Occurrence and Mitigation of Bacterial Regrowth in Stored Household Water in Eastern Coastal Madagascar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Context, and Households Sampled
2.2. Collection of Water Samples
2.3. Quantifying Bacterial Concentrations in Water
2.4. Measuring Biofilms on Interior Surfaces of Jerrycans
2.5. Measuring Efficacy of Jerrycan Cleaning Methods
2.6. Statistical Tests
3. Results
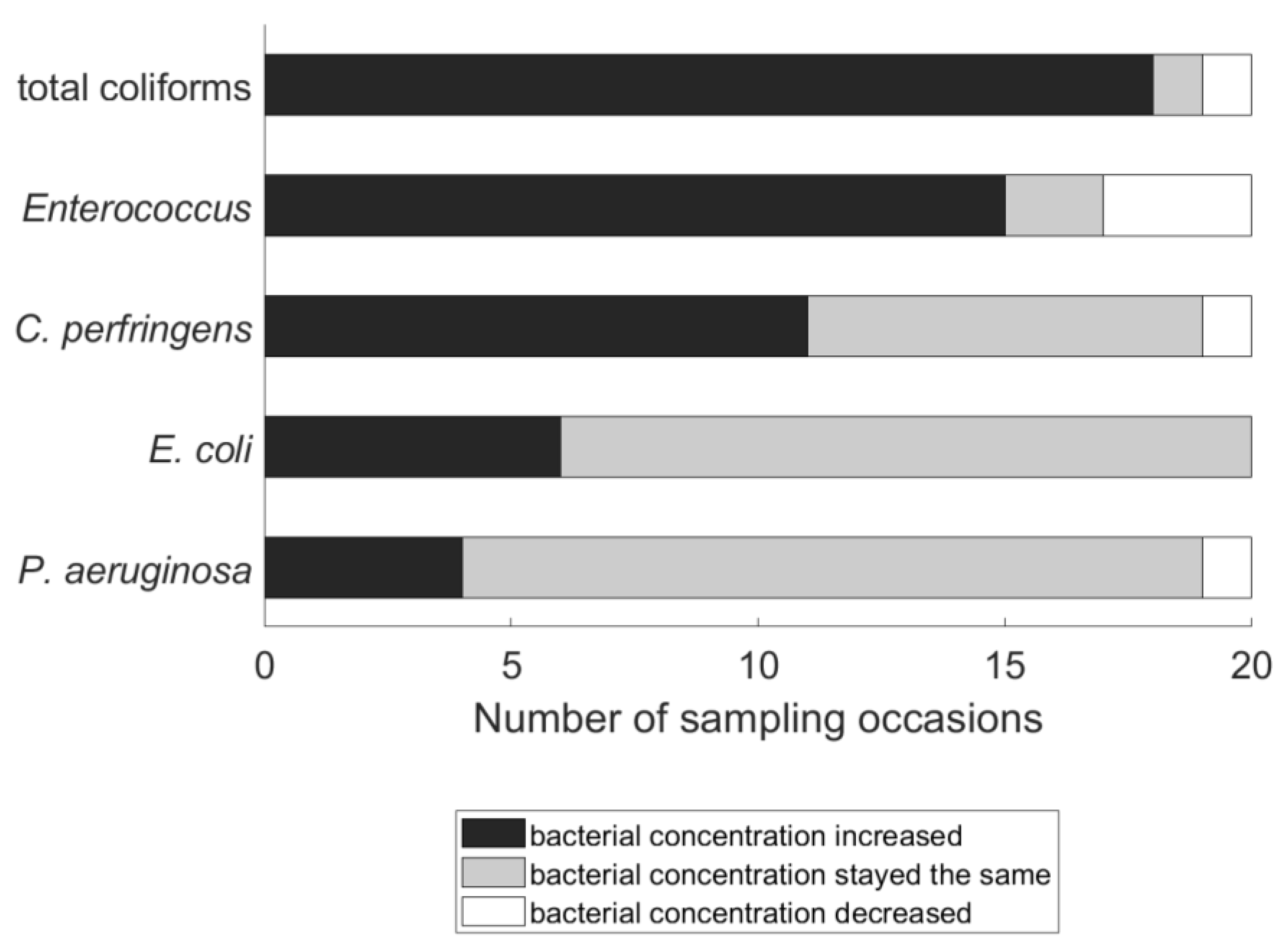
3.1. Frequency of Bacterial Regrowth/Recontamination in Household Water
3.2. Severity of Bacterial Regrowth/Recontamination in Household Water
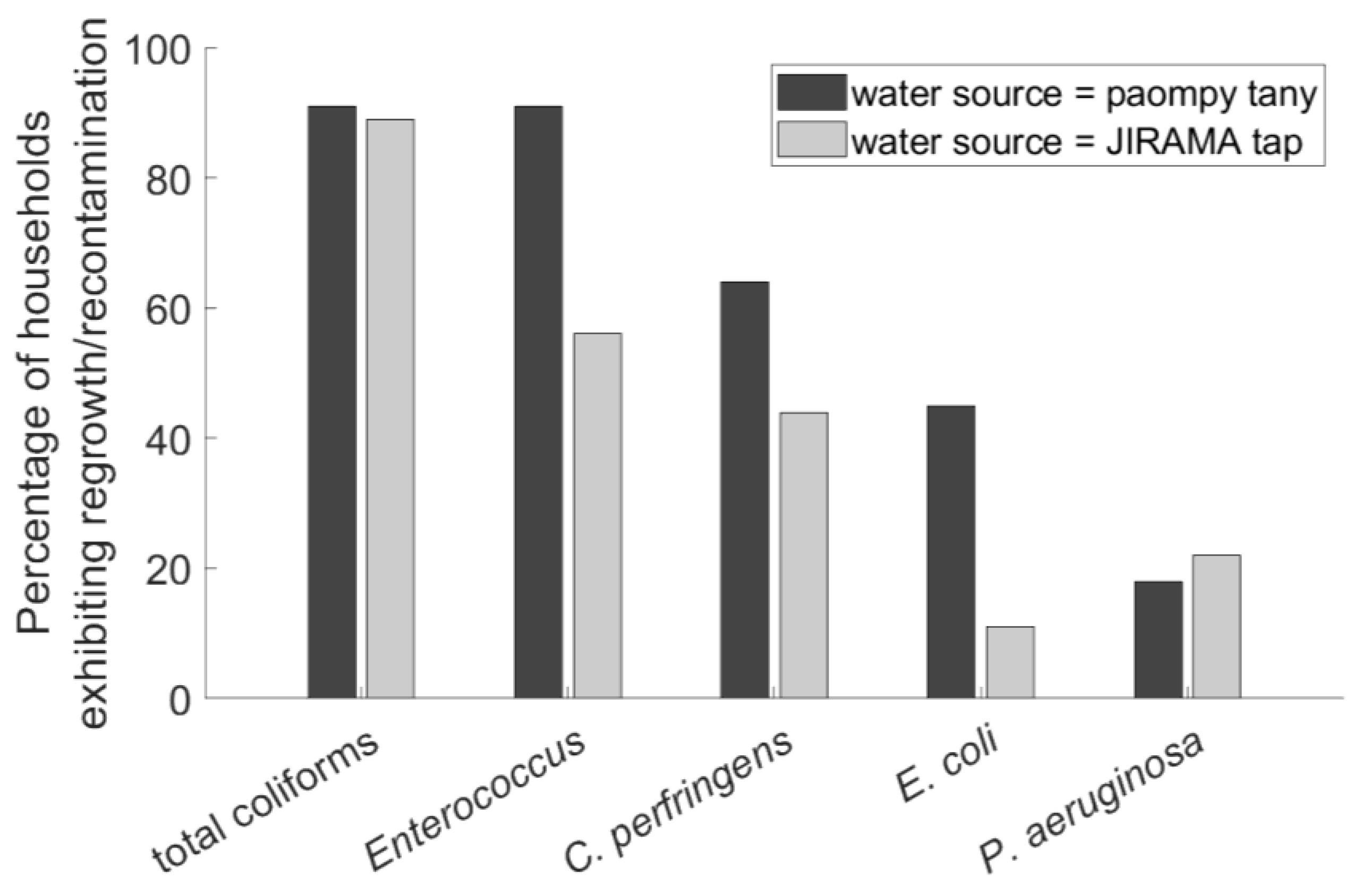
3.3. Correlation of Regrowth/Recontamination with Type of Water Source
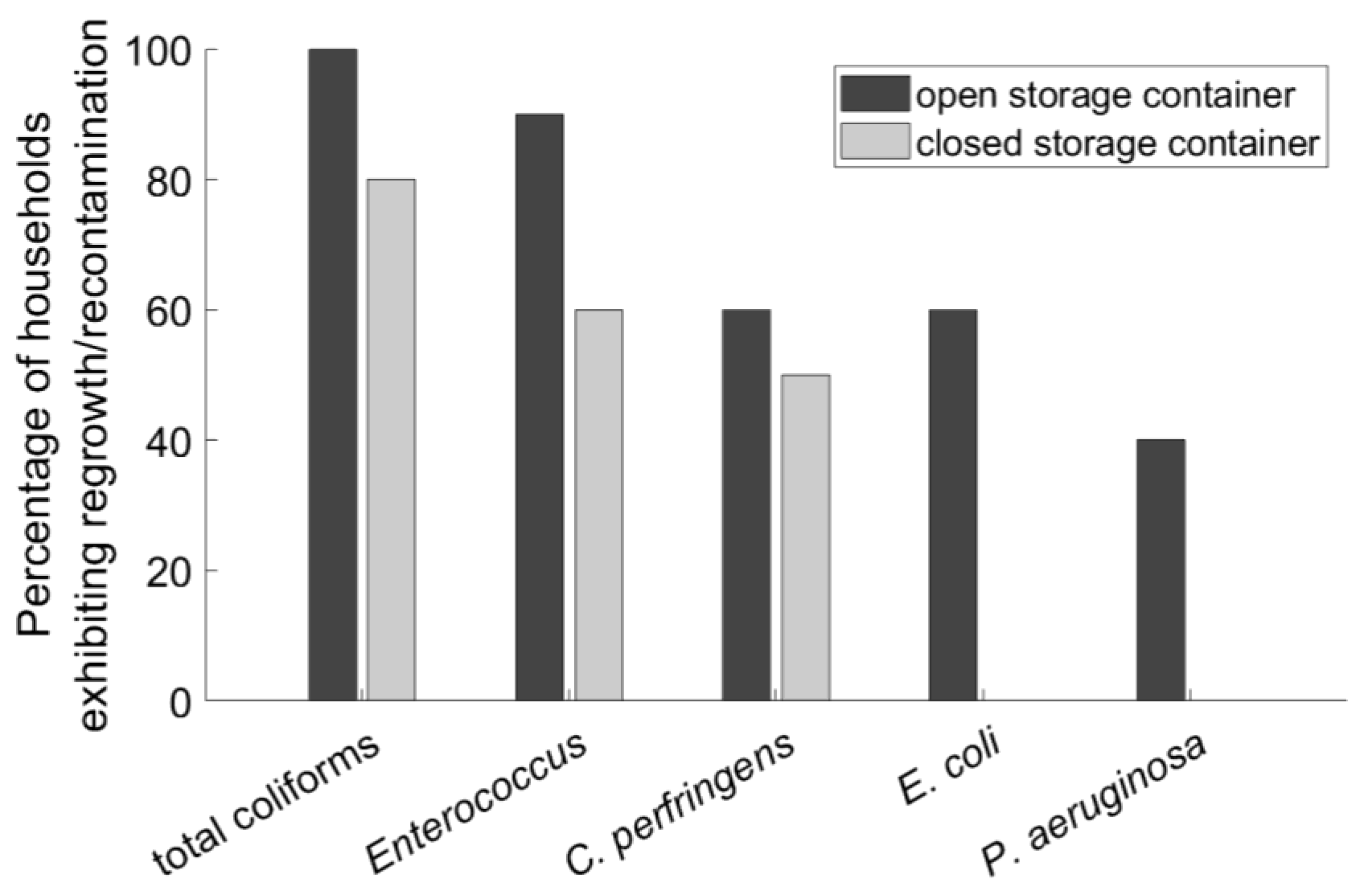
3.4. Correlation of Regrowth/Recontamination with Type of Storage Vessel
3.5. Combined Effects of Water Source and Storage Vessel
3.6. Are Biofilms on Vessel Walls a Source of Recontamination?
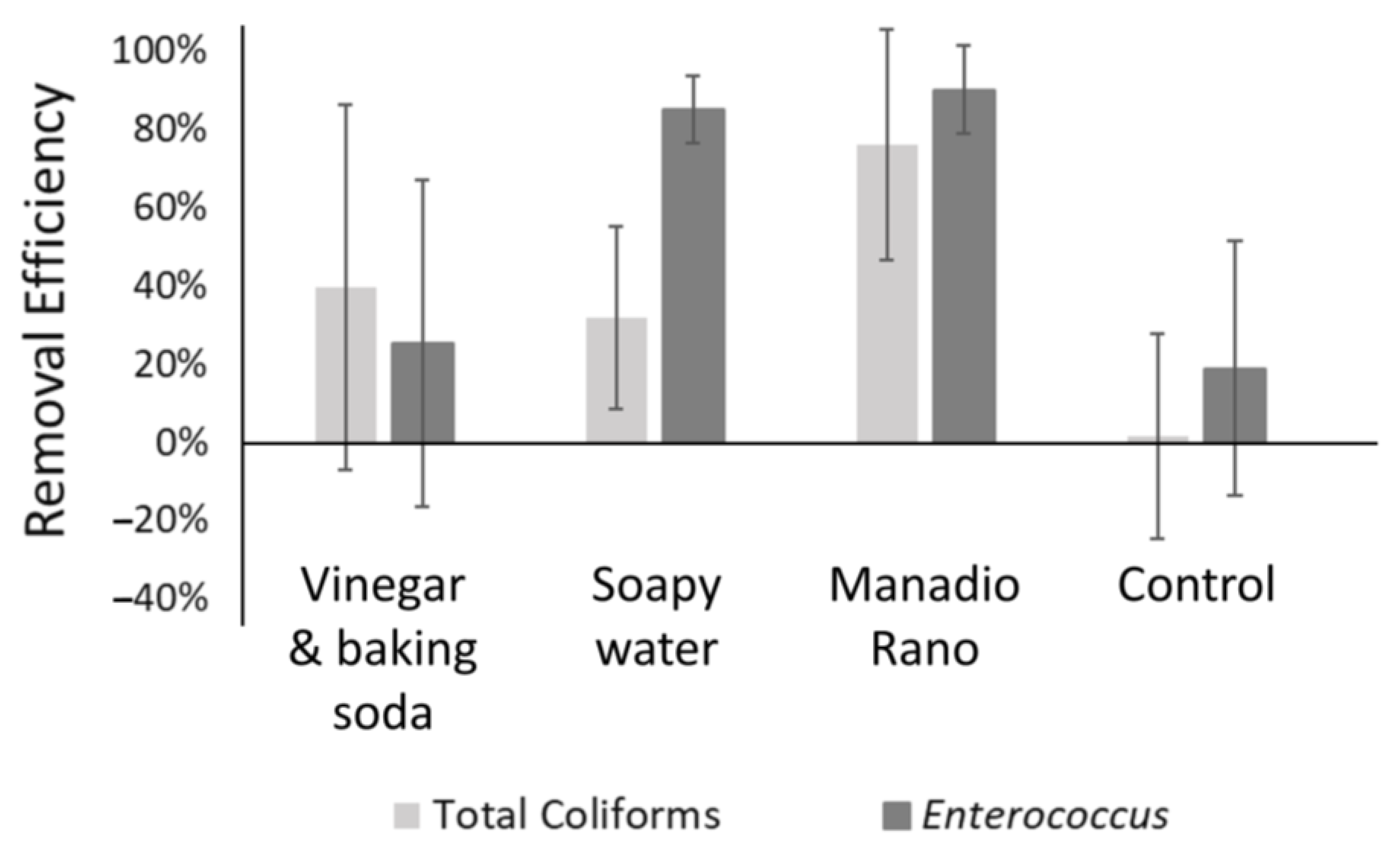
3.7. Efficacy of Locally Available Cleaning Methods
4. Discussion
4.1. Regrowth, Recontamination, or Both?
4.2. Limitations of This Study
4.3. Implications and Recommendations for Household Storage in Toamasina
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oluwasanya, G.; Smith, J.; Carter, R. Self supply systems: Urban dug wells in Abeokuta, Nigeria. Water Sci. Tech. Water Supply 2011, 11, 172–178. [Google Scholar] [CrossRef]
