Evaluation of Algal Control Measures in Eutrophic Reservoirs Based on Aquatic Ecosystem Models
Abstract
1. Introduction
2. Materials and Methods
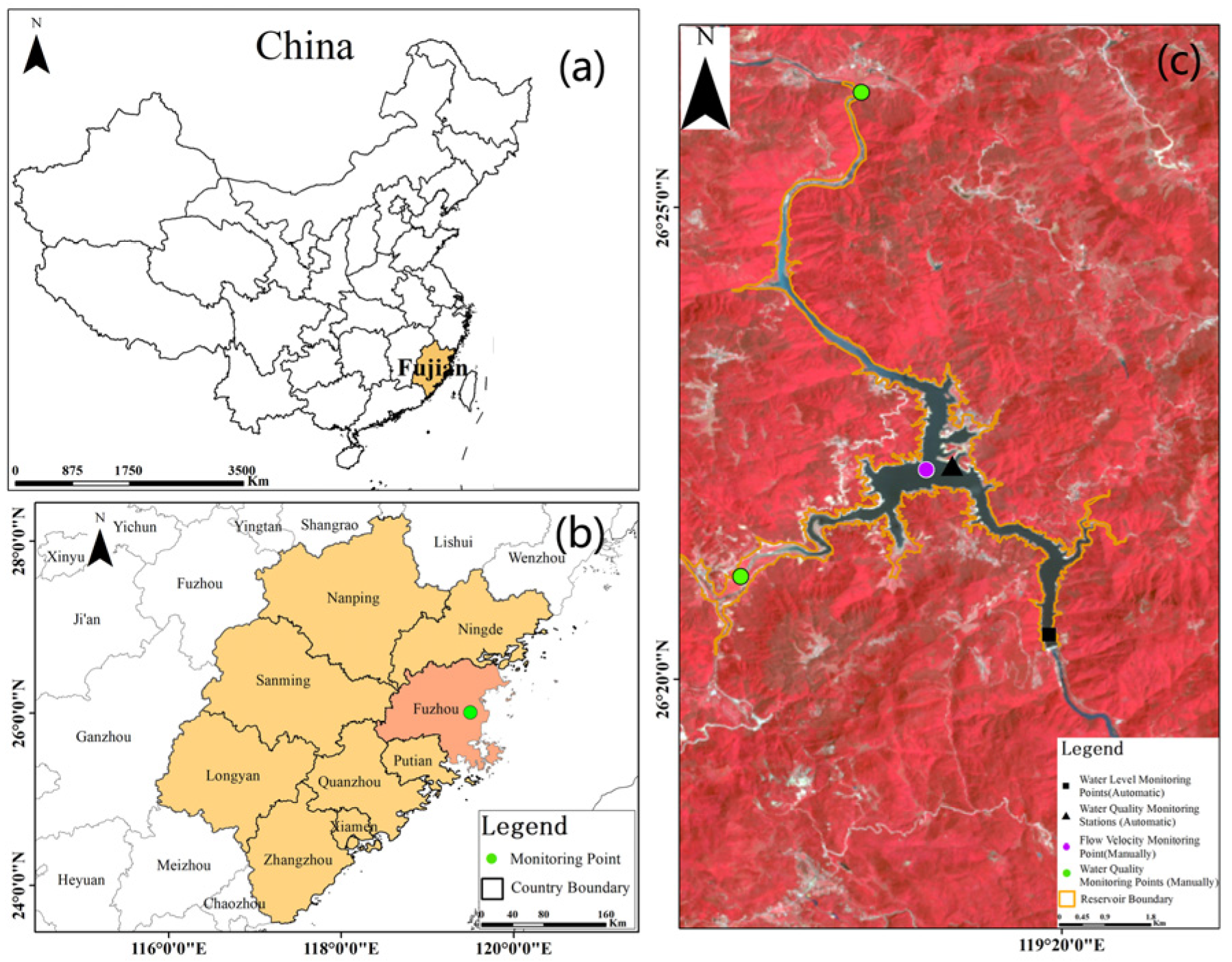
2.1. Overview of the Study Area
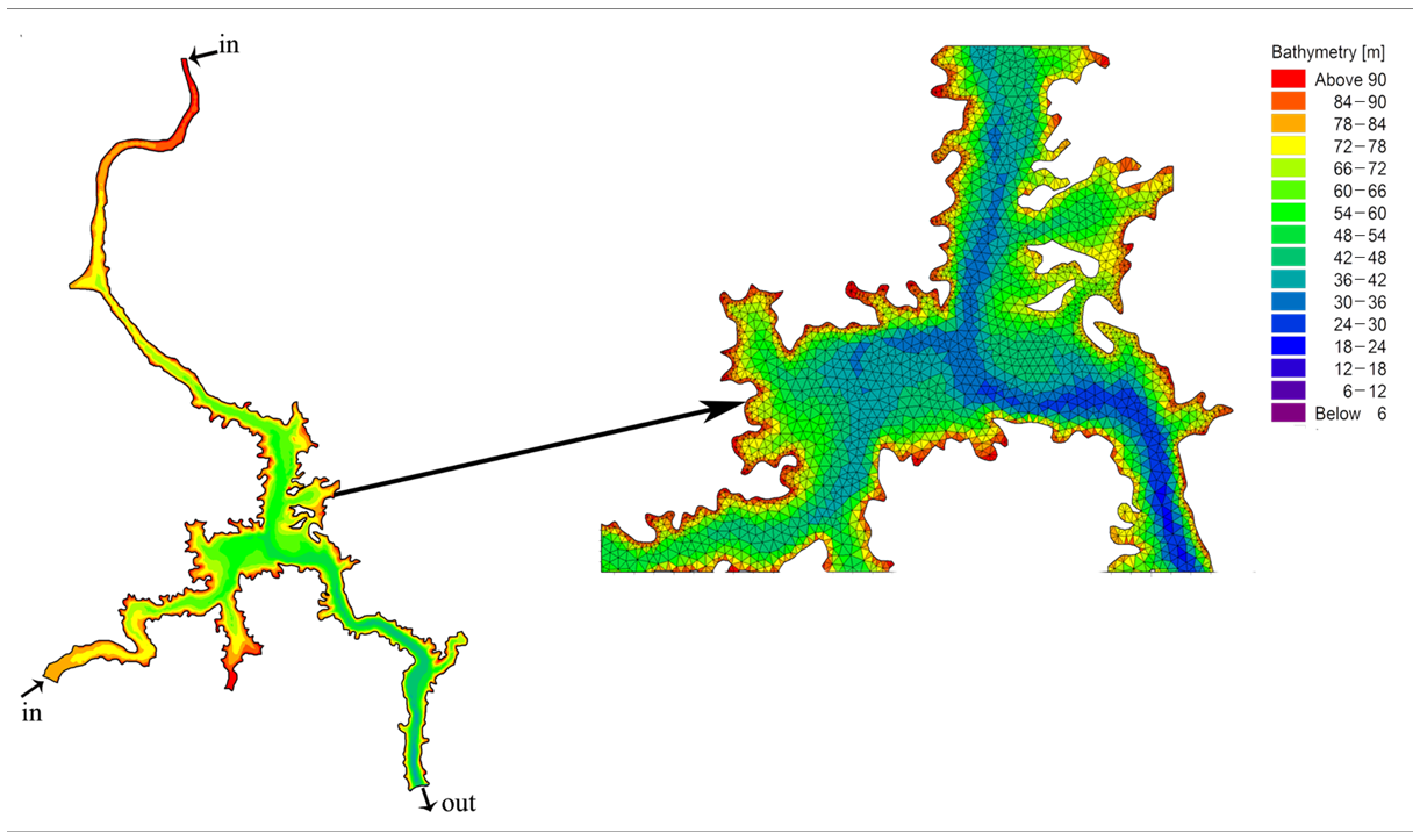
2.2. Principles of Hydrodynamic Modeling
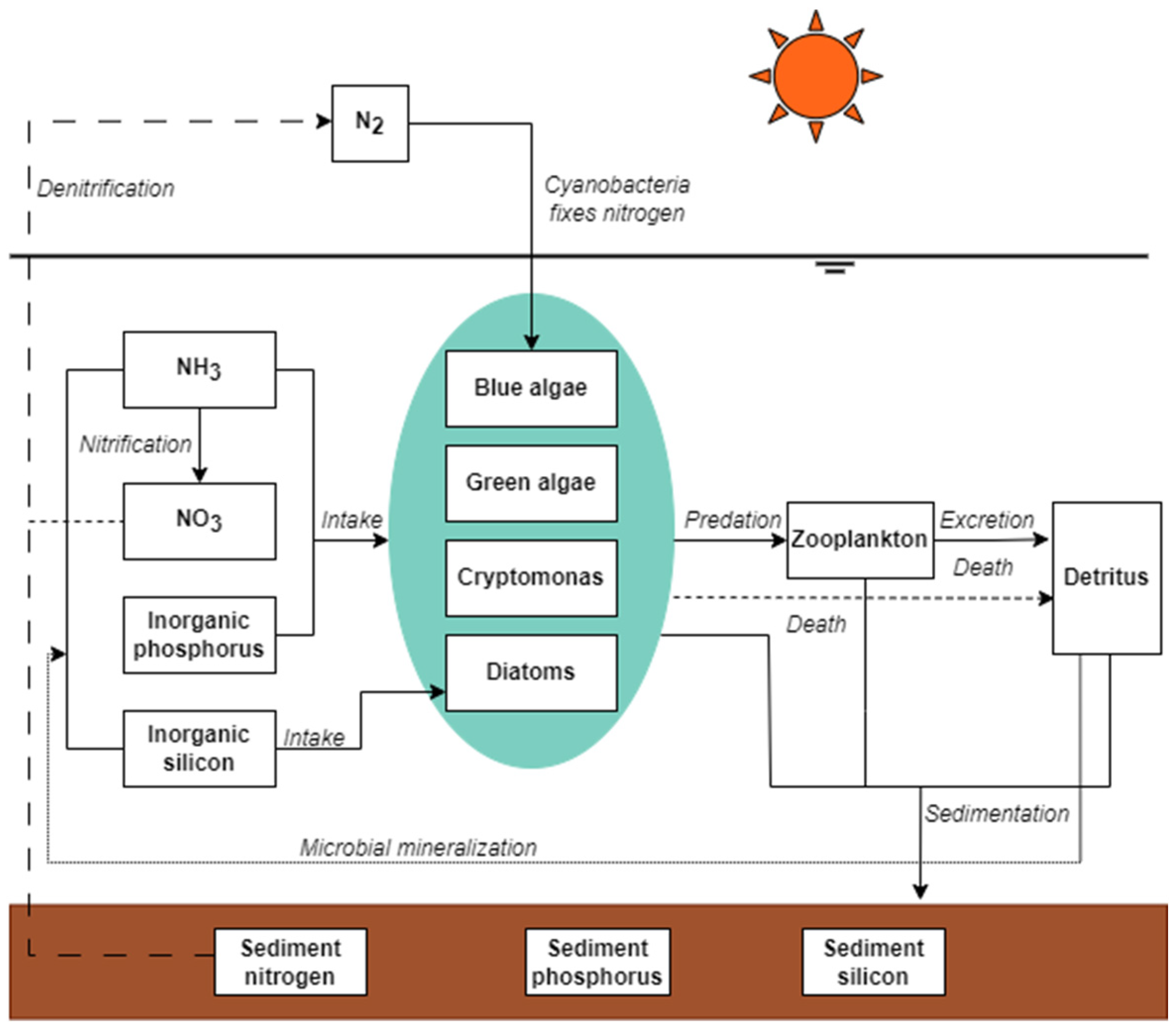
2.3. Aquatic Ecological Model Framework
2.4. Data Collection
2.5. Model Setup and Simulation Scenarios
2.5.1. Initial Model Parameters
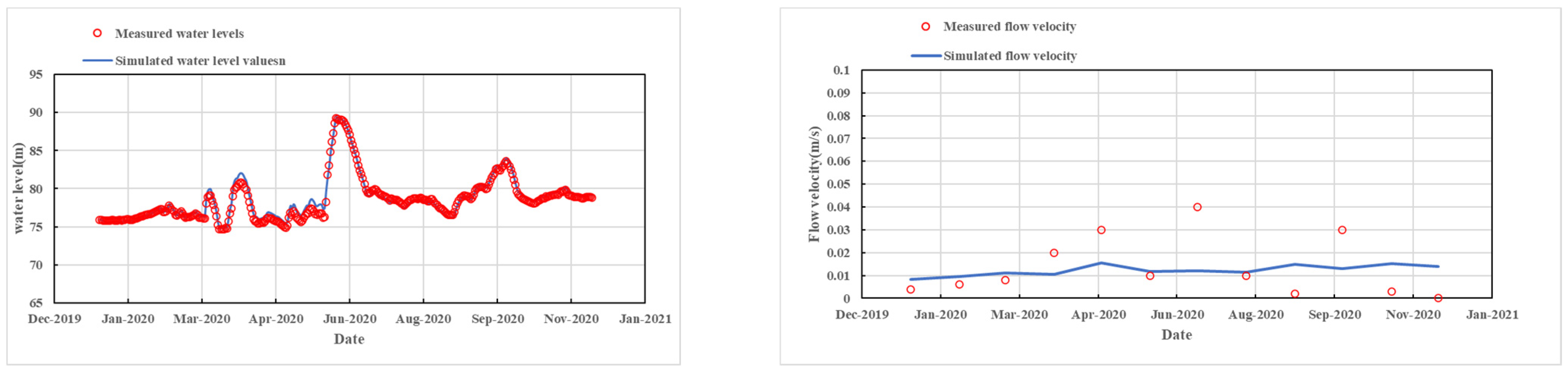
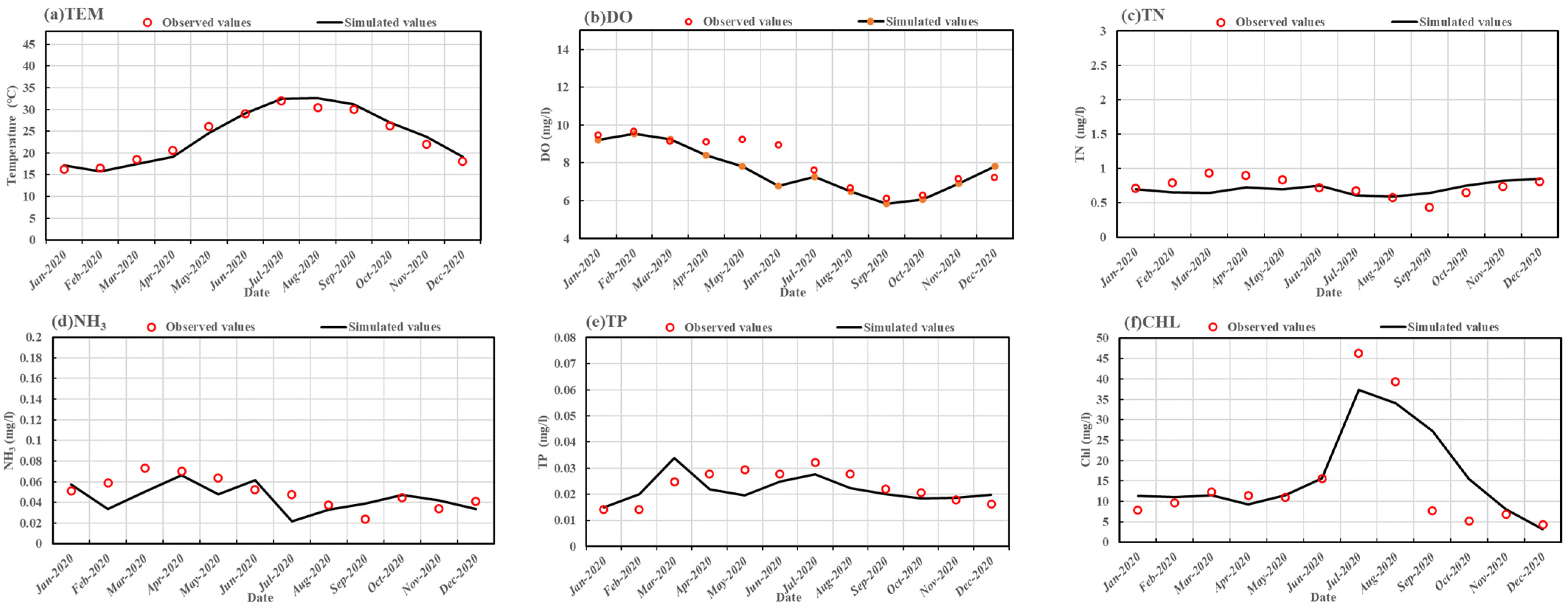
2.5.2. Model Verification and Validation
2.5.3. Simulation Scenarios
3. Results and Discussions
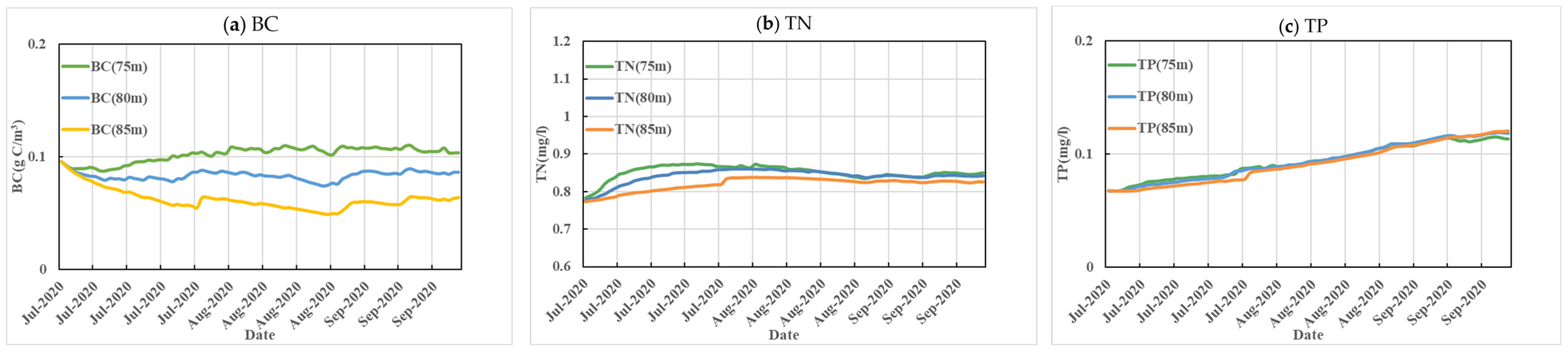
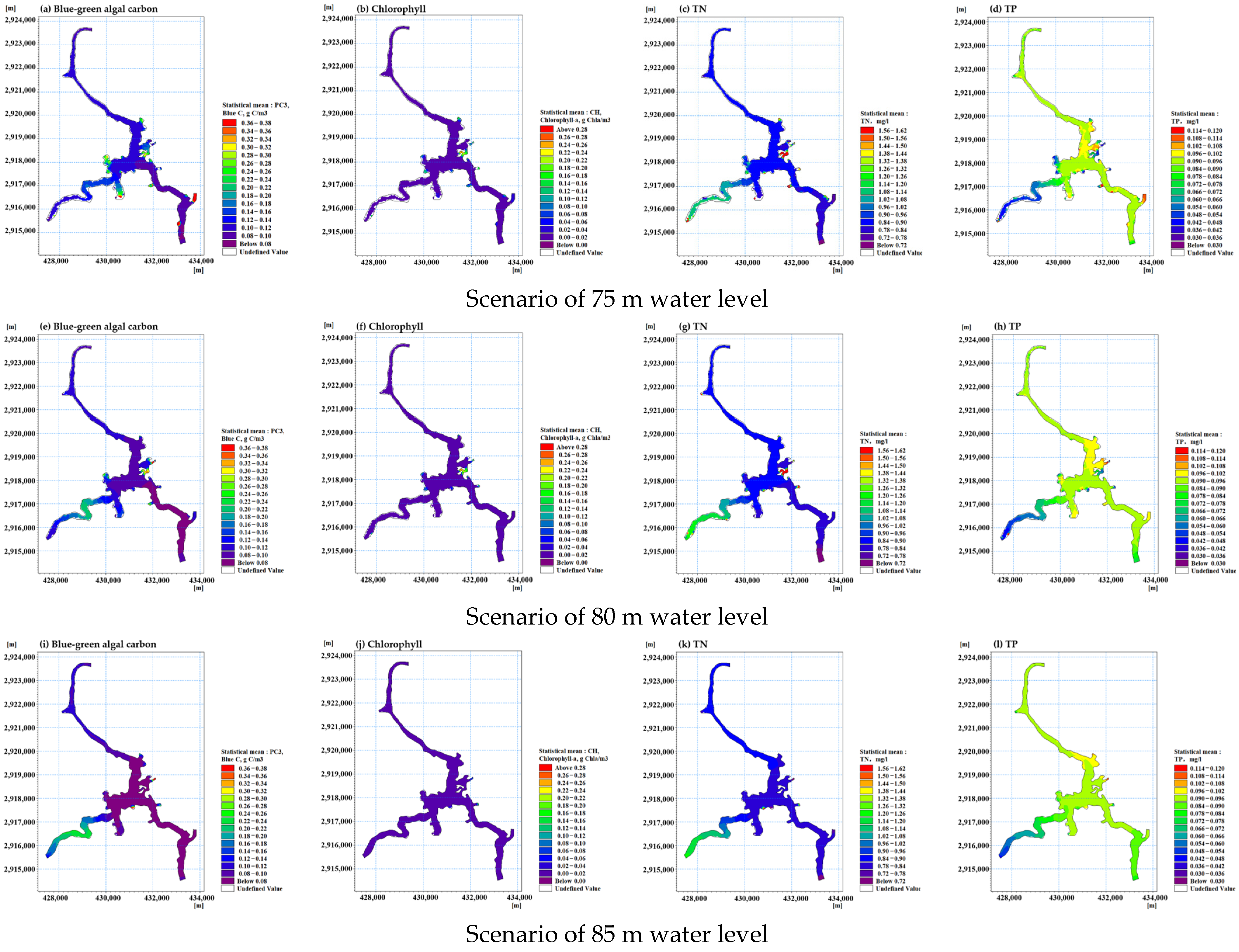
3.1. Reservoir Water Level Scheme Analysis
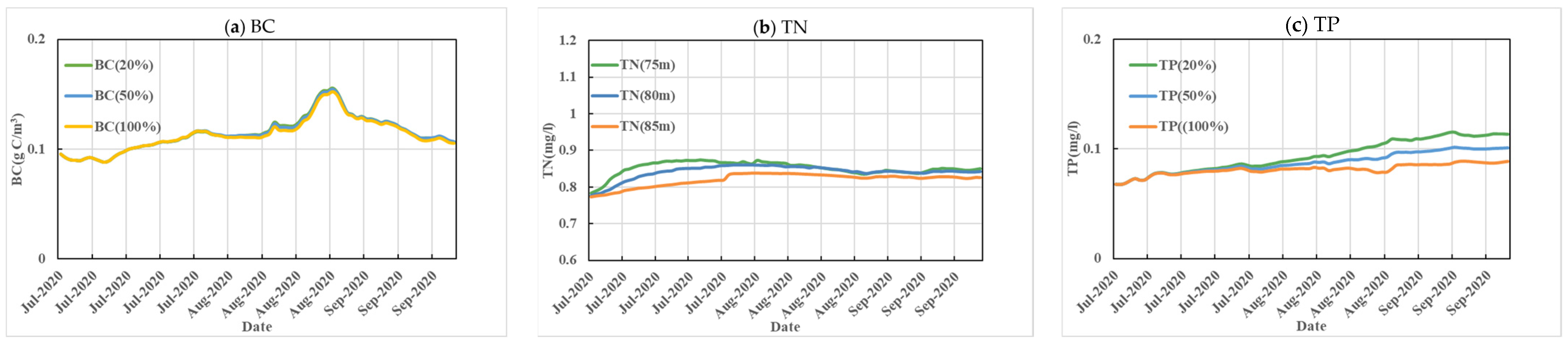
3.2. Analysis of the Impact of the Sediment Pollution Reduction Plan
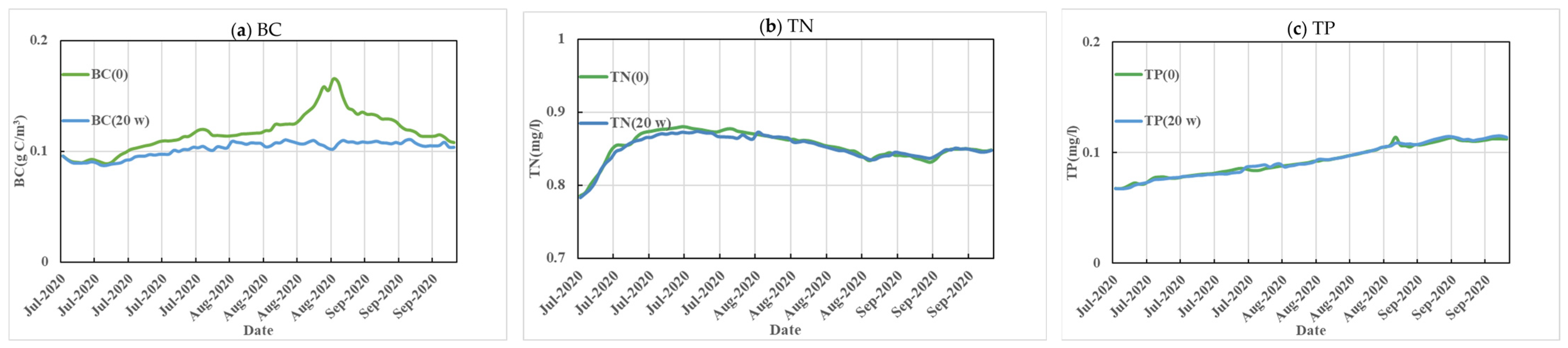
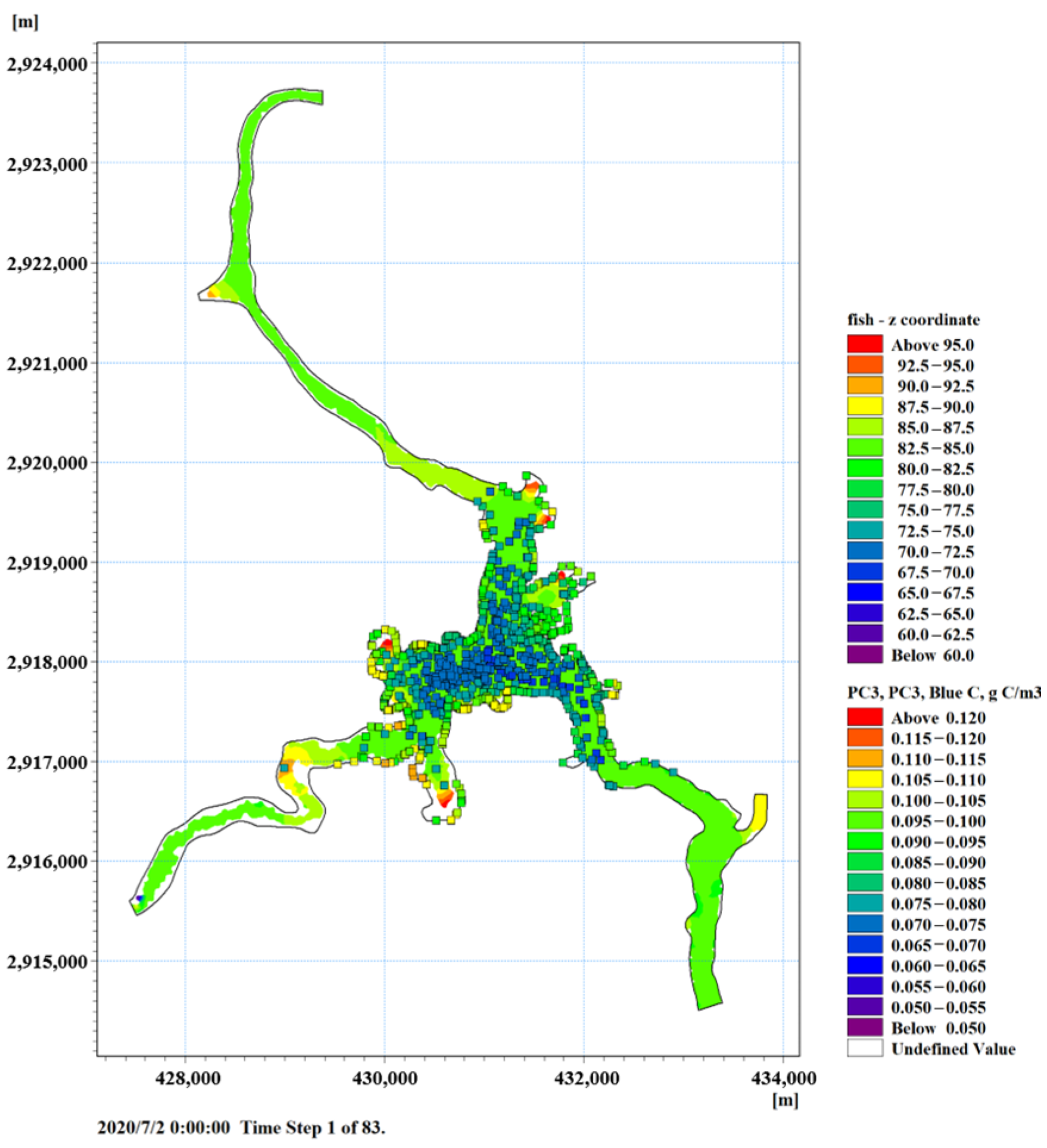
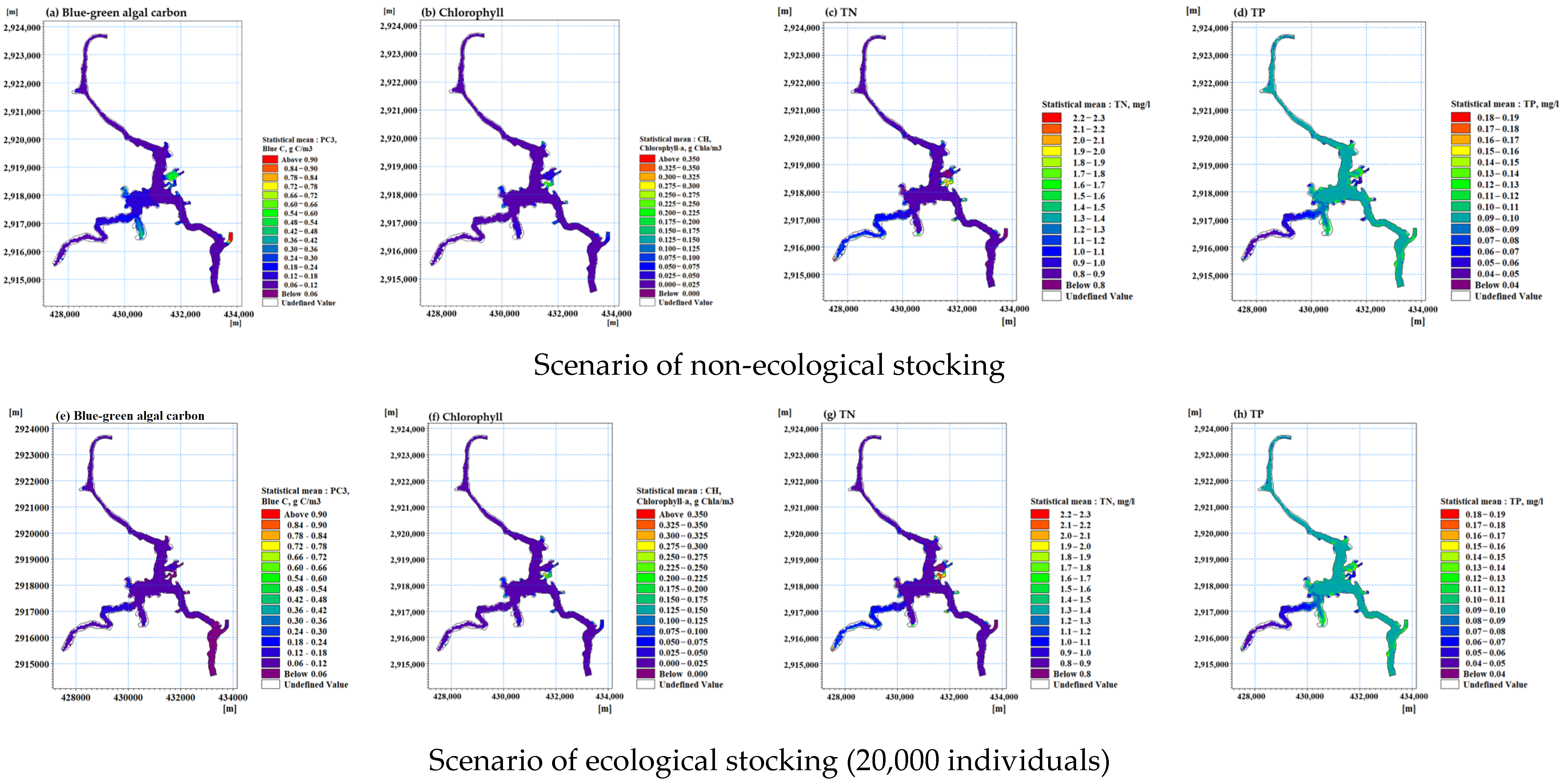
3.3. Ecological Fish Farming
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vorosmarty, C.J.; Mcintyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Zhao, Z.; Wang, L.; Xu, J.; Yu, J. A novel water quality mechanism modeling and eutrophication risk assessment method of lakes and reservoirs. Nonlinear Dyn. 2019, 96, 1037–1053. [Google Scholar] [CrossRef]
- Istvánovics, V. Eutrophication of Lakes and Reservoirs. In Lake Ecosystem Ecology; Elsevier: San Diego, CA, USA, 2010; pp. 47–55. [Google Scholar]
- Chislock, M.F.; Sarnelle, O.; Olsen, B.K.; Doster, E.; Wilson, A.E. Large effects of consumer offense on ecosystem structure and function. Ecology 2013, 94, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Area, W.R.M. Nutrients and Eutrophication. 3 March 2019. Available online: https://www.usgs.gov/mission-areas/water-resources/science/nutrients-and-eutrophication (accessed on 3 March 2019).
- Dey, I.; Banerjee, S.; Bose, R.; Pal, R. Spatiotemporal variations in the composition of algal mats in wastewater treatment ponds of tannery industry. Environ. Monit. Assess. 2021, 193, 359. [Google Scholar] [CrossRef]
