Demonstration of Proactive Algaecide Treatments Targeting Overwintering Cyanobacteria in Sediments of an Urban Pond
Abstract
1. Introduction
2. Materials and Methods
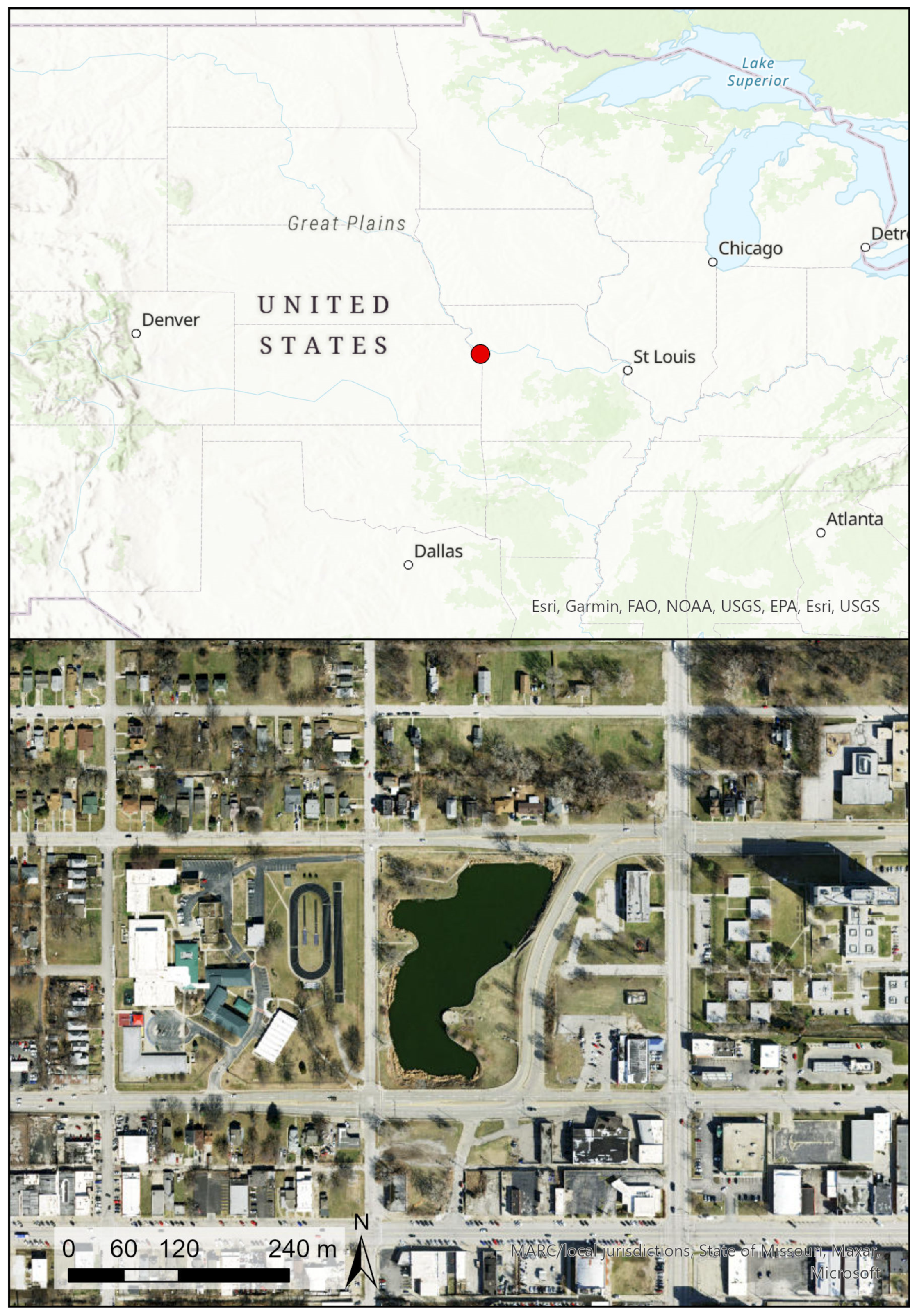
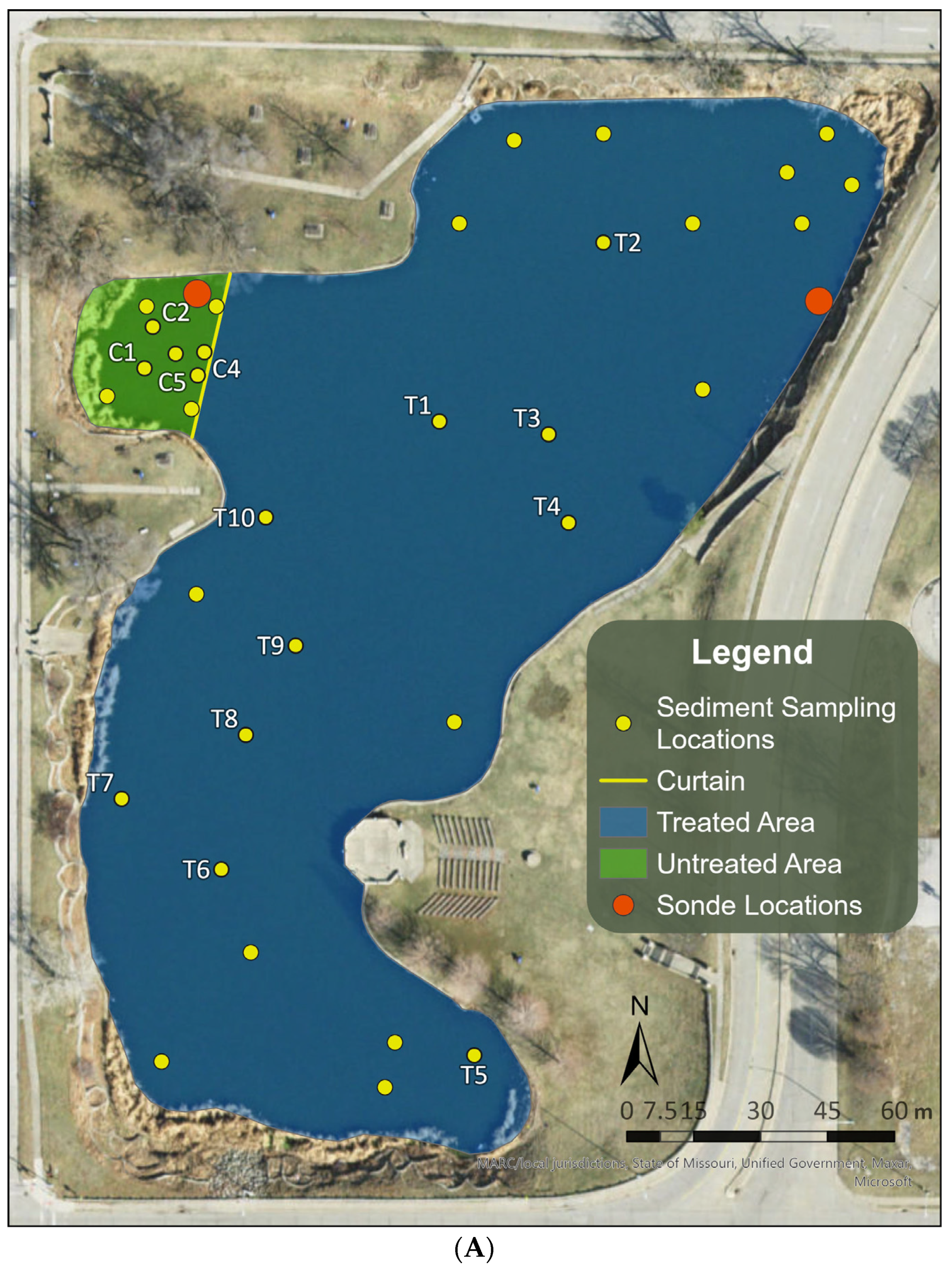
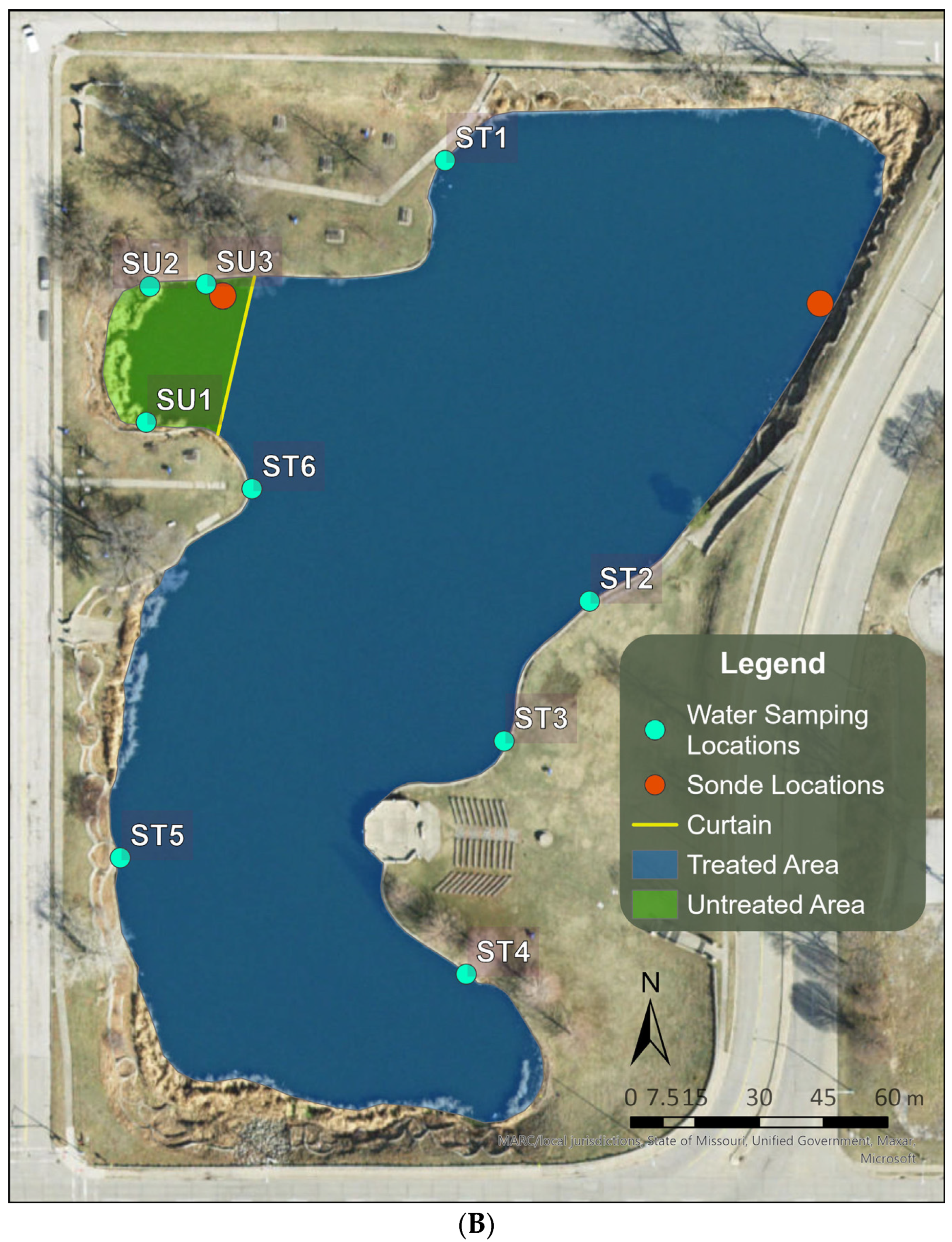
2.1. Selection and Description of the Study Site
2.2. Proactive Algaecide Treatment
2.3. Short-Term Responses of Overwintering Cells in Sediments
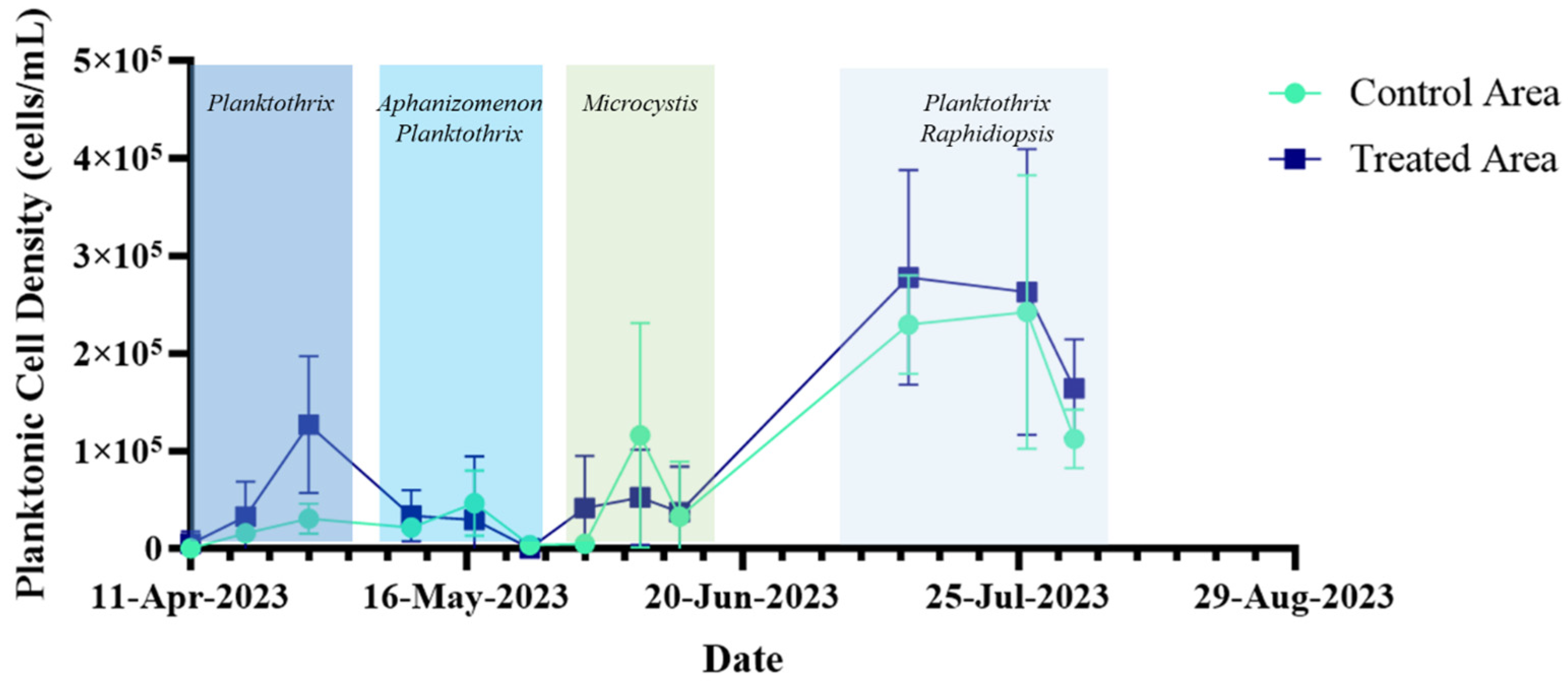
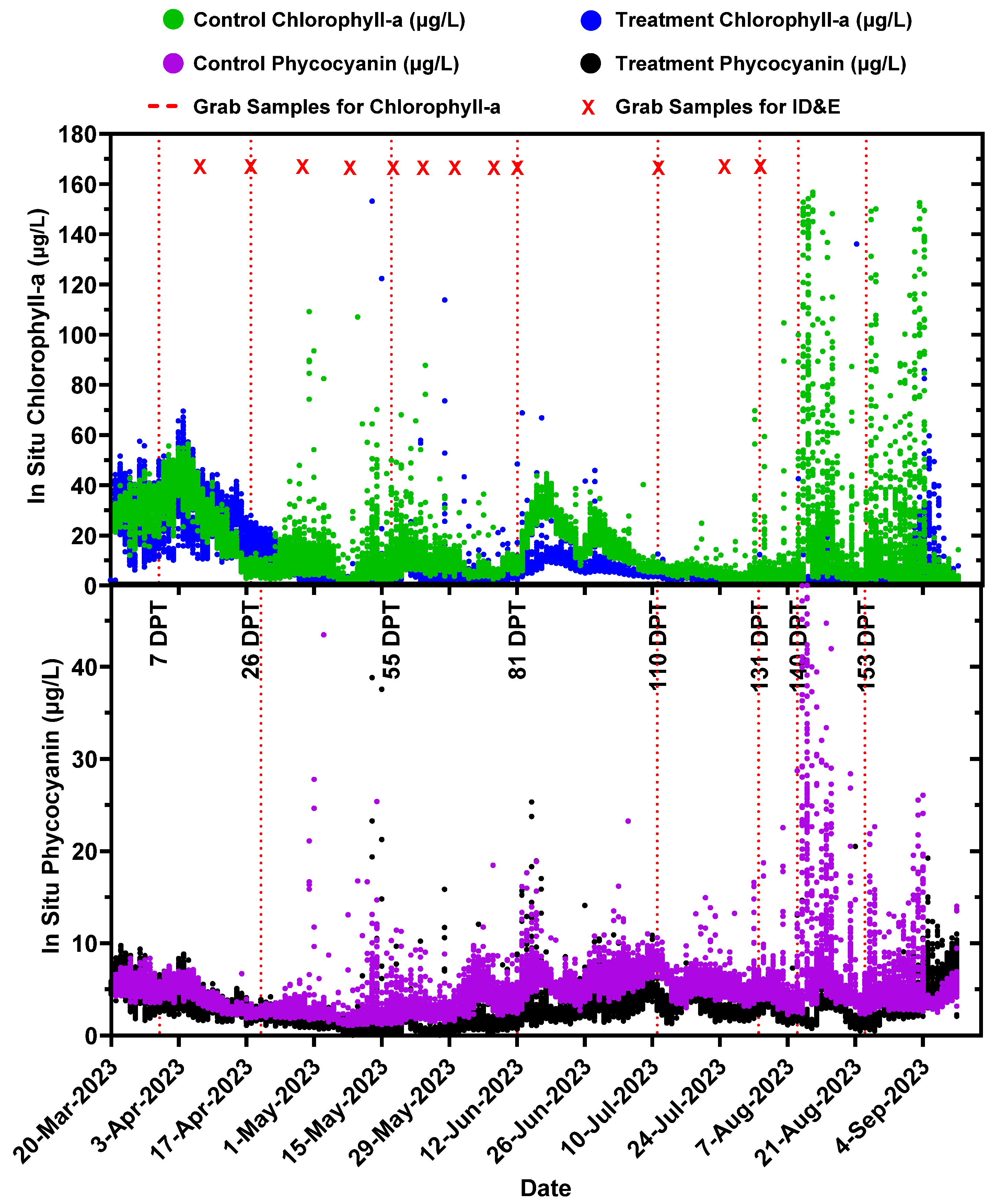
2.4. Long-Term Performance Monitoring of Planktonic HAB Intensity and Duration
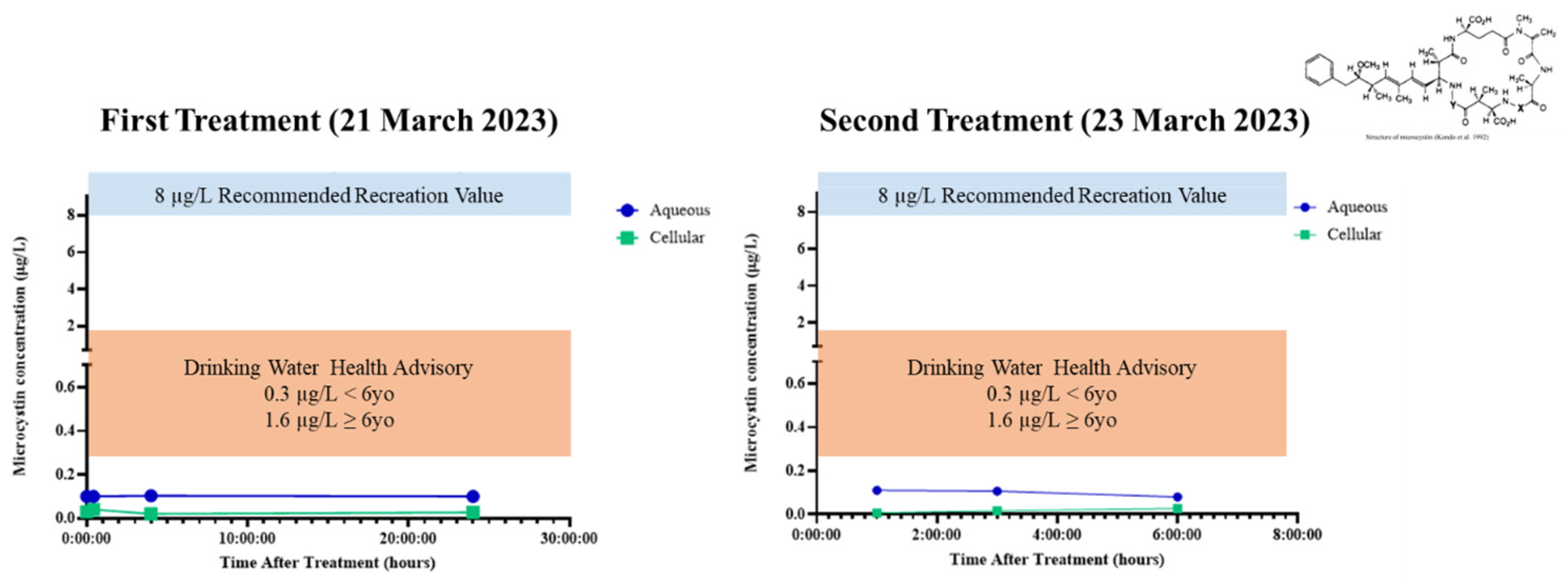
2.5. Microcystin Release and Delayed Degradation
2.6. Statistical Analyses
3. Results and Discussion
3.1. Algaecide Treatments
3.2. Short-term Overwintering Cell Responses in Sediment
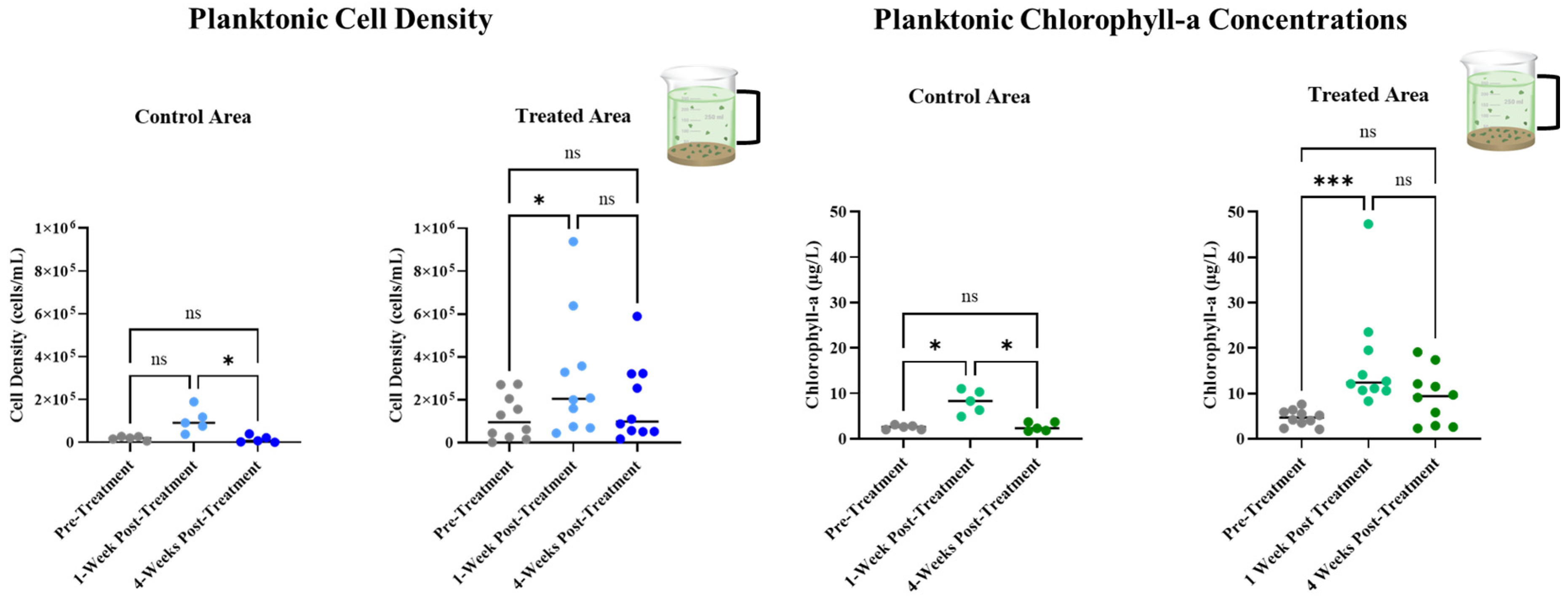
3.3. Long-Term Monitoring of Planktonic HAB Duration and Intensity
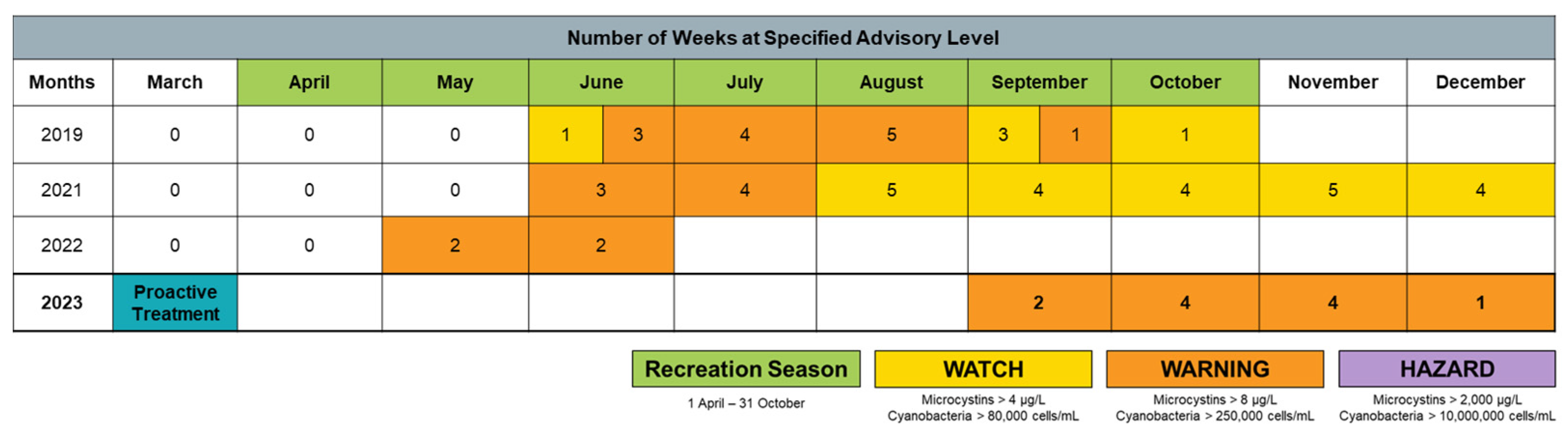
3.4. Weight-of-Evidence—Performance of Proactive Algaecide Application
- Planktonic cyanobacteria densities, chlorophyll-a, and microcystin concentrations from shoreline grab samples collected throughout the active HAB season;
