The Impact of Plant Spatial Patterns on Nitrogen Removal in the Naolihe Wetlands of Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Sampling and Determination
2.3. Remote Sensing Interpretation and Spatial Indices Extraction
2.4. Data Analysis
2.4.1. The Calculation of Removal Rates for TN, NH4-N, and NO3-N
2.4.2. The Step-by-Step Elimination
2.4.3. Function Fitting
3. Results and Discussion
3.1. The Effect of Plant Area on Nitrogen Removal
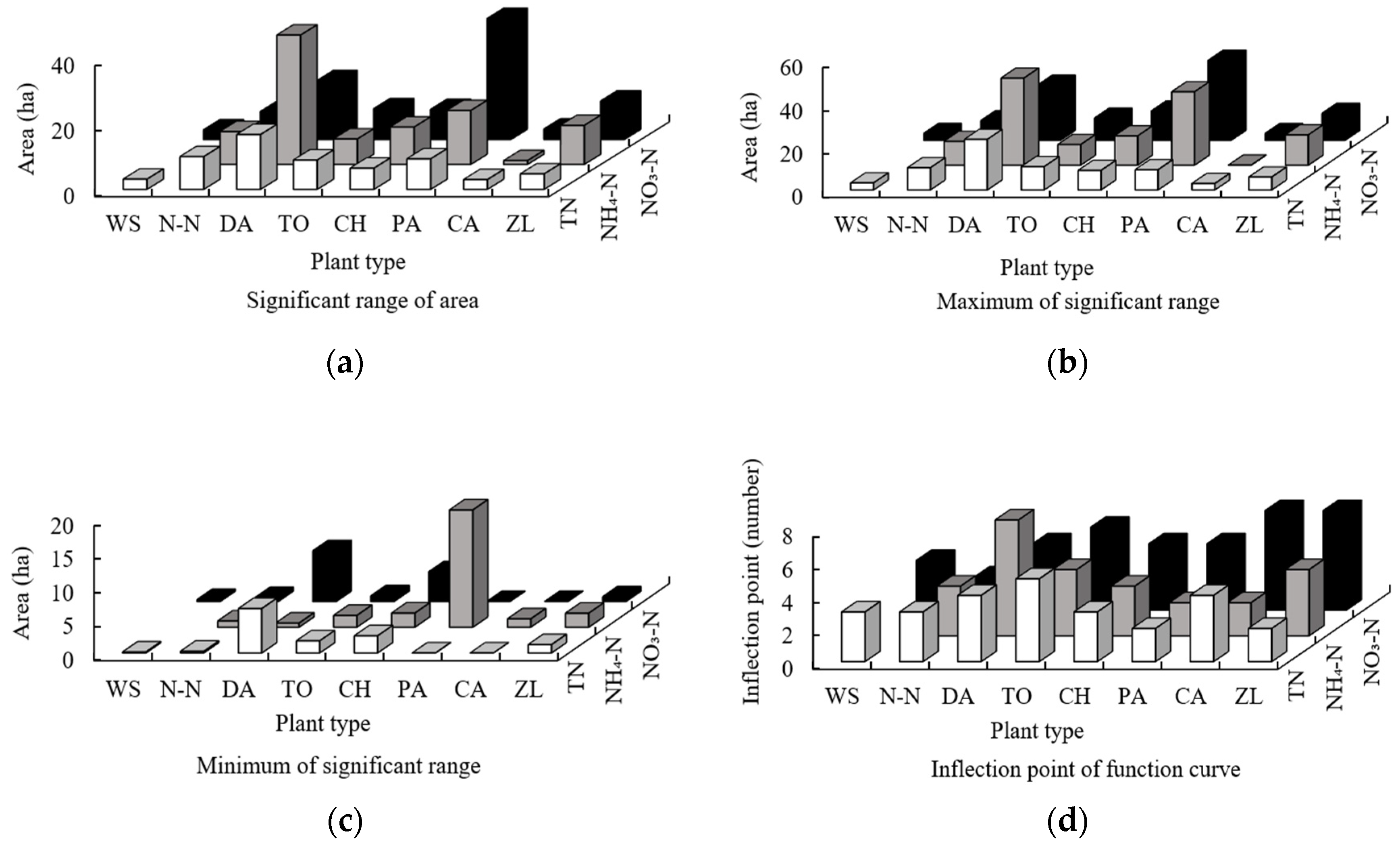
3.1.1. The Impact of Patch Area
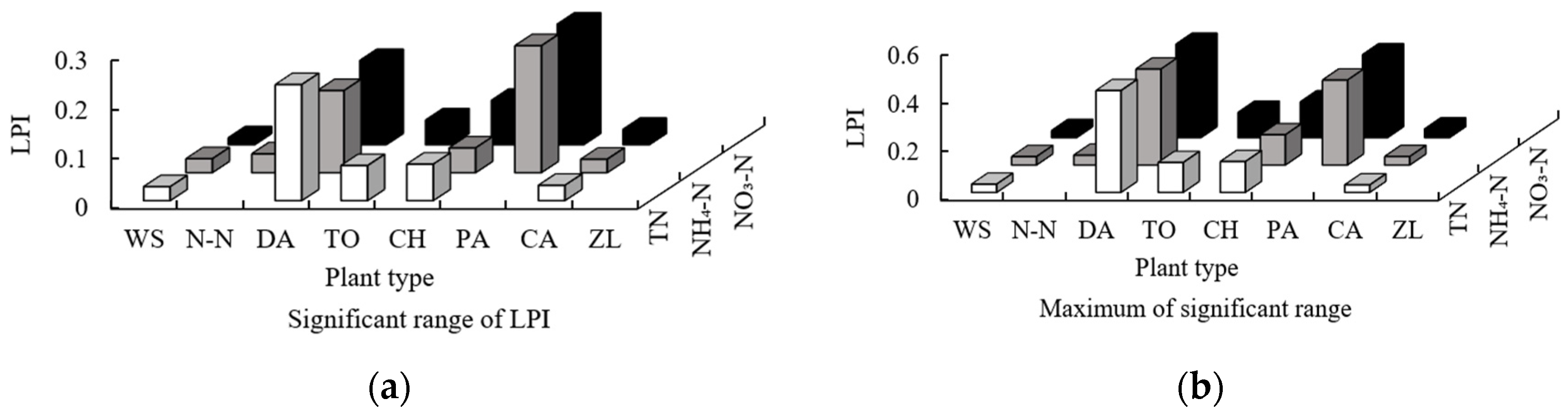
3.1.2. The Impact of Patch Fragmentation
3.2. The Effect of Plant Patch Shape on Nitrogen Removal
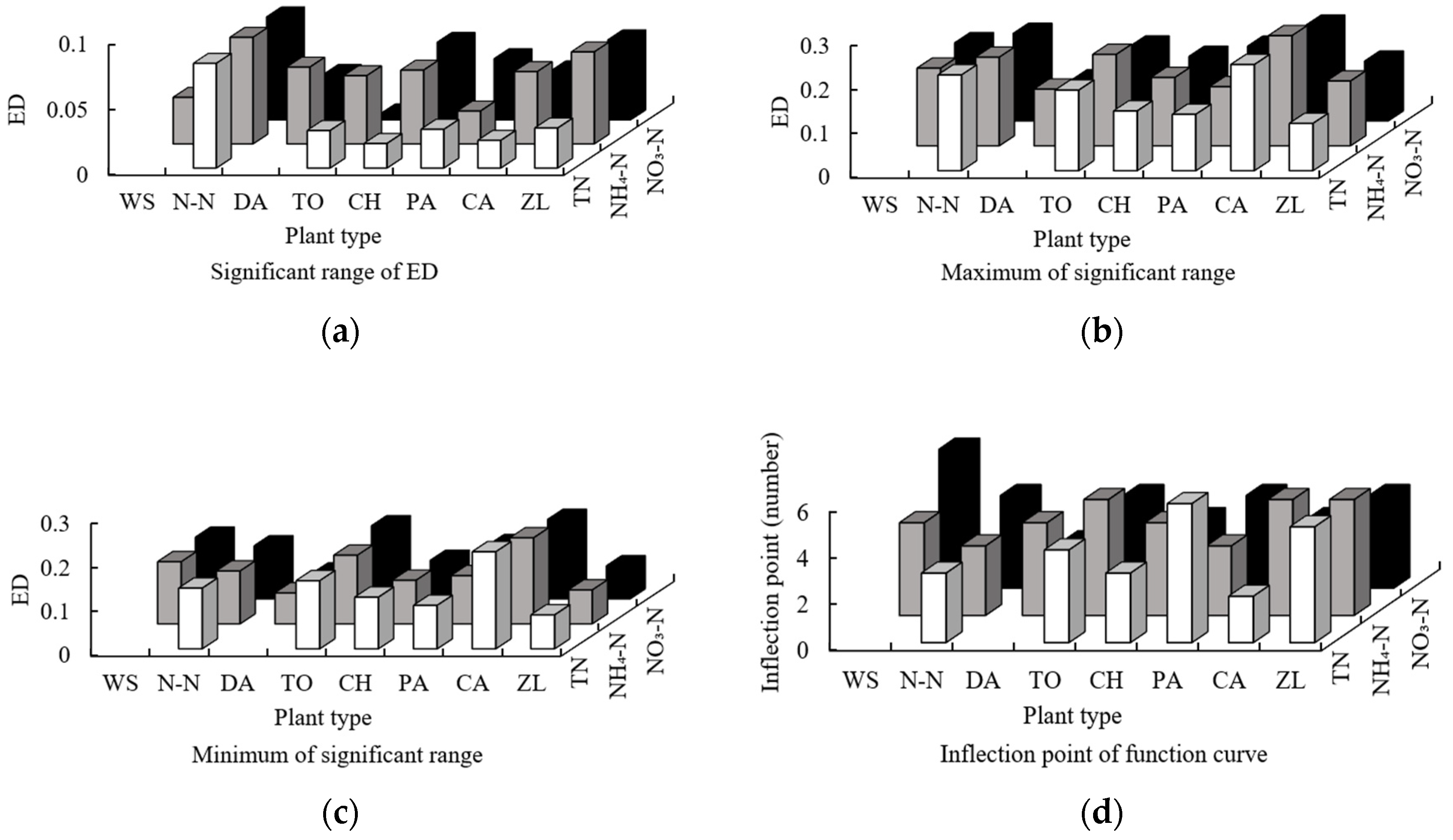
3.2.1. The Impact of Contact Density between Different Plants
3.2.2. The Influence of the Contact of Internal Patches on Plants
3.2.3. The Influence of Patch Shape on Plants
3.3. The Particularity of Floating Leaf Plants
3.4. The Effect of Fragmentation on Nitrogen Removal Ability
3.5. The Collaborative Effects of Wetland Plants on Nitrogen Removal
4. Conclusions
- (1)
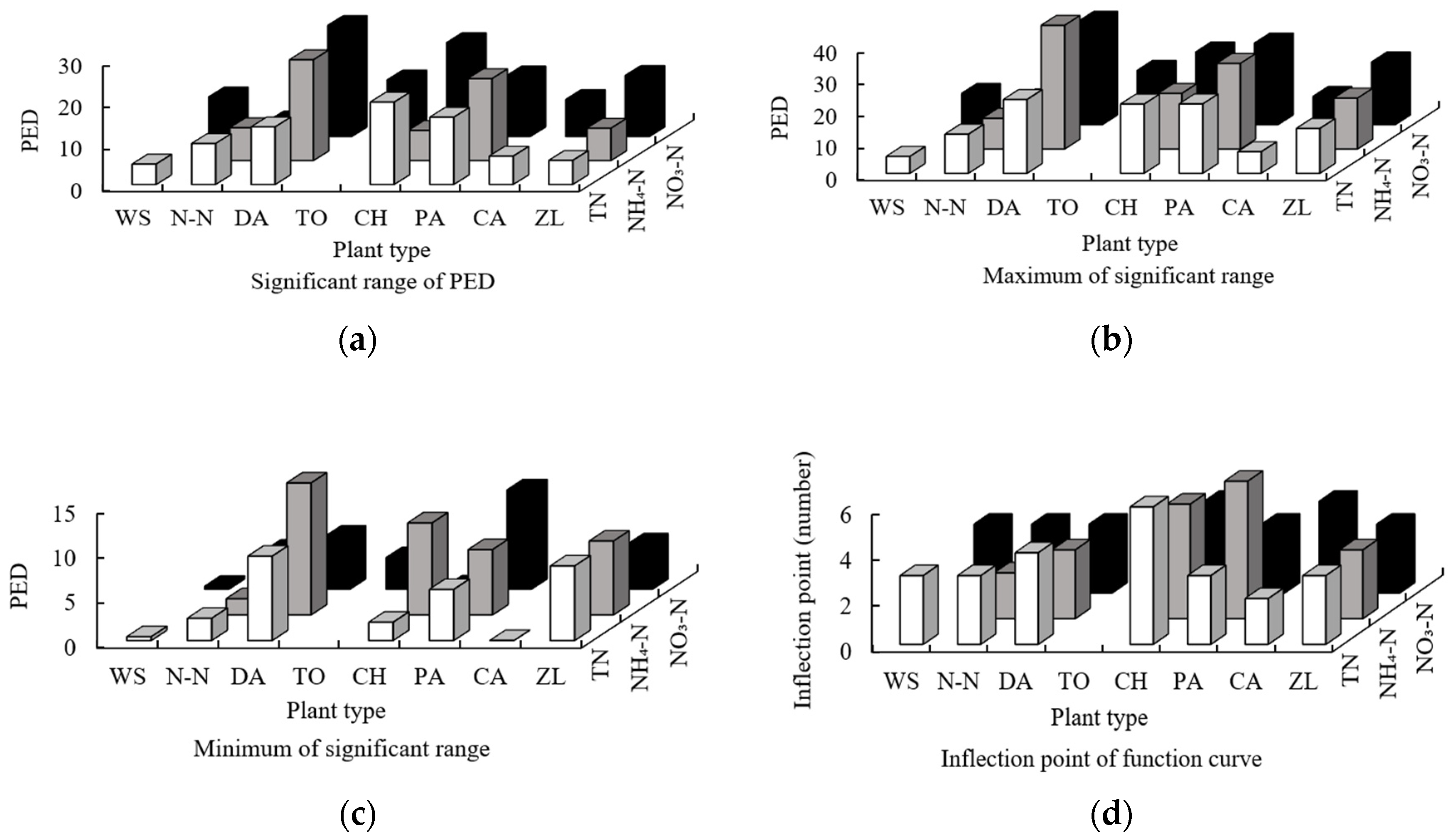
- DA and PA ranked first in terms of the significant range, maximum, and minimum of the functions between the order of sampling points of the area of all plants and the three forms of nitrogen. The main force for nitrogen removal was dependent on the plant with the largest area (DA: 31.2%; PA: 24.3%), and the N-N plant also ranks highly, indicating that floating leaf plants have strong nitrogen removal ability despite their small area (5%). The results for the significant range, maximum, and minimum of the functions between the order of sampling points of the PLI of all plants and the three forms of nitrogen indicate that the fragmentation of plant patches reduces the nitrogen removal ability (the mean for the PLI of 8 plants is 0.10).
- (2)
