Assessing the Feasibility of Sustainable Materials to Boost the Sorption of Pharmaceutical Active Compounds When Included in Reactive Barriers in Soil Aquifer Treatment for Water Reuse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sustainable Materials Selection
2.2. Sustainable Materials Preparation
2.3. Selected PhACs Physicochemical Properties
2.4. Speciation and pH-Dependent n-Octanol/Water Distribution Coefficient (Log Dow) Calculations
2.5. Sorption Batch Setup
2.6. Sample Preparation and Analysis
3. Results
3.1. Influence of Experimental Parameters in the Sorption of PhACs on Sand, Compost, and Clay
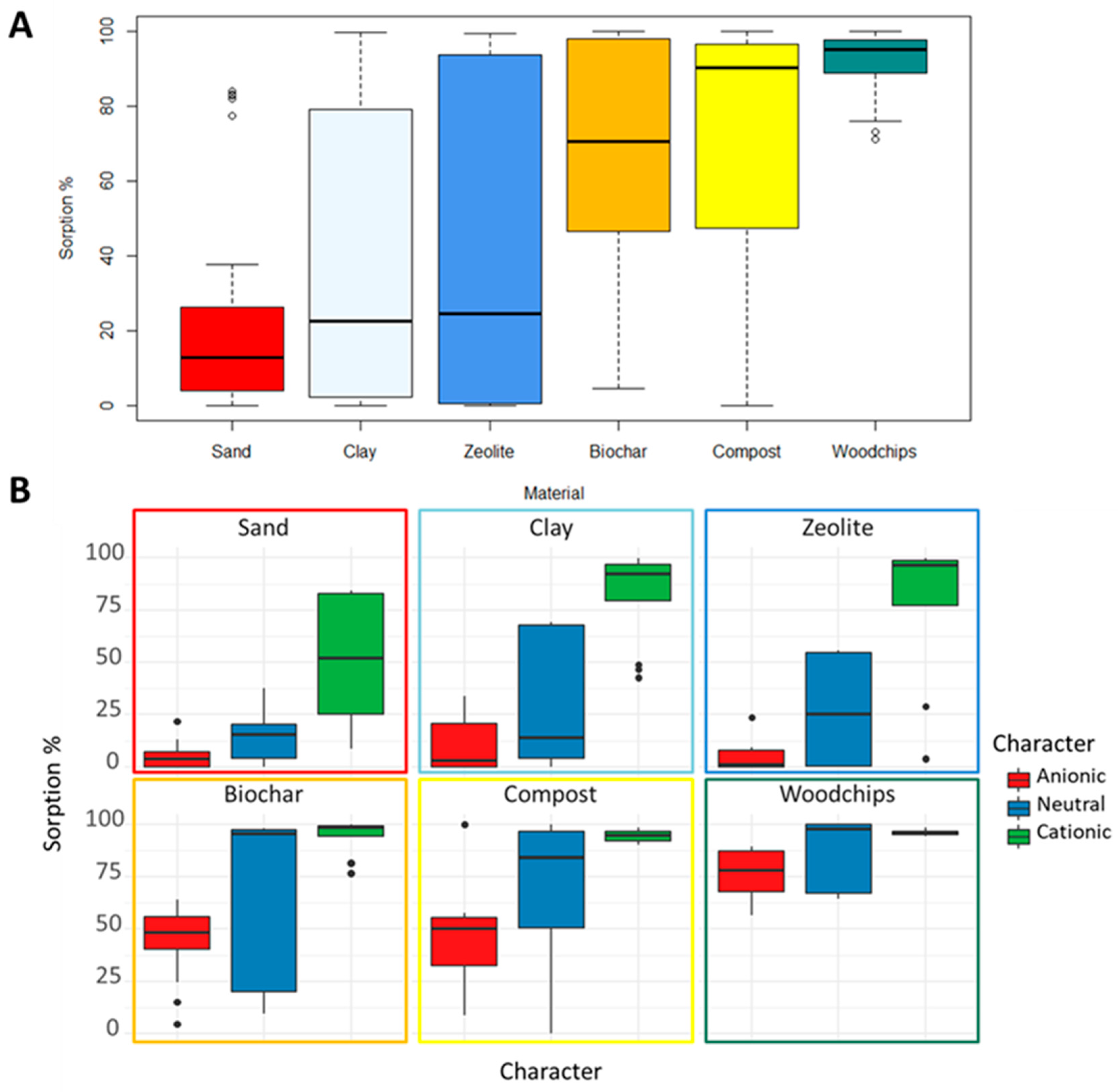
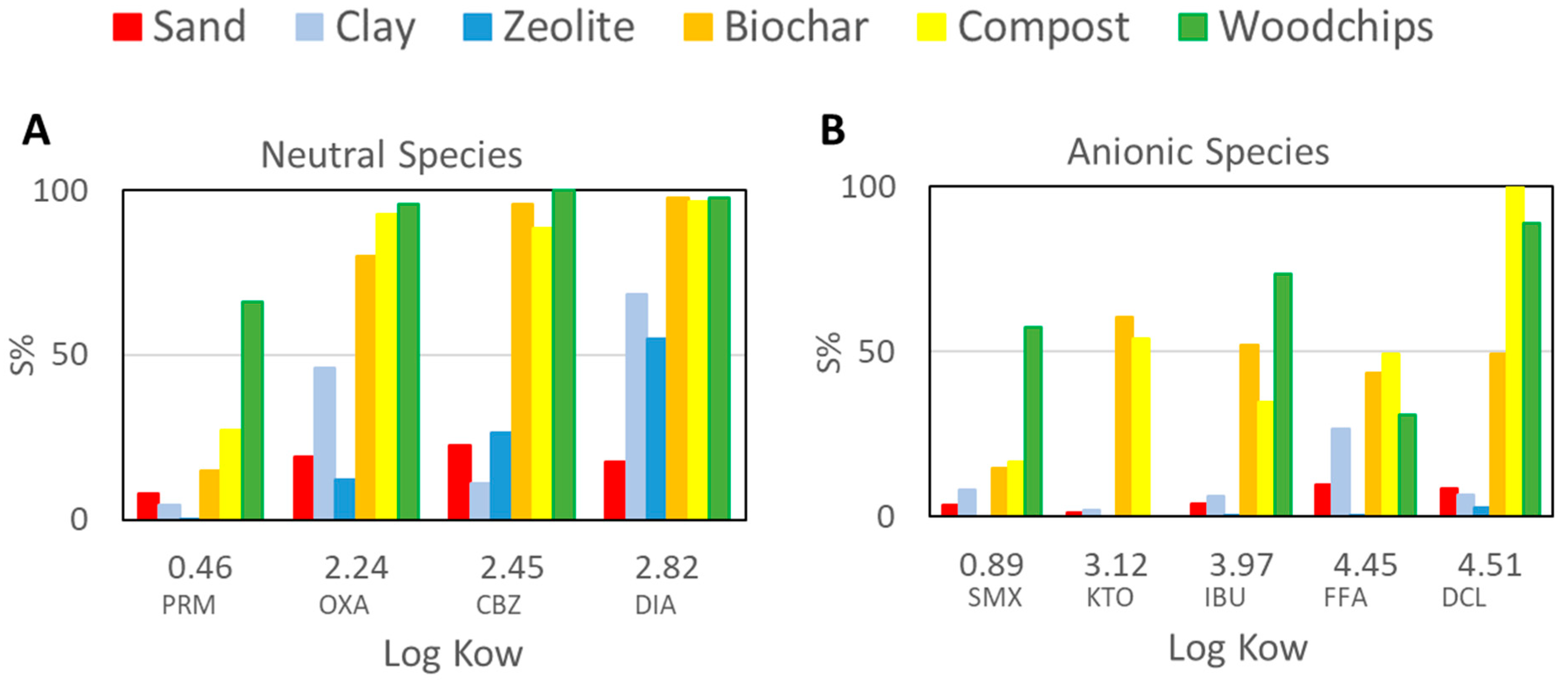
3.2. PhACs Sorption Capacity of the Five Selected Materials Compared to Sand
4. Discussion
4.1. Role of Experimental Parameters in Sorption
4.2. What Materials Should Be Included in Reactive Barriers to Boost PhACs Sorption?
5. Conclusions
- All five materials displayed a higher capacity for PhACs sorption than sand;
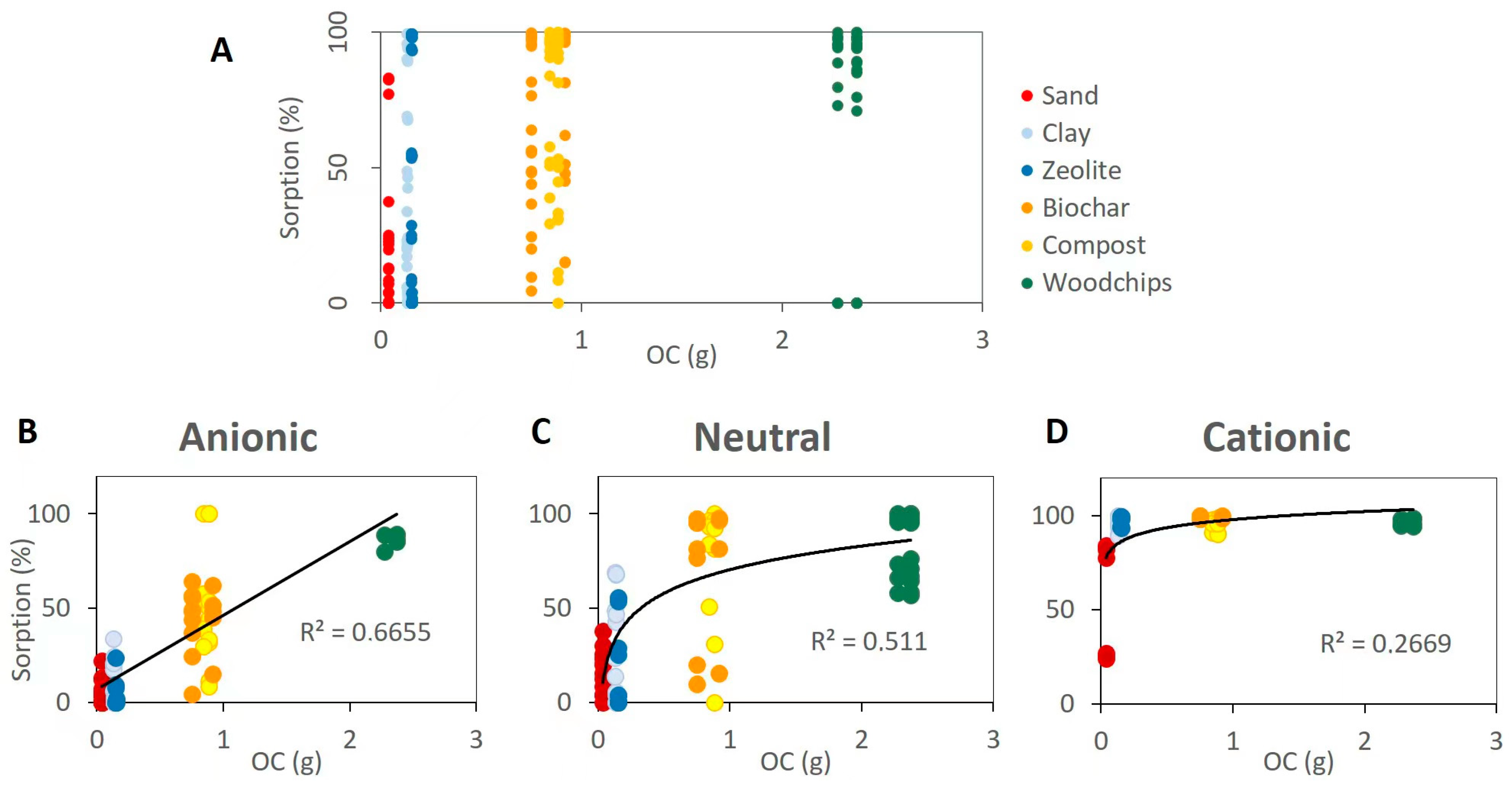
- The organic carbon content is the key parameter for the sorption performance of the materials;
- The PhACs speciation determines the mechanism of interaction with the surfaces and therefore the degree of retention;
- Cationic species displayed a high potential to be retained even in sand (13% of clay). Neutral species interact with materials showing a high organic carbon content, while their sorption on sand, clay, and zeolites was limited or moderate. Anionic species are the ones with the greatest risk of reaching the aquifer since their sorption is moderate even in materials with a high organic carbon content;
- The incorporation of a mixture of woodchips, compost, and biochar in reactive barriers will favor the retention of PhACs, increasing the time during which they are bioavailable for degradation, and therefore increasing the efficiency of SAT systems in water renaturalization.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgot, M.; Huertas, E.; Weber, S.; Dott, W.; Hollender, J. Wastewater Reuse and Risk: Definition of Key Objectives. Desalination 2006, 187, 29–40. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Urík, J.; Beretsou, V.G.; Kampouris, I.; Galani, A.; Oswaldova, M.; Berendonk, T.; Oswald, P.; Thomaidis, N.S.; Slobodnik, J.; et al. Evaluation of Chemical and Biological Contaminants of Emerging Concern in Treated Wastewater Intended for Agricultural Reuse. Environ. Int. 2020, 138, 105597. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Marques, R.; Noronha, J.P.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the Removal of Pharmaceuticals and Personal Care Products in a Full-Scale Activated Sludge Plant. Environ. Sci. Pollut. Res. 2012, 19, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, Fate, and Removal of Pharmaceutical Residues in the Aquatic Environment: A Review of Recent Research Data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, Sources and Fate of Pharmaceuticals and Personal Care Products in the Groundwater: A Review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Benteke Momba, M.N. Endocrine-Disruptive Chemicals as Contaminants of Emerging Concern in Wastewater and Surface Water: A Review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Fent, K. Estrogenic Activity of Ternary UV Filter Mixtures in Fish (Pimephales Promelas)—An Analysis with Nonlinear Isobolograms. Toxicol. Appl. Pharmacol. 2009, 234, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Yeh, A.; Gallagher, E.P. Adverse Metabolic Effects in Fish Exposed to Contaminants of Emerging Concern in the Field and Laboratory. Environ. Pollut. 2018, 236, 850–861. [Google Scholar] [CrossRef]
- Comer, J.E.A. 5.16—Ionization Constants and Ionization Profiles. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Oxford, UK, 2007; ISBN 978-0-08-045044-5. [Google Scholar]
- Manallack, D.T. The pKa Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar] [CrossRef]
- Ràfols, C.; Subirats, X.; Rubio, J.; Rosés, M.; Bosch, E. Lipophilicity of Amphoteric and Zwitterionic Compounds: A Comparative Study of Determination Methods. Talanta 2017, 162, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Peyret, T.; Poulin, P.; Krishnan, K. A Unified Algorithm for Predicting Partition Coefficients for PBPK Modeling of Drugs and Environmental Chemicals. Toxicol. Appl. Pharmacol. 2010, 249, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Strope, C.L.; Mansouri, K.; Clewell, H.J., 3rd; Rabinowitz, J.R.; Stevens, C.; Wambaugh, J.F. High-Throughput in-Silico Prediction of Ionization Equilibria for Pharmacokinetic Modeling. Sci. Total Environ. 