Oil–Water Separation on Hydrophobic and Superhydrophobic Membranes Made of Stainless Steel Meshes with Fluoropolymer Coatings
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Reagents
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Emulsion Preparation
2.5. Emulsion Separation Arrangement and Mechanism
3. Results and Discussion
3.1. Effect of Wettability on Separation Efficiency and Rate
3.2. Influence of Mesh Pore Size on Separation Efficiency
3.3. Evaluation of Separation Efficiency on Membranes
3.4. Resource Tests
4. Conclusions
- (1)
- The HW CVD method can be applied to the fabrication of highly efficient hydrophobic separation membranes by depositing fluoropolymer coatings onto the surfaces of metal meshes. Depending on the deposition parameters, it is possible to obtain membranes with different surface-wetting properties; specifically, in this work, the WCA ranges from 130 to 170°, while the OCA remains constant and is about 80° ± 2°.
- (2)
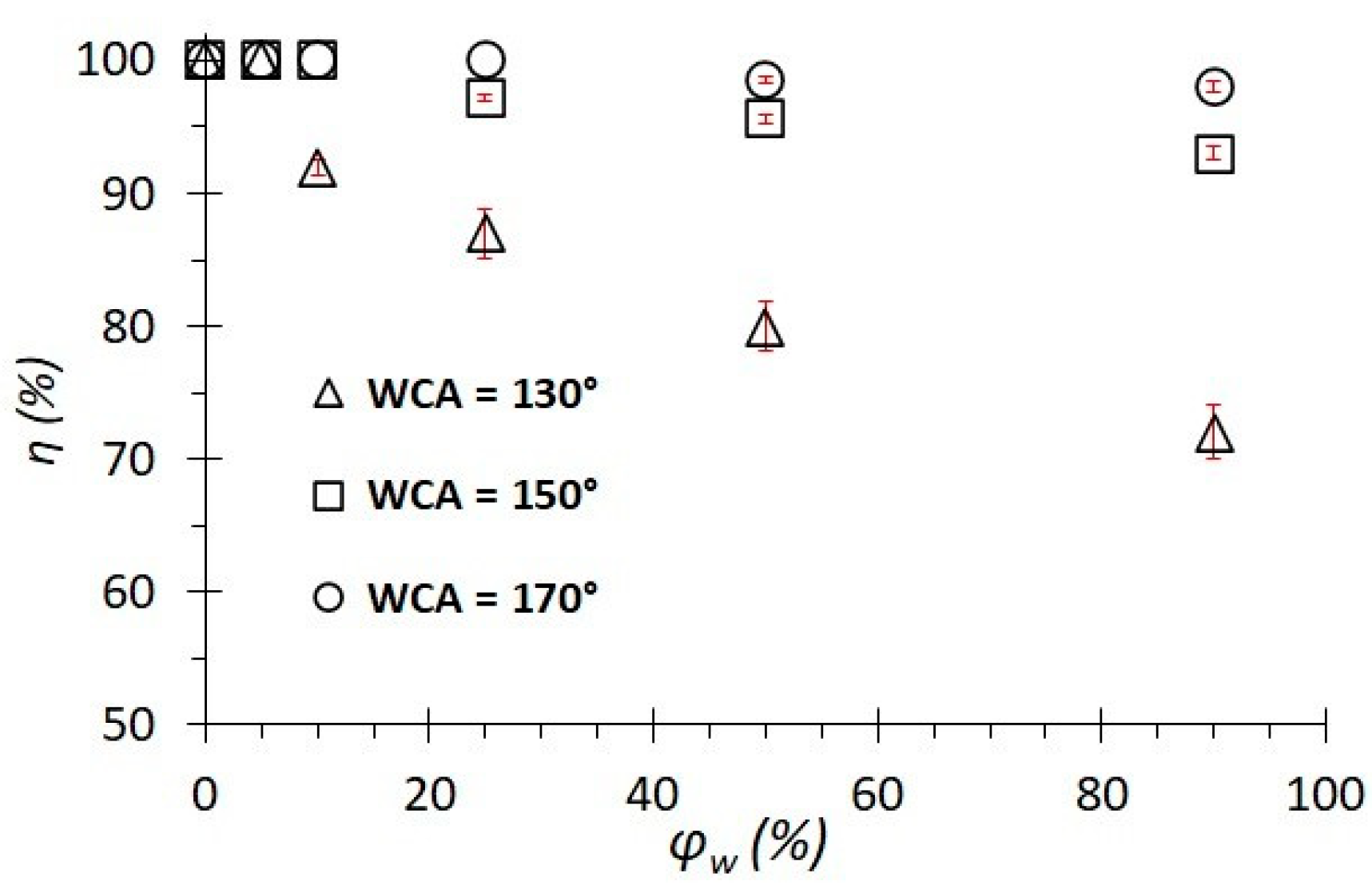
- Studies have shown the effectiveness of the use of the obtained membranes for the separation of emulsions of water and commercial crude oil, with separation efficiency values that can reach over 99%. The membrane-wetting properties affect the rate and efficiency of separation. The higher the WCA value of the membrane surface, the more efficient the separation. It has been established that emulsions with a lower water concentration (5%) are most effectively separated.
- (3)
- The pore size of the membrane significantly affects the rate and efficiency of separation. The smaller the pore size of the membranes, the higher the separation efficiency, but the lower its rate.
- (4)
- The use of the proposed coefficient of separation efficiency made it possible to determine the optimal parameters for the use of membranes for separating emulsions. The highest efficiency is achieved when separating membranes with a superhydrophobic coating (WCA = 170°) and a minimum pore size (40 µm).
- (5)
- The experiments were performed to explore whether hydrophobic coated membranes produced by the HW CVD method can be used for several separation cycles. The used membranes can be easily washed and reused without significant reduction in separation efficiency.
- (6)
