A Review of Organophosphate Esters in Aquatic Environments: Levels, Distribution, and Human Exposure
Abstract
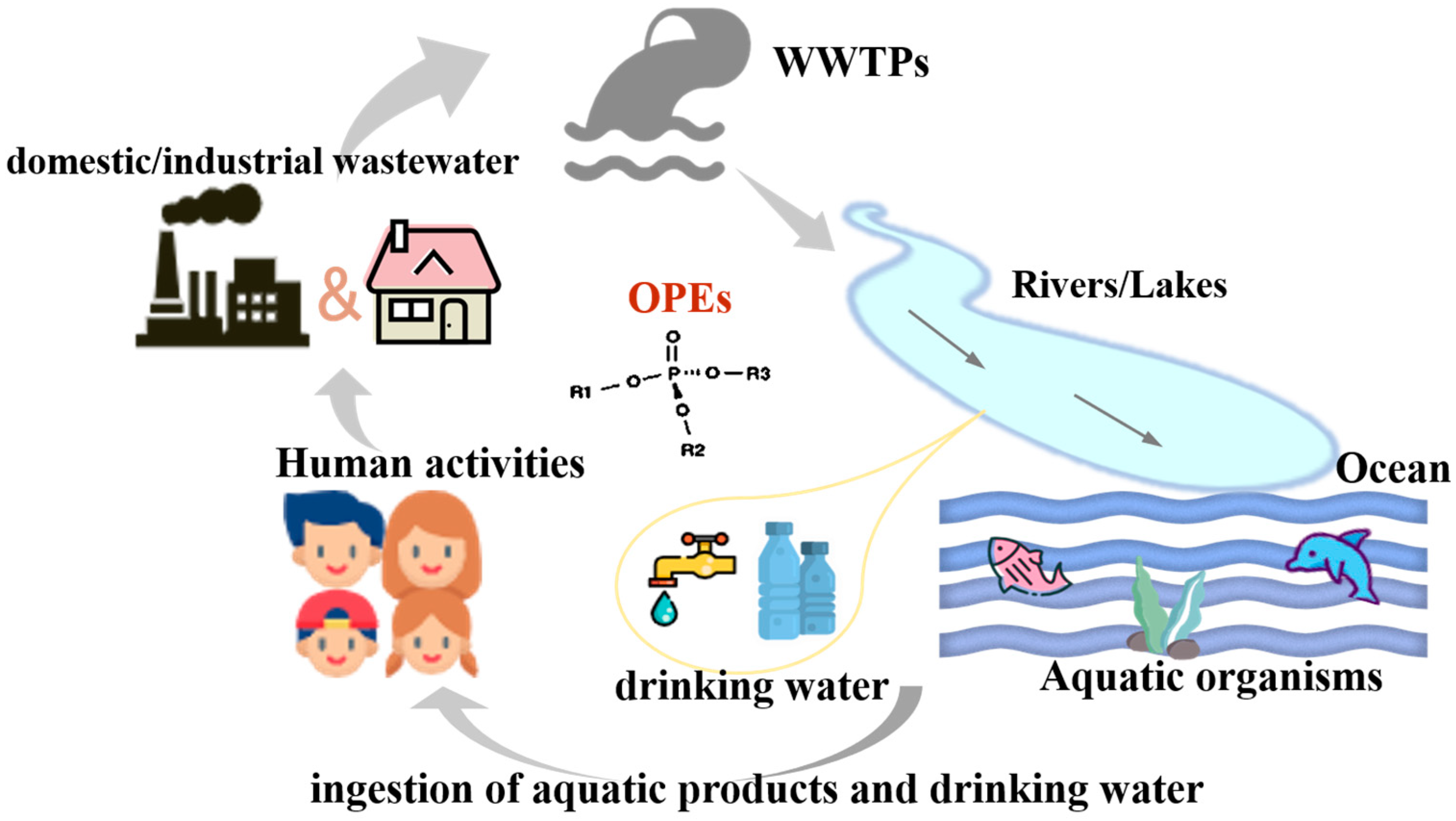
1. Introduction
2. OPEs in Aquatic Environment
2.1. Wastewater
2.2. Surface Water (Rivers, Lakes, and Coastal Seawater)
2.3. Drinking Water (Tap Water, Bottled Water, and Barreled Water)
2.4. Aquatic Organisms
3. Human Exposure
4. Conclusions and Future Perspectives
- (1)
- Thus far, most studies have mainly focused on the commonly used monomeric OPEs, while scant research is available on oligomeric OPEs and their metabolites. Therefore, further studies should be encouraged to study their fate and the process of metabolic/degradation in aquatic environments. More specific studies on OPEs in water are needed;
- (2)
- In addition to the various water bodies, sediments are the final sink of OPEs in water sources. More monitoring studies on the multimedia analysis associated with OPEs are needed, which is extremely important for us to understand the origin and migration of OPEs. More specifically, the previous studies involved in the detection of OPEs in aquatic environments were only conducted in one or two environmental media simultaneously. Only a minority of studies have confirmed the accumulation of OPEs in aquatic organisms and scant research is available on the partitioning of OPEs between water and aquatic organisms. In view of the current research status and existing limitations of OPEs, future relevant research should focus on tracing the pollution sources and environmental behaviors of various OPEs in different environmental media;
- (3)
- For the human exposure risk assessments of OPEs, most studies ignored OPE bioaccessibility, which possibly overestimated or underestimated the risk posed by OPEs by not taking into account their specific chemical fractions. Systematic studies on the toxicity of OPEs and their metabolites should be carried out to lay a scientific foundation for the accurate assessment of their potential ecological and health risks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, L.; Tao, M.; Zhang, Q.; Kong, M.; Sun, J.; Jia, S.; Liu, C.H. Occurrence, spatial distribution and risk assessment of organophosphate esters in surface water from the lower Yangtze River Basin. Sci. Total Environ. 2020, 734, 139380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, M.; Wu, F.; Zhao, X.; Wang, L.; Wei, Y.; Xie, S.; Cui, H. Determination of Organophosphorus Esters in Fall Protection Equipment by Accelerated Solvent Extraction and Solid-Phase Extraction Coupled with LC-MS/MS Detection. J. Anal. Methods Chem. 2021, 2021, 8878247. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-L.; Li, D.-Q.; Zhuo, M.-N.; Liao, Y.-S.; Xie, Z.-Y.; Guo, T.-L.; Li, J.-J.; Zhang, S.-Y.; Liang, Z.-Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.J.; Hardy, E.M.; Beranger, R.; Mezzache, S.; Bourokba, N.; Bastien, P.; Li, J.; Zaros, C.; Chevrier, C.; Palazzi, P.; et al. Human exposure to PCBs, PBDEs and bisphenols revealed by hair analysis: A comparison between two adult female populations in China and France. Environ. Pollut. 2020, 267, 115425. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Stubbings, W.A.; Abdallah, M.A.; Cline-Cole, R.; Harrad, S. Formal waste treatment facilities as a source of halogenated flame retardants and organophosphate esters to the environment: A critical review with particular focus on outdoor air and soil. Sci. Total Environ. 2022, 807 Pt 1, 150747. [Google Scholar] [CrossRef]
- Zou, X.; Hou, S.; Wu, S.; Liu, K.; Huang, R.; Zhang, W.; Yu, J.; Zhan, Z.; Pang, H. The first detection of organophosphate esters (OPEs) of a high altitude fresh snowfall in the northeastern Tibetan Plateau. Sci. Total Environ. 2022, 838 Pt 1, 155615. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, L.; Wen, X.; Jiang, C.; Long, Y.; Zhang, J.; Liu, R. Organophosphate Esters in China: Fate, Occurrence, and Human Exposure. Toxics 2021, 9, 310. [Google Scholar] [CrossRef]
- Zeng, Y.; Ke, C.; Liu, Q.; Huang, K. Simultaneous Determination of Organophosphate Ester Flame Retardants in Water and Sediments by Gas Chromatography–Tandem Mass Spectrometry (GC–MS/MS). Anal. Lett. 2022, 55, 2928–2943. [Google Scholar] [CrossRef]
- Huang, J.; Ye, L.; Fang, M.; Su, G. Industrial Production of Organophosphate Flame Retardants (OPFRs): Big Knowledge Gaps Need to Be Filled? Bull. Environ. Contam. Toxicol. 2022, 108, 809–818. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, X.; Wang, Y.; Liao, C.; Jiang, G. A review of organophosphate flame retardants and plasticizers in the environment: Analysis, occurrence and risk assessment. Sci. Total Environ. 2020, 731, 139071. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhang, X.; Shi, C.; Ma, L.; Zhang, X.; Wang, G. Similarities and differences among the responses to three chlorinated organophosphate esters in earthworm: Evidences from biomarkers, transcriptomics and metabolomics. Sci. Total Environ. 2022, 815, 152853. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Mi, C.; Feng, C.; Zhang, L.; Yang, L.; Zhou, B. The adverse effect of TCIPP and TCEP on neurodevelopment of zebrafish embryos/larvae. Chemosphere 2019, 220, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.; Yu, L.; Liu, C.; Wang, J. Gonadal impairment and parental transfer of tris (2-butoxyethyl) phosphate in zebrafish after long-term exposure to environmentally relevant concentrations. Chemosphere 2019, 218, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wang, Y.; Zhou, S.; Zhou, L.; Yuan, Y.; Xu, Y. Aerobic degradation of nonhalogenated organophosphate flame esters (OPEs) by enriched cultures from sludge: Kinetics, pathways, bacterial community evolution, and toxicity evaluation. Sci. Total Environ. 2021, 760, 143385. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Chen, R.; Yuan, L.; Zha, J. Global microRNA and isomiR expression associated with liver metabolism is induced by organophosphorus flame retardant exposure in male Chinese rare minnow (Gobiocypris rarus). Sci. Total Environ. 2019, 649, 829–838. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhao, Y.Y.; Li, M.H.; Du, M.J.; Li, X.X.; Li, Y. A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- Castro-Jimenez, J.; Sempere, R. Atmospheric particle-bound organophosphate ester flame retardants and plasticizers in a North African Mediterranean coastal city (Bizerte, Tunisia). Sci. Total Environ. 2018, 642, 383–393. [Google Scholar] [CrossRef]