2018, 615, 150–160. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. European Commission Decision (EU) 2020/1161; The European Commission: Brussel, Belgium, 2020; pp. 32–35. [Google Scholar]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Valhondo, C.; Carrera, J. Chapter 1—Water as a Finite Resource: From Historical Accomplishments to Emerging Challenges and Artificial Recharge. In Sustainable Water and Wastewater Processing; Galanakis, C.M., Agrafioti, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. ISBN 978-0-12-816170-8. [Google Scholar]
- Shaikh, I.N.; Mansoor Ahammed, M.; Sukanya Krishnan, M.P. Chapter 2—Graywater Treatment and Reuse. In Sustainable Water and Wastewater Processing; Galanakis, C.M., Agrafioti, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–54. ISBN 978-0-12-816170-8. [Google Scholar]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical Treatment Technologies for Waste-Water Recycling—An Overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Dillon, P.; Toze, S.; Page, D.; Vanderzalm, J.; Bekele, E.; Sidhu, J.; Rinck-Pfeiffer, S. Managed Aquifer Recharge: Rediscovering Nature as a Leading Edge Technology. Water Sci. Technol. 2010, 62, 2338–2345. [Google Scholar] [CrossRef]
- Valhondo, C.; Martínez-Landa, L.; Carrera, J.; Díaz-Cruz, S.M.; Amalfitano, S.; Levantesi, C. Six Artificial Recharge Pilot Replicates to Gain Insight into Water Quality Enhancement Processes. Chemosphere 2020, 240, 124826. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Guidelines for Water Reuse; US Environmental Protection Agency: Washington, DC, USA, 2012; Volume 26.
- Regnery, J.; Wing, A.D.; Alidina, M.; Drewes, J.E. Biotransformation of Trace Organic Chemicals during Groundwater Recharge: How Useful Are First-Order Rate Constants? J. Contam. Hydrol. 2015, 179, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Vanderzalm, J.L.; La Salle, C.L.G.; Dillon, P.J. Fate of Organic Matter during Aquifer Storage and Recovery (ASR) of Reclaimed Water in a Carbonate Aquifer. Appl. Geochem. 2006, 21, 1204–1215. [Google Scholar] [CrossRef]