- The work is of great economic and practical importance for improving the efficiency of the membrane separation of oil–water emulsions. It lays the foundation for future research on the use of hydrophobic membranes for the separation of various emulsions of water and oil products (diesel fuel, gasoline, kerosene, etc.).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joye, S.B. Deepwater Horizon, 5 Years On. Science 2015, 349, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Schrope, M. Oil Spill: Deep Wounds. Nature 2011, 472, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, M.L. State of the Art Review and Future Directions in Oil Spill Modeling. Mar. Pollut. Bull. 2017, 115, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Sobolčiak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Some Theoretical Aspects of Tertiary Treatment of Water/Oil Emulsions by Adsorption and Coalescence Mechanisms: A Review. Water 2021, 13, 652. [Google Scholar] [CrossRef]
- Paul, D.; Friedrich, E.; Schwarz, H.H.; Bartsch, D.; Hicke, H.G.; Neustadt, W.; Morgenstern, S.; Sawatzki, P.; Tietze, R. Membrane filtration for oil-water emulsion separation. Chem. Tech. 1979, 31, 24–26. [Google Scholar]
- Srijaroonrat, P.; Julien, E.; Aurelle, Y. Unstable Secondary Oil/Water Emulsion Treatment Using Ultrafiltration: Fouling Control by Backflushing. J. Membr. Sci. 1999, 159, 11–20. [Google Scholar] [CrossRef]
- Pervez, M.N.; Mishu, M.R.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. Sustainable Treatment of Food Industry Wastewater Using Membrane Technology: A Short Review. Water 2021, 13, 3450. [Google Scholar] [CrossRef]
- Tian, L.; Li, W.; Ye, H.; Zhu, L.; Chen, H.; Liu, H. Environmentally Benign Development of Superhydrophilic and Underwater Superoleophobic Mesh for Effective Oil/Water Separation. Surf. Coat. Technol. 2019, 377, 124892. [Google Scholar] [CrossRef]
- Tudu, B.K.; Kumar, A. Robust and Durable Superhydrophobic Steel and Copper Meshes for Separation of Oil-Water Emulsions. Prog. Org. Coat. 2019, 133, 316–324. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Gao, Q.; Zhao, J.; Wang, Y.; Liu, C.; Shen, C.; Liu, X. Facile Fabrication of Durable Superhydrophobic Mesh via Candle Soot for Oil-Water Separation. Prog. Org. Coat. 2019, 136, 105253. [Google Scholar] [CrossRef]