- Wagner, T.; Erickson, L.E. Sustainable Management of Eutrophic Lakes and Reservoirs. J. Environ. Prot. Ecol. 2017, 8, 436–463. [Google Scholar] [CrossRef]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Hambaryan, L.; Khachikyan, T.; Ghukasyan, E. Changes in the horizontal development of phytoplankton of the littoral of Lake Sevan (Armenia) in conditions of water level fluctuations. Limnol. Freshw. Biol. 2020, 3, 662–664. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, R.; Liang, D.; Wang, F.; Han, Y.; Luo, Y.; Gao, P.; Wang, Q.; Wang, Q.; Yu, C.; et al. Comparison of the Advantages and Disadvantages of Algae Removal Technology and Its Development Status. Water 2023, 15, 1104. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J. Cyanobacteria dynamics in a small tropical reservoir: Understanding spatio-temporal variability and influence of environmental variables. Sci. Total Environ. 2018, 643, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Ma, X.; Zhang, Y.; Zhang, Y.; Shi, Y. Modeling the interaction of light and nutrients as factors driving lake eutrophication. Ecol. Model. 2019, 400, 41–52. [Google Scholar] [CrossRef]
- Pers, B.C. Modeling the Response of Eutrophication Control Measures in a Swedish Lake. Ambio 2005, 34, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Karul, C.; Soyupak, S.; Çilesiz, A.F.; Akbay, N.; Germen, E. Case studies on the use of neural networks in eutrophication modeling. Ecol. Model. 2000, 134, 145–152. [Google Scholar] [CrossRef]
- Heikonen, S.; Heikkilä, M.Y.; Heino, M. Modeling the drivers of eutrophication in Finland with a machine learning approach. Ecosphere 2023, 14, e4522. [Google Scholar] [CrossRef]
