Temporal Environmental Status of a Shallow Lake Using Alpha and Beta Diversity on Phytoplankton Communities
Abstract
1. Introduction
2. Materials and Methods
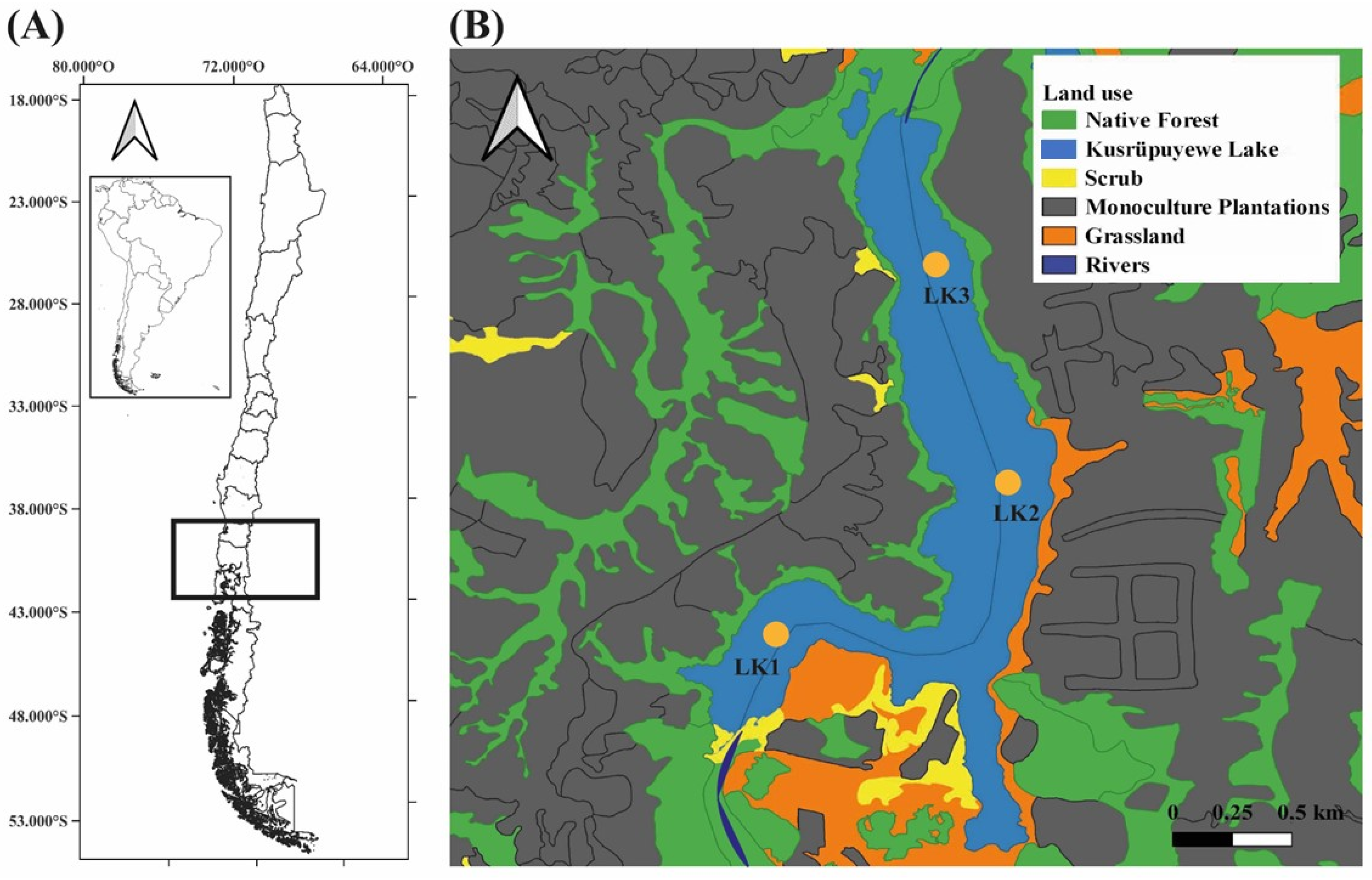
2.1. Study Area
2.2. Sampling, Phytoplankton Analysis and Physicochemical Parameters
2.3. Alpha and Beta Diversities Metrics
2.4. Statistical Analyses
3. Results
3.1. Physicochemical Parameters in Kusrüpuyewe Lake
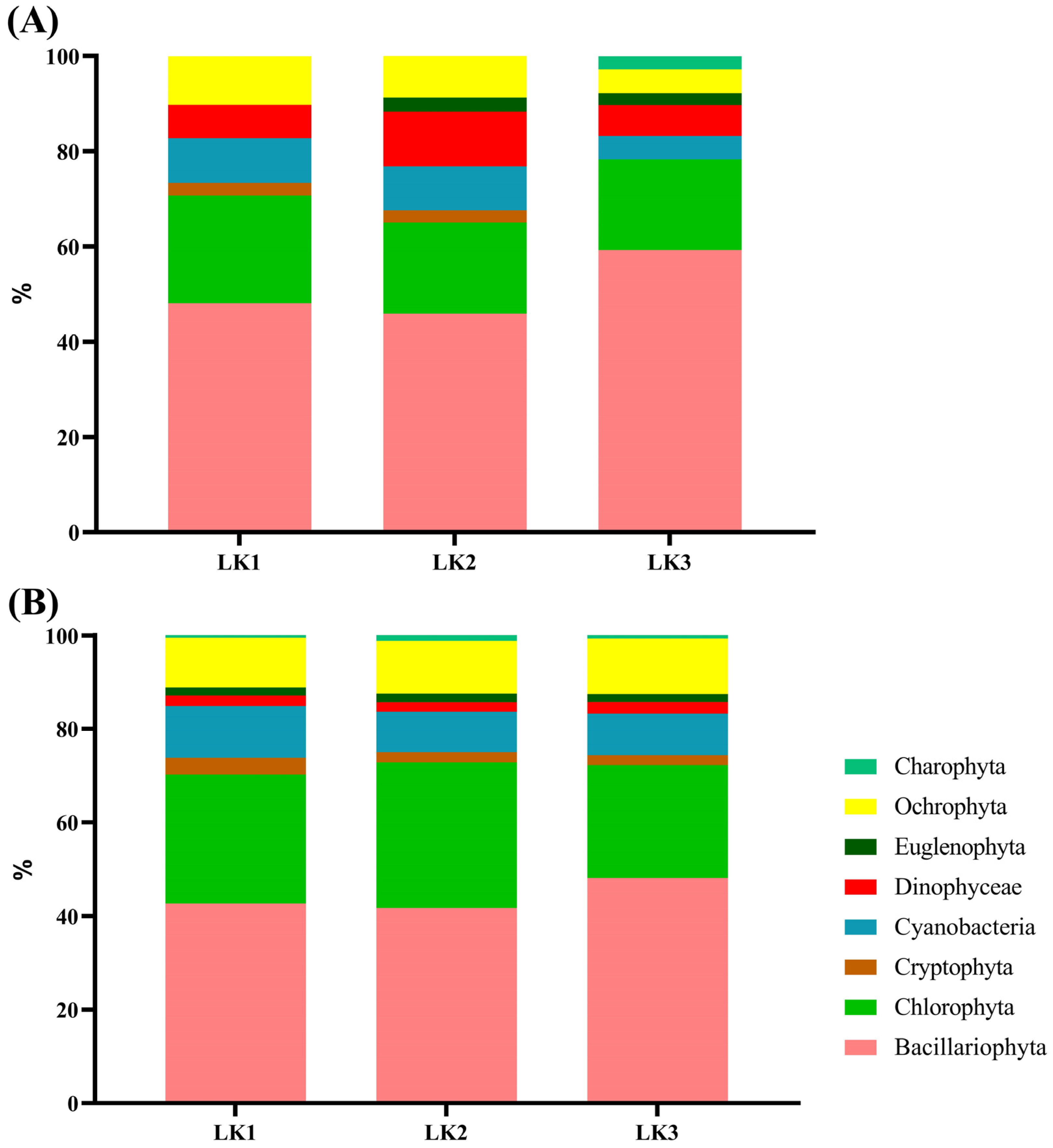
3.2. Spatial Environmental Stability and Phytoplankton Assemblage in Kusrüpuyewe Lake
3.3. Spatial Environmental Heterogeneity and Species Turnover in Kusrüpuyewe Lake
3.4. Temporal Variability of the Phytoplankton Assemblage
4. Discussion
4.1. Environmental Conditions and Heterogeneity
4.2. Temporal Variability and Species Turnover of Phytoplankton Communities
4.3. Application of Alpha and Beta Diversities in Shallow Ecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Özkan, K.; Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Liboriussen, L.; Svenning, J.C. Contrasting roles of water chemistry, lake morphology, land-use, climate and spatial processes in driving phytoplankton richness in the Danish landscape. Hydrobiologia 2013, 710, 173–187. [Google Scholar] [CrossRef]
- Phillips, G.; Lyche-Solheim, A.; Skjelbred, B.; Mischke, U.; Drakare, S.; Free, G.; Järvinen, M.; de Hoyos, C.; Morabito, G.; Poikane, S.; et al. A phytoplankton trophic index to assess the status of lakes for the Water Framework Directive. Hydrobiologia 2013, 704, 75–95. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Simões, N.R.; Bovo-Scomparin, V.M.; Jati, S.; Santana, N.F.; Roberto, M.C.; Train, S. Phytoplankton alpha diversity as an indicator of environmental changes in a neotropical floodplain. Ecol. Indic. 2015, 48, 334–341. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.J.; He, W.; Liu, W.X.; Kong, X.Z.; Jørgensen, S.E.; Xu, F.-L. The tempo-spatial variations of phytoplankton diversities and their correlation with trophic state levels in a large eutrophic Chinese lake. Ecol. Indic. 2016, 66, 153–162. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y.; Yang, Z.; Kong, F. Deterministic diversity changes in freshwater phytoplankton in the Yunnan-Guizhou Plateau lakes in China. Ecol. Indic. 2016, 63, 273–281. [Google Scholar] [CrossRef]
- Fuentes, N.; Arriagada, A. Long-term responses of macroinvertebrate assemblages to the 2011 eruption of the Puyehue-Cordón Caulle volcanic complex, Chile. Sci. Total Environ. 2022, 807, 150978. [Google Scholar] [CrossRef]
- Carvalho, L.; Poikane, S.; Lyche, S.A.; Phillips, G.; Borics, G.; Catalan, J.; De Hoyos, C.; Drakare, S.; Dudley, B.J.; Järvinen, M.; et al. Strength and uncertainty of phytoplankton metrics for assessing eutrophication impacts in lakes. Hydrobiologia 2013, 704, 127–140. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Maloufi, S.; Catherine, A.; Mouillot, D.; Louvard, C.; Couté, A.; Bernard, C.; Troussellier, M. Environmental heterogeneity among lakes promotes hyper β-diversity across phytoplankton communities. Freshw. Biol. 2016, 61, 633–645. [Google Scholar] [CrossRef]
- Siqueiros-Beltrones, D.A. Una paradoja sobre uniformidad vs. Orden y estabilidad en la medida de la diversidad de especies según la teoría de la información. Ludus Vitalis 2005, 13, 83–92. [Google Scholar]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Baselga, A.; Gómez-Rodríguez, C. Diversidad alfa, beta y gamma: ¿cómo medimos diferencias entre comunidades biológicas? Nov. Acta Científica Compostel. 2019, 26, 39–45. [Google Scholar]
- Alves-De-Souza, C.; Benevides, T.S.; Santos, J.B.O.; Von Dassow, P.; Guillou, L.; Menezes, M. Does environmental heterogeneity explain temporal β diversity of small eukaryotic phytoplankton? Example from a tropical eutrophic coastal lagoon. J. Plankton Res. 2017, 39, 698–714. [Google Scholar] [CrossRef]