- Dillon, P.; Page, D.; Vanderzalm, J.; Pavelic, P.; Toze, S.; Bekele, E.; Sidhu, J.; Prommer, H.; Higginson, S.; Regel, R.; et al. A Critical Evaluation of Combined Engineered and Aquifer Treatment Systems in Water Recycling. Water Sci. Technol. 2008, 57, 753–762. [Google Scholar] [CrossRef]
- Elkayam, R.; Aharoni, A.; Vaizel-Ohayon, D.; Katz, Y.; Negev, I.; Marano, R.B.; Cytryn, E.; Shtrasler, L.; Lev, O. Viral and Microbial Pathogens, Indicator Microorganisms, Microbial Source Tracking Indicators, and Antibiotic Resistance Genes in a Confined Managed Effluent Recharge System. J. Environ. Eng. 2018, 144, 05017011. [Google Scholar] [CrossRef]
- Fernández Escalante, E.; Henao Casas, J.D.; San Sebastián Sauto, J.; Calero Gil, R. Monitored and Intentional Recharge (MIR): A Model for Managed Aquifer Recharge (MAR) Guideline and Regulation Formulation. Water 2022, 14, 3405. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, X.; Xu, B.; Peng, D.; Guo, X. Sorption of Pharmaceuticals and Personal Care Products on Soil and Soil Components: Influencing Factors and Mechanisms. Sci. Total Environ. 2021, 753, 141891. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and Partitioning of Eight Selected Pharmaceuticals in the Aquatic Environment: Laboratory Photolysis, Biodegradation, and Sorption Experiments. Water Res. 2009, 43, 351–362. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, X.; Xing, B.; Chen, B. PH-Dependent Sorption of Sulfonamide Antibiotics onto Biochars: Sorption Mechanisms and Modeling. Environ. Pollut. 2019, 248, 48–56. [Google Scholar] [CrossRef]
- Franco, A.; Trapp, S. Estimation of the Soil-Water Partition Coefficient Normalized Organic Carbon for Ionizable Organic Chemicals. Environ. Toxicol. Chem. 2008, 27, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Asim, M.; Khan, T.A. Low Cost Adsorbents for the Removal of Organic Pollutants from Wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of Emerging Contaminants from the Environment by Adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Ibarra-Cabrera, R.; Tecante, A.; Gimeno, M.; Parra, R.; Garcia-Arrazola, R. Removal Strategies for Endocrine Disrupting Chemicals Using Cellulose-Based Materials as Adsorbents: A Review. J. Environ. Chem. Eng. 2016, 4, 3122–3142. [Google Scholar] [CrossRef]
- Franco, A.; Fu, W.; Trapp, S. Influence of Soil PH on the Sorption of Ionizable Chemicals: Modeling Advances. Environ. Toxicol. Chem. 2009, 28, 2018. [Google Scholar] [CrossRef] [PubMed]
- Valhondo, C.; Martinez-Landa, L.; Carrera, J.; Ayora, C.; Nödler, K.; Licha, T. Evaluation of EOC Removal Processes during Artificial Recharge through a Reactive Barrier. Sci. Total Environ. 2018, 612, 985–994. [Google Scholar] [CrossRef]
- Hellman, M.; Valhondo, C.; Martínez-Landa, L.; Carrera, J.; Juhanson, J.; Hallin, S. Nitrogen Removal Capacity of Microbial Communities Developing in Compost- and Woodchip-Based Multipurpose Reactive Barriers for Aquifer Recharge with Wastewater. Front. Microbiol. 2022, 13, 877990. [Google Scholar] [CrossRef]
- Abu, A.; Carrey, R.; Valhondo, C.; Domènech, C.; Soler, A.; Martínez-Landa, L.; Diaz-Cruz, S.; Carrera, J.; Otero, N. Pathways and Efficiency of Nitrogen Attenuation in Wastewater Effluent through Soil Aquifer Treatment. J. Environ. Manag. 2022, 321, 115927. [Google Scholar] [CrossRef]
- Valhondo, C.; Carrera, J.; Martínez-Landa, L.; Wang, J.; Amalfitano, S.; Levantesi, C.; Diaz-Cruz, M.S. Reactive Barriers for Renaturalization of Reclaimed Water during Soil Aquifer Treatment. Water 2020, 12, 1012. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ma, W.; Lu, G.; Meng, F.; Duan, S.; Zhang, Z.; Wei, L.; Pan, Y. Adsorption of Levofloxacin onto Mechanochemistry Treated Zeolite: Modeling and Site Energy Distribution Analysis. Sep. Purif. Technol. 2019, 222, 30–34. [Google Scholar] [CrossRef]
- Espinosa, N.J.; Moore, D.J.P.; Rasmussen, C.; Fehmi, J.S.; Gallery, R.E. Woodchip and Biochar Amendments Differentially Influence Microbial Responses, but Do Not Enhance Plant Recovery in Disturbed Semiarid Soils. Restor. Ecol. 2020, 28, S381–S392. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Belhachemi, M. Chapter 14—Adsorption of Organic Compounds on Activated Carbons. In Sorbents Materials for Controlling Environmental Pollution; Núñez-Delgado, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 355–385. ISBN 978-0-12-820042-1. [Google Scholar]
- Boehm, A.B.; Bell, C.D.; Fitzgerald, N.J.M.; Gallo, E.; Higgins, C.P.; Hogue, T.S.; Luthy, R.G.; Portmann, A.C.; Ulrich, B.A.; Wolfand, J.M. Biochar-Augmented Biofilters to Improve Pollutant Removal from Stormwater-Can They Improve Receiving Water Quality? Environ. Sci. Water Res. Technol. 2020, 6, 1520–1537. [Google Scholar] [CrossRef] [Green Version]
- Paradelo, R.; Al-Zawahreh, K.; Barral, M.T. Utilization of Composts for Adsorption of Methylene Blue from Aqueous Solutions: Kinetics and Equilibrium Studies. Materials 2020, 13, 2179. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, R.A.; Howard, S.M. Use of Distribution Coefficients in Quantitative Structure-Activity Relationships. J. Med. Chem. 1977, 20, 53–58. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Zhang, M. Chapter 8—Biochar for Anionic Contaminants Removal from Water. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–160. ISBN 978-0-12-811729-3. [Google Scholar]
- ter Laak, T.L.; Gebbink, W.A.; Tolls, J. The Effect of PH and Ionic Strength on the Sorption of Sulfachloropyridazine, Tylosin, and Oxytetracycline to Soil. Environ. Toxicol. Chem. 2006, 25, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.; Kröger, K.F.; Nödler, K.; Ayora, C.; Carrera, J.; Hernández, M.; Licha, T. Influence of a Compost Layer on the Attenuation of 28 Selected Organic Micropollutants under Realistic Soil Aquifer Treatment Conditions: Insights from a Large Scale Column Experiment. Water Res. 2015, 74, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Sunyer-Caldú, A.; Benedetti, B.; Valhondo, C.; Martínez-Landa, L.; Carrera, J.; Di Carro, M.; Magi, E.; Diaz-Cruz, M.S. Using Integrative Samplers to Estimate the Removal of Pharmaceuticals and Personal Care Products in a WWTP and by Soil Aquifer Treatment Enhanced with a Reactive Barrier. Sci. Total Environ. 2023, 867, 161466. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Taoufik, N.; García, A.M.; Korili, S.A. Comparative Removal of Emerging Contaminants from Aqueous Solution by Adsorption on an Activated Carbon. Environ. Technol. 2019, 40, 3017–3030. [Google Scholar] [CrossRef]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis Tef) Agricultural Waste. J. Thermodyn. 2013, 2013, 375830. [Google Scholar] [CrossRef] [Green Version]