- Lavrik, V.I.; Dobrynskiy, V.A.; Rogal, I.V. Application of Simulation Mathematical Modeling to the Problems of Management of Eutrophication Processes in Lakes and Reservoirs. Hydrobiol. J. 2002, 38, 10. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Ji, D.; Cheng, Y.; Nwankwegu, A.S.; Paerl, H.W.; Tang, C.; Yang, Z.; Zhao, X.; Chen, Y.; et al. Storm and floods increase the duration and extent of phosphorus limitation on algal blooms in a tributary of the Three Gorges Reservoir, China. J. Hydrol. 2022, 607, 127562. [Google Scholar] [CrossRef]
- Roy, K.; Vrba, J.; Kajgrova, L.; Mraz, J. The concept of balanced fish nutrition in temperate European fishponds to tackle eutrophication. J. Clean. Prod. 2022, 364, 132584. [Google Scholar] [CrossRef]
- Zeng, Q.; Qin, L.; Li, X. The potential impact of an inter-basin water transfer project on nutrients (nitrogen and phosphorous) and chlorophyll a of the receiving water system. Sci. Total Environ. 2015, 536, 675–686. [Google Scholar] [CrossRef]
- Pu, P.; Wang, G.; Hu, C.; Hu, W.; Fan, C. Can We Control Lake Eutrophication by Dredging? Hu Po Ke Xue 2000, 12, 279–287. [Google Scholar]
- Anagnostou, E.; Gianni, A.; Zacharias, I. Ecological modeling and eutrophication—A review. Nat. Resour. Model. 2017, 30, e12130. [Google Scholar] [CrossRef]
- Wu, G.; Xu, Z. Prediction of algal blooming using EFDC model: Case study in the Daoxiang Lake. Ecol. Model. 2011, 222, 1245–1252. [Google Scholar] [CrossRef]
- Dang, T.D.; Arias, M.E.; Tarabih, O.; Phlips, E.J.; Ergas, S.J.; Rains, M.C.; Zhang, Q. Modeling temporal and spatial variations of biogeochemical processes in a large subtropical lake: Assessing alternative solutions to algal blooms in Lake Okeechobee, Florida. J. Hydrol. Reg. Stud. 2023, 47, 101441. [Google Scholar] [CrossRef]
- Cui, J.; Xu, H.; Cui, Y.; Song, C.; Qu, Y.; Zhang, S.; Zhang, H. Improved eutrophication model with flow velocity-influence function and application for algal bloom control in a reservoir in East China. J. Environ. Manag. 2023, 348, 119209. [Google Scholar] [CrossRef]
- Kuang, C.; Wang, D.; Wang, G.; Liu, J.; Han, X.; Li, Y. Impact of reclamation projects on water quality in jinmeng bay, China. Estuar. Coast. Shelf Sci. 2024, 300, 108719. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, J.; Zhang, Z.; Tian, Z. Assessment and a review of research on surface water quality modeling. Ecol. Model. 2022, 466, 109888. [Google Scholar] [CrossRef]