- The results for the eigenvalues of the functions between the order of sampling points of the DE of all plants and the three forms of nitrogen showed that the plants had a higher independent nitrogen removal ability, and the plants with low nitrogen removal ability may only perform a nitrogen removal function when mixed with other plants (the mean for the DE of 8 plants is 9.25). The results for the eigenvalues of the functions between the order of sampling points of the PDE of all plants and the three forms of nitrogen showed that plants with strong nitrogen removal abilities may also play a role in nitrogen removal, even in situations of poor connectivity; however, if connectivity was strong (DA: 16.79; PD: 15.70), this function would increase. The results for the eigenvalues of the functions between the order of sampling points of the FD of all plants and the three forms of nitrogen indicated that different plants have obviously different capacities for removing the different forms of nitrogen. Nitrogen removal involved all wetland plants in a collaborative manner, resulting in the completion of the division.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yargholi, B.; Kanani, E.; Sepehri, S. A long-term assessment of the effectiveness of a semi-artificial wetland in removing organic materials and nutrients from agricultural drainage water. J. Water Process Eng. 2023, 55, 104117. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, G.F.; Fu, Z.S.; Qiao, H.X.; Liu, F.X. Assessing wetland nitrogen removal and reed (Phragmites australis) nutrient responses for the selection of optimal harvest time. J. Environ. Manag. 2021, 280, 111783. [Google Scholar] [CrossRef] [PubMed]
- Khormali, A. Effect of water cut on the performance of an asphaltene inhibitor package: Experimental and modeling analysis. Pet. Sci. Technol. 2022, 40, 2890–2906. [Google Scholar] [CrossRef]