- Foster, T.; Priadi, C.; Kotra, K.K.; Odagiri, M.; Rand, E.C.; Willetts, J. Self-supplied drinking water in low- and middle-income countries in the Asia-Pacific. npj Clean Water 2021, 4, 37. [Google Scholar] [CrossRef]
- Foster, S.; Hirata, R.; Eichholz, M.; Alam, M.-F. Urban self-supply from groundwater—An analysis of management aspects and policy needs. Water 2022, 14, 575. [Google Scholar] [CrossRef]
- Rosa, G.; Kelly, P.; Clasen, T. Consistency of use and effectiveness of household water treatment practices among urban and rural populations claiming to treat their drinking water at home: A case study in Zambia. Am. J. Trop. Med. Hyg. 2016, 94, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Mintz, E.D.; Reiff, F.M.; Tauxe, R.V. Safe water treatment and storage in the home: A practical new strategy to prevent waterborne disease. JAMA 1995, 273, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.E.; Lescano, A.G.; Bern, C.; Calderon, M.M.; Cabrera, L.; Gilman, R.H. Fecal contamination of drinking water within peri-urban households, Lima, Peru. Am. J. Trop. Med. Hyg. 2007, 77, 699–704. [Google Scholar] [CrossRef] [PubMed]
- WEDC (Water Engineering and Development Centre). An Engineer’s Guide to Domestic Water Containers; Loughborough University: Loughborough, UK, 2011. [Google Scholar]
- Mellor, J.E.; Smith, J.A.; Samie, A.; Dillingham, R.A. Coliform sources and mechanisms for regrowth in household drinking water in Limpopo, South Africa. J. Environ. Eng. (ASCE) 2013, 139, 1152–1161. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality, 2nd ed.; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Clasen, T.F.; Bastable, A. Faecal contamination of drinking water during collection and household storage: The need to extend protection to the point of use. J. Water Health 2003, 1, 109–115. [Google Scholar] [CrossRef]
- Trevett, A.F.; Carter, R.; Tyrrel, S. Water quality deterioration: A study of household drinking water quality in rural Honduras. Int. J. Environ. Health Res. 2004, 14, 273–283. [Google Scholar] [CrossRef]
- Wright, J.; Gundry, S.; Conroy, R. Household drinking water in developing countries: A systematic review of microbiological contamination between source and point-of-use. Trop. Med. Int. Health 2004, 9, 106–117. [Google Scholar] [CrossRef]
- Trevett, A.F.; Carter, R.C. Targeting appropriate interventions to minimize deterioration of drinking-water quality in developing countries. J. Health Popul. Nutr. 2008, 26, 125–138. [Google Scholar]
- Levy, K.; Anderson, L.; Robb, K.A.; Cevallos, W.; Trueba, G.; Eisenberg, J.N.S. Household effectiveness vs. laboratory efficacy of point-of-use chlorination. Water Res. 2014, 54, 69–77. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Kaleni, P. Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2002, 36, 3023–3028. [Google Scholar] [CrossRef]
- Murphy, J.L.; Ayers, T.L.; Knee, J.; Oremo, J.; Odhiambo, A.; Faith, S.H.; Nyagol, R.O.; Stauber, C.E.; Lantagne, D.S.; Quick, R.E. Evaluating four measures of water quality in clay pots and plastic safe storage containers in Kenya. Water Res. 2016, 104, 312–319. [Google Scholar] [CrossRef]
- Roberts, L.; Chartier, Y.; Chartier, O.; Malenga, G.; Toole, M.; Rodka, H. Keeping clean water clean in a Malawi refugee camp: A randomized intervention trial. Bull. World Health Organ. 2001, 79, 280–287. [Google Scholar]
- Trevett, A.F.; Carter, R.C.; Tyrrel, S.F. The importance of domestic water quality management in the context of faecal-oral disease transmission. J. Water Health 2005, 3, 259–270. [Google Scholar] [CrossRef]
- Pickering, A.J.; Davis, J.; Walters, S.P.; Horak, H.M.; Keymer, D.P.; Mushi, D.; Strickfaden, R.; Chynoweth, J.S.; Liu, J.; Blum, A.; et al. Hands, water, and health: Fecal contamination in Tanzanian communities with improved, non- networked water supplies. Environ. Sci. Technol. 2010, 44, 3267–3272. [Google Scholar] [CrossRef]
- Jagals, P.; Jagals, C.; Bokako, T.C. The effect of container-biofilm on the microbiological quality of water used from plastic household containers. J. Water Health 2003, 1, 101–108. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Notshe, T.L. The microbiological quality of groundwater-derived drinking water after long storage in household containers in a rural community of South Africa. Aqua 2003, 52, 67–77. [Google Scholar] [CrossRef]
- Bae, S.; Lyons, C.; Onstad, N. A culture-dependent and metagenomic approach of household drinking water from the source to point of use in a developing country. Water Res. X 2019, 2, 100026. [Google Scholar] [CrossRef]
- Jensen, P.K.; Ensink, J.H.J.; Jayasinghe, G.; van der Hoek, W.; Cairncross, S.; Dalsgaard, A. Domestic transmission routes of pathogens: The problem of in-house contamination of drinking water during storage in developing countries. Trop. Med. Int. Health 2002, 7, 604–609. [Google Scholar] [CrossRef]
- CDC (United States Centers for Disease Control and Prevention). Safe Water Systems for the Developing World: A Handbook for Implementing Household-Based Water Treatment and Safe Storage Projects; Department of Health & Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2006.
- About Safe Water Storage. Available online: https://www.cdc.gov/global-water-sanitation-hygiene/about/about-safe-water-storage.html (accessed on 30 May 2024).
- Maraj, S.; Rodda, N.; Jackson, S.; Buckley, C.; Macleod, N. Microbial deterioration of stored water for users supplied by stand-pipes and ground-tanks in a peri-urban community. Water SA 2006, 32, 693–699. [Google Scholar] [CrossRef]
- Harris, A.R.; Davis, J.; Boehm, A.B. Mechanisms of post-supply contamination of drinking water in Bagamoyo, Tanzania. J. Water Health 2013, 11, 543–554. [Google Scholar] [CrossRef]
- Meierhofer, R.; Rubli, P.; Dreyer, K.; Ouma, H.; Wanyama, K.; Peter-Varbanets, M. Membrane filtration reduces recontamination risk in chlorinated household water containers. In Proceedings of the 40th WEDC International Conference, Loughborough, UK, 24–28 July 2017; p. 2595. Available online: https://wedc-knowledge.lboro.ac.uk/resources/conference/40/Meierhofer-2595.pdf (accessed on 5 April 2024).
- Budeli, P.; Moropeng, R.C.; Mpenyana-Monyatsi, L.; Momba, M.N.B. Inhibition of biofilm formation on the surface of water storage containers using biosand zeolite silver-impregnated clay granular and silver impregnated porous pot filtration systems. PLoS ONE 2018, 13, e0194715. [Google Scholar] [CrossRef]
- String, G.M.; Domini, M.; Badr, H.; Brodsky, H.; Kamal, Y.; Tatro, T.; Johnston, M.; Ogudipe, A.; Vu, T.N.; Wolfe, M.K.; et al. Efficacy of locally available cleaning methods and household chlorination at inhibiting biofilm development in jerricans used to store household drinking water. Environ. Sci. Water Res. Technol. 2021, 7, 367–383. [Google Scholar] [CrossRef]
- Burkowska-But, A.; Kalwasinska, A.; Swiontek-Brzezinska, M. Bacterial growth and biofilm formation in household-stored groundwater collected from public wells. J. Water Health 2015, 13, 353–361. [Google Scholar] [CrossRef]
- Meierhofer, R.; Wietlisbach, B.; Matiko, C. Influence of container cleanliness, container disinfection with chlorine, and container handling on recontamination of water collected from a water kiosk in a Kenyan slum. J. Water Health 2019, 17, 308–317. [Google Scholar] [CrossRef]
- Meierhofer, R.; Rubli, P.; Oremo, J.; Odhiambo, A. Does activated silver reduce recontamination risks in the reservoirs of ceramic water filters? Water 2019, 11, 1108. [Google Scholar] [CrossRef]
- String, G.; Domini, M.; Mirindi, P.; Brodsky, H.; Kamal, Y.; Tatro, T.; Johnston, M.; Badr, H.; Lantagne, D. Efficacy of locally available cleaning methods in removing biofilms from taps and surfaces of household water storage containers. npj Clean Water 2020, 3, 13. [Google Scholar] [CrossRef]
- Steele, A.; Clarke, B.; Watkins, O. Impact of jerry can disinfection in a camp environment—Experiences in an IDP camp in northern Uganda. J. Water Health 2008, 6, 559–564. [Google Scholar] [CrossRef]
- Gärtner, N.; Germann, L.; Wanyama, K.; Ouma, H.; Meierhofer, R. Keeping water from kiosks clean: Strategies for reducing recontamination during transport and storage in eastern Uganda. Water Res. X 2021, 10, 100079. [Google Scholar] [CrossRef]
- Poulin, C.; Trimmer, J.; Delaire, C.; Peletz, R. Pathogen Pathways Study for Children Under Two Years in the USAID Fiovana Intervention Areas of Southeastern Madagascar; The Aquaya Institute: Nairobi, Kenya, 2022. [Google Scholar]
- Allali, I.; Abotsi, R.E.; Tow, L.A.; Thabane, L.; Zar, H.J.; Mulder, N.M.; Nicol, M.P. Human microbiota research in Africa: A systematic review reveals gaps and priorities for future research. Microbiome 2021, 9, 241. [Google Scholar] [CrossRef]
- Muhammad, F. The 21st-century microbiology profession and professionals’ challenges in African countries. J. Microbiota 2024, e145808. [Google Scholar] [CrossRef]
- WHO (World Health Organization); UNICEF (United Nations Children’s Fund). Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- USAID (United States Agency for International Development). Madagascar Water and Sanitation Profile. 2010. Available online: https://www.washplus.org/sites/default/files/madagascar2010.pdf (accessed on 8 April 2024).
- WHO (World Health Organization); UNICEF (United Nations Children’s Fund). Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Diorano-WASH. Available online: https://www.comminit.com/global/content/diorano-wash (accessed on 3 March 2024).
- WSSCC (Water Supply and Sanitation Collaborative Council). Madagascar: WaSH Case Study Series; WSSCC: Geneva, Switzerland, 2008; Available online: https://www.ircwash.org/sites/default/files/WSSCC-2008-Madagascar.pdf (accessed on 8 April 2024).
- Judah, L. Assessment and Prevention of Bacterial Regrowth in Stored Household Water in Eastern Coastal Madagascar. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2022. Available online: https://digitalcommons.usf.edu/etd/9786/ (accessed on 20 May 2024).
- The Public Health Laboratory Service Water Sub-Committee. The effect of sodium thiosulphate on the coliform and Bacterium coli counts of non-chlorinated water samples. J. Hyg. 1953, 51, 572–577. [Google Scholar]
- Murray, A.L.; Kumpel, E.; Peletz, R.; Khush, R.S.; Lantagne, D.S. The effect of sodium thiosulfate dechlorination on fecal indicator bacteria enumeration: Laboratory and field data. J. Water Health 2018, 16, 70–77. [Google Scholar] [CrossRef]
- Momba, M.; Edbon, J.; Kamika, I.; Verbyla, M. Using indicators to assess microbial treatment and disinfection efficacy. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Rose, J.B., Jiménez-Cisneros, B., Eds.; Part 2: Indicators and Microbial Source Tracking Markers; UNESCO: Paris, France; Michigan State University: East Lansing, MI, USA, 2019. [Google Scholar]
- Kohn, T.; Decrey, L.; Vinneras, B. Chemical disinfectants. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Rose, J.B., Jiménez-Cisneros, B., Eds.; Part 4: Management of Risk from Excreta and Wastewater—Section: Disinfection; UNESCO, Michigan State University:: East Lansing, MI, USA, 2019. [Google Scholar]
- Pruden, A.; Ashbolt, N.; Miller, J. Overview of issues for water bacterial pathogens. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Rose, J.B., Jiménez-Cisneros, B., Eds.; Part 3: Specific Excreted Pathogens: Environmental and Epidemiology Aspects—Section 2: Bacteria; UNESCO: Paris, France; Michigan State University: East Lansing, MI, USA, 2019. [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B.; Eaton, A.D.; Rice, E.W. (Eds.) American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- MacWilliams, M.P. Indole Test Protocol; American Society for Microbiology: Washington, DC, USA, 2009; Available online: https://asm.org/getattachment/200d3f34-c75e-4072-a7e6-df912c792f62/indole-test-protocol-3202.pdf (accessed on 3 March 2023).
- Smith, A.C.; Hussey, M.A. Gram Stain Protocols; American Society for Microbiology: Washington, DC, USA, 2005; Available online: https://asm.org/getattachment/5c95a063-326b-4b2f-98ce-001de9a5ece3/gram-stain-protocol-2886.pdf (accessed on 3 March 2023).