- Shi, T.; Liang, J.; Li, X.; Zhang, C.; Yang, H. Improving the Corrosion Resistance of Aluminum Alloy by Creating a Superhydrophobic Surface Structure through a Two-Step Process of Etching Followed by Polymer Modification. Polymers 2022, 14, 4509. [Google Scholar] [CrossRef]
- Shang, Y.; Si, Y.; Raza, A.; Yang, L.; Mao, X.; Ding, B.; Yu, J. An in Situ Polymerization Approach for the Synthesis of Superhydrophobic and Superoleophilic Nanofibrous Membranes for Oil–Water Separation. Nanoscale 2012, 4, 7847. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y. Oil-Water Separation Capability of Superhydrophobic Fabrics Fabricated via Combining Polydopamine Adhesion with Lotus-Leaf-like Structure. J. Appl. Polym. Sci. 2015, 132, e42614. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, F.; Xue, L. Under Seawater Superoleophobic PVDF Membrane Inspired by Polydopamine for Efficient Oil/Seawater Separation. J. Membr. Sci. 2015, 476, 321–329. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem. 2004, 116, 2046–2048. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Han, Y. A Composite Polymer Film with Both Superhydrophobicity and Superoleophilicity. Macromol. Rapid Commun. 2006, 27, 804–808. [Google Scholar] [CrossRef]
- Halake, K.; Bae, S.; Lee, J.; Cho, Y.; Jo, H.; Heo, J.; Park, K.; Kim, H.; Ju, H.; Kim, Y.; et al. Strategies for Fabrication of Hydrophobic Porous Materials Based on Polydimethylsiloxane for Oil-Water Separation. Macromol. Res. 2019, 27, 109–114. [Google Scholar] [CrossRef]
- Xiong, X.; Xie, F.; Meng, J. Preparation of Superhydrophobic Porous Coating Film with the Matrix Covered with Polydimethylsiloxane for Oil/Water Separation. Prog. Org. Coat. 2018, 125, 365–371. [Google Scholar] [CrossRef]
- Caldona, E.B.; De Leon, A.C.C.; Thomas, P.G.; Naylor, D.F.; Pajarito, B.B.; Advincula, R.C. Superhydrophobic Rubber-Modified Polybenzoxazine/SiO 2 Nanocomposite Coating with Anticorrosion, Anti-Ice, and Superoleophilicity Properties. Ind. Eng. Chem. Res. 2017, 56, 1485–1497. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Tian, H.; Wu, Y.; Zha, F.; Feng, H.; Tang, X. Facile Fabrication of Anti-Corrosive Superhydrophobic Diatomite Coatings for Removal Oil from Harsh Environments. Sep. Purif. Technol. 2017, 189, 335–340. [Google Scholar] [CrossRef]