- Zorzal-Almeida, S.; Bini, L.M.; Bicudo, D.C. Beta diversity of diatoms is driven by environmental heterogeneity, spatial extent and productivity. Hydrobiologia 2017, 800, 7–16. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Sci. Compass 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Gonzalez, A.; Loreau, M. The causes and consequences of compensatory dynamics in ecological communities. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 393–414. [Google Scholar] [CrossRef]
- Ríos-Henríquez, C.; Fuentes, N.; Tobar, C.N.; Rau, J.R.; Cruces, F. Planktonic diatom assemblage seasonal diversity used to assess environmental health in a coastal wetland of southern Chile. Gayana Bot. 2020, 77, 139–151. [Google Scholar] [CrossRef]
- Tavares-Costa, A.P.; Oliveira-Crossetti, L.; Hartz, S.M.; Becker, F.G.; Hepp, L.U.; Bohnenberger, J.E.; Lima, M.S.; Guimarães, T.; Schneck, F. Land cover is the main correlate of phytoplankton beta diversity in subtropical coastal shallow lakes. Aquat. Ecol. 2020, 54, 1015–1028. [Google Scholar] [CrossRef]
- Frau, D.; Pineda, A.; Mayora, G.; Devercelli, M. Phytoplankton taxonomic and functional diversity in two shallow alluvial lakes with contrasting river connectivity. Aquat. Sci. 2022, 84, 26. [Google Scholar] [CrossRef]
- Fuentes, N.; Arriagada, A. New records of the endangered southern river otter Lontra provocax, with notes on its diet, in threatened wetlands of southern Chile. Oryx 2023, 57, 76–79. [Google Scholar] [CrossRef]
- Santibáñez, F. El cambio climático y los recursos hídricos de Chile. In Agricultura Chilena, Reflexiones y Desafíos Al 2030, 1st ed.; Apey, A., Barrera, D., Rivas, T., Eds.; Oficina de Estudios y Políticas Agrarias (ODEPA): Santiago, Chile, 2016; pp. 147–177. [Google Scholar]
- Lemmens, P.; Mergeay, J.; de Bie, T.; Van Wichelen, J.; de Meester, L.; Declerck, S.A.J. How to Maximally Support Local and Regional Biodiversity in Applied Conservation? Insights from Pond Management. PLoS ONE 2013, 8, e72538. [Google Scholar] [CrossRef] [PubMed]
- Celewicz-Goldyn, S.; Kuczynska-Kippen, N. Ecological value of macrophyte cover in creating habitat for microalgae (diatoms) and zooplankton (rotifers and crustaceans) in small field and forest water bodies. PLoS ONE 2017, 12, e0177317. [Google Scholar] [CrossRef] [PubMed]
- Kollár, J.; Fránková, M.; Hašler, P.; Letáková, M.; Poulíčková, A. Epiphytic diatoms in lotic and lentic waters-Diversity and representation of species complexes. Fottea 2015, 15, 259–271. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Céréghino, R.; Boix, D.; Cauchie, H.M.; Martens, K.; Oertli, B. The ecological role of ponds in a changing world. Hydrobiologia 2014, 723, 1–6. [Google Scholar] [CrossRef]
- Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Weatherby, A. 15 Years of pond assessment in Britain: Results and lessons learned from the work of Pond Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 693–714. [Google Scholar] [CrossRef]
- Bella, V.D.; Mancini, L. Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. Hydrobiologia 2009, 634, 25–41. [Google Scholar] [CrossRef]
- Pereira, H.M.; Navarro, L.M.; Martins, I.S. Global biodiversity change: The Bad, the good, and the unknown. Annu. Rev. Environ. Resour. 2012, 37, 25–50. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Cermeño, P.; Teixeira, I.G.; Branco, M.; Figueiras, F.G.; Marañón, E. Sampling the limits of species richness in marine phytoplankton communities. J. Plankton Res. 2014, 36, 1135–1139. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton methodic. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Parra, O.O.; González, M.A.; Dellarossa, V.; Rivera, P.; Orellana, M. Manual Taxonómico del Fitoplancton de Agua Continentales Con Especial Referencia al Fitoplancton en Chile; Editorial Universidad de Concepción: Concepción, Chile, 1982; pp. 1–20. [Google Scholar]
