Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants
Abstract
:1. Introduction
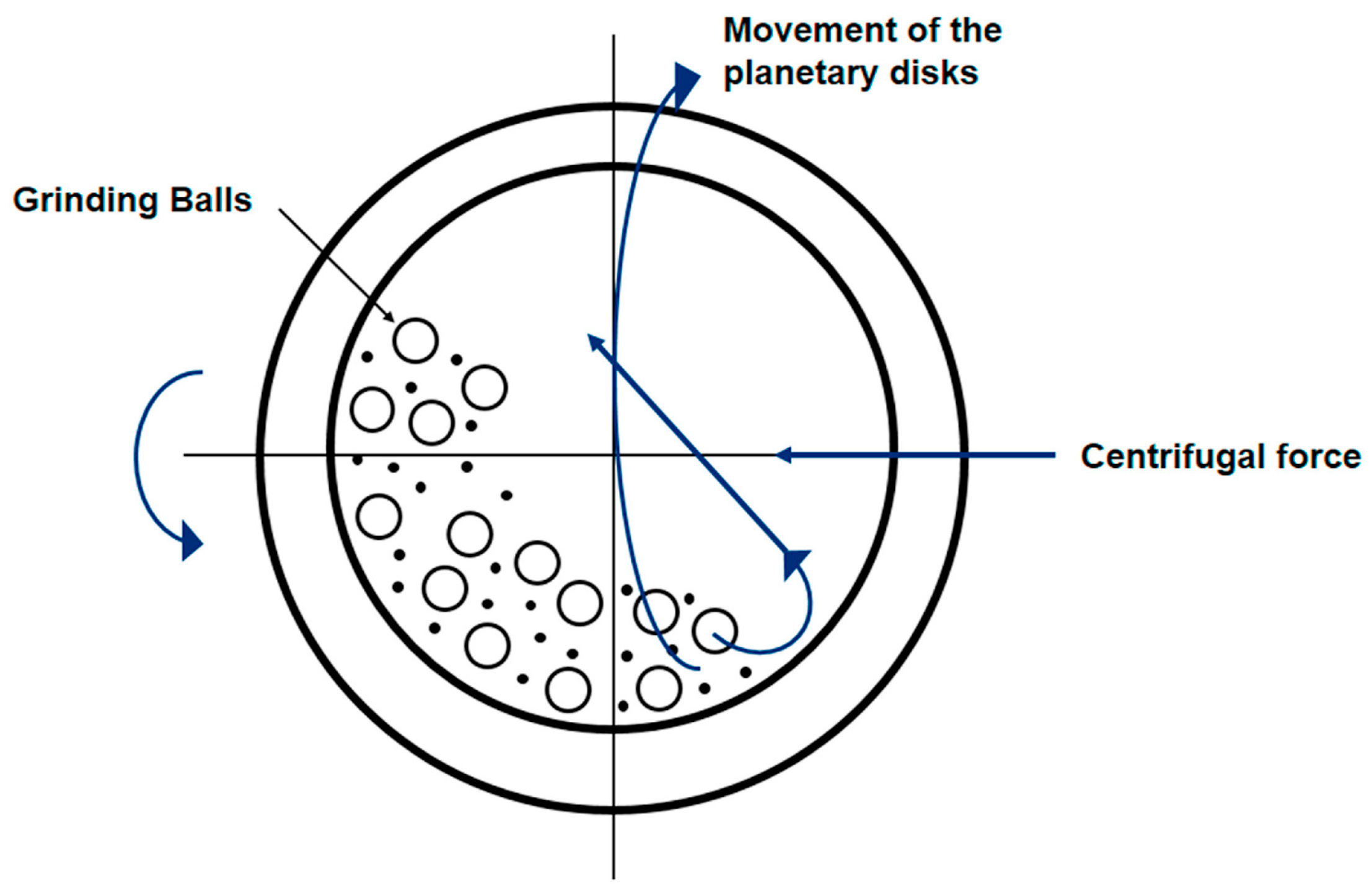
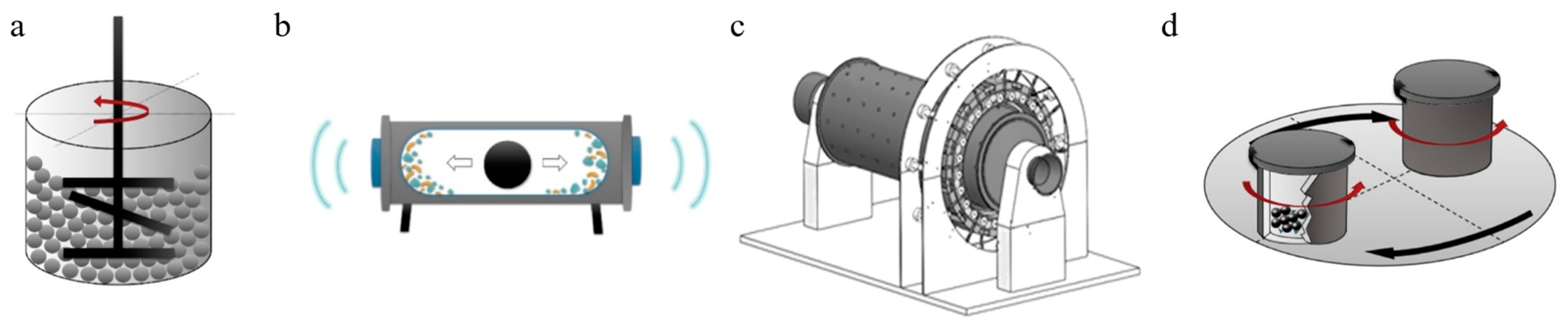
2. Principles and Mechanisms of Ball Milling
3. Ball-Milled Materials
3.1. Ball-Milled Commercial Activated Carbons
3.2. Ball-Milled Biochar-Based Materials
3.3. Ball-Milled CNTs-Based Materials
3.4. Ball-Milled Graphene-Based Materials
3.5. Ball-Milled ZVIs-Based Materials
3.6. Ball-Milled ZVAIs-Based Materials
4. Application of Ball-Milled Adsorbents for Water Purification
4.1. Removal of Inorganic Pollutants
| Materials | Ball Milling Treatment | Material Characterization | Pollutants | Adsorption Experiment | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Condition | Isotherm Model | Adsorption Capacity | Kinetic Model | Equilibrium Time | Adsorption Mechanism | |||||
| Ball-milled ACs-based materials | ||||||||||
| ZVIs-ACs | P, BM: ACs (300 mg) +ZVIs (5.6 mg), ZrO2 balls (Φ = 6 and 10 mm, MR = 4:1), RS = 300 rpm, MT = 30 min, air atmosphere | S = 16.9 m2/g, Fe = 69.4%, C = 27.3%, O = 2.9%, P = 0.4% | Cr(VI) | T = 25 ± 2 °C, pH = 3.93 | Langmuir model | 14.35 mg/g a | PSO | 2 h | Pore filling | [8] |
| HSACs | P, BM: ACs (10 g), steel balls (Φ = 5 mm), RS = 300 rpm, MT = 60 min | S = 929 m2/g, APS = 4 μm, D = 15.3 Å, AFGs = 1.84 mmol/g, carboxyl = 0.97 mmol/g, phenolic, hydroxyl, and lactols = 0.87 mmol/g, C = 91.24%, N = 0.93%, O = 6.86%, S = 0.97%, zeta potential pH = 2–10≈−22.5~−37.5 mV ACs: S = 846 m2/g, APS = 20 μm, D = 19.0 Å, AFGs = 1.31 mmol/g, carboxyl = 0.31 mmol/g, phenolic, hydroxyl, lactols = 1.00 mmol/g, zeta potential (pH = 2–10) ≈−15~−23 mV | Cr(VI) | T = 295–313 K, pH = 6 | Freundlich model | 3.843–5.523 (mg/g)/(mg/L)1/n b ACs: 0.002–0.441 (mg/g)/(mg/L)1/n b | PSO | 2 h | Complexation | [9] |
| Ball-milled biochar-based materials | ||||||||||
| BM-BCs | P, BM: biochars (1.8 g), agate balls (Φ = 6 mm, 180 g), RS = 300 rpm; MT = 3–24, TA = 0.5 h, air atmosphere | S = 364 m2/g, V = 0.125 cm3/g, D = 3.4 nm, hydrodynamic radius = 140 nm, TAG = 2.5 mmol/g, carboxyl = 1.1 mmol/g, lactols groups = 0.5 mmol/g; phenolic hydroxyl = 0.9 mmol/g, pHpzc < 1.6 Biochars: S = 359 m2/g, V = 0.009 cm3/g, D = 3.6 nm, grain size = 0.5–1 mm; TAG = 0.8 mmol/g, carboxyl = 0.5 mmol/g, lactols groups = 0 mmol/g, phenolic hydroxyl = 0.3 mmol/g, pHpzc ≈ 4.0 | Ni(II) | T = 20 ± 2 °C, pH = 6.0 | Redlich–Peterson model | 1949 mmol/kg a Biochars: 211 mmol/kg a | Elovich mode | 24 h Biochars: 30 h | Physical adsorption, electrostatic interaction, complexation | [86] |
| BM-BCs | BM: biochars (10 g), ZrO2 balls, RS = 1600 rpm, MT = 60 s, T = 30 | S = 74.39 m2/g, V = 0.1540 cm3/g, D = 8.3741 nm, C = 54.36%, N = 2.80%, O = 30.65%, pHpzc = 2.3 Biochars: S = 46.20 m2/g, V = 0.1274 cm3/g, D = 10.9337 nm, C = 63.22%, N = 3.19%, O = 23.94% | Pb(II) | T = 298–308 K | Langmuir model | 163.63–170.09 mg/g a | PSO | 60 min | - | [167] |
| BM-BCs | V, BM: biochars (150 g), ZrO2 balls (Φ = 6–10 mm, 1500 g), MT = 20 min | S = 53.82 ± 5.82–217.03 ± 4.36 m2/g, V = 0.030 ± 0.006–0.113 ± 0.005 cm3/g, Vmicro = 0.013 ± 0.002–0.027 ± 0.005 cm3/g, D = 7.882 ± 1.797–13.04 ± 3.427 nm, carboxyl = 0.34 ± 0.01–0.36 ± 0.02 mmol/g, lactones = 0.16 ± 0.02–0.23 ± 0.02 mmol/g, phenolic hydroxyl = 0.12 ± 0.02–0.26 ± 0.03 mmol/g, OFGs = 0.62 ± 0.05–0.84 ± 0.03, CEC = 6.44 ± 0.92–15.07 ± 0.89 cmol/kg, pH = 9.31 ± 0.10–9.67 ± 0.11 Biochars: S = 14.37 ± 0.75–198.11 ± 2.61 m2/g, V = 0.010 ± 0.001–0.094 ± 0.002 cm3/g, Vmicro = 0.009 ± 0.001–0.009 ± 0.002 cm3/g, D = 4.708 ± 0.252–6.072 ± 0.535 nm, carboxyl = 0.20 ± 0.03–0.24 ± 0.02 mmol/g, lactones = 0.09 ± 0.02–0.15 ± 0.01 mmol/g, phenolic hydroxyl = 0.04 ± 0.01–0.18 ± 0.03 mmol/g, OFGs = 0.33 ± 0.01–0.57 ± 0.02 mmol/g, CEC = 6.27 ± 0.87–14.60 ± 1.21 cmol/kg, pH = 10.03 ± 0.14–10.28 ± 0.16 | Pb(II) | T = 25 °C, pH = 5 ± 0.05 | Langmuir model | 103.99–210.90 mg/g a Biochars: 73.50–164.23 mg/g a | PSO | Equilibrium time shortened Biochars: 8–16 h | Co-precipitation, π electronic interactions, and complexation | [168] |
| BM-BCs | V, BM: biochars (8 g), ZrO2 balls (Φ = 6–10 mm, 800 g), MT = 5 min | S = 130.14 ± 3.48 m2/g, V = (22.49 ± 4.12) × 10−3 cm3/g, PS10 = 1.30 ± 0.02 μm, PS50 = 4.32 ± 0.06 μm, PS90 = 14.20 ± 0.99 μm, H/C = 0.22 ± 0.00, O/C = 0.06 ± 0.00, CEC = 3.25 ± 0.05 mmol/g, AFGs = 0.57 ± 0.02 mmol/g, pHpzc = 9.77 ± 0.02. Biochars: S = 6.89 ± 1.28 m2/g; V = (7.04 ± 2.25) × 10−3 cm3/g; PS10 = 14.65 ± 0.92 μm, PS50 = 71.20 ± 4.38 μm, PS90 = 256.00 ± 36.77 μm, H/C = 0.19 ± 0.00, O/C = 0.04 ± 0.00, CEC = 3.31 ± 0.06 mmol/g, AFGs = 0.36 ± 0.01 mmol/g, pHpzc = 9.87 ± 0.01 | Pb(II) | T = 25 °C, pH = 5.0 | - | 100.00 ± 0.00–134.68 ± 0.95 mg/g c Biochars: 99.45 ± 0.49–119.55 ± 0.64 mg/g c | - | - | Ion exchange, precipitation, and complexation | [178] |
| BM-NBBCs | BM: biochars (3.30 g) + DW(60 g), agate spheres, RS = 300 rpm, MT= 12 h, TA = 3 h | S = 35.49–313.09 m2/g, Smicro = 0–193.89 m2/g, Sexternal = 35.49–119.20 m2/g, V = 0.1635–0.4538 cm3/g, D = 6.46–11.74 nm, pHpzc = −2.0–3.1 Biochars: S = 2.76–52.78 m2/g, Smicro = 0–24.32 m2/g, Sexternal = 2.76–28.46 m2/g, V = 0.0175–0.0975 cm3/g, D = 8.22–14.48 nm | Cd(II) | T = 298 K, pH = 5.0 | Langmuir model | 66.33–165.77 mg/g a Biochars: 31.12–75.15 mg/g a | PSO | 200 min | Surface complexation, cation exchange, precipitation, electrostatic attraction, and cation–π interaction | [169] |