- Peiyu, L.; Xuan, W.; Fangbing, M.A. Effect of hydrodynamic conditions on water eutrophication: A review. J. Lake Sci. 2013, 25, 455–462. [Google Scholar] [CrossRef]
- Deng, J.; Chen, F.; Liu, X.; Peng, J.; Hu, W. Horizontal migration of algal patches associated with cyanobacterial blooms in an eutrophic shallow lake. Ecol. Eng. 2016, 87, 185–193. [Google Scholar] [CrossRef]
- Ranjbar, M.H.; Hamilton, D.P.; Etemad-Shahidi, A.; Helfer, F. Impacts of atmospheric stilling and climate warming on cyanobacterial blooms: An individual-based modelling approach. Water Res. 2022, 221, 118814. [Google Scholar] [CrossRef]
- Attayde, J.L.; Hansson, L. Effects of Nutrient Recycling by Zooplankton and Fish on Phytoplankton Communities. Oecologia 1999, 121, 47–54. [Google Scholar] [CrossRef]
- Rast, W.; Thornton, J.A. Trends in eutrophication research and control. Hydrol. Process. 1996, 10, 295–313. [Google Scholar] [CrossRef]
- Qin, B.; Yang, L.; Chen, F.; Zhu, G.; Zhang, L.; Chen, Y. Mechanism and control of lake eutrophication. Chin. Sci. Bull. 2006, 51, 2401–2412. [Google Scholar] [CrossRef]
- Ansari, A.A.; Singh, G.S.; Lanza, G.R.; Rast, W. Eutrophication: Causes, Consequences and Control; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1. [Google Scholar]
- Zhou, Q.; Chen, J.; Liu, S.; Zhou, F.; Wen, H. Pollution Control and Well-Being in Rural Areas: A Study Based on Survey Data. Sustainability 2024, 16, 1334. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Huang, X.; Liu, Y.; Guan, M.; Tian, Y. Hydrological conditions can change the effects of major nutrients and dissolved organic matter on phytoplankton community dynamics in a eutrophic river. J. Hydrol. 2024, 628, 130503. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, J.; Zheng, Z.; Lai, Y.; Geng, H. Risk assessment and spatio-temporal characteristics analysis of water bloom in three large-scale eutrophic reservoirs in Fujian Province, China. Ecol. Indic. 