- Mfuh, K.O.; Abanda, N.N.; Titanji, B.K. Strengthening diagnostic capacity in Africa as a key pillar of public health and pandemic preparedness. PLoS Glob. Public Health 2023, 3, e0001998. [Google Scholar] [CrossRef]
- Daniel, D.; Diener, A.; van de Vossenberg, J.; Bhatta, M.; Marks, S.J. Assessing drinking water quality at the point of collection and within household storage containers in the hilly rural areas of mid- and far-western Nepal. Int. J. Environ. Res. Public Health 2020, 17, 2172. [Google Scholar] [CrossRef]
- Dey, A.; Bokka, V.; Sen, S. Dependence of bacterial growth rate on dynamic temperature changes. IET Syst. Biol. 2020, 14, 68–74. [Google Scholar] [CrossRef]
- Vital, M.; Stucki, D.; Egli, T.; Hammes, F. Evaluating the growth potential of pathogenic bacteria in water. Appl. Environ. Microbiol. 2010, 76, 6477–6484. [Google Scholar] [CrossRef]
- MacCarthy, M.F.; Annis, J.; Mihelcic, J.R. Unsubsidised self-supply in eastern Madagascar. Water Altern. 2013, 6, 424–438. [Google Scholar]
- Agensi, A.; Tibyangye, J.; Tamale, A.; Agwu, E.; Amongi, C. Contamination potentials of household water handling and storage practices in Kirundo Subcounty, Kisoro District, Uganda. J. Environ. Public Health 2019, 7932193. [Google Scholar] [CrossRef] [PubMed]
- PROJECT Sur’Eau (PSE), Final Report (September 15, 2000 through December 14, 2002); PSI Madagascar: Antananarivo, Madagascar, 2003. Available online: https://pdf.usaid.gov/pdf_docs/Pdaby045.pdf (accessed on 11 April 2024).
- Ram, P.K.; Kelsey, E.; Rasoatiana; Miarintsoa, R.R.; Rakotomalala, O.; Dunston, C.; Quick, R.E. Bringing safe water to remote populations: An evaluation of a portable point-of-use intervention in rural Madagascar. Am. J. Public Health 2007, 97, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Praz, V.; Morton, A.; Matondo, I. End-of-Project Evaluation of the PSI Social Marketing Project in Madagascar; United States Agency for International Development and International Business & Technical Consultants, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Potgieter, N.; Becker, P.J.; Ehlers, M.M. Evaluation of the CDC safe water-storage intervention to improve the microbiological quality of point-of-use drinking water in rural communities in South Africa. Water SA 2009, 35, 505–516. [Google Scholar] [CrossRef]


| Average Increase in Bacterial Concentration in Households Using Open Storage Vessels (n = 10) | Average Increase in Bacterial Concentration in Households Using Closed Storage Vessels (n = 10) | |
|---|---|---|
| Total coliforms | 7500 CFU/(100 mL) | 16,000 CFU/(100 mL) |
| Enterococcus | 280 CFU/(100 mL) | 2300 CFU/(100 mL) |
| C. perfringens | 2 CFU/(100 mL) | 2 CFU/(100 mL) |
| E. coli * | 270 CFU/(100 mL) | 0 CFU/(100 mL) |
| P. aeruginosa * | 2 CFU/(100 mL) | –1 CFU/(100 mL) |
| Fecal Indicator | Number of Jerrycans with Fecal Indicator Present on Internal Jerrycan Walls (Out of 4 Jerrycans Tested) |
|---|---|
| Total coliforms | 4 |
| Enterococcus | 4 |
| C. perfringens | 3 |
| E. coli | 1 |
| P. aeruginosa | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judah, L.A.; Andriambololonirina, C.; Rakotoarisoa, L.; Barrett, L.J.P.; Khaliq, M.; Mihelcic, J.R.; Cunningham, J.A. Occurrence and Mitigation of Bacterial Regrowth in Stored Household Water in Eastern Coastal Madagascar. Water 2024, 16, 1592. https://doi.org/10.3390/w16111592
Judah LA, Andriambololonirina C, Rakotoarisoa L, Barrett LJP, Khaliq M, Mihelcic JR, Cunningham JA. Occurrence and Mitigation of Bacterial Regrowth in Stored Household Water in Eastern Coastal Madagascar. Water. 2024; 16(11):1592. https://doi.org/10.3390/w16111592
Chicago/Turabian StyleJudah, Lauren A., Cathy Andriambololonirina, Lova Rakotoarisoa, Luke John Paul Barrett, Mahmooda Khaliq, James R. Mihelcic, and Jeffrey A. Cunningham. 2024. "Occurrence and Mitigation of Bacterial Regrowth in Stored Household Water in Eastern Coastal Madagascar" Water 16, no. 11: 1592. https://doi.org/10.3390/w16111592
APA StyleJudah, L. A., Andriambololonirina, C., Rakotoarisoa, L., Barrett, L. J. P., Khaliq, M., Mihelcic, J. R., & Cunningham, J. A. (2024). Occurrence and Mitigation of Bacterial Regrowth in Stored Household Water in Eastern Coastal Madagascar. Water, 16(11), 1592. https://doi.org/10.3390/w16111592