| Cu(II) | 159.27–287.58 mg/g a Biochars: 86.35–163.80 mg/g a | 200 min | ||||||||
| Pb(II) | 339.34–558.88 mg/g a Biochars: 209.35–389.51 mg/g a | 90 min | ||||||||
| BM-MBC | P, BM: MBC (1 g), agate balls (Φ = 5 mm, 100 g), MT = 12 h, TA = 20 min, RP = 10 min | S = 296.3 m2/g, V = 0.091 cm3/g, C = 47.98%, H = 0.88%, O = 27.89%, N = 0.53%, Fe = 12.32%, Na = 0.13%, Mg = 0.61%, Si = 0.13%, Ca = 2.13%, P = 0.12%, K = 0.88%, pHzpc = 4.43, MS = 15.39 emu/g Magnetic biochars: S = 198.6 m2/g, V = 0.006 cm3/g, C = 57.82%, H = 2.48%, O = 21.98%, N = 0.87%, Fe = 1.25%, Na = 0.12%, Mg = 0.58%, Si = 0.11%, Ca = 2.10%, P = 0.11%, K = 0.81%, MS = 10.76 emu/g | Hg(II) | T = 24 ± 2 °C | Langmuir model | 127.4 mg/g a | PSO | 12 h | Electrostatic interactions, Hg–π interaction, and surface complexation | [170] |
| BM-PBCs | P, BM: potassium ferrate-activated biochars, agate balls (MR of large, medium, and small = 2:18:15), RS = 300 rpm, MT = 12 h | S = 284.17–282.47 m2/g, D = 11.62–12.10 nm, pHpzc = 3.2–4.9, MS = 18.94–20.33 emu/g | Cr(VI) | T = 15, 25, 35 °C, pH = 2 | Langmuir model | 75.65–117.49 mg/g a | PSO | 80–150 min | Ion exchange, pore filling, electrostatic attraction, precipitation, and surface complexation | [171] |
| BM-Fe-BCs | P, BM: biochar/iron oxide composites, RS = 500 rpm, MT = 4 h | S = 241 m2/g. Biochar/iron oxide composites: S = 199 m2/g | Cr(VI) | T = 22 ± 0.5 °C, pH = 5 | Langmuir model | 48.1 mg/g a | Elovich mode | 200 min | Electrostatic interaction | [85] |
| CaO-biochars | BM: eggshell and rice straw powder (MR = 1:4–2:1), ZrO2 balls (Φ = 0.8 cm, 60 g), MT = 30 min, pyrolysis (800 °C for 2 h) | S = 8.30–25.8 m2/g, V = 0.0273–0.0467 cm3/g; D = 6.70–13.1 nm, Ca = 19.5–42.2%, C = 4.32–16.7%, O = 15.8–25.4%, H = 1.68–2.42% Biochars: S = 7.87 m2/g, V = 0.0126 cm3/g, D = 6.40 nm, Ca = 1.00%, C = 46.6%, O = 7.59%, H = 1.90% | Phosphate | T = 298 K, pH = 7 | Langmuir model | 96.4–231 mg/g a Biochars: 5.58 mg/g a | PSO Biochars: PFO. | 6 h Biochars: 6 h | Precipitate | [81] |
| MgO-biochars | BM: biochars (1.0 g) + MgO (0.5 g), agate balls (Φ = 6 and 8 mm, 75 g), RS = 500 rpm, MT = 12 h | S = 10.141–49.324 m2/g, V = 3.809–3.820 cm3/g, D = 0.033–0.091 nm, C = 33.72–34.43%, H = 1.71–2.37%, O = 11.33–14.05, N = 1.30–1.89, H/C = 0.05–0.07, O/C = 0.34–0.41, (O + N)/C = 0.38–0.45, pHpzc = 2.14–2.19 Biochars: S = 2.088–2.458 m2/g, V = 3.820–3.836 cm3/g, D = 0.001–0.002 nm, C = 42.58–59.79%, H = 0.88–2.97%, O = 1.63–10.82%, N = 1.82–2.16%, H/C = 0.01–0.05, O/C = 0.03–0.19, (O + N)/C = 0.06–0.23 | Ni(II) | T = 298, 308, 318 K, pH = 6.0 | Freundlich model | 99.3 ± 5.4–239.6 ± 3.7 (mg/g)/(mg/L)1/n b | PSO | 5–20 h | Van der Waals force, metal ion exchange, metal–π interaction, surface functional group complexation | [172] |
| MgO-biochars | BM: biochars (1.8 g) + MgO (10–50% wt/wt), RS = 500 rpm, MT = 12 h, TA = 3 h | S = 140 m2/g, V = 0.100 cm3/g, C = 47.71%, O = 29.77%, Mg = 21.8%, Si = 0.72% Biochars: S = 249.7 m2/g, V = 0.112 m3/g BM-BC: S = 310.7 m2/g, V = 0.140 m3/g | Phosphate | T = 25 ± 2 °C | - | 2–12 mg/g c Biochars and BM-BCs < 0 | - | - | Electrostatic action and surface precipitation | [95] |
| SiO2@C | BM: biochars+ SiO2 | S = 262.39 m2/g, V = 0.1480 cm3/g D = 2.2527 nm, SiO2 = 27.02%, C = 72.98%, zeta potential = −71 mV | Cu(II) | pH = 6 | - | 34.60 mg/g d | PSO | 60 min | Electrostatic interaction | [173] |
| Pb(II) | 23.47 mg/g d | |||||||||
| Zn (II) | 27.55 mg/g d | |||||||||
| Ball-milled biochar–vermiculite nanocomposites | P, BM: biochars (1.8 g)+ expanded vermiculite (MR = 1:9, 1:4, 3:7 and 2:3), beads (Φ = 6 mm, 180 g), RS = 300 rpm, MT = 12 h, TA = 0.5 h | S = 16.078 m2/g, V = 0.047 cm3/g, D = 12.929 nm Biochars: S = 214.622 m2/g, V = 0.009 cm3/g, D = 1.140 nm | As(V) | T = 25 ± 0.5 °C, pH = 6 | Langmuir model | 20.1 mg/g a | PSO | 36 h | Ion exchange and electrostatic attraction | [99] |
| Biochar–attapulgite nanocomposites | P, BM: biochars (1 g)+ attapulgite (0.5–2 g), agate balls (Φ = 2–5 mm, 150–300 g), RS = 550 rpm, MT = 5 h, TA = 0.5 h | S = 16.1–17.12 m2/g, V = 0.0536–0.0613 cm3/g, D = 13.32–14.32 nm, C = 24.58–45.79%, N = 0.30–0.41%, H = 2.32–3.22%. Biochars: S = 4.46 m2/g, V = 0.0056 cm3/g, D = 5.04 nm, C = 65.54%, N = 0.88%, H = 4.79%, C/O = 2.88 | Cd(II) | T = 25 °C | Freundlich model | 5.9916–17.8571 L/g b Biochars: 2.1513 L/g b | PSO | 4 h | Silicate precipitate, acid-oxygenated groups complexation, and electrostatic interaction | [98] |
| FeS2-BCs | P, BM: biochars (0.6 g) + FeS2 (2 g), ZrO2 balls (Φ = 3, 5, 15 mm, 200 g, MR = 3:5:2), RS = 400 rpm, MT = 24 h, AT = 6 h, purged with N2 ( > 99%) for 30 min | S = 82.9 m2/g, V = 0.021 cm3/g, D = 3.53 nm, C = 13.7%, H = 1.74%, O = 38.1%, N = 0.06%, S = 24.7%, Fe = 21.7%, H/C = 0.13, O/C = 2.78, (N + O)/C = 2.79, pHpzc = 6.4 Biochars: S = 455 m2/g, V = 0.015 cm3/g, D = 1.65 nm, C = 85.7%, H = 1.90%, O = 12.2%, N = 0.20%, H/C = 0.022, O/C = 0.14, (N + O)/C = 0.15 BM-BCs: S = 568 m2/g, V = 0.141 cm3/g, D = 2.33 nm, C = 78.3%, H = 2.41%, O = 19.1%, N = 0.19%, H/C = 0.031, O/C = 0.24, (N + O)/C = 0.25 | Cr(VI) | pH = 4.7 | Langmuir model | 134 ± 1.32 mg/g a | PSO | - | Electrostatic attraction and surface complexation | [100] |