- Historical active advisory data;
- Planktonic algal pigment (phycocyanin and chlorophyll-a) concentrations from in situ sondes;
- Planktonic transfer potential results of the 14 d incubation of site-collected sediments containing overwintering cells post-treatment;
- Overwintering cell densities in sediments.
3.5. Microcystin Release and Delayed Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Summary Report—One Health Harmful Algal Blooms System (OHHABS); CDC: Atlanta, GA, USA, 2021.
- de la Cruz, A.; Logsdon, R.; Lye, D.; Guglielmi, S.; Rice, A.; Kannan, M.S. Harmful Algae Bloom Occurrence in Urban Ponds: Relationship of Toxin Levels with Cell Density and Species Composition. J. Earth Environ. Sci. 2017, 25, 704–726. [Google Scholar] [CrossRef]
- Grogan, A.E.; Alves-de-Souza, C.; Cahoon, L.B.; Mallin, M.A. Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds. Water 2023, 15, 2436. [Google Scholar] [CrossRef]
- Interstate Technology& Regulatory Council (ITRC). Strategies for Preventing and Managing Harmful Cyanobacterial Blooms (HCB-1); Interstate Technology& Regulatory Council, HCB Team: Washington, DC, USA, 2020. [Google Scholar]
- Calomeni, A.J.; McQueen, A.D.; Kinley-Baird, C.M.; Clyde, G.A. Identification and Preventative Treatment of Overwintering Cyanobacteria in Sediments: A Literature Review; RDC/EL TR-22-10; U.S. Army Engineer Research and Development Center (U.S.): Vicksburg, MI, USA, 2022. [Google Scholar]
- Kim, B.H.L. Relationship between akinete germination and vegetative population of Anabaena flos-aquae (Nostocales, Cyanobacteria) in Seokchon reservoir (Seoul, Korea). Arch. Hydrobiol. 2005, 163, 49–64. [Google Scholar] [CrossRef]
- Cirés, S.; Wörmer, L.; Agha, R.; Quesada, A. Overwintering populations of Anabaena, Aphanizomenon and Microcystis as potential inocula for summer blooms. J. Plankton Res. 2013, 35, 1254–1266. [Google Scholar] [CrossRef]
- Kitchens, C.M.; Johengen, T.H.; Davis, T.W. Establishing spatial and temporal patterns in Microcystis sediment seed stock viability and their relationship to subsequent bloom development in Western Lake Erie. PLoS ONE 2018, 13, e0206821. [Google Scholar] [CrossRef]