- Lee, S.; Cho, H.J.; Choi, W.; Moon, H.B. Organophosphate flame retardants (OPFRs) in water and sediment: Occurrence, distribution, and hotspots of contamination of Lake Shihwa, Korea. Mar. Pollut. Bull. 2018, 130, 105–112. [Google Scholar] [CrossRef]
- Pantelaki, I.; Voutsa, D. Organophosphate esters in inland and coastal waters in northern Greece. Sci. Total Environ. 2021, 800, 149544. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Liu, M.; Zhao, W.; Li, Y.; Meng, X.Z.; Cai, M. Occurrence of organophosphate esters in surface water and sediment in drinking water source of Xiangjiang River, China. Sci. Total Environ. 2021, 781, 146734. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, Z.; Lohmann, R.; Mi, W.; Gao, G. Organophosphate Ester Flame Retardants and Plasticizers in Ocean Sediments from the North Pacific to the Arctic Ocean. Environ. Sci. Technol. 2017, 51, 3809–3815. [Google Scholar] [CrossRef] [PubMed]
- Fauvelle, V.; Castro-Jiménez, J.; Schmidt, N.; Carlez, B.; Panagiotopoulos, C.; Sempéré, R. One-Single Extraction Procedure for the Simultaneous Determination of a Wide Range of Polar and Nonpolar Organic Contaminants in Seawater. Front. Mar. Sci. 2018, 5, 295. [Google Scholar] [CrossRef]
- Huang, J.; Li, R.; Shi, T.; Ye, J.; Zhang, H.; Jin, S.; Gao, H.; Wang, Q.; Na, G. Determination of multiple organic flame retardants in maricultural water using High-volume/High-throughput Solid-phase extraction followed by liquid/gas chromatography tandem mass spectrometry. J. Chromatogr. A 2022, 1663, 462766. [Google Scholar] [CrossRef]
- Pantelaki, I.; Voutsa, D. Organophosphate flame retardants (OPFRs): A review on analytical methods and occurrence in wastewater and aquatic environment. Sci. Total Environ. 2019, 649, 247–263. [Google Scholar] [CrossRef]
- Kim, U.J.; Oh, J.K.; Kannan, K. Occurrence, Removal, and Environmental Emission of Organophosphate Flame Retardants/Plasticizers in a Wastewater Treatment Plant in New York State. Environ. Sci. Technol. 2017, 51, 7872–7880. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, L.; Li, W.; Wang, Y.; Liu, J.; Cai, Y. Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ. Pollut. 2016, 209, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, Z.; He, L.; Cao, S.; Song, H.; Yu, Z.; Sheng, G.; Fu, J. The occurrence and removal of organophosphate ester flame retardants/plasticizers in a municipal wastewater treatment plant in the Pearl River Delta, China. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2015, 50, 1291–1297. [Google Scholar] [CrossRef]
- Liang, K.; Liu, J. Understanding the distribution, degradation and fate of organophosphate esters in an advanced municipal sewage treatment plant based on mass flow and mass balance analysis. Sci. Total Environ. 2016, 544, 262–270. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.; Liang, K.; Liu, R. Tris(pentafluoroethyl)trifluorophosphate-basd ionic liquids as advantageous solid-phase micro-extraction coatings for the extraction of organophosphate esters in environmental waters. J. Chromatogr. A 2016, 1447, 9–16. [Google Scholar] [CrossRef]
- Cristale, J.; Ramos, D.D.; Dantas, R.F.; Machulek Junior, A.; Lacorte, S.; Sans, C.; Esplugas, S. Can activated sludge treatments and advanced oxidation processes remove organophosphorus flame retardants? Environ. Res. 2016, 144 Pt A, 11–18. [Google Scholar] [CrossRef]
- Wang, S.; Qian, J.; Zhang, B.; Chen, L.; Wei, S.; Pan, B. Unveiling the Occurrence and Potential Ecological Risks of Organophosphate Esters in Municipal Wastewater Treatment Plants across China. Environ. Sci. Technol. 2023, 57, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Zhu, F.; Li, C.; Xu, Y.; Qing, D.; Wang, J. The influence of an upgrade on the reduction of organophosphate flame retardants in a wastewater treatment plant. Chemosphere 2020, 256, 126895. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, M.; Wu, Q.; Kuo, D.T.F.; Zeng, L.; Wang, Z.; Zhang, Y.; Liu, X.; Liu, S.; Liang, J.; et al. Occurrence, seasonal variation and environmental impact of phosphorus flame retardants in a large scale wastewater treatment plant. Environ. Sci. Pollut. Res. 2019, 26, 36333–36342. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Kuo, D.T.F.; Wu, Q.; Zhang, Y.; Liu, X.; Liu, S.; Hu, X.; Mai, B.; Liu, Z.; Zhang, H. Organophosphorus flame retardants and heavy metals in municipal landfill leachate treatment system in Guangzhou, China. Environ. Pollut. 2018, 236, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pantelaki, I.; Voutsa, D. Occurrence and removal of organophosphate esters in municipal wastewater treatment plants in Thessaloniki, Greece. Environ. Res. 2022, 214, 113908. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Lin, C.; Xin, M.; Gu, X.; Lu, S.; Wang, B.; Ouyang, W.; Liu, X.; He, M. Organophosphate esters in surface waters of Shandong Peninsula in eastern China: Levels, profile, source, spatial distribution, and partitioning. Environ. Pollut. 2022, 297, 118792. [Google Scholar] [CrossRef]
- Hao, C.; Helm, P.A.; Morse, D.; Reiner, E.J. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere 2018, 191, 288–295. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Q.; Liu, J.; Liu, S.; Liu, C.; Deng, Q.; Zeng, X.; Yu, Z. Occurrence of organophosphate esters and their diesters degradation products in industrial wastewater treatment plants in China: Implication for the usage and potential degradation during production processing. Environ. Pollut. 2019, 250, 559–566. [Google Scholar] [CrossRef]