| ZVIs-BCs | BM: cotton husk + ZVIs, pyrolysis (800 °C for 1 h), stainless balls (Φ = 5 mm, 40 g), RS = 350 rpm, MT = 2.5 h, TA = 10 min | S = 378.66 m2/g, V = 0.1704 cm3/g, D = 1.7996 nm, H/C = 0.01, O/C = 0.09; (O + N)/C = 0.11, Fe = 8.99% Biochars: S = 4.32 m2/g, V = 0.008217 cm3/g, D = 7.6157 nm, H/C = 0.01, O/C = 0.07, (O + N)/C = 0.08, Fe =0.04% | Cd(II) | T = 298 K | Langmuir model | 96.40 mg/g a Biochars: 84.19 mg/g a | PSO | 4 h | Physical adsorption, electrostatic attraction, and complexation | [79] |
| BM-Fe3O4-BC | P, BM: biochars + Fe3O4 (MS = 1:100), agate balls (Φ = 6, 10, and 15 mm), MM =1:2, RS= 500 rpm, MT = 12 h, TA = 3 h | S = 10.1178 m2/g, V = 0.0015 cm3/g, pHpzc = 5.3, MS = 5.29 emu/g. Biochars: S = 82.10 m2/g | Pb(II) | T = 10–50 °C | Langmuir model | 183.99–339.39 mg/g a | Avrami fractional-order model | 20 min | Electrostatic attraction, precipitation, complexation, cation exchange, and co-precipitation. | [174] |
| BM-NaOH-BC | BM: NaOH-modified biochars (2 g) + Fe3O4 (2 g), agate balls (Φ = 6 mm, 200 g), RS = 500 rpm, MT = 12 h, TA = 3 h | S = 148.41 m2/g, V = 0.178 cm3/g, D = 1.985 nm, pHpzc = 10.52, MS = 37.09 emu/g NaOH-modified biochars: S = 288.91 m2/g, V = 0.315 cm3/g, D = 3.061 nm | Cd(II) | T = 25 °C, pH = 7.0 | Freundlich model | 183.59 mg/g a NaOH-modified biochars: 101.51 mg/g a | PSO | 60 min NaOH-modified biochars: 120 min | Pore adsorption, precipitation, ion exchange, complexation, and Cd–π interaction | [179] |
| BM-SnZVI@BC | P, BM: biochars (3 g) + S (1 g) + Fe (1 g), ZrO2 balls (Φ = 5, 10, 15 mm, 150 g, MR = 1:1:1), RS = 400 rpm, MT = 12 h, TA = 30 min, N2 purging for 20 min | pHpzc = 9.49, MS = 11.91 emu/g | Phosphorus | T = 298, 308, 318 K, pH ≈ 6 | Langmuir model | 25.00–39.72 mg/g a | PFO | 240 min | Electrostatic attraction, surface precipitation, hydrogen bonding, and ligand effects | [106] |
| BM-FeS@NBCs | BM: biochars (1 g) + NH3·H2O (15 g), agate balls (Φ = 15, 10, 6 mm, 45 g, MR = 2:20:22), RS = 300 rpm, MT = 12 h, AT = 3 h, N2 purging for 30 min BM: N-biochars (1 g) + FeS (0.5 g), agate balls (Φ = 15, 10, 6 mm, 27 g, MR = 1:10:11), RS = 300 rpm, MT = 12 h, AT = 3 h, N2 purging for 30 min | pHpzc = 3.9 | Cr(VI) | T = 15–25 °C | Langmuir model | 149.38–194.69 mg/g a | Avrami fractional-order model | 250 min | Electrostatic attraction, ion exchange, and complexation | [10] |
| BM-LDH-BCs | P, BM: biochars + LDHs + water (MR = 10:1:1), agate balls, RS = 500 rpm, MT = 4 h, TA = 5 min | S = 226 m2/g, V = 0.140 cm3/g, D = 3.51 nm, zeta potential (pH = 5.5) = −17.5 mV Biochars: S = 122 m2/g, V = 0.108 cm3/g, D = 3.86 nm, zeta potential (pH = 5.5) = −15.7 mV BM-BCs S = 246 m2/g, V = 0.101 cm3/g, D = 3.52 nm, zeta potential (pH = 5.5) = −26.3 mV | Cd(II) | T = 25 °C, pH = 5.5 | Freundlich model | 41.0 (mg/g)/(mg/L)1/n b Biochars: 7.82 (mg/g)/(mg/L)1/n b BM-BCs 26.9 (mg/g)/(mg/L)1/n b | PFO | 8 h | Surface complexation, chelation, precipitation, and physical adsorption | [108] |
| Mg/Al-BCs | BM: biochars (1.5 g) + Mg(OH)2 (0.897 g) + Al(OH)3 (0.603 g), agate balls (300 g), RS = 300 rpm, MT = 8 h, TA = 0.5 h | S = 17.577 m2/g, V = 0.0885 cm3/g, D = 12.56 nm, C = 12.51%, H = 2.79%, N = 1.54%, pHpzc = 4.56 Biochars: S = 14.108 m2/g, V = 0.0383 cm3/g, D = 14.26 nm, C = 25.87%, H = 1.23%, N = 3.51%, pHpzc = 3.42 BM-BCs S = 16.199 m2/g, V = 0.0749 cm3/g, D = 12.74 nm, C = 24.17%, H = 1.69%, N = 3.27%, pHpzc = 3.66 | As(V) | T = 25–35 °C, pH = 7.0 | Freundlich model | TM: 24.49 mg/g c Biochars: 0.48 mg/g c BM-BCs 6.73 mg/g c | PSO | 20 h | Precipitation, ion exchange, surface complexation, and electrostatic interaction. | [107] |
| Fe/Mn-BCs | P, BM: biochars, agate balls, MM = 1:100, RS = 300 rpm, MT = 6 h | S = 226.50–331.5 m2/g, Vmeso = 0.32–0.36 cm3/g, D = 4.16–5.21 nm, pHpzc = 1.73–3.06 Biochars: S = 14.02–30.35 m2/g, Vmicro = 0.003–0.006 cm3/g, Vmeso = 0.006–0.03 cm3/g, D = 6.49–7.49 nm | Cd(II) | T = 298 K, pH = 5 | Langmuir model | 65.3–100.9 mg/g a Biochars: 12.9–20.9 mg/g a | PSO Before: PFO | 3 h Biochar: 4 h | Surface complexation, cation exchange, Cd–π interaction, precipitation, and electrostatic attraction. | [175] |
| BM-PBCs | P, BM: biochars (3.3 g) and phytic acid (0–50% wt% solution), agate balls (Φ = 6 mm, 330 g), RS = 12 h, MT = 12 h, TA = 3 h, RP = 30 min | S = 66–285 m2/g, Smicro = 36–205 m2/g; V = 0.089–0.273 cm3/g, Vmicro = 0.017–0.092 cm3/g, APS = 307–615 nm, C = 73.5–80.0%, O = 15.9–21.8%, N = 2.7–2.9%, P = 0.9–1.9%, pHpzc ≈ 2.00–2.61 Biochars: S = 7 m2/g, V = 0.026 cm3/g, APS = 1353 nm, C = 85.3%, O = 12.2%, N = 2.5%, pHpzc = 3.14 BM-BCs: S = 433 m2/g, Smicro = 356 m2/g, V = 0.379 cm3/g, Vmicro = 0.158 cm3/g, APS = 414 nm, C = 78.3%, O = 18.0%, N = 3.7%, pHpzc < 2.0 | U(VI) | T = 298.15–338.15 K, pH = 4.0 | Langmuir model | 78.6–114.9 mg/g a Biochars: 23.2–43.3 mg/g a BM-BCs: 73.4–100.5 mg/g a | PSO | 60 min | Complexation, electrostatic attraction, cation–π bonding, and coordination | [89] |