- Nilsson, J.E.; Liess, A.; Ehde, P.E.; Weisner, S.E.B. Mature wetland ecosystems remove nitrogen equally well regardless of initial planting. Sci. Total Environ. 2020, 716, 137002. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.S.; Chen, D.Y.; Wu, F.; Tang, L.; He, S.B.; Zhou, W.L. Function of aquatic plants on nitrogen removal and greenhouse gas emission in enhanced denitrification constructed wetlands: Iris pseudacorus for example. J. Clean. Prod. 2022, 330, 129842. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, W.L.; Wu, S.Q.; He, S.B.; Huang, J.C.; Zhang, X. Nitrogen removal by thiosulfate-driven denitrification and plant uptake in enhanced floating treatment wetland. Sci. Total Environ. 2018, 621, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.C.; Wang, H.F.; Zhou, J.; Lu, D.P. Purification effects of aquatic plants on nitrogen and phosphorus in eutrophic water bodies. J. Dali Univ. 2021, 12, 65–68. (In Chinese) [Google Scholar] [CrossRef]
- Bai, X.M.; He, L.S.; Li, B.C.; Meng, R.; Meng, F.L.; Huang, C.H.; Dong, J.; Li, G. Application of combined aquatic plants to control eutrophic water in Baiyangdian lake. Wetl. Sci. 2013, 11, 495–498. (In Chinese) [Google Scholar] [CrossRef]
- Białowiec, A.; Davies, L.; Albuquerque, A.; Randerson, P.F. The influence of plants on nitrogen removal from landfill leachate in discontinuous batch shallow constructed wetland with recirculating subsurface horizontal flow. Ecol. Eng. 2012, 40, 44–52. [Google Scholar] [CrossRef]
- Wang, Y.N.; Guo, X.C.; Lu, S.Y.; Liu, X.H.; Wang, X.H. Review of nitrogen removal in low-polluted water by constructed wetlands: Performance, mechanism, and influencing. J. Agric. Resour. Environ. 2021, 38, 722–734. (In Chinese) [Google Scholar] [CrossRef]
- Dong, C.; Li, M.T.; Zhuang, L.L.; Zhang, J.; Shen, Y.H.; Li, X.Z. The improvement of pollutant removal in the ferric-carbon micro-electrolysis constructed wetland by partial aeration. Water 2020, 12, 389. [Google Scholar] [CrossRef]