- Karnik, A.; Shu, B.; Habib, J.; Galvan, J.; Rappuhn, N. Quantifying the Role of Microcystis Resuspension on Harmful Algal Blooms in Coastal Lake Erie Using Multidisciplinary Approaches. Master’s Thesis, University of Michigan, Ann Arbor, MI, USA, 2023. [Google Scholar]
- Calomeni, A.; McQueen, A.; Kinley-Baird, C.; Clyde Jr, G.; Gusler, G.; Boyer, M.; Smith, E.F. Efficacy of algaecides for the proactive treatment of overwintering cyanobacteria. Ecotoxicol. Environ. Saf. 2023, 262, 115187. [Google Scholar] [CrossRef] [PubMed]
- Kinley-Baird, C.M.; Smith, E.F.; Calomeni, A.J.; McQueen, A.D.; Gusler, G.O.; Boyer, M.; Decker, K.N.; Clyde, G.A. Evaluation of preventative algaecide treatments for cyanobacterial resting cells in sediments of a central US lake. Lake Reserv. Manag. 2023, 39, 340–355. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, Z.; Su, W.; Johnson, D.; Kong, F. Controlling of cyanobacteria bloom during bottleneck stages of algal cycling in shallow Lake Taihu (China). J. Freshw. Ecol. 2013, 29, 129–140. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.; Hadas, O.; Summers, M.; Rucker, J.; Sukenik, A. Akinetes: Dormant Cells of Cyanobacteria. In Dormancy and Resistance in Harsh Environments; Lubsens, E., Clark, M., Cerda, J., Eds.; Springer: New York, NY, USA, 2010; pp. 5–28. [Google Scholar]