- Xue, C.-H.; Ji, P.-T.; Zhang, P.; Li, Y.-R.; Jia, S.-T. Fabrication of Superhydrophobic and Superoleophilic Textiles for Oil–Water Separation. Appl. Surf. Sci. 2013, 284, 464–471. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Zhang, H.; Liu, J.; Wu, S.; Fan, Q.; Xue, H. Highly Efficient and Robust Oil/Water Separation Materials Based on Wire Mesh Coated by Reduced Graphene Oxide. Langmuir 2017, 33, 9590–9597. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, B.O.; Ahmed, E.; Al Abdulgader, H.; Alghunaimi, F.; Saleh, T.A. Facile Fabrication of Hydrophobic Alkylamine Intercalated Graphene Oxide as Absorbent for Highly Effective Oil-Water Separation. J. Mol. Liq. 2021, 325, 115057. [Google Scholar] [CrossRef]
- Cao, Q.; Zheng, S.; Wong, C.-P.; Liu, S.; Peng, Q. Massively Engineering the Wettability of Titanium by Tuning Nanostructures and Roughness via Laser Ablation. J. Phys. Chem. C 2019, 123, 30382–30388. [Google Scholar] [CrossRef]
- Baig, U.; Dastageer, M.A.; Gondal, M.A. Facile Fabrication of Super-Wettable Mesh Membrane Using Locally-Synthesized Cobalt Oxide Nanoparticles and Their Application in Efficient Gravity Driven Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130793. [Google Scholar] [CrossRef]
- Said, A.; Al Abdulgader, H.; Alsaeed, D.; Drmosh, Q.A.; Baroud, T.N.; Saleh, T.A. Hydrophobic Tungsten Oxide-Based Mesh Modified with Hexadecanoic Branches for Efficient Oil/Water Separation. J. Water Process Eng. 2022, 49, 102931. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Z.; Shen, Y.; Mu, P.; Zhu, G.; Li, J. Facile Fabrication of Superhydrophobic Copper Hydroxide Coated Mesh for Effective Separation of Water-in-Oil Emulsions. Sep. Purif. Technol. 2020, 230, 115856. [Google Scholar] [CrossRef]
- Huang, X.; Wen, X.; Cheng, J.; Yang, Z. Sticky Superhydrophobic Filter Paper Developed by Dip-Coating of Fluorinated Waterborne Epoxy Emulsion. Appl. Surf. Sci. 2012, 258, 8739–8746. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Li, H.; Li, J.; Zha, F.; Lei, Z. A Facile One-Step Spray-Coating Process for the Fabrication of a Superhydrophobic Attapulgite Coated Mesh for Use in Oil/Water Separation. RSC Adv. 2015, 5, 53802–53808. [Google Scholar] [CrossRef]