| Thiol-modified biochars | BM: biochars (2 g) + 3-trimethoxysilylpropanethiol (1.6 mL with strong nitrogen purging) + DW (2.4 mL) + ethanol (76 mL) + NH4OH, agate balls (Φ = 3, 5, 15 mm, 200 g, MR = 3:5:2), RS = 400 rpm, MT = 30 h, TA= 6 h | S = 56.05—458.94 m2/g, V = 0.271–0.635 cm3/g; D = 5.53–19.34 nm, C = 59.15–71.24%, O = 18.45–27.95%, N = 0–2.25%, S = 2.98–5.63%, Si = 7.03–10.77%, O/C = 0.259–0.473, pHpzc < 2. BM-BC: S = 3.78–385.80 m2/g, V = 0.0163–0.182 cm3/g, D = 2.59–17.22 nm, C = 73.12–87.49%, O = 12.51–25.88%, Si = 1–1.00%, O/C = 0.143–0.354, pHpzc < 2 | Hg(II) | T = 25± 0.2 °C, pH = 7.0 ± 0.2 | Langmuir model | 270.60 ± 2.67–401.8 ± 2.27 mg/g a BM-BC:163.70 ± 8.45–386.34 ± 23.45 mg/g a | PSO | 4 h Ball-milled biochar: 1 h | Surface adsorption, electrostatic attraction, surface complexation, and ligand exchange. | [55] |
| Ball-milled CNTs-based materials | ||||||||||
| HA-MWCNTs | V, BM: CNTs (0.01 g) + HA (1.0 g), stainless steel ball (Φ = 30.0 mm, 112.0 g), RS = ~ 617 rpm, MT = 15 min | C = 74.2%, O = 20.2%, Si = 3.1%, Al = 2.5%, Zeta potential (DW) = −42.5 ± 1.0 mV | Cu(II) | Reconstituted water (Daphnia magna medium), pH = 7.0 | - | 68.5 ± 3.5 mg/g a | - | 3 h | Chemical complexation | [116] |
| CeO2-CNTs | BM: CNTs | — | Cr(VI) | T = 25 °C, pH = 3–11 | Langmuir model | 23.26–31.55 mg/g a | - | - | Specific affinity between hydrous oxides of Ce and Cr(VI) | [176] |
| FeOx@CNTs | P, BM: CNTs (0.2 g) + FeCl3·6H2O (0.6 g) + KOH (1.25 g), a spherical planetary ball mill, agate ball (Φ = 5 mm, 200 g), RS = 300 rpm, MT = 12 h, TA = 3 h | S = 242 m2/g, V = 0.523 cm3/g, D = 3.42 nm, pHpzc = 4.3 CNTs: S = 228 m2/g, V = 1.86 cm3/g, D = 30.5 nm, pHpzc = 5.7 | Sb(III) | T = 298 K, pH = 6.35 | Redlich–Peterson model | 172 mg/g a CNTs: 4.01 mg/g a | PSO | 12 h | Complexation and surface pore adsorption | [66] |
| Ball-milled graphenes-based materials | ||||||||||
| Ball-milled graphene sheets | BM: graphene sheets + N-methylpyrrolidone, stainless steel ball, RS = 300 rpm, MT = 50 h | - | U(VI) | T = 298 K pH = 4.5 | Langmuir model | 71.93 mg/g a | PSO | 2 h | Chemical oxidation | [32] |
| HGO | P, BM: jaggery + graphite + DW, hydrothermal treatment, and further calcination, MR = 100:1, stainless steel balls (Φ = 1 cm and 2 cm), RS = 70 rpm, MT = 30 h | - | Cr(VI) | pH = 1 | - | 5.48 mg/g c | - | 1 h | C–O–C and –OH functionalities, aromatic π network | [127] |
| PGO | P, BM: graphene (2 g) + dry ice (40 g), modification by PCl3, ZrO2 balls, RS = 350 rpm, MT = 48 h, RP = 10 min | S = 25 m2/g, C = 85.4%, O = 14.2% and P = 0.4% | Hg(II) | Room temperature, pH = 7 | Langmuir model | 82.2 mg/g a | PSO | - | Complexation | [121] |
| Ball-milled metal-based materials | ||||||||||
| FeS2-ZVIs | BM: ZVIs + FeS2 (total amount = 5.0 g, MR = 1:0, 4:1, 1:1, 1:4 and 0:1), steel balls (4.0 g), RS = 300 rpm, MT = 30 min | S = 0.912 m2/g, O = 5.87%, S = 27.12%, Fe = 67.00%. Ball-milled FeS2: S = 1.190 m2/g, O = 13.99%, S = 43.48%, Fe = 42.53%. Ball-milled ZVIs: S = 0.800 m2/g, O = 11.77%, S = 0.70%, Fe = 87.52% | As(III) | T = 25 °C, pH = 6.8 | - | 78.3–97% c Ball-milled FeS2: 18.1% c Ball-milled ZVIs: 19.3% c | - | 90 min | - | [151] |
| Sulfidated ZVIs | P, BM: ZVIs + S (MR = 0.1–0.2), ZrO2 balls, RS = 400 rpm, MT = 20 h, N2 atmosphere | S = 1.46–2.08 m2/g Ball-milled ZVIs: S = 0.21 m2/g | Cr(VI) | T = 25 ± 0.5 °C, pH = 6 | - | 3.831mg/g b | PSO | 180 min | - | [177] |
| LS-ZVIs | P, BM: ZVIs (2.5 g) + lignosulfonate (0.025–0.25 g), ZrO2 balls (Φ = 6 mm), RS = 400 rpm, MT = 2–20 h, Ar headspace | S = 2.59 m2/g, Fe(0) = 10.5%, Fe(II) = 59.7%, Fe(III) = 19.8% ZVIs: S = 0.76 m2/g, Fe(0) = 3.4%, Fe(II) = 76.9%, Fe(III) = 19.7% | Cr(VI) | T = 25 °C, pH = 5.5 | - | 4.0–100% c ZVIs: ≈0% c | PSO | 60 min | Chemical adsorption | [157] |
| Sulfidated ZVIs | P, BM: ZVIs + S (MR = 0.01–0.2), ZrO2 balls (Φ = 6 mm), RS = 400 rpm, MT = 20 h, Ar headspace | S = 1.5 m2/g Ball-milled mZVIs: S = 0.21 m2/g | As(III) | T = 25 ± 5 °C, pH = 7, oxic condition | - | 174.91–275.10 mg/g d Ball-milled ZVIs: 353.27 mg/g d | PSO | 24 h Ball-milled ZVIs: 72 h | Chemical adsorption | [156] |
| Coffee grounds-modified ZVIs | P, BM: ZVIs + coffee grounds (MR = 2–8%), ZrO2 balls (Φ = 6 and 9 mm, 100 g, MR = 3:2), RS = 550 rpm, MT = 2 h | S = 1.48–1.85 m2/g, APS = 80 μm, Fe = 84.20–95.70%, C = 0.75–3.60%, O = 3.55–12.20% Ball-milled ZVIs: S = 1.45 m2/g, APS = 71 μm, Fe = 98.20%, C = 0.55%, O = 1.25% | Cr(VI) | T = 25 °C, pH = 6.5 | - | 80–100% c Ball-milled ZVIs: <10% c | PFO | 120 min | Complexation | [160] |
| ZVAls/Fe3O4 | P, BM: ZVAls (2 g) + Fe3O4 (1.0 g), ZrO2 balls (3.0g), RS = 300 r/min, MT = 1.5 h | S = 6.5154 ± 0.1963 m2/g, pHpzc = 9.2, SM = 10.03 emu/g ZVAls: S = 4.0427± 0.7390 m2/g | Cr(VI) | T = 25 ± 2 °C, pH = 7 | Langmuir model | 8.10 mg/g a | PFO | 30 min ZVAls: 50 min | Surface adsorption | [34] |
| ZVAls/MFe2O4 (M = Mn, Zn, Ni) | BM: ZVAl (1.0 g) + MFe2O4 powders (0.5 g), ZrO2 balls (Φ = 8, 4, 2, 1 mm, 15 g, MR = 1: 2: 4: 8), RS = 300 rpm, MT = 2 h | S = 17.344–24.646 m2/g, V = 0.067–0.076 cm3/g; D = 5.136–7.443 nm, pHpzc ≈ 9.5, SM = 7.84–51.59 emu/g ZVAls: S = 1.826 m2/g, V = 0.007 cm3/g, D = 4.140 nm | Cr(VI) | T = 298 K, pH = 7 | - | 89.15–100% c ZVAls: 0% c | PSO | 30 min | Surface adsorption and ion exchange | [161] |
4.2. Removal of Organic Pollutants