- Awad, J.; Hewa, G.; Myers, B.R.; Walker, C.; Lucke, T.; Akyol, B.; Duan, X.H. Investigation of the potential of native wetland plants for removal of nutrients from synthetic stormwater and domestic wastewater. Ecol. Eng. 2022, 179, 106642. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.Y.; Li, Y.; Wu, J.S. The phytoremediation of water with high concentrations of nitrogen and phosphorus contamination by three selected wetland plants. J. Water Process Eng. 2021, 40, 101828. [Google Scholar] [CrossRef]
- Zhou, X.H.; Wang, G.X.; Guo, C.C.; Cao, Y.; Ge, X.G.; Yang, W.B.; Pan, G.Q.; Ma, T. Effect of aquatic macrophytes in Tidal wetlands on the total nitrogen and total phosphorus. Res. Soil. Water Conserv. 2007, 14, 137–140. (In Chinese) [Google Scholar]
- Szota, C.; Farrell, C.; Livesley, S.J.; Fletcher, T.D. Salt tolerant plants increase nitrogen removal from biofiltration systems affected by saline stormwater. Water Res. 2015, 83, 195–204. [Google Scholar] [CrossRef]
- Hallin, S.; Hellman, M.; Choudhury, M.I.; Ecke, F. Relative importance of plant uptake and plant associated denitrification for removal of nitrogen from mine drainage in sub-arctic wetlands. Water Res. 2015, 85, 377–383. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tian, K.; Yue, H.T.; Yang, Y. Purification effects of common lakeside plant communities in plateau wetlands of Yunnan on the removal of sewage nitrogen. Ecol. Environ. Sci. 2012, 21, 359–363. (In Chinese) [Google Scholar] [CrossRef]
- Hong, S.C.; Wang, C.E.; Feng, Z.R.; Xue, X.D. Differential study on nitrogen and phosphorus accumulation in different aquatic plant communities. J. Zhejiang Univ. Sci. Technol. 2021, 33, 432–440. (In Chinese) [Google Scholar] [CrossRef]
- Xing, W.M.; Han, Y.G.; Guo, Z.F.; Zhou, Y. Quantitative study on redistribution of nitrogen and phosphorus by wetland plants under different water quality conditions. Environ. Pollut. 2020, 261, 114086. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, G.H.; Xing, W. Variations of multi-elements in wetland plants on the Tibetan Plateau are mainly determined by environmental factors. Ecol. Indic. 