- Pang, L.; Yang, P.; Zhao, J.; Zhang, H. Comparison of wastewater treatment processes on the removal efficiency of organophosphate esters. Water Sci. Technol. 2016, 74, 1602–1609. [Google Scholar] [CrossRef]
- Wang, Y.; Kannan, P.; Halden, R.U.; Kannan, K. A nationwide survey of 31 organophosphate esters in sewage sludge from the United States. Sci. Total Environ. 2019, 655, 446–453. [Google Scholar] [CrossRef]
- Liang, K.; Shi, F.; Liu, J. Occurrence and distribution of oligomeric organophosphorus flame retardants in different treatment stages of a sewage treatment plant. Environ. Pollut. 2018, 232, 229–235. [Google Scholar] [CrossRef]
- Yin, H.; Luo, Y.; Song, J.; Li, S.; Lin, S.; Xiong, Y.; Fang, S.; Tang, J. Pollution characteristics and emissions of typical organophosphate esters of a wastewater treatment plant. Environ. Sci. Pollut. Res. Int. 2022, 29, 25892–25901. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lin, Y.; Li, J.; Xu, Y.; Qian, Z.; Lin, W. Spatial pattern analysis reveals multiple sources of organophosphorus flame retardants in coastal waters. J. Hazard. Mater. 2021, 417, 125882. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Campo, J.; Alfarhan, A.H.; El-Sheikh, M.A.; Barcelo, D. A reconnaissance study of pharmaceuticals, pesticides, perfluoroalkyl substances and organophosphorus flame retardants in the aquatic environment, wild plants and vegetables of two Saudi Arabia urban areas: Environmental and human health risk assessment. Sci. Total Environ. 2021, 776, 145843. [Google Scholar] [CrossRef] [PubMed]
- Propp, V.R.; De Silva, A.O.; Spencer, C.; Brown, S.J.; Catingan, S.D.; Smith, J.E.; Roy, J.W. Organic contaminants of emerging concern in leachate of historic municipal landfills. Environ. Pollut. 2021, 276, 116474. [Google Scholar] [CrossRef]
- Fu, L.; Du, B.; Wang, F.; Lam, J.C.W.; Zeng, L.; Zeng, E.Y. Organophosphate Triesters and Diester Degradation Products in Municipal Sludge from Wastewater Treatment Plants in China: Spatial Patterns and Ecological Implications. Environ. Sci. Technol. 2017, 51, 13614–13623. [Google Scholar] [CrossRef]
- Pang, L.; Yang, P.; Ge, L.; Du, J.; Zhang, H. Accelerated solvent extraction combined with solid phase extraction for the determination of organophosphate esters from sewage sludge compost by UHPLC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 1435–1440. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Mi, W.; Wang, Z.; Lai, S. Organophosphate esters in atmospheric particles and surface seawater in the western South China Sea. Environ. Pollut. 2022, 292, 118255. [Google Scholar] [CrossRef]
- Schmidt, N.; Castro-Jimenez, J.; Fauvelle, V.; Ourgaud, M.; Sempere, R. Occurrence of organic plastic additives in surface waters of the Rhone River (France). Environ. Pollut. 2020, 257, 113637. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, Q.; Sun, X.; Zhu, H.; Zhang, S.; Xu, H. Occurrence, distribution and risk assessment of organophosphate esters in surface water and sediment from a shallow freshwater Lake, China. Sci. Total Environ. 2018, 636, 632–640. [Google Scholar] [CrossRef]
- Lian, M.; Lin, C.; Li, Y.; Hao, X.; Wang, A.; He, M.; Liu, X.; Ouyang, W. Distribution, partitioning, and health risk assessment of organophosphate esters in a major tributary of middle Yangtze River using Monte Carlo simulation. Water Res. 2022, 219, 118559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef]
- Qi, Y.; He, Z.; Yuan, J.; Ma, X.; Du, J.; Yao, Z.; Wang, W. Comprehensive evaluation of organophosphate ester contamination in surface water and sediment of the Bohai Sea, China. Mar. Pollut. Bull. 2021, 163, 112013. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Guo, C.; Luo, Y.; Liu, Y.; Deng, Y.; Sun, S.; Xu, J. Spatial distribution, receptor modelling and risk assessment of organophosphate esters in surface water from the largest freshwater lake in China. Ecotoxicol. Environ. Saf. 2022, 238, 113618. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, H.; Wu, M.; Zhang, J.; Li, D.; Li, J.; Wang, H.; Shi, W.; Xu, G. Occurrence, Distribution, and Potential Sources of Organophosphate Esters in Urban and Rural Surface Water in Shanghai, China. Arch. Environ. Contam. Toxicol. 2019, 77, 115–126. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, Y.; Fu, M.; Zhang, X.; Lei, B.; Huang, X.; Zhao, Z. Organophosphate tri- and diesters in source water supply and drinking water treatment systems of a metropolitan city in China. Environ. Geochem. Health 2022, 45, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, C.; Song, N.; Zhang, S.; Bu, Y.; Xiong, L.; Yin, L.; Pu, Y.; Zhang, J. Seasonal variation and health risk assessment of organophosphate esters in surface and drinking water in Nanjing, China. Int. J. Environ. Sci. Technol. 2022, 20, 411–422. [Google Scholar] [CrossRef]
- Liu, F.; Wei, C.; Zhang, R.; Zeng, W.; Han, M.; Kang, Y.; Zhang, Z.; Wang, R.; Yu, K.; Wang, Y. Occurrence, distribution, source identification, and risk assessment of organophosphate esters in the coastal waters of Beibu Gulf, South China Sea: Impacts of riverine discharge and fishery. J. Hazard. Mater. 2022, 436, 129214. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, L.; Zhu, W.; Yang, B.; Lu, D.; Dan, S.F.; Zhang, S. Organophosphorus flame retardants (OPFRs) in the seawater and sediments of the Qinzhou Bay, Northern Beibu Gulf: Occurrence, distribution, and ecological risks. Mar. Pollut. Bull. 2021, 168, 112368. [Google Scholar] [CrossRef]
- Xiao, K.; Lu, Z.; Yang, C.; Zhao, S.; Zheng, H.; Gao, Y.; Kaluwin, C.; Liu, Y.; Cai, M. Occurrence, distribution and risk assessment of organophosphate ester flame retardants and plasticizers in surface seawater of the West Pacific. Mar. Pollut. Bull. 2021, 170, 112691. [Google Scholar] [CrossRef]
- Suehring, R.; Diamond, M.L.; Bernstein, S.; Adams, J.K.; Schuster, J.K.; Fernie, K.; Elliott, K.; Stern, G.; Jantunen, L.M. Organophosphate Esters in the Canadian Arctic Ocean. Environ. Sci. Technol. 2021, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Fauvelle, V.; Ody, A.; Castro-Jimenez, J.; Jouanno, J.; Changeux, T.; Thibaut, T.; Sempere, R. The Amazon River: A Major Source of Organic Plastic Additives to the Tropical North Atlantic? Environ. Sci. Technol. 2019, 53, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Chen, D.; Sun, J.; Greig, D.J.; Wu, Y. Characterization of brominated, chlorinated, and phosphate flame retardants in San Francisco Bay, an urban estuary. Sci. Total Environ. 2019, 652, 212–223. [Google Scholar] [CrossRef]
- Kim, U.J.; Kannan, K. Occurrence and Distribution of Organophosphate Flame Retardants/Plasticizers in Surface Waters, Tap Water, and Rainwater: Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 5625–5633. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Oliveira Santos, I.; Umbuzeiro, G.d.A.; Fagnani, E. Occurrence and risk assessment of organophosphate esters in urban rivers from Piracicaba watershed (Brazil). Environ. Sci. Pollut. Res. 2021, 28, 59244–59255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Sarvajayakesavalu, S.; Han, Y.; Zhang, H.; Gao, J.; Li, X.; Ma, M. Occurrence, distribution and risk assessment of organophosphate esters (OPEs) in water sources from Northeast to Southeast China. Environ. Pollut. 2022, 307, 119461. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Guo, R.; Xu, H.; Song, N.; Han, Z.; Chen, N.; Zhang, S.; Chen, J. Spatiotemporal distribution and risk assessment of organophosphate esters in sediment from Taihu Lake, China. Environ. Sci. Pollut. Res. Int. 2018, 25, 13787–13795. [Google Scholar] [CrossRef]
- Shi, F.; Liang, K.; Liu, R.; Dong, Q.; He, Z.; Xu, J.; Liu, J. Elevated occupational exposure to chlorinated phosphate esters at a construction materials manufacturing plant. Environ. Int. 2020, 139, 105653. [Google Scholar] [CrossRef]
- Chen, M.; Gan, Z.; Qu, B.; Chen, S.; Dai, Y.; Bao, X. Temporal and seasonal variation and ecological risk evaluation of flame retardants in seawater and sediments from Bohai Bay near Tianjin, China during 2014 to 2017. Mar. Pollut. Bull. 2019, 146, 874–883. [Google Scholar] [CrossRef]
- Lian, M.; Lin, C.; Wu, T.; Xin, M.; Gu, X.; Lu, S.; Cao, Y.; Wang, B.; Ouyang, W.; Liu, X.; et al. Occurrence, spatiotemporal distribution, and ecological risks of organophosphate esters in the water of the Yellow River to the Laizhou Bay, Bohai Sea. Sci. Total Environ. 2021, 787, 147528. [Google Scholar] [CrossRef]
- Zhong, M.; Tang, J.; Guo, X.; Guo, C.; Li, F.; Wu, H. Occurrence and spatial distribution of organophosphorus flame retardants and plasticizers in the Bohai, Yellow and East China seas. Sci. Total Environ. 2020, 741, 140434. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, L.; Guo, C.; Feng, S.; Gao, T. Inter-annual variation and comprehensive evaluation of organophosphate esters (OPEs) in the Yellow Sea, China. Mar. Pollut. Bull. 2022, 176, 113440. [Google Scholar] [CrossRef]
- Castro-Jimenez, J.; Gonzalez-Gaya, B.; Pizarro, M.; Casal, P.; Pizarro-Alvarez, C.; Dachs, J. Organophosphate Ester Flame Retardants and Plasticizers in the Global Oceanic Atmosphere. Environ. Sci. Technol. 2016, 50, 12831–12839. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.; Mi, W.; Lai, S.; Tian, C.; Emeis, K.C.; Ebinghaus, R. Organophosphate Esters in Air, Snow, and Seawater in the North Atlantic and the Arctic. Environ. Sci. Technol. 2017, 51, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Suhring, R.; Diamond, M.L.; Scheringer, M.; Wong, F.; Pucko, M.; Stern, G.; Burt, A.; Hung, H.; Fellin, P.; Li, H.; et al. Organophosphate Esters in Canadian Arctic Air: Occurrence, Levels and Trends. Environ. Sci. Technol. 2016, 50, 7409–7415. [Google Scholar] [CrossRef]
- Na, G.; Hou, C.; Li, R.; Shi, Y.; Gao, H.; Jin, S.; Gao, Y.; Jiao, L.; Cai, Y. Occurrence, distribution, air-seawater exchange and atmospheric deposition of organophosphate esters (OPEs) from the Northwestern Pacific to the Arctic Ocean. Mar. Pollut. Bull. 2020, 157, 111243. [Google Scholar] [CrossRef]
- Sun, Y.; De Silva, A.O.; St Pierre, K.A.; Muir, D.C.G.; Spencer, C.; Lehnherr, I.; MacInnis, J.J. Glacial Melt Inputs of Organophosphate Ester Flame Retardants to the Largest High Arctic Lake. Environ. Sci. Technol. 2020, 54, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Shi, Y.L.; Na, G.S.; Zhao, Z.S.; Cai, Y.Q. Increased Human Exposure to Organophosphate Esters via Ingestion of Drinking Water from Water Dispensers: Sources, Influencing Factors, and Exposure Assessment. Environ. Sci. Technol. Lett. 2021, 8, 884–889. [Google Scholar] [CrossRef]
- Li, J.; Yu, N.; Zhang, B.; Jin, L.; Li, M.; Hu, M.; Zhang, X.; Wei, S.; Yu, H. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 2014, 54, 53–61. [Google Scholar] [CrossRef]
- Ding, J.; Shen, X.; Liu, W.; Covaci, A.; Yang, F. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci. Total Environ. 2015, 538, 959–965. [Google Scholar] [CrossRef]
- Liang, C.; Mo, X.J.; Xie, J.F.; Wei, G.L.; Liu, L.Y. Organophosphate tri-esters and di-esters in drinking water and surface water from the Pearl River Delta, South China: Implications for human exposure. Environ. Pollut. 2022, 313, 120150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment, and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef]
- Khan, M.U.; Li, J.; Zhang, G.; Malik, R.N. First insight into the levels and distribution of flame retardants in potable water in Pakistan: An underestimated problem with an associated health risk diagnosis. Sci. Total Environ. 2016, 565, 346–359. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Choo, G.; Oh, J.E. Seasonal occurrence and removal of organophosphate esters in conventional and advanced drinking water treatment plants. Water Res. 2020, 186, 116359. [Google Scholar] [CrossRef] [PubMed]
- Struzina, L.; Pineda Castro, M.A.; Kubwabo, C.; Siddique, S.; Zhang, G.; Fan, X.; Tian, L.; Bayen, S.; Aneck-Hahn, N.; Bornman, R.; et al. Occurrence of legacy and replacement plasticizers, bisphenols, and flame retardants in potable water in Montreal and South Africa. Sci. Total Environ. 2022, 840, 156581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, J.; Li, Y.; Liu, Y.; Li, W.; Wu, N.; Zhang, L.; Zhang, Y.; Niu, Z. Assessing the threats of organophosphate esters (flame retardants and plasticizers) to drinking water safety based on USEPA oral reference dose (RfD) and oral cancer slope factor (SFO). Water Res. 2019, 154, 84–93. [Google Scholar] [CrossRef]