| Materials | Treatment | Material Characterization | Pollutants | Adsorption Experiment | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Condition | Isotherm Model | Adsorption Capacity | Kinetic Model | Equilibrium Time | Adsorption Mechanism | |||||
| Ball-milled ACs-based materials | ||||||||||
| BM-ACs | BM: ACs (1 g), milling balls (4 g), RS = 350 rpm, MT = 60 min | S = 496.32 m2/g, Vt = 0.278 cm3/g, Vmicro = 0.203 cm3/g, Vmeso = 0.075 cm3/g, D = 2.248 nm, APS = 1.57 μm, pHpzc = 6.46 ACs: S = 526.80 m2/g, Vt = 0.293 cm3/g, Vmicro = 0.182 cm3/g, Vmeso = 0.110 cm3/g, D = 2.225 nm, APS = 5.30 μm | MB | T = 25 °C, pH = 10 | Langmuir model | 505 mg/g a Biochars: 227.14 mg/g c | PSO | 420 min | Electrostatic interaction | [56] |
| AC-COOH | P, BM: ACs (5 g) + NaOH (25 g) + DW (50 mL), RS = 400 rpm, MT = 6 h BM: obtained materials + chloroacetic acid (25 g), RS = 400 rpm, MT = 6 h | C = 79.00%, O = 21.00%, pHpzc = 6.20 | MB | T = 20 °C, pH = 7 | Langmuir model | 123.02 mg/g a | PSO | 15 min | Electrostatic interaction | [77] |
| CV | 120.3 mg/g a | |||||||||
| Magnetic ACs | P, BM: ACs (0.45 g) + Fe3O4 (1.35 g), agate balls (Φ = 6 mm, 180 g), RS = 500 rpm, MT = 12 h | S = 75.4 m2/g, V = 0.05 cm3/g, D = 2.6 nm, APS = 609 nm, C = 47.4%, H = 0.71%, O = 25.0%, N = 0.02%, Fe = 21.6%, pHpzc < 3, MS = 33.8 emu/g ACs: S = 743.7 m2/g, V = 0.39 cm3/g, D = 2.1 nm, APS = 0.5–1 mm, C = 70.8%, H = 2.97%, O = 18.7%, N = 0.05%, Fe = 0.04%, pHpzc < 3 BM-ACs: S = 544.9 m2/g, V = 0.31 cm3/g, D = 2.3 nm, APS = 479 nm, C = 72.7%, H = 2.63%, O = 17.4%, N = 0.09%, Fe = 0.03%, pHpzc < 3 | MB | T = 25 ± 2 °C | Langmuir model | 304.2 mg/g a BM-ACs: 298.7 mg/g a ACs: 111.9 mg/g a | PSO | 8 h | - | [104] |
| Magnetic ACs | P, BM: ACs (2.25 g) + Fe3O4 (0.75 g), milling balls (Φ = 5.60 mm, 120 g), RS = 550 rpm, MT = 2 h, TA = 0.5 h | C = 66.3%, O = 17.4%, Si = 0.3%, Fe = 16.1%, APS ≈ 1 μm, pHpzc ≈ 6.46, MS = 24.2 emu/g ACs: C = 95.5%, O = 3.8%, Si = 0.7%, Fe = 0.0%, APS = 48 μm | Potassium perfluorooctane sulfonate | T = 28 °C | Langmuir model | 1.63 mmol/g a | PFO | <2 h | Hydrophobic interaction | [180] |
| Perfluorooctanoic acid | 0.90 mmol/g a | |||||||||
| Potassium perfluorohexane sulfonate | 0.33 mmol/g a | |||||||||
| Potassium perfluorobutane sulfonate | 0.21 mmol/g a | |||||||||
| Ball-milled biochar-based materials | ||||||||||
| Graphite-like biochars | BM: aqueous mixture of biomass (newspaper or maize straw raw), oxidation and pyrolysis, agate balls (200 g), RS = 300 rpm, MT = 2 h | S = 871.5–1065 m2/g, V = 1.46–4.45 cm3/g, Vmacro = 0.031–0.145 cm3/g, Vmeso = 1.13–3.53 cm3/g, Vmicro = 0.304–0.768 cm3/g, D = 4.9–8.5 nm, C = 68.5 ± 0.16–84.9 ± 0.23%, H = 1.4 ± 0.06–1.5 ± 0.14%, O = 4.7 ± 0.17–6.4 ± 0.01%, N = 0.5 ± 0.07–0.7 ± 0.05%, (O + N)/C = 1.53–1.65, H/C = 0.20–0.26 Biochars derived from biomass: S = 144.8–545.8 m2/g, V = 1.52–1.89 cm3/g, Vmacro = 0.015–0.034 cm3/g, Vmeso = 1.25–1.52 cm3/g, Vmicro = 0.257–0.330 cm3/g, D = 7.8–11.4 nm, C = 72.0 ± 0.10–85.5 ± 0.17%, H = 1.8 ± 0.01–1.9 ± 0.10%, O = 2.6 ± 0.60–4.3 ± 0.35%, N = 0.7 ± 0.02–1.3 ± 0.07%, (O + N)/C = 1.66–1.68, H/C = 0.25–0.32 Biochars derived from ball-milled biomass: S = 277.0–407.5 m2/g, V = 0.78–1.11 cm3/g, Vmacro = 0.025–0.028 cm3/g, Vmeso = 0.57–0.89 cm3/g, Vmicro = 0.179–0.195 cm3/g, D = 7.0–9.6 nm, C = 71.8 ± 0.18–85.3 ± 0.25%, H = 1.8 ± 0.01–1.9 ± 0.03%, O = 2.6 ± 0.29–4.2 ± 0.61%, N = 0.7 ± 0.02–1.4 ± 0.08%, (O + N)/C = 1.68–1.69, H/C = 0.25–0.32 | IMI | T = 25 ± 1 °C, pH = 8.0–8.7 | Freundlich model | 67.8–181.1 (mg/g)/(mg/L)n b Biochars: 4.74–19.8 (mg/g)/(mg/L)n b Biochars derived from ball-milled biomass: 13.3–16.2 (mg/g)/(mg/L)n b | - | - | Pore filling, H-bonding, and cation/p/π–π EDA interactions | [33] |
| SUL | 40.2–43.1 (mg/g)/(mg/L)n b Biochars derived from biomass: 8.41–12.6 (mg/g)/(mg/L)n b Biochars derived from ball-milled biomass: 10.8–16.0 (mg/g)/(mg/L)n b | Pore filling, H-bonding, cation/p/π–π EDA interactions, and electrostatic interactions | ||||||||
| BCs-CBM | P, BM: cellulose+ montmorillonite, pyrolysis, RS = 1000 r/min, MT = 4 h | S = 95.472 m2/g, V = 0.123 cm3/g, D = 2.2–4.2 nm | MB | T = 25 °C | Freundlich model | 11.489 ± 1.516 (mg/g)/(mg/L)n b | PSO | 8 h | Cation exchange | [82] |
| Acidic ball-milled biochars | P, BM: hickory chips (1 g) + H₂SO₄ (20 mL, 9.2 mol/L, pH = 1.265) + DW (20 mL), agate balls (Φ = 6 mm, 100 g) RS = 300 rpm, MT = 12 h, RT = 3 h, ambient air | S = 5.619 m2/g, V = 0.012 cm3/g, APS = 0.3–4 μm | TY | T = 25 °C | Freundlich model | 182.3 mg/g a | PSO | 12 h | Ion exchange and electrostatic interaction | [109] |
| Biosorbents derived from acidic and alkaline one-step ball milling of hickory wood | P, BM: hickory wood (1 g) + H₂SO₄ (20 mL, 9.2 mol/L)/NaOH (20 mL, 3.75 mol/L), agate balls (Φ = 6 mm, 100 g), RS = 300 rpm, MT = 24 h, RT = 3 h | S = 5.191–5.619 m2/g, V = 0.023–0.030 cm3/g | CR | T = 25 °C | Freundlich model | 0.68 ± 0.20–311.01 ± 2.13 (mg/g)/(mg/L)n b | PSO | >8 h | Surface complexation | [53] |
| CV | 149.02 ± 4.62 (mg/g)/(mg/L)n b | >3 h | ||||||||
| BM-BCs | BM: biochars (10 g), ZrO2 balls, RS = 1600 rpm, MT = 60 s, T = 30 | S = 74.39 m2/g, V = 0.1540 cm3/g, D = 8.3741 nm, C = 54.36%, N = 2.80%, O = 30.65%, pHpzc = 2.3 Biochars: S = 46.20 m2/g, V = 0.1274 cm3/g, D = 10.9337 nm, C = 63.22%, N = 3.19%, O = 23.94% | MB | T = 298–308 K | Langmuir model | 408.79–419.11 mg/g a | PSO | 120 min | - | [167] |