2024, 158, 111539. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, J.; Zheng, Y.; Wang, F.; Wu, C.; Xie, R. Effect of a dam on the optical properties of different-sized fractions of dissolved organic matter in a mid-subtropical drinking water source reservoir. Sci. Total Environ. 2017, 598, 704–712. [Google Scholar] [CrossRef]
- Warren, I.R.; Bach, H.K. MIKE 21: A modelling system for estuaries, coastal waters and seas. Environ. Softw. 1992, 7, 229–240. [Google Scholar] [CrossRef]
- Ahn, J.; Na, Y.; Park, S.W. Development of two-dimensional inundation modelling process using MIKE21 model. KSCE J. Civ. Eng. 2019, 23, 3968–3977. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Wang, R. Numerical simulation of Donghu Lake hydrodynamics and water quality based on remote sensing and MIKE 21. ISPRS Int. J. Geo-Inf. 2020, 9, 94. [Google Scholar] [CrossRef]
- Xu, C.; Ren, Z.; Huang, S.; Li, J.; Zi, Y.; Hu, X. Simulation Study on the Impact of Water Flow Regulation Based on the MIKE 21 Model in a River Water Environment. Sustainability 2023, 15, 10313. [Google Scholar] [CrossRef]
- Engin, G.O.; Gurbulak, E.; Celen, M.; Ulu, F.; Oncel, M.S. Nutrient Modelling in Coastal Waters of Izmit Bay, Turkey. Fresenius Environ. Bull. 2014, 23, 3345–3352. [Google Scholar]
- Global Modeling and Assimilation Office (GMAO). MERRA-2 tavg1_2d_rad_Nx: 2d, 1-Hourly, Time-Averaged, Single-Level, Assimilation, Radiation Diagnostics V5.12.4; Goddard Earth Sciences Data and Information Services Center (GES DISC): Greenbelt, MD, USA, 2015. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Men, C.; Guo, L.; Miao, Y. Effects of dynamic land use inputs on improvement of SWAT model performance and uncertainty analysis of outputs. J. Hydrol. 2018, 563, 874–886. [Google Scholar] [CrossRef]
- Brient, L.; Lengronne, M.; Bertrand, E.; Rolland, D.; Sipel, A.; Steinmann, D.; Baudin, I.; Legeas, M.; Le Rouzic, B.; Bormans, M. A phycocyanin probe as a tool for monitoring cyanobacteria in freshwater bodies. J. Environ. Monit. 2008, 10, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Fadel, A.; Sharaf, N.; Siblini, M.; Slim, K.; Kobaissi, A. A simple modelling approach to simulate the effect of different climate scenarios on toxic cyanobacterial bloom in a eutrophic reservoir. Ecohydrol. Hydrobiol. 2019, 19, 359–369. [Google Scholar] [CrossRef]
- Bhagowati, B.; Ahamad, K.U. A review on lake eutrophication dynamics and recent developments in lake modeling. Ecohydrol. Hydrobiol. 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Liu, H. Assessing the eutrophication risk of the Danjiangkou Reservoir based on the EFDC model. Ecol. Eng. 2016, 96, 117–127. [Google Scholar] [CrossRef]
- Pereira, A.C.; Mulligan, C.N. Practices for Eutrophic Shallow Lake Water Remediation and Restoration: A Critical Literature Review. Water 2023, 15, 2270. [Google Scholar] [CrossRef]
- Kakade, A.; Salama, E.; Han, H.; Zheng, Y.; Kulshrestha, S.; Jalalah, M.; Harraz, F.A.; Alsareii, S.A.; Li, X. World eutrophic pollution of lake and river: Biotreatment potential and future perspectives. Environ. Technol. Innov. 2021, 23, 101604. [Google Scholar] [CrossRef]
- Jilbert, T.; Couture, R.M.; Huser, B.J.; Salonen, K. Preface: Restoration of eutrophic lakes: Current practices and future challenges. Hydrobiologia 2020, 847, 4343–4357. [Google Scholar] [CrossRef]
- Sellergren, M.; Li, J.; Drakare, S.; Thöns, S. Decision Support for Lake Restoration: A Case Study in Swedish Freshwater Bodies. Water 2023, 15, 668. [Google Scholar] [CrossRef]
- Drenner, R.W.; Hambright, K.D. Biomanipulation of fish assemblages as a lake restoration technique. Arch. Hydrobiol. 1999, 146, 129–165. [Google Scholar] [CrossRef] [PubMed]
- Beklioğlu, M.; Bucak, T.; Coppens, J.; Bezirci, G.; Tavşanoğlu, Ü.; Çakıroğlu, A.; Levi, E.; Erdoğan, Ş.; Filiz, N.; Özkan, K.; et al. Restoration of eutrophic lakes with fluctuating water levels: A 20-year monitoring study of two inter-connected lakes. Water 2017, 9, 127. [Google Scholar] [CrossRef]
- Kim, J.; Lee, T.; Seo, D. Algal bloom prediction of the lower han river, korea using the EFDC hydrodynamic and water quality model. Ecol. Model. 2017, 366, 27–36. [Google Scholar] [CrossRef]
- Horppila, J. Sediment nutrients, ecological status and restoration of lakes. Water Res. 2019, 160, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qiu, H.; Chen, Y.; Ma, X.; Yu, G.; Hong, Y.; Hu, B. Fish-mussel-algae-bacteria model remedied eutrophication pollution: Application in Dongxiang district reservoir. Environ. Pollut. 2024, 342, 123011. [Google Scholar] [CrossRef] [PubMed]

| State Variable | Description | Initial Value | Unit |
|---|---|---|---|
| BAC | Blue algae carbon content | 0.1 | g C/m3 |
| BAN | Blue algae nitrogen content | 0.014 | g N/m3 |
| BAP | Blue algae phosphorus content | 0.002 | g P/m3 |
| BACH | Blue algae chlorophyll content | 0.001 | g CH/m3 |
| Diatoms Si | Diatoms silicon content | 0.01 | g Si/m3 |
| Zooplankton C | Zooplankton carbon | 0.003 | g C/m3 |
| DC | Detritus carbon content | 0.5 | g C/m3 |
| DN | Detritus nitrogen content | 0.3 | g N/m3 |
| Detritus P | Detritus phosphorus content | 0.02 | g P/m3 |
| Detritus Si | Detritus silicon content | 0.1 | g Si/m3 |
| Dissolved oxygen | Dissolved oxygen | 11.05 | g DO/m3 |
| Ammonium | Ammonium nitrogen | 4.05 | g N/m3 |
| Nitrate | Nitrate nitrogen | 1.6 | g N/m3 |
| Phosphate | Phosphate | 0.1 | g P/m3 |
| Silicate | Silicate | 0.1 | g Si/m3 |
| Statistical Indicators | Water Level (m) | Flow Velocity (m/s) | Tem (°C) | DO (mg/L) | TN (mg/L) | NH3 (mg/L) | TP (mg/L) | CHL (mg/L) |
|---|---|---|---|---|---|---|---|---|
| RMSE | 1.12 | 0.001 | 1.24 | 1.55 | 0.13 | 0.52 | 0.005 | 7.18 |
| NSE | 0.98 | 0.01 | 0.96 | 0.65 | 0.08 | 0.16 | 0.32 | 0.71 |
| Scenario | Water Level in Front of the Dam | Aquatic Ecological Environment Background | Nutrient |
|---|---|---|---|
| 1 | 75 | The inflow is the average of the last five years, including 35 m3/s in Huokou and 5 m3/s in Rixi. Water quality uses average data of 2022. | The nitrogen and phosphorus loads entering the reservoir include inflow and internal sources of pollution. All parameters are set using the model parameters validated in 2020. |
| 2 | 80 | ||
| 3 | 85 |
| Scenario | Water Level in Front of the Dam | Aquatic Ecological Environment Background |
|---|---|---|
| 1 | 20% | Idealized setting for sediment reduction to quantify the benefits of dredging. Actual projects should consider safe dredging with negative effects on sediment disturbance and consider zoning, staging, and negative impact. All settings are based on the 2020 modeling parameters. |
| 2 | 50% | |
| 3 | 100% |
| Scenario | Survival Quantity (Ten Thousand) | Fish Fry (g/piece) | Feed Intake (Daily Weight Percentage, 1/d) | Feed Intake (Daily Weight Percentage, 1/d) | Hydraulic Conditions |
|---|---|---|---|---|---|
| Crucian carp | 20 | 15 | 0.05 | Actual inflow and outflow and water quality from July to September 2020 | Idealized setting for sediment reduction to quantify the benefits of dredging. Actual projects should consider safe dredging with negative effects on sediment disturbance and consider zoning, staging, and negative impact. All settings are based on the 2020 modeling parameters. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Liao, T.; Lin, Y.; Zhu, X.; Meng, H. Evaluation of Algal Control Measures in Eutrophic Reservoirs Based on Aquatic Ecosystem Models. Water 2024, 16, 1494. https://doi.org/10.3390/w16111494
Zheng Z, Liao T, Lin Y, Zhu X, Meng H. Evaluation of Algal Control Measures in Eutrophic Reservoirs Based on Aquatic Ecosystem Models. Water. 2024; 16(11):1494. https://doi.org/10.3390/w16111494
Chicago/Turabian StyleZheng, Zhen, Tingting Liao, Yafeng Lin, Xueyi Zhu, and Haobin Meng. 2024. "Evaluation of Algal Control Measures in Eutrophic Reservoirs Based on Aquatic Ecosystem Models" Water 16, no. 11: 1494. https://doi.org/10.3390/w16111494
APA StyleZheng, Z., Liao, T., Lin, Y., Zhu, X., & Meng, H. (2024). Evaluation of Algal Control Measures in Eutrophic Reservoirs Based on Aquatic Ecosystem Models. Water, 16(11), 1494. https://doi.org/10.3390/w16111494