2023, 146, 109807. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.X. Degradation characteristics, patterns, and processes of lakeside wetland in Napahai of northwest Yunnan Plateau, Southwest China. Chin. J. Appl. Ecol. 2012, 23, 3257–3265. (In Chinese) [Google Scholar] [CrossRef]
- Sosa, L.L.D.; Glanville, H.C.; Marshall, M.R.; Williams, A.P.; Abadie, M.; Clark, I.M.; Blaud, A.; Jones, D.L. Spatial zoning of microbial functions and plant-soil nitrogen dynamics across a riparian area in an extensively grazed livestock system. Soil. Biol. Biochem. 2018, 120, 153–164. [Google Scholar] [CrossRef]
- Li, Q.R.; Liu, J.X.; Su, J.H.; Chai, B.F. Diversity distribution pattern and maintenance mechanism of epiphytic fungi communities on leaves of Nymphaea tetragona and Vallisneria natans. Nat. Sci. Ed. 2023. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Tian, K.; Xiao, D.R.; Yang, Q.; Xie, W.Y. Effects of lakeside plant spatial allocation in plateau wetlands of Yunnan, Southwest China on the removal of sewage nitrogen and phosphorus. Chin. J. Ecol. 2012, 31, 1425–1431. (In Chinese) [Google Scholar] [CrossRef]
- Wei, O.Y.; Yang, W.X.; Tysklind, M.; Xu, Y.X.; Lin, C.Y.; Gao, X.; Hao, Z.C. Using river sediments to analyze the driving force difference for nonpoint source pollution dynamics between two scales of watersheds. Water Res. 2018, 139, 311e320. [Google Scholar] [CrossRef]
- Wang, G.D.; Jiang, M.; Wang, M.; Xue, Z.S. Natural revegetation during restoration of wetlands in the Sanjiang Plain, Northeastern China. Ecol. Eng. 2019, 132, 49–55. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Sun, J.H.; Zhang, G.X.; Liu, X.M.; Wu, Y.F.; Sun, J.X.; Hu, B.T. Spatiotemporal dynamics of water supply–demand patterns under large-scale paddy expansion: Implications for regional sustainable water resource management. Agr. Water Manag. 2023, 285, 108388. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.D.; Chu, L.J.; Wang, G.D.; Sun, G.Z.; Sun, M.Y.; Wang, J.Y.; Jiang, M. Is there any correlation between landscape characteristics and total nitrogen in wetlands receiving agricultural drainages? Chin. Geogra. Sci. 2019, 29, 712–724. [Google Scholar] [CrossRef]