| BM-BCs | P, BM: biochars (1.8 g), agate balls (Φ = 6 mm, 180 g), RS = 300 rpm, MT = 12 h, TA = 3 h | S = 331 m2/g, V = 0.099 cm3/g, APS = 170nm, AFGs = 1.35 mmol/g, -COOH = 0.45 mmol/g, lactonic groups = 0.05 mmol/g, –OH = 0.85 mmol/g, pHpzc = 2.7 Biochars: S = 51 m2/g, V = 0.008 cm3/g, APS = 0.5–1 nm, AFGs = 0.30 mmol/g, lactonic groups = 0.08 mmol/g, –OH = 0.23 mmol/g, pHpzc = 4.2 | MB | T = 20 ± 2 °C, pH = 4.5 and 7.5 | Dual Langmuir model | 213 ± 19–354 ± 20 mg/g a Biochars: 14.4 ± 1.3–17.2 ± 3.5 mg/g a | PSO | 8 h Biochars: 16 h | pH = 4.5: π–π interaction pH = 7.5: π–π interaction and electrostatic effect | [181] |
| BM-BCs | P, BM: biochars (1.8 g), steel balls (Φ = 5 mm, 180 g), RS = 300 rpm, MT = 24 h, TA = 3 h, N2/vacuum environment | S = 300–452 m2/g, D = 140–223 nm, C = 77.3–82.8%, O = 15.2–19.9%, Si = 1.50–3.00%, O/C = 0.184–0.257, –C–O = 13.6–15.9%, –C=O = 1.61–4.09%, pHpzc < 2.2 Biochars: S = 2.60–343 m2/g, C = 86.5–92.8%, O = 6.70–12.5%, Si = 0.0–0.90%, O/C = 0.072–0.145, –C–O = 6.37–10.9%, –C=O = 1.33–1.56%, pHpzc ≈ 2.2 | RR | T = 25 ± 2 °C, pH = 6± 0.1 | Langmuir model | 9.2–34.8 mg/g a Before: 1.70–3.60 mg/g a | PSO | 120 min | Electrostatic adsorption | [87] |
| PWNBCs | P, BM: biochars, stainless steel balls (Φ = 2.4 mm, 45 g), RS = 575 rpm. MT = 100 min, ambient conditions | S = 47.25 m2/g, APS = 60 ± 20 nm, C = 83.1%, H = 3.5%, N < 1%, CEF = 14.8 ± 1.2 meq/100, zeta potential (6.61) = −31.3 mV | CBZ | T = 25 ± 1 °C, pH = 6.0 | Freundlich model | 0.068 (ng/mg)(L/ng)1/n b | PSO | 2 d | Hydrogen bonding | [182] |
| BM-BCs | P, BM: biochars, agate balls (Φ = 5 mm), MM = 1:100, RS = 300 rpm, MT = 24 h TA = 3 h | S = 10.8–401 m2/g, V = 0.043–0.076 cm3/g, D = 15.1–48.1 nm, C = 58.1–62.7%, H = 1.50–6.80%, O = 30.8–34.6%, N = <0.01–0.50%, O/C = 0.46–0.60, H/C = 0.02–0.12, (O + N)/C = 0.59–0.75, total organic carbon = 541–666 mg/g Biochars: S = 1.25–328 m2/g, V = 0.002–0.031 cm3/g, D = 3.42–23.0 nm, C = 61.0–70.0%, H = 0.64–4.61%, O = 29.4–33.9%, N = <0.01–0.52%, O/C = 0.42–0.56, H/C = 0.01–0.08, (O + N)/C = 0.42–0.56, total organic carbon = 553–565 mg/g | Galaxolide | T = 20 ± 2 °C | Freundlich model | 588 ± 31.2f 1955 ± 157 (mg/kg)/(mg/L)n b Biochars: 247 ± 3.78- 579 ± 43 (mg/kg)/(mg/L)n b | - | 48 h | Hydrophobic effect, π–π interaction, and micropore filling | [183] |
| BM-BCs | P, BM: biochars, milling balls, MM = 1:100, RS = 300 rpm, MT = 24 h, TA = 3 h | S = 13.5–139.89 m2/g, V = 0.0706–0.3366 cm3/g, Vmeso = 0.0706–0.3366 cm3/g, D = 2.27–4.03 nm, AFGs = 0.48–1.62 mmol/g Biochars: S = 3.9–211.56 m2/g, V = 0.0174–0.1370 cm3/g, Vmeso = 0.0172–0.0473 cm3/g, D = 2.28–4.84 nm, AFGs = 0.01–1.48 mmol/g | TC | T = 25°C, pH = 7.0 | Langmuir model | 51.04–96.69 mg/g a Biochars: 17.19–21.29 mg/g a | PSO | 60 h | Surface adsorption and pore filling | [83] |
| BM-BCs | P, BM: biochars, milling balls (Φ = 6 mm, 180 g), MM = 1:100, RS = 300 rpm, MT = 12 h, RT = 3 h | S = 309.0 m2/g, zeta potential (pH = 3.5–8.5) = −36–43 mV Biochars: S = 9.8 m2/g | SMX | T = 25 ± 0.5 °C, pH = 6.0 | Langmuir model | 100.30 mg/g a | Elovich model | 8–12 h | Hydrophobic interaction, π–π interaction, hydrogen bonding, and electrostatic interaction | [184] |
| Sulfapyridine | 57.90 mg/g a | 12 h | ||||||||
| BM-BCs | P, BM: MBC (1 g), agate balls (Φ = 5 mm, 100 g), MT = 12 h, TA = 20 min, RP = 10 min | S = 296.3 m2/g, V = 0.091 cm3/g, C = 47.98%, H = 0.88%, O = 27.89%, N = 0.53%, Fe = 12.32%, Na = 0.13%, Mg = 0.61%, Si = 0.13%, Ca = 2.13%, P = 0.12%, K = 0.88%, pHzpc = 4.43, MS = 15.39 emu/g Magnetic biochars: S = 198.6 m2/g, V = 0.006 cm3/g, C = 57.82%, H = 2.48%, O = 21.98%, N = 0.87%, Fe = 1.25%, Na = 0.12%, Mg = 0.58%, Si = 0.11%, Ca = 2.10%, P = 0.11%, K = 0.81, MS = 10.76 emu/g | TC | T = 24 ± 2 °C | Langmuir model | 268.3 mg/g a | PSO | 12 h | Electrostatic interactions, hydrogen bonds, and π–π interaction | [170] |
| BM-BCs | BM: MBC (1 g), stainless steel balls (Φ = 6 and 10 mm, 100 g, MR = 2: 8), RS = 400 rpm, MT = 24 h, RT = 6 h | S = 124.96 m2/g, C = 28.22%, H = 1.656%, O = 21.69%, N = 0.76%, O/C = 0.58, H/C = 0.70, (N + O)/C = 0.60, MS = 55.15 emu/g Magnetic biochars: S = 211.18 m2/g, C = 31.33%, H = 0.892%, O = 11.06%, N = 0.79%, O/C = 0.26, H/C = 0.34, (N + O)/C = 0.29 | Fuconazole | T = 293–313 K, pH = 5.6 | Langmuir model | 12.19–15.90 mg/g a Magnetic biochars: 2.21–3.30 mg/g a | Elovich model | 6 h | π–π interactions, hydrogen bonding, and surface complexation | [187] |
| BC-SBCs | P, BM: MBC, RS = 500 rpm, MT = 60 min | S = 73.4 m2/g, V = 0.186 cm3/g, D = 10.1 nm, C = 11.42%, O = 26.71%, Si = 11.66%, Fe = 50.22%, pHpzc = 3.75, MS = 12.9 emu/mg | SMX | T = 25 °C | Freundlich model | 851 (ug/g)/(ug/L)n b | PSO | 200 min | π–π conjugation, pore filling, H-bonding, Fe–O complexation, and electrostatic interaction | [185] |
| BM-PBCs | P, BM: potassium ferrate-activated biochars, agate balls (MR of large, medium, and small balls = 2:18:15), RS = 300 rpm, T = 12 h | S = 284.17–282.47 m2/g, D = 11.62–12.10 nm, pHpzc = 3.2–4.9, MS = 18.94–20.33 emu/g | TC | T = 15, 25, 35 °C, pH = 4.2 | Langmuir model | 56.35–90.31 mg/g a | Avrami fractional-order model | 80–150 min | Hydrogen bonding force, complexation, pore filling, and π–π stacking | [171] |