- Bahls, L.; Boynton, B.; Johnston, B. Atlas of diatoms (Bacillariophyta) from diverse habitats in remote regions of western Canada. PhytoKeys 2018, 186, 1–186. [Google Scholar] [CrossRef] [PubMed]
- Nusch, E. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol. 1980, 14, 14–36. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2005; p. 541. [Google Scholar]
- Siqueiros-Beltrones, D.A.; Argumedo-Hernández, U.; Hernández-Almeida, O.U. High species diversity (H) of benthic diatoms in a coastal lagoon located within a natural protected area. Hidrobiologica 2017, 27, 293–300. [Google Scholar] [CrossRef]
- Brown, J.H.; Ernest, S.K.M.; Parody, J.M.; Haskell, J.P. Regulation of diversity: Maintenance of species richness in changing environments. Oecologia 2001, 126, 321–332. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.; Chen, F.; Yang, Z.; Yu, Y. The underlying causes and effects of phytoplankton seasonal turnover on resource use efficiency in freshwater lakes. Ecol. Evol. 2021, 11, 8897–8909. [Google Scholar] [CrossRef]
- Secretaría de la Convención de Ramsar. Designación de Sitios Ramsar: Marco Estratégico y Lineamientos Para el Desarrollo Futuro de la Lista de Humedales de Importancia Internacional, 4th ed.; Manuales Ramsar Para el Uso Racional de Los Humedales: Ramsar Gland, Switzerland, 2010; Volume 17, p. 130. [Google Scholar]
- Rahel, F.J. Homogenization of freshwater faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef]
- Lougheed, V.L.; Mcintosh, M.D.; Parker, C.A.; Stevenson, R.J. Wetland degradation leads to homogenization of the biota at local and landscape scales. Freshw. Biol. 2008, 53, 2402–2413. [Google Scholar] [CrossRef]
- Fuentes, N.; Gómez, L.; Venegas, H.; Rau, J.R. Total devastation of river macroinvertebrates following a volcanic eruption in southern Chile. Ecosphere 2020, 11, e03105. [Google Scholar] [CrossRef]
- Jindal, R.; Thakur, R.K.; Singh, U.B.; Ahluwalia, A.S. Phytoplankton dynamics and species diversity in a shallow eutrophic, natural mid-altitude lake in Himachal Pradesh (India): Role of physicochemical factors. Chem. Ecol. 2014, 30, 328–338. [Google Scholar] [CrossRef]
- Downing, A.L.; Leibold, M.A. Species richness facilitates ecosystem resilience in aquatic food webs. Freshw. Biol. 2010, 55, 2123–2137. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Seidl, R. β-Diversity, Community Assembly, and Ecosystem Functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef]
- Andermann, T.; Antonelli, A.; Barrett, R.L.; Silvestro, D. Estimating Alpha, Beta, and Gamma Diversity through Deep Learning. Front. Plant Sci. 2022, 13, 839407. [Google Scholar] [CrossRef]
- Roelke, D.L.; Spatharis, S. Phytoplankton Succession in Recurrently Fluctuating Environments. PLoS ONE 2015, 10, e0121392. [Google Scholar] [CrossRef]
- Gering, J.C.; Crist, T.O.; Veech, J.A. Additive partitioning of species diversity across multiple spatial scales: Implications for regional conservation of biodiversity. Conserv. Biol. 2003, 17, 488–499. [Google Scholar] [CrossRef]
- Bussard, A.; Corre, E.; Hubas, C.; Duvernois-Berthet, E.; Le Corguillé, G.; Jourdren, L.; Coulpier, F.; Claquin, P.; Lopez, P.J. Physiological adjustments and transcriptome reprogramming are involved in the acclimation to salinity gradients in diatoms. Environ. Microbiol. 2017, 19, 909–925. [Google Scholar] [CrossRef]
