Regional Trends of Biodiversity Indices in the Temperate Mesic United States: Testing for Influences of Anthropogenic Land Use on Stream Fish while Controlling for Natural Landscape Variables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Stream Layer
2.3. Natural Landscape Variables
2.4. Anthropogenic Land Uses
2.5. Fish Data
2.6. Biodiversity Indices
2.7. Data Analysis
2.7.1. Comparing Landscape Features across Regions
2.7.2. Pearson’s Correlation among Fish Biodiversity Indices
2.7.3. Regression Analysis
3. Results
3.1. Study Area
3.2. Regional Patterns in Biodiversity Indices
3.3. Regional Patterns in the Correlation of Biodiversity Indices
3.4. Predicting Biodiversity Indices from Landscape Variables with Linear Regression Models
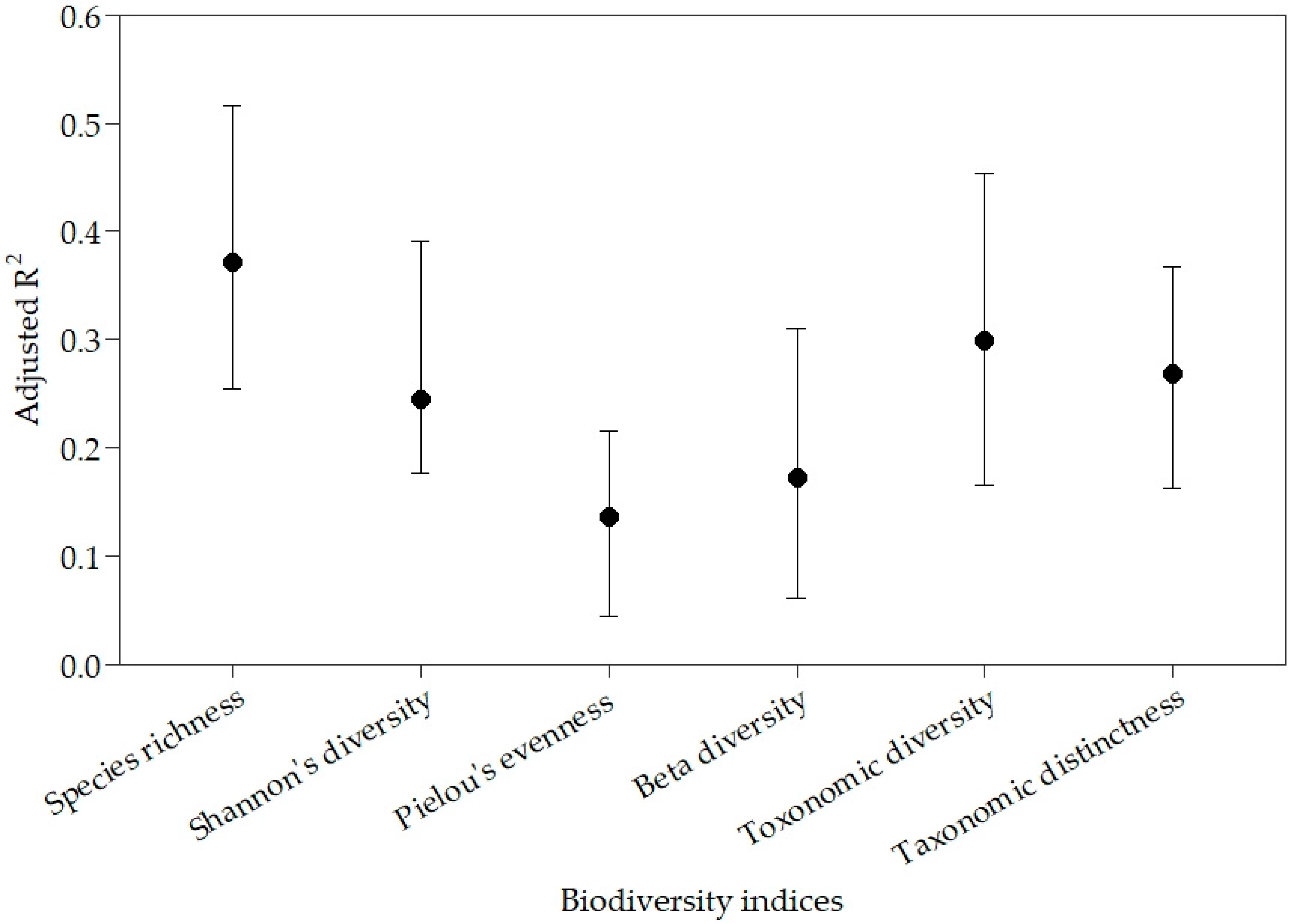
3.5. Regional Differences in Predicting Biodiversity Indices from Landscape Variables
4. Discussion
4.1. Correlation among Biodiversity Indices
4.2. Natural Landscape Variables Predicting Biodiversity Indices
4.3. Anthropogenic Land Uses Predicting Biodiversity Indices
4.4. Spatial Extent of Study and Scope of Natural and Anthropogenic Environmental Gradients Captured
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Helfman, G.S. Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources; Island Press: Washington, DC, USA, 2007. [Google Scholar]
- Jelks, H.L.; Walsh, S.J.; Burkhead, N.M.; Contreras-Balderas, S.; Díaz-Pardo, E.; Hendrickson, D.A.; Lyons, J.; Mandrak, N.E.; McCormick, F.; Nelson, J.S.; et al. Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries 2008, 33, 372–407. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, N.M. Extinction rates in North American freshwater fishes 1900–2010. BioScience 2012, 62, 798–808. [Google Scholar] [CrossRef]
- Dudgeon, D. Threats to freshwater biodiversity in a changing world. In Global Environmental Change; Springer: Berlin/Heidelberg, Germany, 2014; pp. 243–253. [Google Scholar]
- Rahel, F.J. Homogenization of fish faunas across the United States. Science 2000, 288, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.C.; Helfman, G.S. Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries 2001, 26, 6–15. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Ecological processes driving biotic homogenization: Testing a mechanistic model using fish fauna. Ecology 2004, 85, 1867–1875. [Google Scholar] [CrossRef]
- Scott, M.C. Winners and losers among stream fishes in relation to land use legacies and urban development in the southeastern US. Biol. Conserv. 2006, 127, 301–309. [Google Scholar] [CrossRef]
- Villéger, S.; Blanchet, S.; Beauchard, O.; Oberdorff, T.; Brosse, S. Homogenization patterns of the world’s freshwater fish faunas. Proc. Natl. Acad. Sci. USA 2011, 108, 18003–18008. [Google Scholar] [CrossRef]
- Marr, S.M.; Olden, J.D.; Leprieur, F.; Arismendi, I.; Ćaleta, M.; Morgan, D.L.; Nocita, A.; Šanda, R.; Tarkan, A.S.; García-Berthou, E. A global assessment of freshwater fish introductions in Mediterranean-climate regions. Hydrobiologia 2013, 719, 317–329. [Google Scholar] [CrossRef]
- Toussaint, A.; Beauchard, O.; Oberdorff, T.; Brosse, S.; Villéger, S. Historical assemblage distinctiveness and the introduction of widespread non-native species explain worldwide changes in freshwater fish taxonomic dissimilarity. Glob. Ecol. Biogeogr. 2014, 23, 574–584. [Google Scholar] [CrossRef]
- Schweiger, O.; Klotz, S.; Durka, W.; Kühn, I. A comparative test of phylogenetic diversity indices. Oecologia 2008, 157, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Magurran, A.E. Measuring Biological Diversity, 2nd ed.; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Wang, L.; Lyons, J.; Kanehl, P. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environ. Manag. 2001, 28, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Esselman, P.C.; Infante, D.M.; Wang, L.; Wu, D.; Cooper, A.R.; Taylor, W.W. An index of cumulative disturbance to river fish habitats of the conterminous United States from landscape anthropogenic activities. Ecol. Restor. 2011, 29, 133–151. [Google Scholar] [CrossRef]
- Hughes, R.M.; Kaufmann, P.R.; Herlihy, A.T.; Kincaid, T.M.; Reynolds, L.; Larsen, D.P. A process for developing and evaluating indices of fish assemblage integrity. Can. J. Fish. Aquat. Sci. 1998, 55, 1618–1631. [Google Scholar] [CrossRef]
- Pont, D.; Hugueny, B.; Beier, U.; Goffaux, D.; Melcher, A.; Noble, R.; Rogers, C.; Roset, N.; Schmutz, S. Assessing river biotic condition at the continental scale: A European approach using functional metrics and fish assemblages. J. Appl. Ecol. 2006, 43, 70–80. [Google Scholar] [CrossRef]
- Hocutt, C.H.; Wiley, E.O. (Eds.) The Zoogeography of North American Freshwater Fishes; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Mayden, R.L. Vicariance biogeography, parsimony, and evolution in North American freshwater fishes. Syst. Zool. 1988, 37, 329–355. [Google Scholar] [CrossRef]
- Schlosser, I.J. Stream fish ecology: A landscape perspective. BioScience 1991, 41, 704–712. [Google Scholar] [CrossRef]
- Goldstein, R.M.; Meador, M.R. Comparisons of fish species traits from small streams to large rivers. Tran. Am. Fish. Soc. 2004, 133, 971–983. [Google Scholar] [CrossRef]
- Stirling, G.; Wilsey, B. Empirical relationships between species richness, evenness, and proportional diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Podani, J.; Ricotta, C.; Schmera, D. A general framework for analyzing beta diversity, nestedness and related community-level phenomena based on abundance data. Ecol. Complex. 2013, 15, 52–61. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Magurran, A.E.; Phillip, D.A.T. Implications of species loss in freshwater fish assemblages. Ecography 2001, 24, 645–650. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. New ‘biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 21, 265–278. [Google Scholar] [CrossRef]
- Hughes, R.M.; Whittier, C.M.R.; Larsen, D.P. A regional framework for establishing recovery criteria. Environ. Manag. 1990, 14, 673–683. [Google Scholar] [CrossRef]
- Angermeier, P.L.; Winston, M.R. Characterizing fish community diversity across Virginia landscapes: Prerequisite for conservation. Ecol. Appl. 1999, 9, 335–349. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Edwards, C.J.; Jensen, M.E.; Paustian, S.J.; Parrott, H.; Hill, D.M. A Hierarchical Framework of Aquatic Ecological Units in North America (Nearctic Zone). In General Technical Report NC–176; USDA Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1995. [Google Scholar]
- Matthews, W.J. Patterns in Freshwater Fish Ecology; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Abell, R.; Olsen, D.M.; Dinerstein, E.; Hurley, P.; Diggs, J.T.; Eichbaum, W.; Walters, S.; Wettengel, W.; Allnutt, T.; Loucks, C.J.; et al. Freshwater Ecoregions of North America: A Conservation Assessment; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Contreras Balderas, S.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Deweber, J.T.; Sleezer, L.; Frimpong, E.A. A new regionalization framework to quantify how physiography mediates the effect of land use on stream fishes 2019. In Advances in Understanding Landscape Influences on Freshwater Habitats and Biological Assemblages; Hughes, R.M., Infante, D.M., Wang, L., Chen, K., Terra, B.F., Eds.; American Fisheries Society Symposium: Bethesda, MD, USA, 2019; pp. 321–350. [Google Scholar]
- Herlihy, A.T.; Sifneos, J.C.; Hughes, R.M.; Peck, D.V.; Mitchell, R.M. Lotic fish assemblage clusters across the conterminous United States and their associations with environmental variables 2019. In Advances in Understanding Landscape Influences on Freshwater Habitats and Biological Assemblages; Hughes, R.M., Infante, D.M., Wang, L., Chen, K., Terra, B.F., Eds.; American Fisheries Society Symposium: Bethesda, MD, USA, 2019; pp. 385–408. [Google Scholar]
- U.S. Environmental Protection Agency. U.S. Geological Survey (USEPA & USGS) National Hydrography Dataset Plus, NHDPlusV1. 2005. Available online: www.horizon-systems.com/nhdplus/ (accessed on 12 November 2014).
- Wang, L.; Infante, D.; Esselman, P.; Cooper, A.; Wu, D.; Taylor, W.; Beard, D.; Whelan, G.; Ostroff, A. A hierarchical spatial framework and database for the national river fish habitat condition assessment. Fisheries 2011, 36, 436–449. [Google Scholar] [CrossRef]
- Gesch, D.B. The National Elevation Dataset. In Digital Elevation Model Technologies and Applications: The DEM User’s Manual, 2nd ed.; Maune, D., Ed.; ASPRS: Bethesda, MD, USA, 2007; pp. 99–118. [Google Scholar]
- Daly, C.; Halbleib, M.; Smith, J.I.; Gibson, W.P.; Doggett, M.K.; Taylor, G.H.; Curtis, J.; Pasteris, P.P. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int. J. Climatol. 2008, 28, 2031–2064. [Google Scholar] [CrossRef]
- PRISM Climate Group. Oregon State University. 2013. Available online: http://www.prism.oregonstate.edu/ (accessed on 14 March 2023).
- Tsang, Y.; Wieferich, D.; Fung, K.; Infante, D.M.; Cooper, A.R. An approach for aggregating upstream catchment information to support research and management of fluvial systems across large landscapes. SpringerpPlus 2014, 23, 589. [Google Scholar] [CrossRef] [PubMed]
- Soller, D.R.; Reheis, M.C. Surficial Materials in the Conterminous United States: U.S. Geological Survey 2004. Open–File Report 03–275, Scale 1:5,000,000. Available online: http://pubs.usgs.gov/of/2003/of03–275/ (accessed on 12 November 2014).
- Cress, J.; Soller, D.; Sayre, R.; Comer, P.; Warner, H. Terrestrial Ecosystems, Surficial Lithology of the Conterminous United States: U.S. Geological Survey Scientific Investigations Map 3126, scale 1:5,000,000, 1 Sheet. 2010. Available online: http://pubs.usgs.gov/sim/3126 (accessed on 12 November 2014).
- Wolock, D.M. Base-Flow Index Grid for the Conterminous United States: U.S. Geological Survey Open-File Report 03–263, Digital Data Set. 2003. Available online: http://water.usgs.gov/lookup/getspatial?bfi48grd (accessed on 12 November 2014).
- Perkin, J.S.; Troia, M.J.; Shaw, D.C.R.; Gerken, J.E.; Gido, K.B. Multiple watershed alterations influence fish community structure in Great Plains prairie streams. Ecol. Freshw. Fish 2016, 25, 141–155. [Google Scholar] [CrossRef]
- Cheng, S.T.; Herricks, E.E.; Tsai, W.P.; Chang, F.J. Assessing the natural and anthropogenic influences on basin-wide fish species richness. Sci. Total Environ. 2016, 572, 825–836. [Google Scholar] [CrossRef]
- Cooper, A.R.; Infante, D.M.; Wehrly, K.E.; Wang, L.; Brenden, T.O. Identifying indicators and quantifying large-scale effects of dams on fishes. Ecol. Indic. 2016, 61, 646–657. [Google Scholar] [CrossRef]
- Cooper, A.R.; Infante, D.M.; Daniel, W.M.; Wehrly, K.E.; Wang, L.; Brenden, T.O. Assessment of dam effects on streams and fish assemblages of the conterminous USA. Sci. Total Environ. 2017, 586, 879–889. [Google Scholar] [CrossRef]
- Thornbrugh, D.J.; Infante, D.M. Landscape effects on steam fishes: Broad-scale responses to anthropogenic land use across temperate mesic regions of the United States. In Advances in Understanding Landscape Influences on Freshwater Habitats and Biological Assemblages; Hughes, R.M., Infante, D.M., Wang, L., Chen, K., Terra, B.F., Eds.; American Fisheries Society Symposium: Bethesda, MD, USA, 2019; p. 90. [Google Scholar]
- Bouska, W.W.; Paukert, C.P. Road crossing designs and their impact on fish assemblages of Great Plains streams. Trans. Am. Fish. Soc. 2010, 139, 214–222. [Google Scholar] [CrossRef]
- Januchowski–Hartley, S.R.; McIntyre, P.B.; Diebel, M.; Doran, P.J.; Infante, D.M.; Joseph, C.; Allan, J.D. Restoring aquatic ecosystem connectivity requires expanding barrier inventories. Fron. Ecol. Environ. 2013, 11, 211–217. [Google Scholar] [CrossRef]
- Homer, C.; Huang, C.; Yang, L.; Wylie, B.; Coan, M. Development of a 2001 National Landcover Database for the United States. PERS 2004, 70, 829–840. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) United States Environmental Protection Agency Multi-Resolution Land Characteristics Consortium. 2008. Available online: www.epa.gov/mrlc/ (accessed on 12 October 2021).
- U.S. Geological Survey (USGS) National Anthropogenic Barrier Dataset (NABD). 2012. Available online: https://www.sciencebase.gov/catalog/item/512cf142e4b0855fde669828 (accessed on 12 November 2014).
- U.S. Census Bureau, Redistricting Census 2000 TIGER/ Line Files [Machine-Readable Data Files]. Washington, DC: U.S. Census Bureau. 2002. Available online: www.census.gov/geo/maps-data/data/tiger.html (accessed on 12 November 2014).
- Esselman, P.C.; Infante, D.M.; Wang, L.; Cooper, A.R.; Wieferich, D.; Tsang, Y.; Thornbrugh, D.J.; Taylor, W.W. Regional fish community indicators of landscape disturbance to catchments of conterminous United States. Ecol. Indic. 2013, 26, 163–173. [Google Scholar] [CrossRef]
- Daniel, W.M.; Infante, D.M.; Hughes, R.M.; Tsang, Y.; Esselman, P.C.; Wieferich, D.; Herreman, K.; Cooper, A.R.; Wang, L.; Taylor, W.W. Characterizing coal and mineral mines as a regional source of stress to stream fish assemblages. Ecol. Indic. 2015, 50, 50–61. [Google Scholar] [CrossRef]
- ITIS Retrieved [04, 26, 2010], from the Integrated Taxonomic Information System. 2010. Available online: http://www.itis.gov (accessed on 12 October 2021).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; Wiley and Sons: New York, NY, USA, 1975. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. ‘Vegan’: Community Ecology Package, R Package Version 2.0–9. 2020. Available online: http://CRAN.R-project.org/package=vegan (accessed on 12 January 2023).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: http://www.R-project.org/ (accessed on 12 January 2023).
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. Adespatial: Multivariate Multiscale Spatial Analysis, R Package Version 0.3-16. 2022. Available online: https://CRAN.R-project.org/package=adespatial (accessed on 12 January 2023).
- Little, J.D.C.; Murty, K.G.; Sweeney, D.W.; Karel, C. An algorithm for the traveling salesman problem. Oper. Res. 1963, 11, 972–989. [Google Scholar] [CrossRef]
- Miller, A.J. Subset Selection in Regression, 2nd ed.; Chapman and Hall: New York, NY, USA, 2002. [Google Scholar]
- Lumley, T. Based on Fortran Code by Alan Miller. Leaps: Regression Subset Selection, R Package Version 3.1; 2020. Available online: https://CRAN.R-project.org/package=leaps (accessed on 12 January 2023).
- McCuen, R.H.; Snyder, W.M. Hydrologic Modelling: Statistical Method and Applications; Prentice-Hall, A Division of Simon & Schuster, Inc.: Englewood Cliffs, NJ, USA, 1985. [Google Scholar]
- Tsang, Y.; Felton, G.K.; Moglen, G.E.; Paul, M. Region of influence method improves macroinvertebrate predictive models in Maryland. Ecol. Modell. 2011, 222, 3473–3485. [Google Scholar] [CrossRef]
- Fletcher, T.D. QuantPsyc: Quantitative Psychology Tools, R Package Version 1.6. 2022. Available online: https://CRAN.R-project.org/package=QuantPsyc (accessed on 12 January 2023).
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd English ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Heino, J.; Mykrä, H.; Hämäläinen, H.; Aroviita, J.; Muotka, T. Responses of taxonomic distinctness and species diversity indices to anthropogenic impacts and natural environmental gradients in stream macroinvertebates. Freshw. Biol. 2007, 52, 1846–1861. [Google Scholar] [CrossRef]
- Heino, J.; Soininen, J.; Lappalainen, J.; Virtanen, R. The relationship between species richness and taxonomic distinctness in freshwater organisms. Limnol. Oceanogr. 2005, 5, 978–986. [Google Scholar] [CrossRef]
- Eadie, J.M.; Hurly, T.A.; Montgomerie, R.D.; Teather, K.L. Lakes and rivers as islands: Species—Area relationships in the fish faunas of Ontario. Environ. Biol. Fishes 1986, 15, 81–89. [Google Scholar] [CrossRef]
- Angermeier, P.L.; Schlosser, I.J. Species-area relationship for stream fishes. Ecology 1989, 70, 1450–1462. [Google Scholar] [CrossRef]
- Thornbrugh, D.J.; Gido, K.B. Influence of spatial positioning within stream networks on fish assemblage structure in the Kansas River basin, USA. Can. J. Fish. Aquat. Sci. 2010, 67, 143–156. [Google Scholar] [CrossRef]