| BM-LDOs-BC | P, BM: Fe-Mg-LDOs biochar, MM = 1:100, RS = 700 rpm, MT = 2 h | S = 155.90 m2/g, V = 0.0513 cm3/g, D = 1.316 nm, C = 56.73%, O = 27.95%, Mg = 6.60%, Fe = 8.72%, pHpzc < 3. Biochars: S = 67.676 m2/g, V = 0.000852 cm3/g, D = 0.050 nm, C = 84.81%, O = 14.55%, Mg = 0.17%, Fe = 0.47% LDOs-BC: S = 464.89 m2/g, V = 0.156 cm3/g, D = 1.342 nm, C = 65.86%, O = 19.81%, Mg = 6.78%, Fe = 7.56% | CIP | T = 298 K | Freundlich model | 56.80 (mg/g mg/L)−1/n b | PSO | 720 min | Pore filling, electrostatic interaction, H-bonding, complexation, and π–π conjugation | [186] |
| Ball-milling iron-loaded biochars | BM: iron-loaded biochars (1.8 g), agate balls (180 g), RS = 300 rpm, MT = 12 h, RT = 3 h, air atmosphere | S = Sexternal = 48.3 m2/g, C = 40.4%, O = 32.8%, Fe = 11.3%, Cl = 15.5%, pHpzc = 9.19–9.48. Iron-loaded biochars: S = 24.9 m2/g, Sexternal = 24.1 m2/g, Sinternal = 0.774 m2/g, C = 60.8%, O = 22.0%, Fe = 6.50%, Cl = 10.7%, pHpzc < 2.3 | RR | T = 25 ± 2 °C, pH = 3 and 7.5 | Freundlich model | 39.2–53.8 mg1−n Ln g−1 b Iron-loaded biochars: 18.1–20.2 mg1−n Ln g−1 b | Elovich model | 1000 min | Surface adsorption and electrostatic interaction | [193] |
| BP-SBCs | BM: phosphoric acid-modified biochars, stainless steel balls, MM = 1:25, RS = 500 rpm, MT = 60 min | S = 146 m2/g, V = 0.327 cm3/g, D = 8.95 nm, CEC = 41.5 cmol/kg, C = 40.5%, H = 0.089%, O = 48.3%, N = 1.3% Biochars: S = 39.2 m2/g, V = 0.147 cm3/g, D = 15 nm, CEC = 7.6 cmol/kg, C = 45.3%, H = 0.084%, O = 42.5%, N = 1.4% | SMZ | T = 25 °C | Langmuir model | 46.1 mg/g a Biochars: 7.32 mg/g a | PSO | 720 min | Pore filling, π–π conjugation, H-bonding, and P–O complexation | [84] |
| Mg/Al-BCs: | P, BM: Fe-Al bimetallic oxides functionalized biochars, MM = 1:100, RS = 700 rpm, MT = 2 h | S = 91.357 m2/g, V = 0.105 cm3/g, D = 4.579 nm, pHzpc = 3.0, MS = 4.36 emu/g Biochars: S = 23.159 m2/g, V = 0.031 cm3/g, D = 5.327 nm Fe-Al bimetallic oxides functionalized biochars: S = 191.85 m2/g, V = 0.206 cm3/g, D = 4.289 nm, pHzpc ≈ 6.5 | TC | T = 298 K | Langmuir model | 116.59 mg/g a | Elovich model | 1440 min | π–π interaction, hydrogen bonding, complexation, and pore filling | [194] |
| H2O2-modified ball-milled biochars | P, BM: biochars (1.8 g), modification by H2O2, agate balls (Φ = 6 mm, 180 g) RS = 300 rpm, MT = 12 h, RT = 3 h | S = 9.2 m2/g, C = 77.1%, O = 21.4%, N = 1.4% Biochars: S = 3.8 m2/g | MB | - | Langmuir model | 310.115 mg/g a Biochars: 6.780 mg/g a | PSO | 6 h | Electrostatic interaction and ion exchange | [188] |
| N-doped biochars | P, BM: biochars (1.8 g) + NH3·H2O (18 mL), agate balls (Φ = 6 mm, 180 g), RS = 300 rpm, MT = 12 h, RT = 3 h | S = 441–548 m2/g, V = 0.302–0.415 cm3/g, Vmicro = 0.171–0.215 cm3/g, D = 2.55–3.34 nm, C = 89.2–94.6%, O = 4.51–9.10%, N = 0.87–1.68% | RR | T = 25 ± 2 °C | - | 22.0–37.4 mg/g c | - | - | Electrostatic interaction | [90] |
| Thiol-modified biochars | BM: biochars (2 g) + 3-trimethoxysilylpropanethiol (1.6 mL with strong nitrogen purging) + water (2.4 mL) + ethanol (76 mL) + NH4O, agate balls (Φ = 3, 5, 15mm, 200 g, MR = 3:5:2), RS = 400 rpm, MT = 30 h, TA = 6 h | S = 56.05–458.94 m2/g, V = 0.271–0.635 cm3/g, D = 5.53–19.34 nm, C = 59.15–71.24%, O = 18.45–27.95%, N = 0–2.25%, S = 2.98–5.63%, Si = 7.03–10.77%, O/C = 0.259–0.473, pHpzc < 2. BM-BCs: S = 3.78–385.80 m2/g, V = 0.0163–0.182 cm3/g; D = 2.59–17.22 nm, C = 73.12–87.49%, O = 12.51–25.88%, Si = 1–1.00%, O/C = 0.143–0.354, pHpzc < 2 | MeHg | T = 25 °C, pH = 7.0 ± 0.2 | Langmuir model | 39.14 ± 1.46–108.16 ± 3.11 mg/g a BM-BCs: 19.53 ± 1.03–25.54 ± 4.12 mg/g a | PSO | 24 h Ball-milled biochar: 9 h | Surface adsorption, electrostatic attraction, surface complexation, and ligand exchange | [55] |
| BM-FeS@NBCs | BM: biochars (1 g) + NH3·H2O (15 g), agate balls (Φ = 15, 10, 6 mm, 45 g, MR = 2:20:22), RS = 300 rpm, MT = 12 h, TA = 3 h, N2 purging for 30 min BM: N-biochars (1 g) + FeS (0.5 g), agate balls (Φ = 15, 10, 6 mm, 27 g, MR = 1:10:11), RS = 300 rpm, MT = 12 h, TA = 3 h, N2 purging for 30 min | pHpzc = 3.9 | TC | T = 15–25 °C | Langmuir model | 174.82–371.29 mg/g a | Avrami fractional-order model | 350 min | Pore filling, hydrogen bonding, and π–π stacking interactions | [10] |
| MBCs | P, BM: biochars (0.45 g) + Fe3O4 (1.35 g), agate balls (Φ = 6 mm, 180 g), RS = 500 rpm, MT = 12 h | S = 362.4 m2/g, V = 0.09 cm3/g, D = 3.82 nm, APS = 482 nm, C = 49.5%, H = 1.29%, O = 19.7%, N = 0.04%, Fe = 20.3%, pHpzc < 3, MS = 34.9 emu/g Biochars: S = 227.4 m2/g, V = 0.14 cm3/g, D = 2.09 nm, APS = 0.5–1 mm, C = 83.5%, H = 2.73%, O = 4.9%, N = 0.27%, Fe = 0.02%, pHpzc < 3. BM-BCs: S = 319.1 m2/g, V = 0.23 cm3/g, D = 2.82 nm, APS = 335 nm, C = 73.7%, H = 3.38%, O = 14.0%, N = 0.18%, Fe = 0.03%, pHpzc < 3 | MB | T = 25 ± 2 °C | Langmuir model | 500.5 mg/g a | PSO | 8 h | π electronic interaction, electrostatic attraction, and/or ion exchange | [104] |
| BM-Fe3O4-BC | P, BM: biochars + Fe3O4 (MS = 1:100), agate balls (Φ = 6, 10, and 15 mm), MM = 1:2, RS = 500 rpm, MT = 12 h, TA = 3 h | S = 10.1178 m2/g, V = 0.0015 cm3/g, pHpzc = 5.3, MS = 5.29 emu/g Biochars: S = 82.10 m2/g | TC | T = 10–50 °C | Langmuir model | 102.91–237.51 mg/g a | Avrami fractional-order model | 100 min | Pore filling, hydrogen bonding, and π–π stacking | [174] |