- Fu, J.; Liu, J.; Wang, X.W.; Zhang, M.D.; Chen, W.W.; Chen, B. Ecological risk assessment of wetland vegetation under projected climate scenarios in the Sanjiang Plain, China. J. Environ. Manag. 2020, 273, 111108. [Google Scholar] [CrossRef]
- Zhang, S.B.; Qin, W.; Xia, X.H.; Xia, L.Z.; Li, S.L.; Zhang, L.W.; Bai, Y.B.; Wang, G.Q. Ammonia oxidizers in river sediments of the Qinghai-Tibet Plateau and their adaptations to high-elevation conditions. Water Res. 2020, 173, 115589. [Google Scholar] [CrossRef]
- Jin, X.G.; Tu, Q.Y. The Standard Methods in Lake Eutrophication Investigation, 2nd ed.; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Allen, D.J.; Farrell, M.; Huang, J.Y.; Reynolds, C.; Rupasinghe, M.; Mosley, L.M. Long-term water quality response to increased hydraulic loadings in a field-scale free water surface constructed wetland treating domestic effluent. J. Environ. Manag. 2022, 311, 114858. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yang, T. Analysis of the removal effects of nitrogen and phosphorus on reaping wetland plants in Autumn by Loushijiang wetland as an example. Environ. Sci. Surv. 2018, 37, 30–33. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.J.; Du, Y.L.; Bi, E.P.; Wang, L.; Chen, C.B. Simulation of water quality response of Guishui river wetland plants and water diversion. Environ. Sci. 2020, 41, 4096–4104. (In Chinese) [Google Scholar] [CrossRef]
- Houlahan, J.E.; Findlay, C.S. The effects of adjacent land use on wetland amphibian species richness and community composition. Can. J. Fish. Aquat. Sci. 2003, 60, 1078–1094. [Google Scholar] [CrossRef]
- Peng, W.T.; Zou, L.; Duan, W.B.; Li, Y.X.; Pan, Y.Z.; Zeng, D.G. Efficiency of nitrogen and phosphorus removal from sewage by various combinations of wetland plants. Acta Sci. Circumstantiae 2012, 32, 612–617. (In Chinese) [Google Scholar] [CrossRef]
- Hao, M.X.; Yang, L.; Kong, X.H.; Xu, X.; Lu, W.; Li, Z.Q. Diversity and community succession of macrophytes in Lake Changhu, Hubei Province. J. Lake Sci. 2015, 27, 94–102. (In Chinese) [Google Scholar]
- Song, Y.Z.; Zhu, G.W.; Qin, B.Q. Applicability analysis of aquatic macrophytes on controlling nitrogen and phosphorus from water in the Kangshan Bay demonstration area of Lake Taihu. J. Lake Sci. 2013, 25, 259–265. [Google Scholar][Green Version]
- Duan, J.J.; Shu, T.; Xue, L.H.; He, S.Y.; Petropoulos, E.; Feng, Y.Y.; Zhou, B.B.; Yang, L.Z. Nitrogen removal efficiency in sustainable eco-ditches with floating ryegrass mats: The effect of loading (hydraulic and nitrogen) and water level on N removal. Ecol. Eng. 2023, 187, 106872. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Z.H.; Xu, Z.J.; Gu, D.Q.; Zheng, W. Landscape pattern change and its cumulative environmental effects of coastal wetlands in southern Laizhou Bay wetlands. Chin. J. Ecol. 2009, 28, 2437–2443. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, H.S.; Jiao, Y.M.; Zhang, H.; Zhang, L.X.; Tao, Y.; Xu, Q.; Zhang, Z.N. Effects of Landscape Patterns on Surface Water Quality and Its Characteristic Scale in the Lashihai Basin of Yunnan Province. Chin. J. Ecol. 2023. (In Chinese). Available online: https://kns.cnki.net/kcms2/article/abstract?v=xBNwvqFr00I_tdF_wPjKiIVmFUKrMp3K6jSwzTYtNoQCwMiP4OjdiBqTsnvCyoY82jaJFWdWkMOWXyTsg02DfGN8s9VvU27IlqYcZxvE7sg4jC6Xxzqwgg1XVVo1J8WNZpHkgEDpQ_Y=&uniplatform=NZKPT&=CHS (accessed on 25 November 2023).
- Liu, Y.N.; Kong, L.Q.; Xiao, Y.; Zheng, H. Relationships between landscape pattern and ecosystem water purification service in the Yangtze River Basin. Acta Ecol. Sin. 2019, 39, 844–852. (In Chinese) [Google Scholar] [CrossRef]
- Garcia-Lledo, A.; Ruiz-Rueda, O.; Vilar-Sanz, A.; Sala, L.; Baneras, L. Nitrogen removal efficiencies in a free water surface constructed wetland in relation to plant coverage. Ecol. Eng. 2011, 37, 678–684. [Google Scholar] [CrossRef]
- Han, W.J.; Chang, J.; Du, Y.Y.; Chang, S.X.; Zhang, C.B.; Ge, Y. Plant species diversity impacts nitrogen removal and nitrous oxide emissions as much as carbon addition in constructed wetland microcosms. Ecol. Eng. 2016, 93, 144–151. [Google Scholar] [CrossRef]
- Zuo, X.J.; Zhang, H.S.; Yu, J.H. Microbial diversity for the improvement of nitrogen removal in stormwater bioretention cells with three aquatic plants. Chemosphere 2020, 244, 125626. [Google Scholar] [CrossRef]
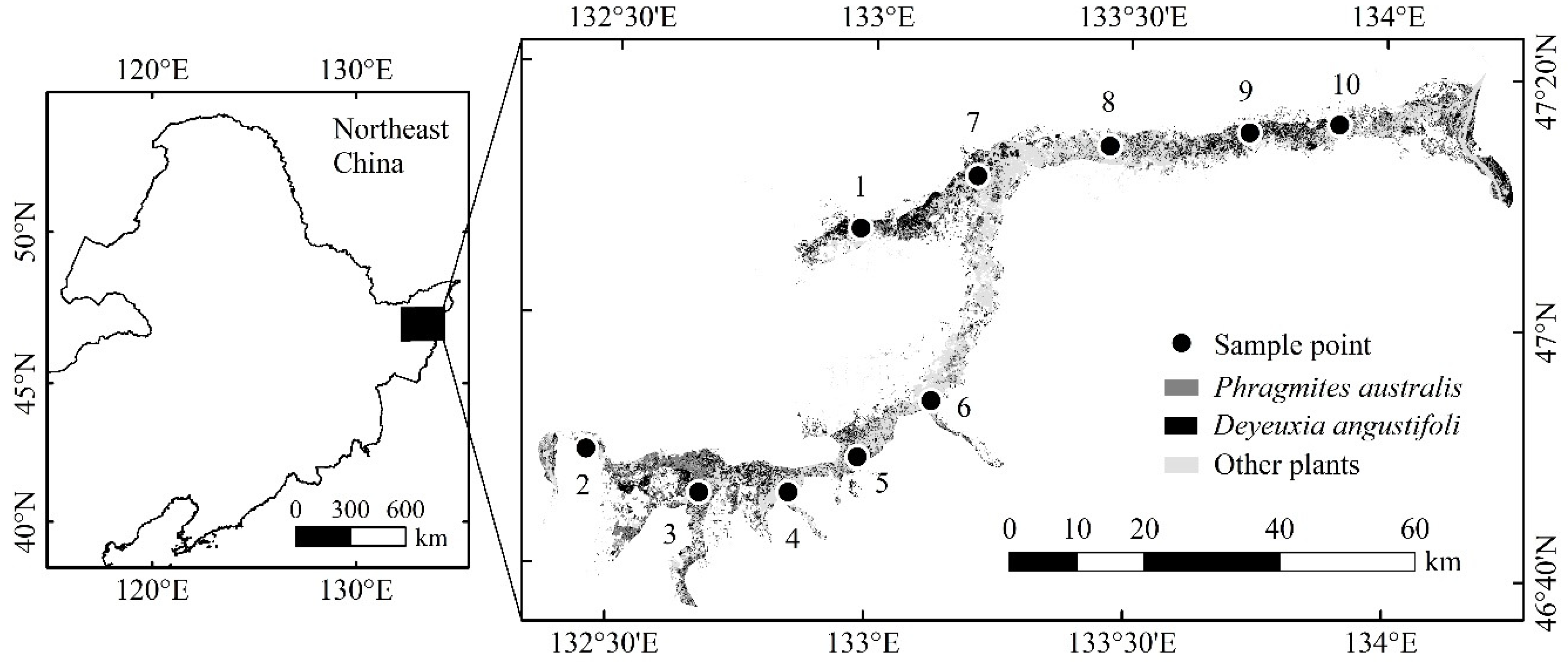



| Sampling Points | Longitude (E) | Latitude (N) |
|---|---|---|
| 1 | 132.98° | 47.12° |
| 2 | 132.46° | 46.82° |
| 3 | 132.68° | 46.77° |
| 4 | 132.85° | 46.77° |
| 5 | 132.98° | 46.82° |
| 6 | 133.12° | 46.90° |
| 7 | 133.20° | 47.20° |
| 8 | 133.46° | 47.24° |
| 9 | 133.73° | 47.26° |
| 10 | 133.91° | 47.27° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, Y.; An, Y.; Zhang, M.; Wang, X. The Impact of Plant Spatial Patterns on Nitrogen Removal in the Naolihe Wetlands of Northeast China. Water 2024, 16, 128. https://doi.org/10.3390/w16010128
Ma J, Wang Y, An Y, Zhang M, Wang X. The Impact of Plant Spatial Patterns on Nitrogen Removal in the Naolihe Wetlands of Northeast China. Water. 2024; 16(1):128. https://doi.org/10.3390/w16010128
Chicago/Turabian StyleMa, Jinfeng, Yuting Wang, Yu An, Mei Zhang, and Xiaodong Wang. 2024. "The Impact of Plant Spatial Patterns on Nitrogen Removal in the Naolihe Wetlands of Northeast China" Water 16, no. 1: 128. https://doi.org/10.3390/w16010128
APA StyleMa, J., Wang, Y., An, Y., Zhang, M., & Wang, X. (2024). The Impact of Plant Spatial Patterns on Nitrogen Removal in the Naolihe Wetlands of Northeast China. Water, 16(1), 128. https://doi.org/10.3390/w16010128