- Stefanidou, N.; Katsiapi, M.; Tsianis, D.; Demertzioglou, M.; Michaloudi, E.; Moustaka-Gouni, M. Patterns in Alpha and Beta Phytoplankton Diversity along a Conductivity Gradient in Coastal Mediterranean Lagoons. Diversity 2020, 12, 38. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Li, Y.; Lin, Q.; Chao He, C.; Huang, S.; Li, H.; Zhang, X.; Liu, B.; Ge, F.; et al. The changing characteristics of phytoplankton community and biomass in subtropical shallow lakes: Coupling effects of land use patterns and lake morphology. Water Res. 2021, 200, 117235. [Google Scholar] [CrossRef]
- Fuentes, N.; Arriagada, A.; Araos, F.; Ríos-Henríquez, C.; Riquelme, W.; Iwama, A.; Cárdenas, L.; Soto, R.; Escobar, A.; Henríquez, G. Caracterización Ambiental y Social de Laguna Trinidad y Estero Pucopio, Comunas de San Pablo y San Juan de la Costa, Provincia de Osorno, 1st ed.; Universidad de Los Lagos y Corporación Nacional de Desarrollo Indígena: Osorno, Chile, 2021; p. 128. [Google Scholar]
- Santana, L.M.; Weithoff, G.; Ferragut, C. Seasonal and spatial functional shifts in phytoplankton communities of five tropical reservoirs. Aquat. Ecol. 2017, 51, 531–543. [Google Scholar] [CrossRef]
- Simões, N.R.; Lansac-Tôha, F.A.; Velho, L.F.M.; Bonecker, C.C. Intra and inter-annual structure of zooplankton communities in floodplain lakes: A long-term ecological research study. Rev. Biol. Trop. 2012, 60, 1819–1836. [Google Scholar] [CrossRef]
- Pineda, A.; Peláez, Ó.; Dias, J.D.; Segovia, B.T.; Bonecker, C.C.; Velho, L.F.M.; Rodrigues, L.C. The El Niño Southern Oscillation (ENSO) is the main source of variation for the gamma diversity of plankton communities in subtropical shallow lakes. Aquat. Sci. 2019, 81, 49. [Google Scholar] [CrossRef]
- Nabout, J.C.; Nogueira, I.S.; Oliveira, L.G. Phytoplankton community of floodplain lakes of the Araguaia River, Brazil, in the rainy and dry seasons. J. Plankton Res. 2006, 28, 181–193. [Google Scholar] [CrossRef]
- Rasconi, S.; Gall, A.; Winter, K.; Kainz, M.J. Increasing water temperature triggers dominance of small freshwater plankton. PLoS ONE 2015, 10, e0140449. [Google Scholar] [CrossRef]
- Train, S.; Rodrigues, L.C. Temporal fluctuations of the phytoplankton community of the Baia River, in the upper Parana River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 1997, 361, 125–134. [Google Scholar] [CrossRef]
- Angeler, D.G. Revealing a conservation challenge through partitioned long-term beta diversity: Increasing turnover and decreasing nestedness of boreal Lake Metacommunities. Divers. Distrib. 2013, 19, 772–781. [Google Scholar] [CrossRef]
- Cardoso, S.J.; Roland, F.; Loverde-Oliveira, S.M.; Huszar, V.L. Phytoplankton abundance, biomass and diversity within and between Pantanal wetland habitats. Limnologica 2012, 42, 235–241. [Google Scholar] [CrossRef]
- Leon-Múñoz, J.; Echeverría, C.; Marcé, R.; Riss, W.; Sherman, B.; Iriarte, J.L. The combined impact of land use change and aquaculture on sediment and water quality in oligotrophic Lake Rupanco (North Patagonia, Chile, 40.8 S). J. Environ. Manag. 2013, 128, 283–291. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, S.; Zhou, Z.; Wang, Y.; Wang, S. Effects of Land Use and Physicochemical Factors on Phytoplankton Community Structure: The Case of Two Fluvial Lakes in the Lower Reach of the Yangtze River, China. Diversity 2023, 15, 180. [Google Scholar] [CrossRef]
- Siqueira, T.; Lacerda, C.G.L.T.; Saito, V.S. How Does Landscape Modification Induce Biological Homogenization in Tropical Stream Metacommunities? Biotropica 2015, 47, 509–516. [Google Scholar] [CrossRef]
- Winegardner, A.K.; Legendre, P.; Beisner, B.E.; Gregory-Eaves, I. Diatom diversity patterns over the past c. 150 years across the conterminous United States of America: Identifying mechanisms behind beta diversity. Glob. Ecol. Biogeogr. 2017, 26, 1303–1315. [Google Scholar] [CrossRef]