- Long, M.; Peng, S.; Deng, W.; Yang, X.; Miao, K.; Wen, N.; Miao, X.; Deng, W. Robust and Thermal-Healing Superhydrophobic Surfaces by Spin-Coating of Polydimethylsiloxane. J. Colloid Interface Sci. 2017, 508, 18–27. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, D.; Wei, Z.; Chen, J.; Jing, J. Fabrication of Superhydrophobic Nano-Aluminum Films on Stainless Steel Meshes by Electrophoretic Deposition for Oil-Water Separation. Appl. Surf. Sci. 2018, 427, 253–261. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, F.; Zhao, B.; Ning, Y.; Lai, Y.; Wang, L. Acid/Base Treatment of Monolithic Activated Carbon for Coating Silver with Tunable Morphology. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 760–765. [Google Scholar] [CrossRef]
- Tang, K.; Yu, J.; Zhao, Y.; Liu, Y.; Wang, X.; Xu, R. Fabrication of Super-Hydrophobic and Super-Oleophilic Boehmite Membranes from Anodic Alumina Oxide Film via a Two-Phase Thermal Approach. J. Mater. Chem. 2006, 16, 1741. [Google Scholar] [CrossRef]
- Salapare, H.S.; Suarez, B.A.T.; Cosiñero, H.S.O.; Bacaoco, M.Y.; Ramos, H.J. Irradiation of Poly(Tetrafluoroethylene) Surfaces by CF4 Plasma to Achieve Robust Superhydrophobic and Enhanced Oleophilic Properties for Biological Applications. Mater. Sci. Eng. C 2015, 46, 270–275. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, D.-H. Effect of Irradiation on the Surface Morphology of Nanostructured Superhydrophobic Surfaces Fabricated by Ion Beam Irradiation. Appl. Surf. Sci. 2019, 477, 154–158. [Google Scholar] [CrossRef]
- Saleh, T.A.; Baig, N. Efficient Chemical Etching Procedure for the Generation of Superhydrophobic Surfaces for Separation of Oil from Water. Prog. Org. Coat. 2019, 133, 27–32. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Nagaraja, K.K.; Nagaraja, H.S.; Pattabi, M. Conversion of Microfiltration Membrane into Nanofiltration Membrane by Vapour Phase Deposition of Aluminium for Desalination Application. Desalination 2011, 274, 177–181. [Google Scholar] [CrossRef]
- Liravi, M.; Pakzad, H.; Moosavi, A.; Nouri-Borujerdi, A. A Comprehensive Review on Recent Advances in Superhydrophobic Surfaces and Their Applications for Drag Reduction. Prog. Org. Coat. 2020, 140, 105537. [Google Scholar] [CrossRef]
- Hou, C.; Cao, C. Superhydrophobic Cotton Fabric Membrane Prepared by Fluoropolymers and Modified Nano-SiO 2 Used for Oil/Water Separation. RSC Adv. 2021, 11, 31675–31687. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Sun, X.; Kong, D.; Chu, D.; Hu, Y.; Duan, J.-A. Spatial Light Modulated Femtosecond Laser Ablated Durable Superhydrophobic Copper Mesh for Oil-Water Separation and Self-Cleaning. Surf. Coat. Technol. 2020, 402, 126254. [Google Scholar] [CrossRef]
- Safonov, A.I.; Sulyaeva, V.S.; Gatapova, E.Y.; Starinskiy, S.V.; Timoshenko, N.I.; Kabov, O.A. Deposition Features and Wettability Behavior of Fluoropolymer Coatings from Hexafluoropropylene Oxide Activated by NiCr Wire. Thin Solid Films 2018, 653, 165–172. [Google Scholar] [CrossRef]
- Safonov, A.I.; Bogoslovtseva, A.L.; Sulyaeva, V.S.; Kiseleva, M.S.; Zhidkov, I.S.; Starinskiy, S.V. Effect of Annealing on the Structure and Properties of Thin Fluoropolymer Coatings Prepared by HW CVD. J. Struct. Chem. 2021, 62, 1441–1446. [Google Scholar] [CrossRef]
- Starinskiy, S.V.; Bulgakov, A.V.; Gatapova, E.Y.; Shukhov, Y.G.; Sulyaeva, V.S.; Timoshenko, N.I.; Safonov, A.I. Transition from Superhydrophilic to Superhydrophobic of Silicon Wafer by a Combination of Laser Treatment and Fluoropolymer Deposition. J. Phys. D Appl. Phys. 2018, 51, 255307. [Google Scholar] [CrossRef]
- Martin, T.P.; Lau, K.K.S.; Chan, K.; Mao, Y.; Gupta, M.; Shannan O’Shaughnessy, W.; Gleason, K.K. Initiated Chemical Vapor Deposition (ICVD) of Polymeric Nanocoatings. Surf. Coat. Technol. 2007, 201, 9400–9405. [Google Scholar] [CrossRef]
- Yasuoka, H.; Yoshida, M.; Sugita, K.; Ohdaira, K.; Murata, H.; Matsumura, H. Fabrication of PTFE Thin Films by Dual Catalytic Chemical Vapor Deposition Method. Thin Solid Films 2008, 516, 687–690. [Google Scholar] [CrossRef]
- Safonov, A.; Sulyaeva, V.; Timoshenko, N.; Gatapova, E.; Kabov, O.; Kirichenko, E.; Semenov, A. Deposition and Investigation of Hydrophobic Coatings. MATEC Web Conf. 2015, 37, 01047. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Hydrophobic Materials and Coatings: Principles of Design, Properties and Applications. Russ. Chem. Rev. 2008, 77, 583–600. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Cao, H.; Gu, W.; Fu, J.; Liu, Y.; Chen, S. Preparation of Superhydrophobic/Oleophilic Copper Mesh for Oil-Water Separation. Appl. Surf. Sci. 2017, 412, 599–605. [Google Scholar] [CrossRef]
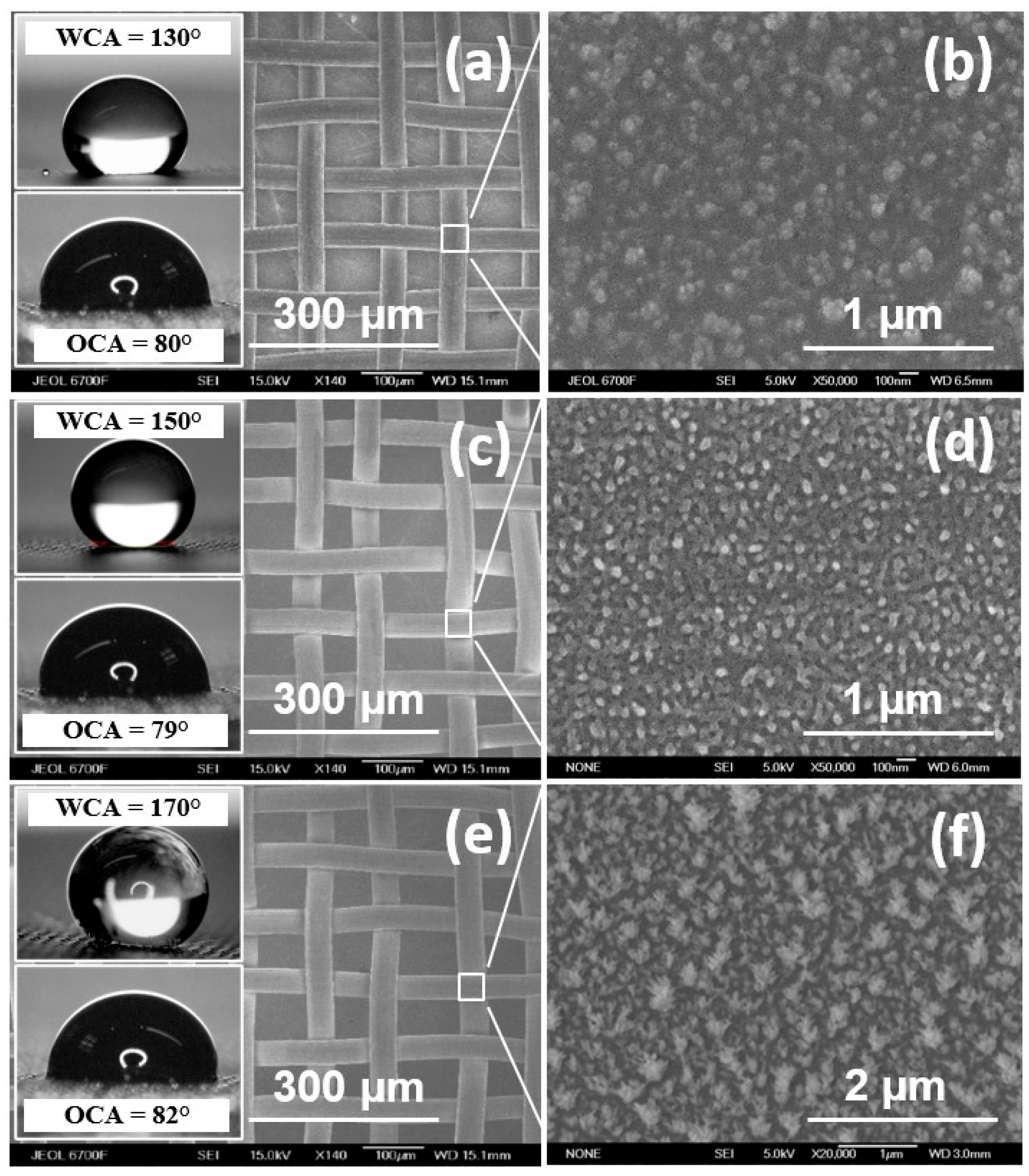





| WCA, ° | Tf, °C | P, Pa | R, mm | Ts, °C | t, min | |
|---|---|---|---|---|---|---|