- Bekele, T.G.; Zhao, H.; Yang, J.; Chegen, R.G.; Chen, J.; Mekonen, S.; Qadeer, A. A review of environmental occurrence, analysis, bioaccumulation, and toxicity of organophosphate esters. Environ. Sci. Pollut. Res. Int. 2021, 28, 49507–49528. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, C.; Zheng, Q.; Yang, W.; Niu, X.; Zhang, Y.; Lu, G. Occurrence and ecological implications of organophosphate triesters and diester degradation products in wastewater, river water, and tap water. Environ. Pollut. 2020, 259, 113810. [Google Scholar] [CrossRef]
- Park, H.; Choo, G.; Kim, H.; Oh, J.E. Evaluation of the current contamination status of PFASs and OPFRs in South Korean tap water associated with its origin. Sci. Total Environ. 2018, 634, 1505–1512. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Liu, B.; Li, Y.; Liu, L.; Wang, L.; Song, L.; Wang, Y.; Hu, L.; Mei, S. Organophosphate esters in children and adolescents in Liuzhou city, China: Concentrations, exposure assessment, and predictors. Environ. Sci. Pollut. Res. 2022, 29, 39310–39322. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, Z.; Hu, G.; Su, G. Non-target screening and risk assessment of organophosphate esters (OPEs) in drinking water resource water, surface water, groundwater, and seawater. Environ. Int. 2022, 168, 107443. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.-Y.; Ruan, Y.; Leung, K.M.Y.; Zeng, E.Y.; Lam, P.K.S. Review on age-specific exposure to organophosphate esters: Multiple exposure pathways and microenvironments. Crit. Rev. Environ. Sci. Technol. 2022, 53, 803–826. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Yang, C.; Meng, X.Z.; Zheng, H.; Gao, Y.; Cai, M. Application of Hi-throat/Hi-volume SPE technique in analyzing occurrence, influencing factors and human health risk of organophosphate esters (OPEs) in drinking water of China. J. Environ. Manag. 2021, 291, 112714. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fu, K.; Gao, K.; Li, H.; Xue, Q.; Chen, Y.; Wang, L.; Shi, J.; Fu, J.; Zhang, Q.; et al. Occurrence and Trophic Magnification of Organophosphate Esters in an Antarctic Ecosystem: Insights into the Shift from Legacy to Emerging Pollutants. J. Hazard. Mater. 2020, 396, 122742. [Google Scholar] [CrossRef] [PubMed]
- Bekele, T.G.; Zhao, H.; Wang, Q.; Chen, J. Bioaccumulation and Trophic Transfer of Emerging Organophosphate Flame Retardants in the Marine Food Webs of Laizhou Bay, North China. Environ. Sci. Technol. 2019, 53, 13417–13426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Miller, P.; von Hippel, F.A.; Buck, C.L.; Carpenter, D.O.; Salamova, A. Legacy and emerging semi-volatile organic compounds in sentinel fish from an arctic formerly used defense site in Alaska. Environ. Pollut. 2020, 259, 113872. [Google Scholar] [CrossRef]
- Castro, V.; Montes, R.; Quintana, J.B.; Rodil, R.; Cela, R. Determination of 18 organophosphorus flame retardants/plasticizers in mussel samples by matrix solid-phase dispersion combined to liquid chromatography-tandem mass spectrometry. Talanta 2020, 208, 120470. [Google Scholar] [CrossRef]
- Aznar-Alemany, O.; Aminot, Y.; Vila-Cano, J.; Kock-Schulmeyer, M.; Readman, J.W.; Marques, A.; Godinho, L.; Botteon, E.; Ferrari, F.; Boti, V.; et al. Halogenated and organophosphorus flame retardants in European aquaculture samples. Sci. Total Environ. 2018, 612, 492–500. [Google Scholar] [CrossRef]
- Choi, Y.; Hao, C.; Helm, P.A.; Bhavsar, S.P.; Kim, S.D. Organophosphate esters in Great Lakes fish: An improved analysis to assess concentrations and human exposure via consumption. Sci. Total Environ. 2022, 807, 150981. [Google Scholar] [CrossRef]
- Guo, J.; Venier, M.; Salamova, A.; Hites, R.A. Bioaccumulation of Dechloranes, organophosphate esters, and other flame retardants in Great Lakes fish. Sci. Total Environ. 2017, 583, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bekele, T.G.; Zhao, H.; Wang, Q. Tissue distribution and bioaccumulation of organophosphate esters in wild marine fish from Laizhou Bay, North China: Implications of human exposure via fish consumption. J. Hazard. Mater. 2021, 401, 123410. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Alemany, O.; Sala, B.; Plon, S.; Bouwman, H.; Barcelo, D.; Eljarrat, E. Halogenated and organophosphorus flame retardants in cetaceans from the southwestern Indian Ocean. Chemosphere 2019, 226, 791–799. [Google Scholar] [CrossRef]
- Sala, B.; Gimenez, J.; de Stephanis, R.; Barcelo, D.; Eljarrat, E. First determination of high levels of organophosphorus flame retardants and plasticizers in dolphins from Southern European waters. Environ. Res. 2019, 172, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.J.; Morris, A.D.; Dyck, M.; Sverko, E.; Reiner, E.J.; Blair, D.A.D.; Chu, S.G.; Shen, L. Legacy and new halogenated persistent organic pollutants in polar bears from a contamination hotspot in the Arctic, Hudson Bay Canada. Sci. Total Environ. 2018, 610–611, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fu, K.; Chen, Y.; Li, X.; Ye, T.; Gao, K.; Pan, W.; Zhang, A.; Fu, J. Long-Range Transport, Trophic Transfer, and Ecological Risks of Organophosphate Esters in Remote Areas. Environ. Sci. Technol. 2021, 55, 10192–10209. [Google Scholar] [CrossRef]
- Giulivo, M.; Capri, E.; Eljarrat, E.; Barcelo, D. Analysis of organophosphorus flame retardants in environmental and biotic matrices using on-line turbulent flow chromatography-liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1474, 71–78. [Google Scholar] [CrossRef]
- Santin, G.; Eljarrat, E.; Barcelo, D. Simultaneous determination of 16 organophosphorus flame retardants and plasticizers in fish by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1441, 34–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, X.; Huang, K.; Sun, Q.; Kong, R.; Chen, S.; Liang, C.; Li, M.; Letcher, R.J.; Liu, C. Tris(1,3-dichloro-2-propyl)phosphate Reduces Growth Hormone Expression via Binding to Growth Hormone Releasing Hormone Receptors and Inhibits the Growth of Crucian Carp. Environ. Sci. Technol. 2021, 55, 8108–8118. [Google Scholar] [CrossRef]
- Rhyu, D.; Lee, H.; Tanguay, R.L.; Kim, K.T. Tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) disrupts zebrafish tail fin development. Ecotoxicol. Environ. Saf. 2019, 182, 109449. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, H.; Huang, Y.; Liu, J.; Yu, L.; Liu, C.; Wang, J. Effects of environmentally relevant concentrations of tris (2-butoxyethyl) phosphate on growth and transcription of genes involved in the GH/IGF and HPT axes in zebrafish (Danio rerio). Chemosphere 2018, 212, 376–384. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Alimohamadi, M.; Khataee, A.; Dragoi, E.N. A global systematic review on the concentration of organophosphate esters in water resources: Meta-analysis, and probabilistic risk assessment. Sci. Total Environ. 2022, 807 Pt 2, 150876. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; Zhang, B.; Zhou, Y. Trophic transfer potential of nTiO(2), nZnO, and triclosan in an algae-algae eating fish food chain. Aquat. Toxicol. 2021, 235, 105824. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Zhao, L.M.; Letcher, R.J.; Zhang, Y.Y.; Jian, K.; Zhang, J.H.; Su, G.Y. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ. Int. 2019, 127, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Mekni, S.; Barhoumi, B.; Touil, S.; Driss, M.R.; Eljarrat, E. Occurrence of Halogenated and Organophosphate Flame Retardants in Sediments and Eels (Anguilla anguilla) From Bizerte Lagoon, Tunisia. Front. Environ. Sci. 2020, 8, 67. [Google Scholar] [CrossRef]
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Tang, S.; Phong, T.; Li, Z.; Baduel, C.; Mueller, J.F. Concentrations of Organophosphate Esters and Their Specific Metabolites in Food in Southeast Queensland, Australia: Is Dietary Exposure an Important Pathway of Organophosphate Esters and Their Metabolites? Environ. Sci. Technol. 2018, 52, 12765–12773. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Deng, T.; Xu, M.; Wang, S.; Yang, F. Residuals of organophosphate esters in foodstuffs and implication for human exposure. Environ. Pollut. 2018, 233, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, K.; Li, A.; Zeng, W.; Lin, T.; Wang, Y. Occurrence, phase distribution, and bioaccumulation of organophosphate esters (OPEs) in mariculture farms of the Beibu Gulf, China: A health risk assessment through seafood consumption. Environ. Pollut. 2020, 263 Pt B, 114426. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, L.; Li, D.; Chen, C.; Cao, Q. Monitoring and exposure assessment of organophosphorus flame retardants in source and drinking water, Nanjing, China. Environ. Monit. Assess. 2019, 191, 119. [Google Scholar] [CrossRef]

| Compound | Abbr. | CAS No. | Molecular Formula | Molecular Mass | LogKow | Solubility (mg/L, 25 °C) |
|---|---|---|---|---|---|---|
| Trimethyl phosphate | TMP | 512-56-1 | C3H9O4P | 140.07 | −0.65 | 3.00 × 105 |
| Triethyl phosphate | TEP | 78-40-0 | C6H15O4P | 182.16 | 0.8 | 5 × 105 |
| Tri-n-butyl phosphate | TnBP | 126-73-8 | C12H27O4P | 266.32 | 4 | 280 |
| Tri-iso-butyl phosphate | TiBP | 126-71-6 | C12H27O4P | 266.31 | 3.60 | 3.72 |
| Tripropyl phosphate | TPP | 513-08-6 | C9H21O4P | 224.23 | 2.35 | 6450 |
| Tris(2-ethylhexyl) phosphate | TEHP | 78-42-2 | C24H51O4P | 434.64 | 9.43 | 2 |
| Tris(2-butoxyethyl) phosphate | TBOEP | 78-51-3 | C18H39O7P | 398.48 | 3.75 | 1100 |
| 2-Ethylhexyl diphenyl phosphate | EHDPP | 1241-94-7 | C20H27O4P | 362.40 | 6.30 | 1.9 |
| Tris(2-chloroethyl) phosphate | TCEP | 115-96-8 | C6H12Cl3O4P | 285.48 | 1.44 | 7000 |
| Tris(1-chloro-2-propyl) phosphate | TCPP | 13674-84-5 | C9H18Cl3O4P | 327.56 | 2.59 | 1200 |
| Tris(2-chloro-1-(chloromethyl)ethyl) phosphate | TDCPP | 13674-87-8 | C9H15Cl6O4P | 430.90 | 3.80 | 1.50 |
| Triphenyl phosphate | TPhP | 115-86-6 | C18H15O4P | 326.29 | 4.59 | 1.9 |
| Tri-3-cresyl phosphate | TCrP | 563-04-2 | C21H21O4P | 368.36 | 5.11 | 1.20 × 10−2 |
| Cresyl diphenyl phosphate | CDPP | 26444-49-5 | C19H17O4P | 340.31 | 4.51 | 0.24 |
| 2,2-bis(chloromethyl)trimethylenebis(bis(2-chloroethyl)phosphate) | V6 | 38051-10-4 | C13H24Cl6O8P2 | 582.99 | 1.92 | 2.1 |
| Bisphenol-A bis(diphenyl phosphate) | BDP | 5945-33-5 | C39H34O8P2 | 692.63 | 7.41 | 0.42 |
| Tetraphenyl resorcinol bis(diphenylphosphate) | RDP | 57583-54-7 | C30H24O8P2 | 574.45 | 4.50 | 1.1 × 104 |
| Location of Sampling | TCPP | TDCPP | TCEP | TBOEP | TNBP | TPhP | TEHP | EHDPP | ΣOPE | Analysis Instrument | Year | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New York (The United States) | Wastewater (ng/L) | Influent | 5120 | 1722 | 1427 | 30,143 | 291 | 491 | 392 | ND | 2230–117,000 | HPLC-MS/MS | 2017 | [25] |
| Effluent | 5949 | 3106 | 1106 | 12,641 | 301 | 293 | 50.5 | ND | - | |||||
| Beijing (China) | Wastewater (ng/L) | Influent | 465 | - | 150 | 398 | 120 | 15.4 | 7.10 | - | - | HPLC-MS/MS | 2016 | [26] |
| Effluent | 605 | - | 254 | 103 | 93.1 | 6.20 | <LOD | - | - | |||||
| The Pearl River Delta (China) | Wastewater (ng/L) | Influent | 299.0 | 60.3 | 438.2 | 4349.4 | 21,271.8 | 149.2 | - | - | - | GC-MS | 2015 | [27] |
| Effluent | 472.9 | 94.5 | 372.2 | 494.5 | 3105.1 | 24.8 | - | - | - | |||||
| Beijing (China) | Wastewater (ng/L) | Influent | 225.0 | 22.8 | 179.1 | 600.3 | 74.4 | 21.3 | ND | 14.0 | 1399 | UPLC-MS | 2016 | [28] |
| Effluent | 338.9 | 15.9 | 232.9 | 39.9 | 29.8 | 4.4 | ND | 0.9 | 833 | |||||
| Beijing (China) | Wastewater (ng/L) | Influent | 440 | 28 | 245 | 31 | 3.8 | 0.6 | ND | ND | - | GC-MS | 2016 | [29] |
| Effluent | 413 | 47 | 250 | 47 | 48 | ND | ND | ND | - | |||||
| Catalonia (Spain) Wastewater (ng/L) | WWTP1 (ng/L) | Influent | 1700 | 129 | 180 | 1560 | <MDL | 101 | <MDL | <MDL | 3670 | GC-MS | 2016 | [30] |
| Effluent | 2400 | 136 | 250 | 207 | 220 | 40 | <MDL | <MDL | 3060 | |||||
| WWTP2 (ng/L) | Influent | 6750 | 290 | 320 | 1200 | 210 | 250 | 131 | 270 | 2170 | ||||
| Effluent | 3000 | 354 | 373 | 620 | 90 | 64 | <MDL | <MDL | 5240 | |||||
| WWTP3 (ng/L) | Influent | 3600 | 111 | 295 | 7000 | 900 | 177 | 120 | 440 | 151,000 | ||||
| Effluent | 3700 | 319 | 570 | 1970 | 174 | 37 | <MDL | <MDL | 29,800 | |||||
| WWTP4 (ng/L) | Influent | 3200 | 220 | 220 | 4600 | 305 | 95 | 12 | 84 | 50,500 | ||||
| Effluent | 2800 | 210 | 240 | 1500 | 136 | 55 | <MDL | <MDL | 5170 | |||||
| WWTP5 (ng/L) | Influent | 3710 | 67 | 320 | 8600 | 135 | 124 | 35 | 240 | 13,500 | ||||
| Effluent | 3100 | 174 | 330 | 3600 | 65 | 70 | <MDL | <MDL | 7530 | |||||
| 25 WWTPs (China) | Wastewater (ng/L) | Influent | 265 | 31 | 56 | 288 | 183 | 8.1 | 8.1 | 5.5 | - | UHPLC-MS/MS | 2023 | [31] |
| Effluent | 238 | 26 | 45 | 71 | 11 | 2.9 | 1.9 | 0.5 | - | |||||
| WWTP Wastewater (ng/L) | Spring (ng/L) | Influent | 435.9 | 70.2 | 216.1 | 91.9 | - | 15 | 2.3 | 3.2 | 838.3 | UPLC-MS | 2020 | [32] |
| Autumn (ng/L) | Influent | 323.3 | 54.7 | 236.9 | 0.7 | - | 10.2 | 7.4 | 8.7 | 595.2 | ||||
| Winter (ng/L) | Influent | 223.5 | 35.1 | 79.9 | 45.7 | - | 7.9 | 3.9 | 6.5 | - | ||||
| Spring (ng/L) | Influent | 368.8 | 44.6 | 184.1 | 115.9 | - | 9 | 2.2 | 1.4 | - | ||||
| Guangzhou (China) | Wastewater (ng/L) | Influent | 756.9 | 92 | 254.2 | - | 353.2 | 20.3 | 192.3 | - | - | GC-MS | 2019 | [33] |
| Effluent | 651.6 | 88.4 | 218.7 | - | 28.36 | 1.63 | 15.44 | - | - | |||||
| Guangzhou (China) | Wastewater (ng/L) | Influent | 3872 | <LOD | 928 | <LOD | - | - | - | - | 4807 | GC-MS | 2018 | [34] |
| Effluent | 54.1 | <LOD | 31.5 | <LOD | - | - | - | - | 103.9 | |||||
| WWTP (Greece) | Wastewater (ng/L) | Influent | 864–3277 | 29.8–310 | 50.3–186 | 476–4037 | 28.9–129.8 | 69.5–1299 | 33.3–376 | - | 2144–9743 | GC-MS | 2022 | [35] |
| Effluent | 460–1444 | 9.9–214 | 46.3–161.4 | 98.9–783 | 4.58–83.3 | 34.1–377 | 28.3–130 | - | 1237–2909 | |||||
| Shandong Peninsula (China) | Wastewater (ng/L) | Effluent | 518.6 | 55.3 | 545.8 | 12.3 | 30.9 | 7.2 | - | - | 1568 | UPLC-MS/MS | 2022 | [36] |
| Ontario (Canada) | Wastewater (ng/L) | Effluent | 1250–2390 | 210–400 | 140–340 | 290–10,200 | - | 5760 | - | - | - | LC-MS/MS | 2018 | [37] |
| The Pearl River Delta (China) | Wastewater (ng/L) | Influent | 14–638 | <LOQ-95.7 | <LOQ-113 | ND-311 | ND-2248 | 10.1–290 | ND-84.9 | - | 65.8–2842 | GC-MS | 2019 | [38] |
| Effluent | 5.4–104 | <LOQ-21.5 | <LOQ-31.9 | ND-23.1 | ND-2541 | 1–108 | <LOQ | - | 6.37–2710 | |||||
| Zhengzhou (China) | Wastewater (ng/L) Sludge (ng/L) | Influent | 204.2 | 15.6 | 172.3 | 648.7 | 50.6 | 15.2 | - | - | 1106.5 | UPLC-MS/MS | 2016 | [39] |
| Effluent | 196 | 7.5 | 171.8 | 96.6 | 35.9 | 3.8 | - | - | 511.7 | |||||
| Sludge | 60.7 | 28 | 21.5 | 48 | 53.2 | 16.9 | - | - | 228.3 | |||||
| Influent | 90.8 | 11.1 | 70 | 294.4 | 53.6 | 8.8 | - | - | 528.6 | |||||
| Effluent | 80.1 | 8.4 | 61.4 | 34.1 | 401 | 3.2 | - | - | 227.4 | |||||
| Sludge | 52.9 | 30.9 | 45.7 | 67 | 31 | 38.7 | - | - | 266.2 | |||||
| 67 WWTPs (The United States) | Sludge (ng/g) | sludge | 61.7 | 101 | 10.6 | 1760 | 127 | 30.4 | 199 | 189 | 3070 | HPLC-MS/MS | 2019 | [40] |
| Location of Sampling | ΣOPE | Alkyl-OPEs | Chlorinated-OPEs | Aryl-OPEs | Analysis Instrument | Year | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMP | TEP | TnBP | TBOEP | TCEP | TCPP | TDCPP | TPhP | TCrP | |||||
| Luoma Lake, China | 0.8–708 | <MDL-127 | <MDL-32.1 | 0.01–5.9 | 0.002–0.2 | 0.01–552 | 0.02–10.8 | 0.03–2.0 | 0.15–8.2 | 0.7–54.6 | HPLC-MS/MS | 2018 | [50] |
| Jiaozhou Bay, China | 474.9–6776.3 | ND-8.1 | 172.0–904.4 | 1.3–328.3 | 3.1–152.8 | ΣChlorinated-OPEs: 206.7–5614.8 | ΣAryl-OPEs: 3.1–20.0 | HPLC-MS/MS | 2022 | [36] | |||
| Laizhou Bay, China | 262.8–2907.3 | <MDL-90 | 55.6–2390.1 | 1.3–86.3 | 2.4–247.2 | ΣChlorinated-OPEs: 143.4–1096.1 | ΣAryl-OPEs: 2.3–25.9 | ||||||
| Zijiang River, China | 18.8–439 | ND-2.89 | 0.3–9.9 | 1.2–58 | 0.97–33.1 | 1.29–26.6 | ND-366 | ND-10.1 | ND-14.8 | - | UPLC-MS | 2022 | [51] |
| Taihu Lake, China | 100–1700 | 2.7–84 | 53–1400 | 0 | ND-2.7 | 14–76 | 12–2900 | ND-6.0 | ND-14 | ND-1.5 | HPLC-MS | 2018 | [52] |
| Bohai Sea, China | 10.9–516.4 | - | 0.7–168.1 | - | - | 4.8–474.0 | ND | - | ND | - | GC-MS/MS | 2021 | [53] |
| Poyang Lake, China | 38.4–428.9 | ND-1.22 | 2.3–60.7 | - | ND-3.52 | 6.4–52.1 | 13.4–143.4 | ND-70.7 | ND-18.9 | ND-10.5 | UPLC-MS/MS | 2022 | [54] |
| The western South China Sea | 2.3–24.4 | - | - | 0.1–2.2 | - | 0.8–22.6 | 0.4–6.2 | ND-0.3 | 0.01–0.1 | - | GC-MS | 2022 | [48] |
| Shanghai, China (Urban/Rural) | 340–1688.7 | - | - | 11.6–63.3 | 15.9–100.6 | 67.5–865.2 | 123.9–523 | <LOQ-45.3 | 1.67–47.7 | - | GC-MS | 2019 | [55] |
| 185.4–321 | - | - | 6.91–44.8 | <LOQ-47.9 | 30–63.3 | 60–154.2 | <LOQ | 5.03–34.3 | - | ||||
| Source water, Shanghai | 415.7–822.6 | - | 100.8–182.0 | 19.8–138.6 | 1.2–27.5 | 70.8–129.5 | 184.3–363.3 | 4.9–18.1 | <MDL-3.6 | <MDL | UPLC-MS/MS | 2022 | [56] |
| Nanjing, China | 4.4–195,269 | ND-18.5 | ND-932.6 | ND-385.3 | 1.1–547.3 | ND-15,483 | ND-244.3 | ND-16273 | ND-290 | ND-126.6 | HPLC-MS/MS | 2022 | [57] |
| Beibu Gulf, China (Summer/winter) | 34.2–1227 | 0.05–14.8 | - | 0.4–1033 | 0.8–10.3 | 1.2–44.9 | 13.4–164 | ND-6.7 | 0–26 | - | GC-MS/MS | 2022 | [58] |
| 20.6–840 | ND | ND-782 | 10–22.8 | 0.7–13.2 | 3.4–112 | 0.7–2.7 | ND | - | |||||
| Xiangjiang River, China | 6.1–25.3 | - | - | 0.1–8.9 | - | ND-0.5 | 2–13.5 | 0.1–8.4 | - | GC-MS/MS | 2021 | [20] | |
| Qinzhou Bay, China | 150–885 | <LOD-2.4 | ND-14 | ND-139.8 | - | 30.9–370.3 | 23.7–568.4 | ND-61.4 | <LOD-11.5 | - | GC-MS | 2021 | [59] |
| The West Pacific Ocean | 3.02–48.4 | - | - | 0.5–16.7 | - | 0.7–26.4 | 0.8–3.3 | 0.5–8.3 | <LOD-0.2 | - | GC-MS | 2021 | [60] |
| The Canadian Arctic | 0.02–306 | - | - | <LOD-8.1 | <LOD | <LOD-246 | 0.2–53 | <LOD-11 | <LOD-63 | - | GC-MS | 2021 | [61] |
| Greece (River water/Coastal water/Streams) | 400–2158 | - | <MDL-134 | 15–374 | <LOD-997 | 18–163 | 59–208 | <LOD-14 | 40–258 | 77–95 | GC-MS/MS | 2021 | [19] |