- Calomeni, A.J.; McQueen, A.; Kinley-Baird, C.; Clyde, G., Jr. Identification and prioritization of sites with overwintering cyanobacteria to inform preventative management of harmful algal blooms. J. Aquat. Plant Manag. 2023, 61, 30–41. [Google Scholar] [CrossRef]
- Calomeni, A.; Rodgers, J.H.; Kinley, C.M. Responses of Planktothrix agardhii and Pseudokirchneriella subcapitata to Copper Sulfate (CuSO4·5H2O) and a Chelated Copper Compound (Cutrine®-Ultra). Water Air Soil Pollut. 2014, 225, 2231. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Ziegler, A.C.; Meyer, M.T. Guidelines for Design and Sampling for Cyanobacterial Toxin and Taste-and-Odor Studies in Lakes and Reservoirs: USGS Scientific Investigations Report; 2008-5038; United States Geological Survey: Reston, VA, USA, 2008; pp. 1–52.
- Cameron, E.S.; Krishna, A.; Emelko, M.B.; Muller, K.M. Sporadic diurnal fluctuations of cyanobacterial populations in oligotrophic temperate systems can prevent accurate characterization of change and risk in aquatic systems. Water Res. 2024, 252, 121199. [Google Scholar] [CrossRef]
- Schaefer, A.M.; Hanisak, M.D.; McFarland, M.; Sullivan, J.M. Integrated observing systems: An approach to studying harmful algal blooms in south Florida. J. Oper. Oceanogr. 2019, 12, S187–S198. [Google Scholar] [CrossRef]
- Sharp, S.L.; Forrest, A.L.; Bouma-Gregson, K.; Jin, Y.; Cortés, A.; Schladow, S.G. Quantifying Scales of Spatial Variability of Cyanobacteria in a Large, Eutrophic Lake Using Multiplatform Remote Sensing Tools. Front. Environ. Sci. 2021, 9, 612934. [Google Scholar] [CrossRef]
- Babica, P.; Kohoutek, J.; Blaha, L.; Adamovsky, O.; Marsalek, B. Evaluation of extraction approaches linked to ELISA and HPLC for analyses of microcystin-LR, -RR and -YR in freshwater sediments with different organic material contents. Anal. Bioanal. Chem. 2006, 385, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Misson, B.; Sabart, M.; Amblard, C.; Latour, D. Benthic survival of Microcystis: Long-term viability and ability to transcribe microcystin genes. Harmful Algae 2012, 13, 20–25. [Google Scholar] [CrossRef]