| Type 1 | 130 | 640 | 67 | 50 | 30 | 180 |
| Type 2 | 150 | 580 | 67 | 50 | 30 | 90 |
| Type 3 | 170 | 680 | 133 | 50 | 100 | 60 |
| φw, % | WCA, ° | ts, min | n, Times | η, % | CSE |
|---|---|---|---|---|---|
| 5 | 130 ± 5 | 6.0 ± 0.5 | 5 | 100 | 3.3 |
| 150 ± 3 | 3.3 ± 0.4 | 6 | 100 | 5.1 | |
| 170 ± 2 | 1.1 ± 0.2 | 4 | 100 | 23.1 | |
| 10 | 130 ± 5 | 9.2 ± 1.1 | 4 | 92.0 ± 0.6 | 2.5 |
| 150 ± 3 | 4.6 ± 0.5 | 6 | 100 | 3.6 | |
| 170 ± 2 | 2.3 ± 0.4 | 5 | 100 | 8.8 | |
| 25 | 130 ± 5 | 14.0 ± 1.2 | 7 | 87.0 ± 1.8 | 0.9 |
| 150 ± 3 | 11.0 ± 1.1 | 6 | 97.0 ± 0.2 | 1.5 | |
| 170 ± 2 | 5.3 ± 1.0 | 4 | 100 | 4.7 | |
| 50 | 130 ± 5 | 17.2 ± 1.2 | 6 | 80.0 ± 1.9 | 0.8 |
| 150 ± 3 | 12.1 ± 1.0 | 5 | 96.0 ± 0.3 | 1.6 | |
| 170 ± 2 | 6.2 ± 1.0 | 5 | 99.0 ± 0.2 | 3.2 | |
| 90 | 130 ± 5 | 21.0 ± 1.2 | 5 | 72.0 ± 2.1 | 0.7 |
| 150 ± 3 | 14.2 ± 1.2 | 4 | 93.0 ± 0.5 | 0.9 | |
| 170 ± 2 | 6.2 ± 1.0 | 4 | 98.0 ± 0.4 | 4.1 |
| WCA, ° | φw, % | D, µm | ts, min | n, Times | η, % | CSE |
|---|---|---|---|---|---|---|
| 130 ± 5 | 25 | 40 | 16.1 ± 0.2 | 3 | 100 | 2.1 |
| 65 | 14.2 ± 0.3 | 4 | 99.0 ± 0.2 | 1.8 | ||
| 130 | 13.5 ± 1.0 | 4 | 94.0 ± 1.3 | 1.7 | ||
| 200 | 14.0 ± 1.2 | 7 | 87.0 ± 1.8 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnik, A.; Bogoslovtseva, A.; Petrova, A.; Safonov, A.; Markides, C.N. Oil–Water Separation on Hydrophobic and Superhydrophobic Membranes Made of Stainless Steel Meshes with Fluoropolymer Coatings. Water 2023, 15, 1346. https://doi.org/10.3390/w15071346
Melnik A, Bogoslovtseva A, Petrova A, Safonov A, Markides CN. Oil–Water Separation on Hydrophobic and Superhydrophobic Membranes Made of Stainless Steel Meshes with Fluoropolymer Coatings. Water. 2023; 15(7):1346. https://doi.org/10.3390/w15071346
Chicago/Turabian StyleMelnik, Alexandra, Alena Bogoslovtseva, Anna Petrova, Alexey Safonov, and Christos N. Markides. 2023. "Oil–Water Separation on Hydrophobic and Superhydrophobic Membranes Made of Stainless Steel Meshes with Fluoropolymer Coatings" Water 15, no. 7: 1346. https://doi.org/10.3390/w15071346
APA StyleMelnik, A., Bogoslovtseva, A., Petrova, A., Safonov, A., & Markides, C. N. (2023). Oil–Water Separation on Hydrophobic and Superhydrophobic Membranes Made of Stainless Steel Meshes with Fluoropolymer Coatings. Water, 15(7), 1346. https://doi.org/10.3390/w15071346