- Saad, D.M.G.; Cukrowska, E.M.; Tutu, H. Phosphonated Cross-Linked Polyethylenimine for Selective Removal of Uranium Ions from Aqueous Solutions. Water Sci. Technol. 2012, 66, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossner, A.; Snyder, S.A.; Knappe, D.R.U. Removal of Emerging Contaminants of Concern by Alternative Adsorbents. Water Res. 2009, 43, 3787–3796. [Google Scholar] [CrossRef]
- Lian, F.; Sun, B.; Song, Z.; Zhu, L.; Qi, X.; Xing, B. Physicochemical Properties of Herb-Residue Biochar and Its Sorption to Ionizable Antibiotic Sulfamethoxazole. Chem. Eng. J. 2014, 248, 128–134. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Seo, Y.-D. Sorption of Halogenated Phenols and Pharmaceuticals to Biochar: Affecting Factors and Mechanisms. Environ. Sci. Pollut. Res. 2016, 23, 951–961. [Google Scholar] [CrossRef]
- Hopkins, M.A.M.; Kuster, A.M.; Vogel, J.R.; Brown, G.O. Pollutant Removal in Stormwater by Woodchips. Int. J. Environ. Sci. Nat. Resour. 2021, 26, 154–160. [Google Scholar] [CrossRef]
- Scheytt, T.J.; Mersmann, P.; Heberer, T. Mobility of Pharmaceuticals Carbamazepine, Diclofenac, Ibuprofen, and Propyphenazone in Miscible-Displacement Experiments. J. Contam. Hydrol. 2006, 83, 53–69. [Google Scholar] [CrossRef]
- Villaescusa, I.; Fiol, N.; Poch, J.; Bianchi, A.; Bazzicalupi, C. Mechanism of Paracetamol Removal by Vegetable Wastes: The Contribution of π–π Interactions, Hydrogen Bonding and Hydrophobic Effect. Desalination 2011, 270, 135–142. [Google Scholar] [CrossRef]
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and Mobility of Pharmaceutical Compounds in Soil Irrigated with Reclaimed Wastewater. Chemosphere 2008, 73, 1335–1343. [Google Scholar] [CrossRef]
- Wang, J.; Carrera, J.; Saaltink, M.W.; Valhondo, C. On the Localization of Chemical Reactions in Multicontinuum Media. Adv. Water Resour. 2022, 167, 104286. [Google Scholar] [CrossRef]
- Lu, L.; Chen, B. Enhanced Bisphenol A Removal from Stormwater in Biochar-Amended Biofilters: Combined with Batch Sorption and Fixed-Bed Column Studies. Environ. Pollut. 2018, 243, 1539–1549. [Google Scholar] [CrossRef] [PubMed]


| Material | %OC | pH (Eq with Water) | CEC (meq/100 g) | Reactive Surface (m2/g) |
|---|---|---|---|---|
| Clay | 3.11 | 8.71 | 20.4 | 23 |
| Sand | 0.73 | 8.5 | 2.3 | 3 |
| Zeolite | 3.76 | 6.36 | 16.6 | 33 |
| Biochar | 83.32 | 9.38 | 6.9 | 98 |
| Vegetable Compost | 21.03 | 7.82 | 43.9 | 5 |