- De Boeck, H.J.; Bloor, J.M.G.; Kreyling, J.; Ransijn, J.C.G.; Nijs, I.; Jentsch, A.; Zeiter, M. Patterns and drivers of biodiversity–stability relationships under climate extremes. J. Ecol. 2018, 106, 890–902. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Shimadzu, H.; Dornelas, M.; McGill, B.; Moyes, F.; Magurran, A.E. Community-level regulation of temporal trends in biodiversity. Sci. Adv. 2017, 3, e1700315. [Google Scholar] [CrossRef]
- Stomp, M.; Huisman, J.; Mittelbach, G.G.; Litchman, E.; Klausmeier, C.A. Large-scale biodiversity patterns in freshwater phytoplankton. Ecology 2011, 92, 2096–2107. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- De Meester, L.; Declerck, S.; Stoks, R.; Louette, G.; Van De Meutter, F.; De Bie, T.; Michels, E.; Brendonck, L. Ponds and pools as model systems in conservation biology, ecology and evolutionary biology. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 715–725. [Google Scholar] [CrossRef]
- Hortal, J.; Nabout, J.C.; Calatayud, J.; Carneiro, F.M.; Padial, A.; Santos, A.M.C.; Siqueira, T.; Bokma, F.; Bini, L.M.; Ventura, M. Perspectives on the use of lakes and ponds as model systems for macroecological research. J. Limnol. 2014, 73, 46–60. [Google Scholar] [CrossRef]
- Almeida-Gomes, M.; Valente-Neto, F.; Pacheco, E.O.; Ganci, C.C.; Leibold, M.A.; Melo, A.S.; Provete, D.B. How Does the Landscape Affect Metacommunity Structure? A Quantitative Review for Lentic Environments. Curr. Landsc. Ecol. Rep. 2020, 5, 68–75. [Google Scholar] [CrossRef]



| Depth | DO% | DO mg L−1 | pH | T °C | Con µS cm −1 | Chl a µg L−1 | TSS mg L−1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Summer | |||||||||
| LK1 | Top | 0–1 m | 100.6 (98.2–102) | 8.6 (8.4–8.7) | 6.9 (6.5–7.2) | 23.3 (23.1–23.4) | 46.5 (46.4–46.5) | 3.9 (2.6–4.9) | 2.1 (1.6–2.7) |
| Bottom | 2–3 m | 98.1 (91.4–100.8) | 8.5 (7.8–9.1) | 7.1 (7.0–7.2) | 22.6 (22.1–23.1) | 45.9 (45.5–46.6) | 3.0 (2.7–3.4) | 2.0 (1.6–2.8) | |
| LK2 | Top | 0–1 m | 97.9 (97.8–98.1) | 8.3 (8.3–8.4) | 6.7 (6.5–7.2) | 23.4 (23.3–23.5) | 43.5 (11.7–51.6) | 1.9 (1.4–2.3) | 2.7 (2.6–3.1) |
| Bottom | 3–4 m | 90.2 (83.4–95.8) | 7.8 (7.2–8.2) | 7.2 (7.1–7.2) | 22.7 (22.4–22.9) | 46.1 (45.9–46.3) | 2.7 (2.4–3.1) | 4.4 (3.3–5.8) | |
| LK3 | Top | 0–1 m | 94.8 (84.4–103.2) | 8.0 (7.2–8.7) | 6.8 (6.3–7.1) | 21.7 (21.5–21.9) | 33.7 (11.5–46.6) | 3.3 (2.3–4.1) | 3.9 (3.7–4.4) |
| Bottom | 4–5 m | 83.5 (76.2–89.9) | 7.9 (5.9–8.7) | 6.9 (6.9–7.1) | 18.7 (18.6–19.1) | 44.4 (43.6–46.1) | 2.5 (1.5–3.4) | 3.9 (3.5–4.2) | |
| Winter | |||||||||
| LK1 | Top | 0–1 m | 85.4 (80.1–92.9) | 8.9 (7.4–10.4) | 6.9 (6.1–7.1) | 10.6 (10.5–10.7) | 20.4 (10.1–25.4) | 0.8 (0.3–1.2) | 2.1 (1.9–2.5) |
| Bottom | 4–5 m | 65.5 (62.0–72.8) | 7.5 (7.1–8.2) | 6.9 (6.8–7.1) | 9.5 (9.3–9.9) | 24.7 (24.6–25.1) | 0.7 (0.5–0.9) | 1.6 (1.5–1.6) | |
| LK2 | Top | 0–1 m | 90.3 (80.9–101.1) | 10.0 (8.9–11.3) | 7.8 (7.5–7.8) | 10.7 (10.6–10.7) | 21.7 (10.1–27.1) | 2.8 (2.3–3.2) | 2.7 (2.6–2.8) |
| Bottom | 6–7 m | 68.4 (66.9–69.8) | 8.1 (7.9–8.2) | 7.9 (7.8–7.9) | 8.1 (8.1–8.2) | 22.6 (22.6–22.7) | 0.9 (0.8–1.1) | 1.5 (1.3–1.8) | |
| LK3 | Top | 0–1 m | 89.2 (80.1–99.3) | 9.9 (9.1–11.5) | 6.5 (6.2–7.1) | 10.2 (10–10.3) | 24.2 (19.1–37.6) | 2.3 (1.7–3.2) | 2.9 (2.5–3.9) |
| Bottom | 5–6 m | 70.4 (69.2–71.7) | 8.1 (8.1–8.2) | 6.9 (6.9–7.1) | 9.0 (8.9–9.7) | 23.4 (23.1–25.4) | 1.4 (1.1–1.8) | 3.2 (3–3.4) | |
| Summer | Winter | SIMPER | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Class | Species | LK1 | LK2 | LK3 | LK1 | LK2 | LK3 | |||||||
| Top | Bottom | Top | Bottom | Top | Bottom | Top | Bottom | Top | Bottom | Top | Bottom | ||||
| Bacillariophyta | Bacillariophyceae | Achnanthes affinis | 6.1 | 6.6 | 5.8 | 9.5 | 9.8 | 8.6 | a | ||||||
| Achnanthes exigua | 6.6 | 7.4 | 7.9 | 9.8 | 8.7 | 10.6 | 2.6 | 3.3 | 1.1 | 2.7 | c | ||||
| Achnanthes hauckiana | 8.8 | 9.3 | 8.7 | a | |||||||||||
| Achnanthes lanceolata | 2.8 | 2.8 | 0.8 | 2.0 | b | ||||||||||
| Amphora veneta | 1.3 | 0.5 | b | ||||||||||||
| Amphipleura lindheimeri | 5.4 | 6.8 | a | ||||||||||||
| Asterionella formosa | 21.6 | 12.8 | 11.4 | 9.6 | 10.2 | 8.8 | 2.4 | 1.8 | 3.1 | 3.2 | 2.8 | c | |||
| Cocconeis placentula | 10.3 | 3.5 | 3.2 | c | |||||||||||
| Cymbella lanceolata | 6.6 | 7.5 | a | ||||||||||||
| Cymbella minuta | 8.0 | 1.9 | 2.0 | 2.2 | 2.8 | 2.4 | c | ||||||||
| Cymbella naviculiforme | 8.2 | 9.1 | 8.0 | a | |||||||||||
| Diatoma tenue | 7.1 | 0.6 | 0.6 | c | |||||||||||
| Epithemia adnata | 8.0 | a | |||||||||||||
| Eunotia bidens | 7.9 | a | |||||||||||||
| Eunotia major | 4.8 | 6.4 | 7.5 | 9.1 | 11.6 | 8.1 | a | ||||||||
| Eunotia juettrerae | 0.8 | 1.4 | b | ||||||||||||
| Fragilaria construens | 4.4 | 2.4 | 2.6 | 1.1 | b | ||||||||||
| Frustulia rhomboides | 7.1 | 6.7 | 1.1 | 1.8 | c | ||||||||||
| Gomphonema affini | 6.2 | a | |||||||||||||
| Gomphonema parvulum | 8.0 | a | |||||||||||||
| Gomphonema subclavatum | 6.3 | 1.5 | 2.0 | 2.2 | 1.2 | 1.3 | c | ||||||||
| Gomphonema tenellum | 4.9 | 8.8 | 9.3 | a | |||||||||||
| Gomphonema angusticephalum | 1.2 | b | |||||||||||||
| Gomphonema herculeanum | 0.7 | a | |||||||||||||
| Gomphonema micropus | 1.5 | 1.3 | b | ||||||||||||
| Gyrosigma terryanum | 6.4 | a | |||||||||||||
| Hannaea arcus | 7.9 | 7.4 | 8.2 | 9.7 | 9.0 | 9.4 | a | ||||||||
| Hantzschia virgata | 7.6 | 5.9 | 8.7 | 8.1 | 1.1 | c | |||||||||
| Meridion circulare | 1.6 | 1.4 | 2.1 | 2.0 | 1.8 | 2.5 | b | ||||||||
| Navicula cryptocephala | 7.1 | 8.1 | 9.4 | 8.1 | a | ||||||||||
| Navicula dicephala | 8.7 | 9.8 | 7.9 | a | |||||||||||
| Navicula directa | 8.5 | 10.8 | 5.9 | 0.8 | c | ||||||||||
| Navicula gotlandica | 9.1 | 8.9 | 8.8 | 2.2 | 1.6 | 1.2 | c | ||||||||
| Navicula lateropunctata | 8.1 | 8.6 | 9.9 | a | |||||||||||
| Navicula pseudoseinhardti | 8.8 | 5.0 | 5.6 | a | |||||||||||
| Navicula ryncocephala | 9.4 | 13.4 | 8.0 | a | |||||||||||
| Navicula viridula var. rotellata | 8.2 | 8.1 | 8.8 | a | |||||||||||
| Neidium bisulcatum | 3.4 | b | |||||||||||||
| Nitzschia apiculata | 7.2 | 9.2 | 8.8 | 2.6 | c | ||||||||||
| Nitzschia ignorata | 7.8 | 9.0 | 8.8 | 8.9 | a | ||||||||||
| Nitzschia rumpens | 5.6 | 8.6 | 1.1 | c | |||||||||||
| Nitzschia parva | 1.3 | b | |||||||||||||
| Nitzschia robusta | 1.0 | 1.6 | b | ||||||||||||
| Opephora martyi | 2.2 | 1.6 | 2.5 | b | |||||||||||
| Pinnularia intermedia | 7.9 | a | |||||||||||||
| Pinnularia major | 11.4 | 8.2 | 8.6 | 8.8 | 9.5 | 7.5 | 0.6 | 1.6 | 0.8 | c | |||||
| Pinnularia minor | 9.9 | 7.5 | 0.6 | 1.2 | 0.8 | 2.5 | 1.4 | 2.3 | c | ||||||
| Pleurosgima sp. | 0.6 | 1.4 | b | ||||||||||||
| Rhoicosphenia curvata | 8.7 | 2.5 | 3.1 | 2.7 | 3.0 | 4.0 | c | ||||||||
| Synedra acicularis | 7.8 | a | |||||||||||||
| Synedra acus | 8.2 | 3.9 | 4.4 | 3.5 | 2.2 | c | |||||||||
| Synedra rumpens | 8.2 | 9.9 | 8.7 | 10.2 | a | ||||||||||
| Tabellaria fenestrata | 8.0 | 2.0 | 2.6 | 3.0 | 4.6 | 4.4 | 5.0 | c | |||||||
| Tabellaria floculosa | 9.2 | 1.5 | 2.4 | 4.4 | 4.8 | 2.2 | c | ||||||||
| Coscinodiscophyceae | Actinocyclus curvatulus | 11.4 | 9.8 | a | |||||||||||
| Actinocyclus subtilis | 9.9 | 8.5 | a | ||||||||||||
| Aulacoseira granulata | 8.7 | 8.9 | 1.8 | 2.0 | 1.2 | 1.7 | 1.5 | 1.6 | c | ||||||
| Melosira hustedttii | 7.5 | 8.6 | 9.9 | 8.7 | 1.4 | 2.4 | c | ||||||||
| Melosira italica | 9.3 | 10.6 | 9.9 | 3.3 | 2.8 | c | |||||||||
| Melosira sol | 6.8 | 7.7 | 7.9 | 10.6 | 9.6 | 10.3 | 0.4 | 0.8 | c | ||||||
| Melosira varians | 8.2 | 8.3 | 10.9 | 8.5 | 2.2 | 2.5 | 1.8 | 2.6 | c | ||||||
| Rhizosolenia longiseta | 9.6 | 8.7 | 7.6 | 9.6 | 11.4 | 9.5 | a | ||||||||
| Mediophyceae | Cyclotella meneghiniana | 5.8 | 6.3 | 5.5 | 8.8 | 8.3 | 8.5 | a | |||||||
| Chlorophyta | Chlorophyceae | Chlamydocapsa bacillus | 6.0 | 1.4 | 3.4 | 1.4 | 1.8 | 1.2 | c | ||||||
| Chlamydocapsa planctonica | 1.6 | 0.8 | b | ||||||||||||
| Chlamydomonas dinobryonii | 7.8 | 2.7 | 2.1 | c | |||||||||||
| Chlamydomonas reinhardtii | 8.8 | 9.2 | a | ||||||||||||
| Coelastrum cambricum | 0.8 | 1.3 | b | ||||||||||||
| Coelastrum crenatum var. cubicum | 1.2 | 1.6 | b | ||||||||||||
| Cylindrocapsa geminella | 1.4 | b | |||||||||||||
| Eudorina elegans | 5.9 | 6.2 | 7.6 | 2.8 | 0.9 | 2.5 | 0.8 | 1.6 | 0.5 | c | |||||
| Gonium sociale | 8.1 | 8.7 | 7.9 | 2.2 | 1.3 | 0.8 | c | ||||||||
| Hemaetococcus pluvialis | 0.7 | 0.8 | 1.1 | b | |||||||||||
| Microspora tumidula | 8.5 | 7.9 | 9.1 | 9.7 | 8.9 | a | |||||||||
| Monoraphidium irregulare | 4.2 | 2.6 | 3.0 | 1.8 | b | ||||||||||
| Monoraphidium saxatile | 6.1 | 1.7 | 2.6 | 1.8 | 1.4 | c | |||||||||
| Pseudosphaerocystis lacustris | 8.4 | a | |||||||||||||
| Radiofilum conjunctivum | 6.7 | 5.9 | 8.2 | 8.7 | a | ||||||||||
| Scenedesmus armatus | 6.8 | 7.5 | a | ||||||||||||
| Scenedesmus brevispina | 7.7 | a | |||||||||||||
| Scenedesmus denticulatus | 7.4 | 0.6 | 0.7 | 0.6 | c | ||||||||||
| Scenedesmus quadriculata var. quadrispina | 7.9 | 5.7 | 7.3 | a | |||||||||||
| Sphaerocystis schroeteri | 10.2 | 2.8 | 0.8 | 3.5 | 1.1 | 0.8 | 0.4 | c | |||||||
| Stauridium tetras | 6.8 | 7.7 | 7.3 | 1.8 | 1.3 | 1.8 | 1.1 | 1.2 | c | ||||||
| Tetraspora lacustris | 7.5 | 7.5 | a | ||||||||||||
| Tetraedron sp. | 0.8 | b | |||||||||||||
| Volvox globator | 8.0 | 8.3 | 0.8 | 1.3 | 0.8 | 0.4 | 1.0 | 0.6 | c | ||||||
| Rhaphidiella fascicularis | 6.9 | a | |||||||||||||
| Trebouxiophyceae | Willea irregularis | 5.9 | a | ||||||||||||
| Botryococcus braunii | 2.4 | 1.7 | 1.2 | b | |||||||||||
| Chlorella fusca | 8.5 | 8.1 | 12.7 | 10.2 | 3.8 | 2.1 | 4.0 | 3.1 | 3.7 | 2.0 | c | ||||
| Chlorella saccharophila | 8.6 | a | |||||||||||||
| Chlorella vugaris | 8.2 | 8.1 | 8.1 | 9.4 | 14.2 | 10.6 | 5.7 | 2.4 | 6.7 | 1.8 | 3.2 | 2.5 | c | ||
| Nephrocytium limneticum | 5.8 | 5.8 | a | ||||||||||||
| Oocystis elliptica | 8.9 | 8.7 | 8.1 | 8.6 | a | ||||||||||
| Oocystis lacustris | 8.0 | 1.2 | 1.9 | 1.5 | 1.2 | 0.8 | 1.6 | c | |||||||
| Oocystis solitaria | 8.6 | a | |||||||||||||
| Ulvophyceae | Ulothrix tenuissima | 0.6 | b | ||||||||||||
| Ulothrix variabilis | 7.9 | 1.0 | c | ||||||||||||
| Ulothrix zonata | 1.5 | 1.2 | b | ||||||||||||
| Cryptophyta | Cryptophyceae | Cryptomonas curvata | 6.2 | 7.6 | 10.2 | a | |||||||||
| Cryptomonas ovata | 8.1 | 3.5 | 3.1 | 2.7 | 1.4 | 1.4 | 1.7 | c | |||||||
| Cyanobacteria | Cyanophyceae | Anabaena constricta | 6.6 | 6.9 | 8.0 | 7.4 | a | ||||||||
| Anabaena solitaria | 5.9 | 6.3 | 6.9 | 5.6 | 0.6 | c | |||||||||
| Aphanocapsa parietina | 7.4 | 8.1 | 1.5 | 1.9 | 0.8 | 0.6 | c | ||||||||
| Aphanothece microscopica | 6.7 | 8.9 | 15.6 | 13.0 | 2.6 | 2.0 | 1.4 | 1.6 | 0.9 | 1.6 | c | ||||
| Chroococcus minutus | 10.6 | 12.2 | 1.3 | 1.8 | 1.4 | c | |||||||||
| Dolicospermum sp. | 2.3 | 0.5 | 0.9 | b | |||||||||||
| Gomphosphaeria aponina | 0.9 | b | |||||||||||||
| Limnothrix redekei | 2.1 | 1.9 | 1.8 | 1.2 | 1.3 | b | |||||||||
| Lyngbya martensiana | 1.1 | b | |||||||||||||
| Merismopedia punctata | 0.5 | b | |||||||||||||
| Microcystis botrys | 0.8 | 0.8 | b | ||||||||||||
| Microcystis flosaquae | 0.4 | 0.4 | 1.4 | b | |||||||||||
| Nodularia spumigena | 5.6 | 4.8 | 5.5 | 6.4 | 0.9 | 0.7 | 0.8 | 1.4 | c | ||||||
| Nostoc kihlman | 5.2 | a | |||||||||||||