| 408–1270 | - | <MDL-126 | 17–92 | <LOD-625 | 17–162 | 67–113 | <LOD-21 | 41–260 | 59–126 | ||||
| 377–30,560 | - | <MDL-352 | 16–5961 | <LOD-11418 | 19–1018 | 55–10,742 | <LOD-1988 | 45–1142 | 77–718 | ||||
| The Rhone River, France | 84.8–264.6 | - | - | 4.4–138.1 | - | ND-25 | 36.6–173.1 | 3.1–8.7 | ND-10.7 | - | GC-MS | 2020 | [49] |
| Lake Shihwa, Korea | 597–16,000 | - | 42.2–3677 | - | 145–839 | 86.5–5963 | 68.3–5102 | <LOQ-325 | 5.1–96.2 | - | GC-MS/MS | 2018 | [18] |
| Amazon River | 74–1341.1 | - | - | <LOQ-6.5 | <LOQ-1.6 | 74–1300 | - | <LOQ-6.9 | - | GC/MS | 2019 | [62] | |
| San Francisco Bay, US | 170–5100 | - | - | 7.8–43 | 24–1000 | 7.4–300 | 46–2900 | 14–450 | 41–360 | - | GC-MS | 2019 | [63] |
| New York State (River/Lake/Seawater) | 37.2–510 | <LOQ-4 | <LOQ-24.8 | - | 2.53–366 | <LOQ-79.5 | 3.3–214 | <LOQ-86.7 | <LOQ-36.5 | - | HPLC-MS/MS | 2018 | [64] |
| 8.2–1280 | <LOQ-5.2 | <LOQ-92.1 | - | 0.6–689 | <LOQ-123 | 4.7–329 | <LOQ-159 | <LOQ-28.7 | - | ||||
| 40–60.8 | <LOQ-0.1 | 0.8–1.8 | - | <LOQ-7.7 | - | 25.8–36.3 | 8.9–25.4 | <LOQ-1.6 | - | ||||
| Location of Sampling | TCPP | TDCPP | TCEP | TBOEP | TNBP | TPhP | ΣOPE | Analysis Instrument | Year | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nanjing, China | Bottled water | 1.3–16.2 | ND | ND-48.8 | 19.5–81.7 | - | - | 165 | UPLC-MS/MS | 2014 | [79] |
| Eastern China | Well water | 1.3–3.8 | ND-1.1 | 0.1–3.5 | 0.01–0.6 | 0.1–0.4 | ND-0.5 | 4.5 | UPLC-MS/MS | 2015 | [80] |
| Barreled Water | ND-48.5 | ND-7.0 | 0.2–44.2 | ND-0.3 | ND-1.6 | 0.05–0.9 | 27.6 | ||||
| Filtered drinking water | 1.6–26.5 | ND-6.6 | 1.9–48.5 | ND-5.3 | 0.2–6.6 | ND-1.8 | 59.2 | ||||
| Tap water | 21.5–109 | 5.4–6.8 | 28.5–139 | 1.4–6.6 | 3.9–76.3 | 0.3–4.0 | 192 | ||||
| Pakistan | Industrial zones | 0.03–85.7 | <MDL-21.4 | 0.09–31.2 | - | - | - | <MDL-71.1 | GC-MS | 2016 | [83] |
| Rural zones | <MDL-13.1 | <MDL-9.2 | <MDL-12.1 | - | - | - | <MDL-12.1 | ||||
| Background zones | <MDL | <MDL | <MDL-0.06 | - | - | - | <MDL-0.08 | ||||
| Korea | Tap water | 67.0 | - | 38.8 | 26.1 | 3.40 | - | 137.4 | GC-MS | 2016 | [84] |
| Purified water | 155 | - | 70.1 | 10.7 | 1.27 | - | 264.7 | ||||
| Bottled water | 79.6 | - | 25.3 | 35.6 | 4.29 | - | 53 | ||||
| The Pearl River Delta, China | Bottled water | <MDL-170 | <MDL-1.9 | <MDL-3.1 | <MDL-2.2 | <MDL-4.5 | 0 | 34 | UPLC-MS/MS | 2022 | [81] |
| Barreled water | 9.8–100 | 65 | <MDL-1.6 | 94 | <MDL-0.6 | <MDL-14 | 24 | ||||
| Tap water | <MDL-350 | 100 | <MDL-180 | 100 | <MDL-120 | <MDL-36 | 72 | ||||
| Shanghai, China | DWTP | 100.5–220.4 | 1.9–16.4 | 33.8–47.6 | <MDL-7.0 | 3.5–39.5 | <MDL-1.6 | 312.1 | UPLC-MS/MS | 2022 | [56] |
| Nakdong River, South Korea | DWTP | 15–35.9 | 2.2–3.2 | 13.5–21.8 | 5.7–20.6 | 0.8–2.7 | 2.8–7.5 | 49.4–86.5 | GC-MS | 2020 | [85] |
| Nanjing, China | Tap water | 78 | 41.4 | 207.6 | 6.7 | 27.7 | 179.7 | 719.8 | HPLC-MS/MS | 2022 | [57] |
| Xiangjiang River, China | Tap water | 9 | - | 0.3 | - | 6.2 | 7.5 | 23.6 | GC-MS/MS | 2021 | [20] |
| New York State, US | Tap water | <LOQ-67.1 | <LOQ-124 | <LOQ-17.4 | <LOQ-109 | - | <LOQ-39.9 | 41.6 | HPLC- MS/MS | 2018 | [64] |
| Hefei China | Tap water | 15.8 | 2.2 | 15.5 | 0.5 | 1.1 | 1.3 | - | UPLC-MS | 2020 | [89] |
| Beijing, China | Barreled Water | ND-6.3 | ND-2.2 | ND-8,2 | ND | ND-1.6 | ND-0.25 | 0.5–23.9 | UPLC-MS/MS | 2021 | [78] |
| Major metropolitan cities, Korea | Tap water | 49.4 | 2 | 39.5 | 43.9 | 11.8 | 23 | 169 | GC-MS | 2018 | [90] |
| Location | Species | Number of OPEs Analyzed | TCPP | TDCPP | TCEP | TBOEP | TNBP | TPhP | ΣOPE | Analysis Instrument | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antarctic | algae | 16 | 23.4 | ND | 25.5 | 1.33 | 9.7 | 2.6 | 88.3 (ng/g lw) | LC-MS/MS | 2020 | [95] |
| Laizhou Bay, China | fish and invertebrate | 20 | - | - | - | - | - | - | 21.1–3510 (ng/g lw) | GC-MS | 2019 | [96] |
| Alaska | sentinel fish | 24 | - | - | - | - | 5.5 | 0.1 | 5.95 (ng/g ww) | UPLC-QQQ MS | 2020 | [97] |
| Spain | mussels | 18 | 3.8–29.6 | ND | <LOQ | 5.6–12.4 | 0.9–9.4 | 23.6–623.6 | -(ng/g dw) | LC-MS/MS | 2020 | [98] |
| 7 European countries | mussel | - | - | - | - | - | - | - | 0.50–102 (ng/g dw) | LC-MS | 2018 | [99] |
| Great Lakes | lake trout | 22 | - | - | - | - | - | - | 9–122 (ng/g ww) | LC-MS/MS | 2022 | [100] |
| Great Lakes | fish | 18 | 6.7 | 9.6 | 13.3 | - | 1.6 | 17.1 | 36.6 (ng/g lw) | GC-MS | 2017 | [101] |
| Laizhou Bay, China | fish muscle | 20 | ND-6.1 | ND-2.5 | ND-5.8 | - | ND-13.1 | ND-8.4 | 6.6–107 (ng/g dw) | GC-MS | 2021 | [102] |
| Indian Ocean | dolphin | 14 | ND | ND | ND | 952–31,841 | ND-1333 | ND | 10,452 ± 11,301 (ng/g lw) | LC-MS/MS | 2019 | [103] |
| Alboran Sea, Spain | dolphin muscle | 16 | ND | - | 32.1 | 66.9 | 1309 | ND | 69.5–2939 (ng/g lw) | LC-MS | 2019 | [104] |
| US | harbor seal | 13 | ND-30 | ND-56 | ND-8.3 | <2.5 | <1.5 | ND-27 | 17–67 (ng/g lw) | LC-MS/MS | 2019 | [63] |
| Canada (in SHB/WHB) | polar bear | 17 | - | - | - | - | - | - | 0.163/0.308 (ng/g lw) | UPLC-MS/MS | 2018 | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, Y.; Han, X.; Wang, J.; Wu, C.; Zhuang, Y.; Liu, J.; Li, W. A Review of Organophosphate Esters in Aquatic Environments: Levels, Distribution, and Human Exposure. Water 2023, 15, 1790. https://doi.org/10.3390/w15091790
Wang Y, Zhao Y, Han X, Wang J, Wu C, Zhuang Y, Liu J, Li W. A Review of Organophosphate Esters in Aquatic Environments: Levels, Distribution, and Human Exposure. Water. 2023; 15(9):1790. https://doi.org/10.3390/w15091790
Chicago/Turabian StyleWang, Yisha, Yanjun Zhao, Xu Han, Jiashuo Wang, Chuandong Wu, Yuan Zhuang, Jiemin Liu, and Wenhui Li. 2023. "A Review of Organophosphate Esters in Aquatic Environments: Levels, Distribution, and Human Exposure" Water 15, no. 9: 1790. https://doi.org/10.3390/w15091790
APA StyleWang, Y., Zhao, Y., Han, X., Wang, J., Wu, C., Zhuang, Y., Liu, J., & Li, W. (2023). A Review of Organophosphate Esters in Aquatic Environments: Levels, Distribution, and Human Exposure. Water, 15(9), 1790. https://doi.org/10.3390/w15091790