| Woodchips | 94.83 | 5.26 | 10.7 | - |
| Molecule | Acronym | Formula a | Solubility in Water a (mg/L) | Molecular Weight a | pKa a (Character) | Log Kowa | Species b at pH= | ||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | |||||||
| Sulfamethoxazole | SMX | C10H11N3O3S | 610 | 253.28 | 1.6 (A), 5.7 (B) | 0.89 | 0 | 0, − | − |
| Oxazepam | OXA | C15H11ClN2O2 | 20 | 286.71 | 1.55 (B), 10.9 (A) | 2.24 | 0 | 0 | 0 |
| Carbamazepine | CBZ | C15H12N2O | 18 | 236.27 | −3.8 (B), 13.9 (A) | 2.45 | 0 | 0 | 0 |
| Diazepam | DIA | C16H13ClN2O | 66 | 284.74 | 3.4 (B) | 2.82 | +, 0 | 0 | 0 |
| Propanolol | PRL | C16H21NO2 | 62 | 259.34 | 9.42 (B) | 3.48 | + | + | + |
| Atenolol | ATL | C14H22N2O3 | 13 | 266.34 | 9.6 (B) | 0.16 | + | + | + |
| Venlafaxine | VLX | C17H27NO2 | 572 | 277.4 | 10.09 (B) | 3.2 | + | + | + |
| Fenofibric Acid | FFA | C17H15ClO4 | 5 | 318.7 | 3.1 (A) | 4.45 | 0, − | − | − |
| Diclofenac | DCL | C14H11Cl2NO2 | 2 | 296.1 | 4.15 (A) | 4.51 | 0, − | − | − |
| Ketoprofen | KTO | C16H14O3 | 51 | 254.28 | 4.45 (A) | 3.12 | 0, − | − | − |
| Ibuprofen | IBU | C13H18O2 | 21 | 206.28 | 5.3 (A) | 3.97 | 0 | 0, − | − |
| Paracetamol | PRM | C8H9NO2 | 14 | 151.16 | 9.38 (A) | 0.46 | 0 | 0 | 0, − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valhondo, C.; Duporté, G.; Cabaret, G.; Rosain, D.; Gomez, E.; Luquot, L. Assessing the Feasibility of Sustainable Materials to Boost the Sorption of Pharmaceutical Active Compounds When Included in Reactive Barriers in Soil Aquifer Treatment for Water Reuse. Water 2023, 15, 1393. https://doi.org/10.3390/w15071393
Valhondo C, Duporté G, Cabaret G, Rosain D, Gomez E, Luquot L. Assessing the Feasibility of Sustainable Materials to Boost the Sorption of Pharmaceutical Active Compounds When Included in Reactive Barriers in Soil Aquifer Treatment for Water Reuse. Water. 2023; 15(7):1393. https://doi.org/10.3390/w15071393
Chicago/Turabian StyleValhondo, Cristina, Geoffroy Duporté, Gaëlle Cabaret, David Rosain, Elena Gomez, and Linda Luquot. 2023. "Assessing the Feasibility of Sustainable Materials to Boost the Sorption of Pharmaceutical Active Compounds When Included in Reactive Barriers in Soil Aquifer Treatment for Water Reuse" Water 15, no. 7: 1393. https://doi.org/10.3390/w15071393