| Fe@MBC | V, BM: biochars + FeCl3, MM = 1: 9, RS = 400 rpm, MT = 130 min, RT = 60 min RP = 10 min, T = 3, air atmosphere | S = 17.19 m2/g, V = 0.13 cm3/g, D = 28.91 nm Biochars: S = 166.95 m2/g, V = 0.07 cm3/g, D = 1.63 nm BM-BCs: S = 219.24 m2/g, V = 0.30 cm3/g, D = 5.26 nm | TC | T = 25 °C | Freundlich Model BM-BCs: Langmuir model | 24.58 mg1–1/n·L1/n·g–1 b BM-BCs: 41.08 mg/g a | PSO | 24 h | Ion exchange, π–π stacking, van der Waals forces, electrostatic interactions, and hydrogen bonding | [189] |
| CuO-biochars | P, BM: biochars (1.8 g) + CuO (0.018 g), agate balls (90 g), RS = 400 rpm, MT = 9 h, RT = 1.5 h, air atmosphere | S = 296.5 m2/g, V = 0.111 cm3/g, CuO size = 11.4 nm, C = 80.96%, O = 13.25%, Ca = 0.91%, K = 0.29%, Cu = 4.60%, pHzp ≈ 3.0 | RR | T= 25 °C | Freundlich model | 4.01 mg(1–n) Ln g–1 b | PSO | 3 h | Electrostatic attraction | [96] |
| Twice-milled magnetic biochars | BM: biomass BM: MBC (1.0 g), RS = 400 rpm, MT = 1 h | S = 139.1 m2/g, AFGs = 0.582 mmol/g, carboxyl = 0.194 mmol/g, lactonic groups = 0.028 mmol/g, phenolic hydroxyl = 0.360 mmol/g, pHpzc≈3.9 | MB | T = 25 ± 1 °C | Freundlich model | 78.96 (mg/g)(L/mg)1/n b | PSO | 24 h | π–π and electrostatic interactions | [190] |
| Ball-milled CNTs-based materials | ||||||||||
| Ball-milled CNTs | P, BM: CNTs (1–2 g), agate balls (Φ = 18, 12, and 6 mm, MR = 1:10:25), RS = 160 rpm, MT = 12–50 h | S = 213–220 m2/g, mean length = 100–800 nm, open end, none, and few tangled phenomena CNTs: S = 198 m2/g, mean length > μm, closed-end, serious tangled phenomena | Aniline | - | - | 22.2–36.2 mg/g c CNTs: 14.9 mg/g c | - | - | Capillary adsorption | [191] |
| Ball-milled CNTs | P, BM: CNTs, milling balls, RS = 140 rpm, T = 6–30 h | Even length = 100–200 nm tens of micron, open tube tips, clearly reduced tangled phenomena, D = 9–15 nm CNTs: even length = tens of microns, closed tube tips, serious tangled phenomena, D = 30 nm | Nitrobenzene | - | - | 24.4–41.5 mg/g c CNTs: 19.8 mg/g c | - | 24 h | Capillary adsorption | [113] |
| Ball-milled carbon/CNTs | P, BM: carbon/CNTs, stainless steel balls (Φ = 3 mm), MM = 1:12, MT = 3 h | S = 358 m2/g, APS = ~500 nm CNTs: S = 78 m2/g | Sodium fluoride | T = 323 K, pH = 2 | Langmuir model | 0.36 mg/g a | PSO | 3.5 h | Physical adsorption and ion exchange | [192] |
| Ball-milled graphene-based materials | ||||||||||
| ECG | P, BM: graphite, stainless steel balls (Φ = 10 mm), MM = 1:7, RS = 600 rpm, MT = 4 and 8 h | S = 316.47–387.69 m2/g, V = 0.46–0.55 cm3/g, APS = 95 ± 5.27–164 ± 8.67 nm, O = 9.37–25.17%, pHpzc < 4.0 Graphite: S = 7.84 m2/g, V = 0.03 cm3/g | MB RB MO CV | T = 27.6 °C, pH = 4–10 | - | 97.7 ± 2–99.7 ± 0.2% c | - | 20 min | Physical absorption and electrostatic interaction | [125] |
| Ni-MOF-GO | BM: GO + nickel acetate + 1,3,5-trimesic acid, stainless steel balls (560 g), RS = 235 rpm, MT = 30 min | S = 69.36 m2/g | CR | T = 298–318 K, pH = 4–10 | Freundlich model | 211.55–385.65 mg/g(L/mg)1/n b | PSO | - | Lewis acid–base interaction and ion exchange | [131] |
| Al–carbon composites | P, BM: ZVAls (5 g) + NaCl (0.1 g) + ACs (0.05–0.5 g), ZrO2 balls (Φ = 5, 8 and 10 mm, 300 g, MR = 6: 3:1), RS = 300 rpm, MT = 1 h | S = 10.400 m2/g, Al = 44.05%, O = 10.42%, C = 44.49%, Na = 0.49%, Cl = 0.56% ZVAl: S = 3.099 m2/g, Al = 66.03%, O = 4.21%, C = 29.75% | Hexabromocyclododecane | T = 25 ± 1 °C, pH = 6.2 | - | >90% c | - | 1 h | - | [164] |
5. Regeneration
6. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Zhang, S.; Pang, H.; Wang, X.; Chen, Z.; Li, M.; Song, G.; Qiu, M.; Yu, S. Recent advances in nanoscale zero-valent iron-based materials: Characteristics, environmental remediation and challenges. J. Clean. Prod. 2021, 319, 128641. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Lin, N.; Wang, R.; Wang, M.; Zhang, X. Iron-based materials for simultaneous removal of heavy metal(loid)s and emerging organic contaminants from the aquatic environment: Recent advances and perspectives. Environ. Pollut. 2022, 299, 118871. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ- ment and human health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Wang, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yang, C. Cr(VI) removal by micron-scale iron-carbon composite induced by ball milling: The role of activated carbon. Chem. Eng. J. 2020, 389, 122633. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, K.; Zhang, Y.; Peng, C.; Robledo-Cabrera, A.; Lopez-Valdivieso, A. Highly surface activated carbon to remove Cr(VI) from aqueous solution with adsorbent recycling. Environ. Res. 2021, 197, 111151. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Bi, F.; Yan, S.; Miao, X.; Zhang, B.; Wang, Y.; Ge, C.; Zhang, Y. Two-step ball milling-assisted synthesis of N-doped biochar loaded with ferrous sulfide for enhanced adsorptive removal of Cr(VI) and tetracycline from water. Environ. Pollut. 2022, 306, 119398. [Google Scholar] [CrossRef]
- Kishore, S.; Malik, S.; Shah, M.P.; Bora, J.; Chaudhary, V.; Kumar, L.; Sayyed, R.Z.; Ranjan, A. A comprehensive review on removal of pollutants from wastewater through microbial nanobiotechnology-based solutions. Biotechnol. