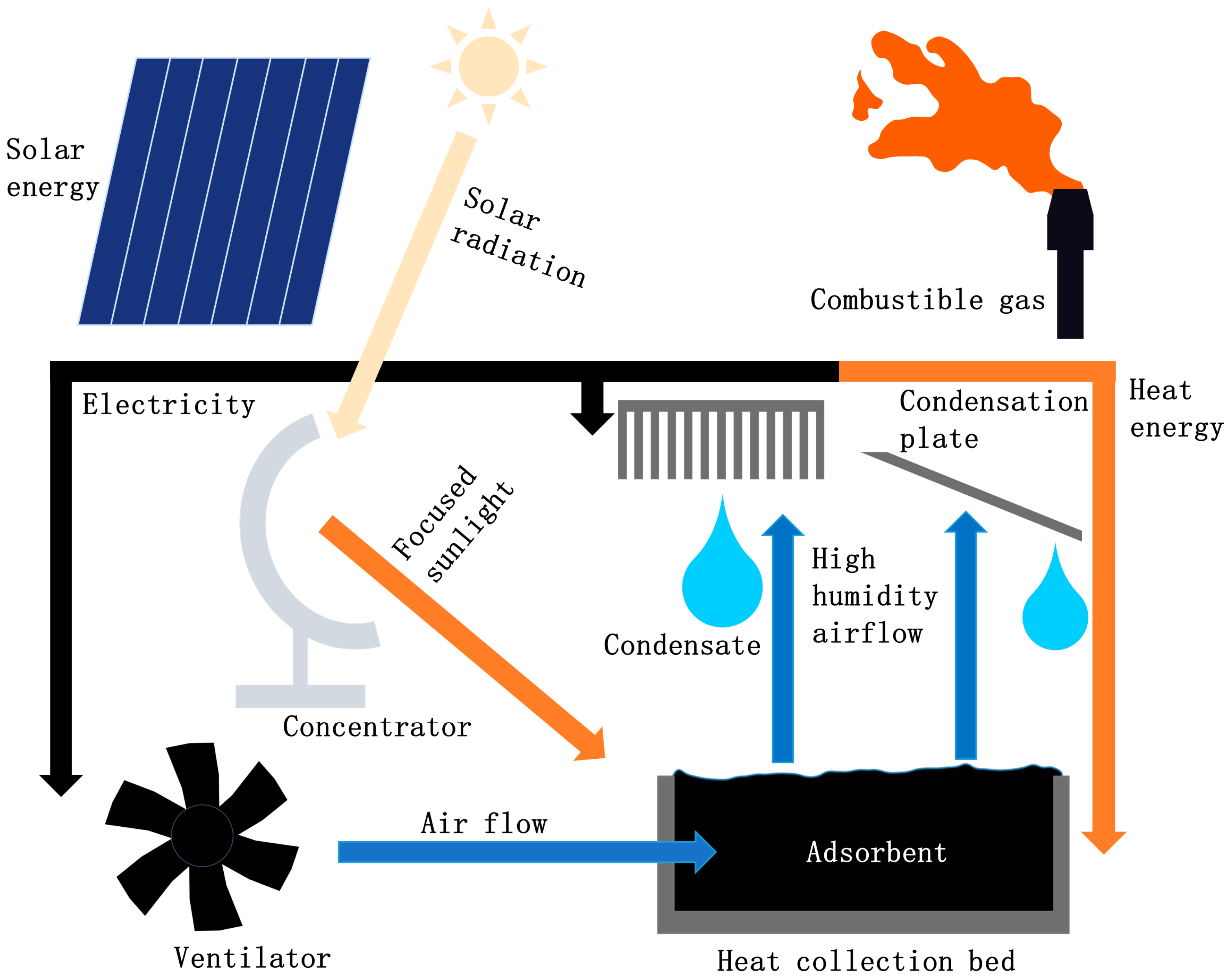
| Water Uptake Mode | Features | Working Condition | Performance | Research Type |
|---|---|---|---|---|
| Single-stage | Varied working conditions [30] | RH = 60–90%, ambient temperature at 24 °C, airflow= 30–70 m3/h | 0.14–0.41 L/kWh | Experiment |
| Thermoelectric cooler array, air channel [37] | Ambient temperature at 318 K, RH = 75% | 1.3 L/kWh | Simulation | |
| Multi-stage | Multi-stage water harvesting unit [34] | Ambient temperature at 20 °C, air moisture content = 8.7 g/kg | 0.16 L/kWh | Experiment |
| Multi-stage dehumidification wheel, air humidification [35] | Ambient temperature at 10 °C, air moisture content = 5 g/kg or Ambient temperature at 20 °C, air moisture content = 6 g/kg | 10.3–27.3 L/kWh | Simulation | |
| Enhanced membrane system [36] | Vacuum pressure = 0.17 bar, inlet air moisture content= 24.3 g/m3, outlet air moisture content= 15.5 g/m3 | 5.24 L/kWh | Simulation |
| Water Uptake Mode | Features | Working Condition | Performance |
|---|---|---|---|
| Multi adsorption/desorption cycle | Two adsorbers, alternating adsorption and desorption [50] | Temperature at 22.2 °C and RH = 44%; temperature at 10.4 °C and RH = 77% | 0.0485–0.089 L/kg/h, 4 h for one cycle |
| Liquid sorbent, moisture diffusion [53] | RH = 80% | 0.5 L/m2/h, 2.8 L/m2/day | |
| Flippable adsorbent stage [51] | RH = 20%, ambient temperature at 298 K | 0.058 L/kg/h, one hour for one cycle | |
| Vertically aligned and hierarchical pores [52] | Ambient temperature at 30 °C | 2.12 L/kg/day, eight cycles per day | |
| Rapid adsorption and subsequent desorption steps [57] | Ambient temperature at 23 °C, RH = 69%, solar radiation = 1 kW/m2 | 0.2545 L/kg/h, one cycle for 46 min | |
| Nano vapor sorbent [54] | RH = 60%, solar radiation = 1 kW/m2 | 1.6 L/kg/day, 3 cycles a day | |
| Nano-enabled photothermal desiccants [55] | RH = 40% | 0.039 L/kg/h, 10 cycles per day | |
| Fluidized metal-organic frameworks [56] | RH = 18–39% | 0.33–0.52 L/kg/h, 40–55 cycles per day | |
| MOF-303 [58] | RH = 10–32%, ambient temperature at 27 °C | 0.7–1.3 L/kg/day | |
| Moisture-indicated Cellulose Aerogels [59] | RH = 25–85% | 2.81 L/kg/day, 3 cycles per day | |
| Scaled-up device [60] | RH = 20–90% | 0.42 L/kg/day, 2 cycles per day |
| Water Uptake Mode | Features | Working Condition | Performance |
|---|---|---|---|
| Active dew collection | Enhanced by artificial cooling [70] | Ambient temperature at 5.7–11.9 °C, RH at 70.1–95.1% | Enhancement of 45% and 150% for Teflon and aluminum collectors, respectively |
| Active fog collection | Magnetically responsive flexible conical arrays [71] | Ambient temperature at 27 °C, RH = 80% | 2 L/m2/h |
| Electrostatically driven [72] | Voltage = 10 kV, RH = 100% | 24 L/m2/h,40 W/m2 | |
| Radial electric field [73] | Voltage = 40 kV, air velocity = 2 m/s | 1.367 L/m2/h | |
| Combining wires with electric field [74] | Voltage = 25 kV, RH = 100% | 0.7–2.7 L/m2/h, 60 W/m2 |
| Type of Atmospheric Water Harvesting | Performance Influencing Factors | Ways of Influence | Lifting Rate of Water Uptake Rate |
|---|---|---|---|
| Direct condensation | Ambient temperature | High temperature is favorable for water harvesting | It determines whether there is condensate [39] |
| Relative humidity | High relative humidity is favorable for water harvesting | It determines whether there is condensate | |
| Airflow | Affecting heat transfer and droplet removal, limited by cooling capacity | 28% [30], 50% [37] | |
| Temperature of condensation surface | Low temperature is better, limited by frost | It determines whether there is condensate [37] | |
| Thermal conductivity of condensation surface material | High thermal conductivity is better, limited by frost | - | |
| Wetting characteristics of condensation surface | Affecting heat transfer and the generation and removal of droplets, limited by other factors | 110% [31] | |
| Installation angle of condensation surface | Affecting droplet removal and system structure, limited by other factors | 680% [33] | |
| Regenerator | Reducing energy consumption | - | |
| Intelligent monitoring and control | Improving the environmental adaptability of the device and reducing energy consumption | - | |
| Adsorption and desorption | Water absorption rate | Affecting water release at desorption stage | 4–6% |
| Adsorption/desorption speed | Affecting the time of adsorption/desorption cycles | Multiplied by the times of absorption/desorption cycles | |
| Desorption temperature | Low temperature, low energy consumption | 7–8% | |
| Specific heat capacity of adsorption material | Low specific heat capacity, low energy consumption | 3% | |
| Desorption heat | Low desorption heat, low energy consumption | 15% | |
| Regeneration stability of adsorbent | Affecting long-term water harvesting | - | |
| Adsorption material cost | Low cost, good practicability | - | |
| Heat transfer performance of adsorption bed | Affecting heat transfer efficiency | - | |
| Enhanced condensation | Improving water vapor condensation rate | - | |
| Heat storage | Respond to changes in energy supply and extend desorption time | - | |
| Active collection of fog and dew | Enhanced by artificial-cooling | Improving water collection rate | 45–150% [70] |
| Magnetically responsive flexible conical arrays | Fog harvesting under windless conditions | It determines whether there is water collected [71] | |
| Electrostatically driven | Fog harvesting under windless conditions | It determines whether there is water collected [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-J.; Cheng, L.; Sun, P.; Li, F.-F.; Qiu, J. Potential Analysis of Atmospheric Water Harvesting Technologies from the Perspective of “Trading-in Energy for Water”. Water 2023, 15, 878. https://doi.org/10.3390/w15050878
Li H-J, Cheng L, Sun P, Li F-F, Qiu J. Potential Analysis of Atmospheric Water Harvesting Technologies from the Perspective of “Trading-in Energy for Water”. Water. 2023; 15(5):878. https://doi.org/10.3390/w15050878
Chicago/Turabian StyleLi, Hou-Jun, Liang Cheng, Peng Sun, Fang-Fang Li, and Jun Qiu. 2023. "Potential Analysis of Atmospheric Water Harvesting Technologies from the Perspective of “Trading-in Energy for Water”" Water 15, no. 5: 878. https://doi.org/10.3390/w15050878
APA StyleLi, H.-J., Cheng, L., Sun, P., Li, F.-F., & Qiu, J. (2023). Potential Analysis of Atmospheric Water Harvesting Technologies from the Perspective of “Trading-in Energy for Water”. Water, 15(5), 878. https://doi.org/10.3390/w15050878