- Iwinski, K.J.; Calomeni, A.J.; Geer, T.D.; Rodgers, J.H., Jr. Cellular and aqueous microcystin-LR following laboratory exposures of Microcystis aeruginosa to copper algaecides. Chemosphere 2016, 147, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, L.; Peng, L.; Wan, N.; Zhang, X.; Gan, N. Reduction in microcystin concentrations in large and shallow lakes: Water and sediment-interface contributions. Water Res. 2008, 42, 763–773. [Google Scholar] [CrossRef]
- Kinley, C.M.; Iwinski-Wood, K.J.; Geer, T.D.; Hendrikse, M.; McQueen, A.D.; Calomeni, A.J.; Liang, J.; Friesen, V.; Simair, M.C.; Rodgers, J.H. Microcystin-LR Degradation Following Copper-Based Algaecide Exposures. Water Air Soil Pollut. 2018, 229, 62. [Google Scholar] [CrossRef]
- Kansole, M.; Lin, T.-F. Microcystin-LR Biodegradation by Bacillus sp.: Reaction Rates and Possible Genes Involved in the Degradation. Water 2016, 8, 508. [Google Scholar] [CrossRef]
- Library, K.C.P. Photograph of Big Eleven Lake from Kansas City Star Collection. Available online: https://kchistory.org/image/big-eleven-lake-0 (accessed on 10 March 2023).