| Oscillatoria lacustris | 0.9 | b | |||||||||||||
| Raphidiopsis curvata | 0.4 | b | |||||||||||||
| Rivularia sp. | 0.8 | 0.9 | 1.8 | b | |||||||||||
| Spirulina platensis | 7.1 | 1.1 | 1.3 | c | |||||||||||
| Miozoa | Dinophyceae | Ceratium furcoide | 7.9 | 8.4 | 12.9 | 13.9 | 18.1 | 19.2 | 0.7 | c | |||||
| Ceratium tetraceros | 1.1 | 0.6 | 0.6 | 0.7 | 0.7 | b | |||||||||
| Gymnodinium fuscum | 5.4 | 15.4 | 16.6 | 11.8 | 19.5 | 14.8 | 1.1 | 0.6 | 0.3 | 0.7 | 0.6 | 0.8 | c | ||
| Peridium cingulum | 15.5 | 12.1 | 0.4 | 0.9 | c | ||||||||||
| Protoperidinium | 0.8 | 1.0 | b | ||||||||||||
| Euglenophyta | Euglenophyceae | Euglena gracilis | 5.3 | 7.8 | 10.2 | 8.4 | 0.6 | 0.8 | 0.9 | 0.4 | 0.7 | 0.5 | c | ||
| Phacus sp. | 1.3 | 0.4 | b | ||||||||||||
| Trachelomonas volvocina | 8.3 | 8.9 | 0.8 | 0.9 | 0.8 | 0.9 | c | ||||||||
| Ochrophyta | Chrysophyceae | Dinobryon divergens | 9.0 | 9.2 | 8.5 | 9.0 | 12.1 | 9.2 | 4.6 | 3.6 | 3.9 | 3.4 | 2.0 | 3.2 | c |
| Ochromonas elegans | 5.6 | 1.7 | 1.3 | 2.2 | 0.9 | c | |||||||||
| Mallomonas alpina | 2.0 | b | |||||||||||||
| Mallomonas longiseta | 4.4 | 8.8 | 8.3 | 1.7 | 1.2 | c | |||||||||
| Mallomonas areolata | 10.6 | 7.2 | 10.5 | 1.3 | 3.8 | 1.8 | 0.8 | c | |||||||
| Mallomonas caudata | 5.4 | 10.1 | 9.7 | 1.2 | 1.6 | 2.6 | 2.9 | c | |||||||
| Mallomonas elongiseta | 8.4 | 10.3 | 7.9 | 1.3 | 1.0 | 1.6 | 1.3 | 1.6 | 1.3 | c | |||||
| Synura uvella | 0.6 | b | |||||||||||||
| Chlorodendrophyceae | Tetraselmis limnetis | 0.4 | 0.4 | 0.5 | 0.8 | b | |||||||||
| Eustigmatophyceae | Goniochloris fallax | 1.4 | b | ||||||||||||
| Xantophyceae | Ophiocytium capitatum | 8.1 | a | ||||||||||||
| Charophyta | Zygnematophyceae | Closterium parvulum | 8.7 | a | |||||||||||
| Staurastrum orbiculare | 7.9 | 0.9 | 1.3 | 1.0 | c | ||||||||||
| Staurastrum paradoxum | 7.9 | a | |||||||||||||
| Staurastrum rotula | 0.8 | b | |||||||||||||
| Klebsormidiophyceae | Elakatothrix gelatinosa | 6.3 | a | ||||||||||||
| BIO-ENV | p = 0.08 | p = 0.51 | |||||||||||||
| ANOSIM between season | p = 0.02 | ||||||||||||||
| H′ | Chao-1 | J′ | N1 | βSØR (%) | βSIM (%) | ||
|---|---|---|---|---|---|---|---|
| Summer | |||||||
| LK1 | Top | 4.5 | 24 | 0.9 | 22 | 60.9 | 87.5 |
| Bottom | 5.5 | 45 | 0.9 | 44 | |||
| LK2 | Top | 5.1 | 36 | 0.9 | 35 | 53.7 | 61.1 |
| Bottom | 5.5 | 46 | 0.9 | 45 | |||
| LT3 | Top | 5.7 | 55 | 0.9 | 53 | 67.2 | 74.6 |
| Bottom | 6.1 | 67 | 0.9 | 66 | |||
| Winter | |||||||
| LK1 | Top | 5.5 | 53 | 0.9 | 45 | 62.7 | 65.3 |
| Bottom | 5.4 | 49 | 0.9 | 42 | |||
| LK2 | Top | 5.4 | 51 | 0.9 | 42 | 55.0 | 66.7 |
| Bottom | 5.4 | 51 | 0.9 | 42 | |||
| LK3 | Top | 5.5 | 54 | 0.9 | 46 | 71.3 | 70.7 |
| Bottom | 5.2 | 41 | 0.9 | 36 | |||
| Between Seasons | |||||||
| Summer | 5.8 | 103 | 0.9 | 98 | 62.9 | 74.4 | |
| Winter | 5.7 | 96 | 0.9 | 87 | 60.6 | 67.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Henríquez, C.; Fuentes, N. Temporal Environmental Status of a Shallow Lake Using Alpha and Beta Diversity on Phytoplankton Communities. Water 2024, 16, 274. https://doi.org/10.3390/w16020274
Ríos-Henríquez C, Fuentes N. Temporal Environmental Status of a Shallow Lake Using Alpha and Beta Diversity on Phytoplankton Communities. Water. 2024; 16(2):274. https://doi.org/10.3390/w16020274
Chicago/Turabian StyleRíos-Henríquez, Catalina, and Norka Fuentes. 2024. "Temporal Environmental Status of a Shallow Lake Using Alpha and Beta Diversity on Phytoplankton Communities" Water 16, no. 2: 274. https://doi.org/10.3390/w16020274
APA StyleRíos-Henríquez, C., & Fuentes, N. (2024). Temporal Environmental Status of a Shallow Lake Using Alpha and Beta Diversity on Phytoplankton Communities. Water, 16(2), 274. https://doi.org/10.3390/w16020274