- Sheldon, A.L. Species diversity and longitudinal succession in stream fishes. Ecology 1968, 49, 193–198. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Marsh-Matthews, E.; Matthews, W.J. Geographic, terrestrial and aquatic factors: Which most influence the structure of stream fish assemblages in the Midwestern United States? Ecol. Freshw. Fish 2000, 9, 9–21. [Google Scholar] [CrossRef]
- Angermeier, P.L.; Winston, M.R. Local vs. regional influences on local diversity in stream fish communities of Virginia. Ecology 1998, 79, 911–927. [Google Scholar] [CrossRef]
- Jackson, D.A.; Peres-Neto, P.R.; Olden, J.D. What controls who is where in freshwater fish communities—The roles of biotic, abiotic, and spatial factors. Can. J. Fish. Aquat. Sci. 2011, 58, 157–170. [Google Scholar]
- Zorn, T.G.; Seelbach, P.W.; Wiley, M.J. Distributions of stream fishes and their relationship to stream size and hydrology in Michigan’s Lower Peninsula. Trans. Am. Fish. Soc. 2002, 131, 70–85. [Google Scholar] [CrossRef]
- Wehrly, K.E.; Wiley, M.J.; Seelbach, P.W. Classifying regional variation in thermal regime based on stream fish community patterns. Trans. Am. Fish. Soc. 2003, 132, 18–38. [Google Scholar] [CrossRef]
- Buisson, L.; Thuiller, W.; Lek, S.; Limp, P.; Grenouillet, G. Climate change hastens the turnover of stream fish assemblages. Glob. Chang. Biol. 2008, 14, 2232–2248. [Google Scholar] [CrossRef]
- Olden, J.D.; Kennard, M.J.; Leprieur, F.; Tedesco, P.A.; Winemiller, K.O.; García-Berthou, E. Conservation biogeography of freshwater fishes: Recent progress and future challenges. Divers. Distrib. 2010, 16, 496–513. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Kanehl, P.; Gatti, R. Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries 1997, 22, 6–12. [Google Scholar] [CrossRef]
- Utz, R.M.; Hilderbrand, R.M.; Raesly, R.L. Regional differences in patterns of fish species loss with changing land use. Biol. Conserv. 2010, 143, 688–699. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Rahel, F.J. Homogenization of freshwater faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, J.L.; Herlihy, A.T.; Peck, D.V.; Hughes, R.M.; Whittier, T.R.; Tarquinio, E. A process for creating multimetric indices for large-scale aquatic surveys. J. N. Am. Benthol. Soc. 2008, 27, 878–891. [Google Scholar] [CrossRef]
- Perkin, J.S.; Gido, K.B. Fragmentation alters stream fish community structure in dendritic ecological networks. Ecol. Appl. 2012, 22, 2176–2187. [Google Scholar] [CrossRef]

| Appalachian Piedmont (n = 1083) | Chesapeake Bay (n = 807) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Landscape Variable Description | Mean | Min | Max | 10% | 90% | Mean | Min | Max | 10% | 90% | |||||
| Natural | |||||||||||||||
| Network catchment area (km2) | 155.16 | 1.42 | 8062.60 | 11.42 | 180.03 | 371.17 | 0.82 | 8483.38 | 3.48 | 560.87 | |||||
| Fine lithology in network catchment (%) | 21.76 | 0.00 | 100.00 | 0.00 | 100.00 | 13.22 | 0.00 | 100.00 | 0.00 | 99.80 | |||||
| Mean local catchment elevation (m) | 157.30 | 1.39 | 937.66 | 20.42 | 298.06 | 288.25 | 2.52 | 1050.68 | 21.71 | 491.14 | |||||
| Base-flow index, network catchment groundwater contribution to baseflow (%) | 45.95 | 25.23 | 78.30 | 30.54 | 60.35 | 46.26 | 26.99 | 64.02 | 38.08 | 54.74 | |||||
| Mean annual air temperature in local catchment (°C) | 15.64 | 9.53 | 19.33 | 14.11 | 17.52 | 9.57 | 6.35 | 14.28 | 7.00 | 13.25 | |||||
| Area weighted average of mean annual precipitation in network catchment (mm) | 1215.13 | 973.82 | 2372.90 | 1125.53 | 1317.59 | 1027.70 | 806.87 | 1239.06 | 914.37 | 1108.89 | |||||
| Anthropogenic land uses | |||||||||||||||
| Urban (%) | |||||||||||||||
| Developed = Low, medium, & high intensity developed and open developed land in network catchment | 14.20 | 0.00 | 98.62 | 3.13 | 37.22 | 8.15 | 0.00 | 98.44 | 1.27 | 14.85 | |||||
| Agriculture (%) | |||||||||||||||
| Pasture/hay & cultivated crops in network catchment | 21.34 | 0.00 | 72.63 | 3.83 | 40.93 | 31.59 | 0.00 | 94.91 | 7.72 | 63.49 | |||||
| Road crossings | |||||||||||||||
| Density of road crossings in network catchment (number/100 km2) | 48.96 | 0.00 | 345.30 | 15.82 | 89.03 | 53.99 | 0.00 | 588.24 | 9.40 | 96.04 | |||||
| Dams | |||||||||||||||
| Dam density (number/100 km2) | 3.32 | 0.00 | 57.56 | 0.00 | 9.06 | 1.10 | 0.00 | 30.53 | 0.00 | 3.04 | |||||
| Laurentian Great Lakes (n = 3773) | Upper Mississippi (n = 3973) | ||||||||||||||
| Landscape Variable Description | Mean | Min | Max | 10% | 90% | Mean | Min | Max | 10% | 90% | |||||
| Natural | |||||||||||||||
| Network catchment area (km2) | 354.81 | 0.23 | 9533.74 | 10.27 | 758.72 | 356.01 | 0.23 | 9964.22 | 9.65 | 740.40 | |||||
| Fine lithology in network catchment (%) | 41.97 | 0.00 | 100.00 | 0.00 | 100.00 | 9.49 | 0.00 | 100.00 | 0.00 | 27.43 | |||||
| Mean local catchment elevation (m) | 277.15 | 74.56 | 680.78 | 180.28 | 430.92 | 288.86 | 114.85 | 589.09 | 184.87 | 382.03 | |||||
| Base-flow index, network catchment groundwater contribution to baseflow (%) | 45.04 | 18.69 | 87.95 | 21.99 | 68.45 | 49.85 | 7.84 | 74.87 | 31.21 | 66.29 | |||||
| Mean annual air temperature in local catchment (°C) | 7.74 | 2.12 | 10.56 | 4.69 | 9.68 | 7.56 | 3.07 | 13.60 | 5.48 | 10.61 | |||||
| Area weighted average mean of annual precipitation in network catchment (mm) | 929.80 | 641.04 | 1566.84 | 764.17 | 1123.50 | 823.55 | 556.81 | 1190.27 | 704.84 | 949.22 | |||||
| Human land uses | |||||||||||||||
| Urban (%) | |||||||||||||||
| Developed = Low, medium, & high intensity developed and open developed land in network catchment | 11.05 | 0.00 | 99.92 | 1.15 | 26.31 | 8.65 | 0.00 | 99.46 | 3.24 | 13.05 | |||||
| Agriculture (%) | |||||||||||||||
| Pasture/hay & cultivated crops in network catchment | 39.85 | 0.00 | 94.26 | 0.47 | 83.07 | 60.21 | 0.00 | 98.06 | 15.54 | 89.80 | |||||
| Road crossings | |||||||||||||||
| Density of road crossings in network catchment (number/100 km2) | 48.49 | 0.00 | 413.37 | 8.35 | 91.15 | 39.58 | 0.00 | 643.09 | 8.88 | 69.17 | |||||
| Dams | |||||||||||||||
| Dam density (number/100 km2) | 1.13 | 0.00 | 73.69 | 0.00 | 2.84 | 0.77 | 0.00 | 39.03 | 0.00 | 1.88 | |||||
| Upper Mississippi (n = 3973) | |||||||||||||||
| Landscape Variable Description | Mean | Min | Max | 10% | 90% | ||||||||||
| Natural | |||||||||||||||
| Network catchment area (km2) | 356.01 | 0.23 | 9964.22 | 9.65 | 740.40 | ||||||||||
| Fine lithology in network catchment (%) | 9.49 | 0.00 | 100.00 | 0.00 | 27.43 | ||||||||||
| Mean local catchment elevation (m) | 288.86 | 114.85 | 589.09 | 184.87 | 382.03 | ||||||||||
| Base-flow index, network catchment groundwater contribution to baseflow (%) | 49.85 | 7.84 | 74.87 | 31.21 | 66.29 | ||||||||||
| Mean annual air temperature in local catchment (°C) | 7.56 | 3.07 | 13.60 | 5.48 | 10.61 | ||||||||||
| Area weighted average mean of annual precipitation in network catchment (mm) | 823.55 | 556.81 | 1190.27 | 704.84 | 949.22 | ||||||||||
| Human land uses | |||||||||||||||
| Urban (%) | |||||||||||||||
| Developed = Low, medium, & high intensity developed and open developed land in network catchment | 8.65 | 0.00 | 99.46 | 3.24 | 13.05 | ||||||||||
| Agriculture (%) | |||||||||||||||
| Pasture/hay & cultivated crops in network catchment | 60.21 | 0.00 | 98.06 | 15.54 | 89.80 | ||||||||||
| Road crossings | |||||||||||||||
| Density of road crossings in network catchment (number/100 km2) | 39.58 | 0.00 | 643.09 | 8.88 | 69.17 | ||||||||||
| Dams | |||||||||||||||
| Dam density (number/100 km2) | 0.77 | 0.00 | 39.03 | 0.00 | 1.88 | ||||||||||
| Freshwater Ecoregion | Species Richness (S) | Shannon’s Diversity (H′) | Pielou’s Evenness (J′) | Taxonomic Diversity (Δ) | Taxonomic Distinctness (Δ*) | Beta Diversity (β) | |
|---|---|---|---|---|---|---|---|
| Appalachian Piedmont | |||||||
| Mean | 15 | 1.89 | 0.51 | 54.05 | 69.98 | 0.0001019 | |
| Range | 1–34 | 0–2.77 | 0.11–1.00 | 0–76.62 | 21.94–96.17 | 0.0000860–0.0001087 | |
| 10% | 8 | 1.28 | 0.33 | 38.79 | 58.57 | 0.0000976 | |
| 90% | 22 | 2.37 | 0.69 | 66.62 | 79.73 | 0.0001056 | |
| Chesapeake Bay | |||||||
| Mean | 10 | 1.46 | 0.61 | 38.51 | 58.35 | 0.0000967 | |
| Range | 1–31 | 0–2.94 | 0.14–1.00 | 0–84.01 | 21.94–100.00 | 0.0000786–0.0001089 | |
| 10% | 2 | 0.36 | 0.37 | 6.55 | 38.80 | 0.0000880 | |
| 90% | 19 | 2.29 | 0.95 | 62.48 | 80.15 | 0.0001052 | |
| Laurentian Great Lakes | |||||||
| Mean | 11 | 1.56 | 0.55 | 37.31 | 54.08 | 0.0000938 | |
| Range | 1–37 | 0–2.87 | 0.10–1.00 | 0–84.01 | 21.94–95.65 | 0.0000759–0.0001091 | |
| 10% | 4 | 0.74 | 0.33 | 18.62 | 38.26 | 0.0000832 | |
| 90% | 19 | 2.23 | 0.82 | 56.98 | 74.39 | 0.0001043 | |
| Middle Missouri | |||||||
| Mean | 10 | 1.38 | 0.50 | 41.89 | 66.40 | 0.0000958 | |
| Range | 1–29 | 0–2.69 | 0.12–1.00 | 0–70.06 | 21.94–84.01 | 0.0000788–0.0001090 | |
| 10% | 4 | 0.63 | 0.25 | 16.05 | 42.83 | 0.0000864 | |
| 90% | 17 | 2.03 | 0.76 | 59.91 | 82.05 | 0.0001043 | |
| Upper Mississippi | |||||||
| Mean | 13 | 1.61 | 0.54 | 38.35 | 54.95 | 0.0000939 | |
| Range | 1–48 | 0–3.11 | 0.07–1.00 | 0–84.01 | 21.94–100.00 | 0.0000759–0.0001091 | |
| 10% | 3 | 0.62 | 0.33 | 15.25 | 39.10 | 0.0000837 | |
| 90% | 24 | 2.38 | 0.78 | 60.74 | 74.21 | 0.0001043 | |
| Freshwater Ecoregion | |||||||
|---|---|---|---|---|---|---|---|
| Variable | S | H′ | J′ | Δ | Δ* | β | |
| Appalachian Piedmont | |||||||
| Species richness (S) | – | ||||||
| Shannon’s diversity (H’) | 0.78 ** | – | |||||
| Pielou’s evenness (J’) | −0.44 ** | 0.15 ** | – | ||||
| Taxonomic diversity (Δ) | 0.54 ** | 0.81 ** | 0.25 ** | – | |||
| Taxonomic distinctness (Δ*) | 0.24 ** | 0.25 ** | −<0.01 | 0.72 ** | – | ||
| Beta diversity (β) | −0.15 ** | −0.23 ** | −0.033 | −0.03 | 0.22 ** | – | |
| Chesapeake Bay | |||||||
| Species richness (S) | – | ||||||
| Shannon’s diversity (H’) | 0.91 ** | – | |||||
| Pielou’s evenness (J’) | −0.65 ** | −0.39 ** | – | ||||
| Taxonomic diversity (Δ) | 0.71 ** | 0.82 ** | −0.25 ** | – | |||
| Taxonomic distinctness (Δ*) | 0.25 ** | 0.28 ** | −<0.01 | 0.79 ** | – | ||
| Beta diversity (β) | −0.45 ** | −0.49 ** | 0.28 ** | −0.12 ** | 0.41 ** | – | |
| Laurentian Great Lakes | |||||||
| Species richness (S) | – | ||||||
| Shannon’s diversity (H’) | 0.84 ** | – | |||||
| Pielou’s evenness (J’) | −0.60 ** | −0.18 ** | – | ||||
| Taxonomic diversity (Δ) | 0.56 ** | 0.76 ** | 0.03 | – | |||
| Taxonomic distinctness (Δ*) | 0.12 ** | 0.12 ** | 0.05 ** | 0.70 ** | – | ||
| Beta diversity (β) | −0.46 ** | −0.50 ** | 0.26 ** | −0.03 | 0.48 ** | – | |
| Middle Missouri | |||||||
| Species richness (S) | – | ||||||
| Shannon’s diversity (H’) | 0.74 ** | – | |||||
| Pielou’s evenness (J’) | −0.61 ** | −0.034 | – | ||||
| Taxonomic diversity (Δ) | 0.63 ** | 0.83 ** | −0.055 | – | |||
| Taxonomic distinctness (Δ*) | 0.35 ** | 0.16 ** | −0.31 ** | 0.62 ** | – | ||
| Beta diversity (β) | −0.26 ** | −0.54 ** | −0.11 ** | −0.22 ** | 0.34 ** | – | |
| Upper Mississippi | |||||||
| Species richness (S) | – | ||||||
| Shannon’s diversity (H’) | 0.88 ** | – | |||||
| Pielou’s evenness (J’) | −0.58 ** | −0.22 ** | – | ||||
| Taxonomic diversity (Δ) | 0.75 ** | 0.86 ** | −0.14 ** | – | |||
| Taxonomic distinctness (Δ*) | 0.44 ** | 0.38 ** | −0.16 ** | 0.76 ** | – | ||
| Beta diversity (β) | −0.40 ** | −0.53 ** | 0.16 ** | −0.22 ** | 0.25 ** | – | |
| Freshwater Ecoregion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | d. f. | Adj R2 | Area_nc | Elev_c | Fine_nc | Baseflow_nc | AirTemp_c | Precip_nc | Urban_nc | Ag_nc | Rd_nc | Dam_nc | |
| Appalachian Piedmont | |||||||||||||
| Species richness (S) | 1077 | 0.25 | +0.42 | – | – | −0.14 | −0.14 | – | −0.10 | +0.10 | – | – | |
| Shannon’s diversity (H’) | 1077 | 0.18 | +0.33 | – | – | −0.14 | −0.11 | – | −0.11 | +0.09 | – | – | |
| Pielou’s evenness (J’) | 1080 | 0.04 | −0.19 | – | +0.14 | – | – | – | – | – | – | – | |
| Taxonomic diversity (Δ) | 1077 | 0.17 | +0.29 | −0.26 | – | – | – | – | – | – | – | – | |
| Taxonomic distinctness (Δ*) | 1073 | 0.16 | +0.22 | −0.30 | – | – | – | – | +0.12 | – | – | – | |
| Beta diversity (β) | 1077 | 0.31 | +0.10 | −0.29 | – | +0.14 | +0.30 | +0.16 | – | – | – | – | |
| Chesapeake Bay | |||||||||||||
| Species richness (S) | 801 | 0.33 | +0.59 | – | – | – | +0.19 | – | – | +0.16 | +0.13 | +0.12 | |
| Shannon’s diversity (H’) | 801 | 0.26 | +0.52 | – | – | – | +0.17 | – | – | +0.14 | +0.13 | +0.12 | |
| Pielou’s evenness (J’) | 803 | 0.12 | −0.31 | – | – | – | – | −0.09 | – | −0.15 | – | – | |
| Taxonomic diversity (Δ) | 794 | 0.37 | +0.52 | −0.29 | – | – | – | +0.14 | +0.10 | +0.16 | – | – | |
| Taxonomic distinctness (Δ*) | 737 | 0.37 | +0.41 | −0.49 | – | +0.17 | – | – | – | – | – | – | |
| Beta diversity (β) | 801 | 0.20 | – | −0.52 | +0.15 | – | −0.25 | – | −0.18 | −0.16 | – | – | |
| Laurentian Great Lakes | |||||||||||||
| Species richness (S) | 3767 | 0.35 | +0.42 | +0.16 | +0.29 | – | +0.31 | −0.13 | – | – | – | – | |
| Shannon’s diversity (H′) | 3767 | 0.20 | +0.34 | – | +0.08 | −0.14 | +0.15 | −0.06 | – | – | – | – | |
| Pielou’s evenness (J′) | 3766 | 0.16 | −0.17 | −0.08 | −0.25 | – | −0.18 | +0.17 | – | – | – | – | |
| Taxonomic diversity (Δ) | 3752 | 0.27 | +0.43 | −0.17 | – | −0.13 | – | – | −0.06 | – | – | +0.05 | |
| Taxonomic distinctness (Δ*) | 3652 | 0.22 | +0.39 | −0.14 | – | – | +0.11 | – | −0.15 | −0.13 | – | – | |
| Beta diversity (β) | 3767 | 0.19 | – | −0.26 | −0.12 | – | −0.24 | +0.18 | – | – | −0.21 | – | |
| Middle Missouri | |||||||||||||
| Species richness (S) | 880 | 0.40 | +0.47 | −0.65 | −0.20 | – | – | – | – | −0.20 | +0.10 | – | |
| Shannon’s diversity (H′) | 882 | 0.20 | +0.22 | −0.43 | −0.18 | – | – | – | – | – | – | – | |
| Pielou’s evenness (J′) | 881 | 0.22 | −0.39 | – | – | – | −0.22 | −0.30 | – | +0.12 | – | – | |
| Taxonomic diversity (Δ) | 879 | 0.24 | +0.27 | −0.48 | – | – | – | – | – | – | – | – | |
| Taxonomic distinctness (Δ*) | 857 | 0.25 | +0.29 | −0.26 | +0.14 | – | – | +0.20 | −0.14 | – | – | – | |
| Beta diversity (β) | 882 | 0.10 | +0.16 | – | +0.22 | – | – | – | – | −0.15 | – | – | |
| Upper Mississippi | |||||||||||||
| Species richness (S) | 3967 | 0.52 | +0.63 | – | – | −0.20 | −0.18 | +0.22 | – | +0.15 | – | – | |
| Shannon’s diversity (H′) | 3967 | 0.39 | +0.53 | +0.21 | – | −0.23 | – | +0.24 | – | +0.10 | – | – | |
| Pielou’s evenness (J′) | 3970 | 0.14 | −0.37 | – | – | – | – | – | – | −0.07 | – | – | |
| Taxonomic diversity (Δ) | 3947 | 0.45 | +0.51 | – | +0.08 | −0.15 | – | +0.19 | – | +0.09 | – | – | |
| Taxonomic distinctness (Δ*) | 3843 | 0.34 | +0.44 | – | +0.07 | – | +0.17 | +0.17 | −0.08 | – | – | – | |
| Beta diversity (β) | 3967 | 0.06 | −0.15 | −0.31 | – | – | – | −0.18 | – | −0.14 | −0.15 | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thornbrugh, D.; Infante, D.; Tsang, Y. Regional Trends of Biodiversity Indices in the Temperate Mesic United States: Testing for Influences of Anthropogenic Land Use on Stream Fish while Controlling for Natural Landscape Variables. Water 2023, 15, 1591. https://doi.org/10.3390/w15081591
Thornbrugh D, Infante D, Tsang Y. Regional Trends of Biodiversity Indices in the Temperate Mesic United States: Testing for Influences of Anthropogenic Land Use on Stream Fish while Controlling for Natural Landscape Variables. Water. 2023; 15(8):1591. https://doi.org/10.3390/w15081591
Chicago/Turabian StyleThornbrugh, Darren, Dana Infante, and Yinphan Tsang. 2023. "Regional Trends of Biodiversity Indices in the Temperate Mesic United States: Testing for Influences of Anthropogenic Land Use on Stream Fish while Controlling for Natural Landscape Variables" Water 15, no. 8: 1591. https://doi.org/10.3390/w15081591
APA StyleThornbrugh, D., Infante, D., & Tsang, Y. (2023). Regional Trends of Biodiversity Indices in the Temperate Mesic United States: Testing for Influences of Anthropogenic Land Use on Stream Fish while Controlling for Natural Landscape Variables. Water, 15(8), 1591. https://doi.org/10.3390/w15081591