- Zamyadi, A.; Choo, F.; Newcombe, G.; Stuetz, R.; Henderson, R. A review of monitoring technologies for real-time mangaement of cyanobacteria: Recent advances and future direction. TrAC Trends Anal. Chem. 2016, 85, 83–96. [Google Scholar] [CrossRef]
- Iwinski, K.J.; Rodgers, J.H., Jr.; Kinley, C.M.; Hendrikse, M.; Calomeni, A.J.; McQueen, A.D.; Geer, T.D.; Liang, J.; Friesen, V.; Haakensen, M. Influence of CuSO4 and chelated copper algaecide exposures on biodegradation of microcystin-LR. Chemosphere 2017, 174, 538–544. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by ADDA Enxyme-Linked Immunosorbent Assay; U.S. EPA: Washington, DC, USA, 2016.
- Geer, T.D.; Calomeni, A.J.; Kinley, C.M.; Iwinski, K.J.; Rodgers, J.H. Predicting In Situ Responses of Taste- and Odor-Producing Algae in a Southeastern US Reservoir to a Sodium Carbonate Peroxyhydrate Algaecide Using a Laboratory Exposure-Response Model. Water Air Soil Pollut. 2017, 228, 53. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS). WaterWatch-Table of Computed Runoff by Water-Year for Kansas. Available online: https://waterwatch.usgs.gov/index.php?r=ks&id=statesum (accessed on 20 February 2024).
- Paerl, H.W.; Havens, K.; Hall, N.; Otten, T.; Zhu, M.; Xu, H.; Zhu, G.; Qin, B. Mitigating a global expansion of toxic cyanobacterial blooms: Confounding effects and challenges posed by climate change. Mar. Freshw. Res. 2019, 71, 579–592. [Google Scholar] [CrossRef]
- Mosley, L. Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci. Rev. 2015, 140, 203–214. [Google Scholar] [CrossRef]
- Linkov, I.; Loney, D.; Cormier, S.; Satterstrom, F.K.; Bridges, T. Weight-of-evidence evaluation in environmental assessment: Review of qualitative and quantitative approaches. Sci. Total Environ. 2009, 407, 5199–5205. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Drinking Water Health Advisory for the Cyanobacterial Microcystin Toxins; EPA_820R15100; Office of Water: Washington, DC, USA, 2015.
- U.S. Environmental Protection Agency (USEPA). Recommended Human Health Recreational Ambient Water Quality Criteria of Swimming Advisories for Microcystins and Cylindrospermopsin; EPA 822-R-19-001; United States Environmental Protection Agency-Office of Water: Washington, DC, USA, 2019; p. 249.


| Lines of Evidence | Outcome |
|---|---|
| Planktonic cyanobacteria densities, chlorophyll-a concentrations, and microcystin concentrations from grab samples collected throughout the active HAB season |  |
| Historical active advisory data |  |
| Planktonic algal pigment concentrations from sondes collected throughout the active HAB season |  |
| Planktonic cell densities following 14d incubation of sediments collected immediately post-treatment |  |
| Overwintering cell densities in sediments collected immediately post-treatment |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calomeni-Eck, A.; McQueen, A.; Kinley-Baird, C.; Smith, E.; Growcock, B.; Decker, K.; Hampton, S.; Stahl, A.; Boyer, M.; Clyde, G., Jr. Demonstration of Proactive Algaecide Treatments Targeting Overwintering Cyanobacteria in Sediments of an Urban Pond. Water 2024, 16, 1624. https://doi.org/10.3390/w16111624
Calomeni-Eck A, McQueen A, Kinley-Baird C, Smith E, Growcock B, Decker K, Hampton S, Stahl A, Boyer M, Clyde G Jr. Demonstration of Proactive Algaecide Treatments Targeting Overwintering Cyanobacteria in Sediments of an Urban Pond. Water. 2024; 16(11):1624. https://doi.org/10.3390/w16111624
Chicago/Turabian StyleCalomeni-Eck, Alyssa, Andrew McQueen, Ciera Kinley-Baird, Elizabeth Smith, Benjamin Growcock, Katlynn Decker, Schad Hampton, Anthony Stahl, Marvin Boyer, and Gerard Clyde, Jr. 2024. "Demonstration of Proactive Algaecide Treatments Targeting Overwintering Cyanobacteria in Sediments of an Urban Pond" Water 16, no. 11: 1624. https://doi.org/10.3390/w16111624
APA StyleCalomeni-Eck, A., McQueen, A., Kinley-Baird, C., Smith, E., Growcock, B., Decker, K., Hampton, S., Stahl, A., Boyer, M., & Clyde, G., Jr. (2024). Demonstration of Proactive Algaecide Treatments Targeting Overwintering Cyanobacteria in Sediments of an Urban Pond. Water, 16(11), 1624. https://doi.org/10.3390/w16111624








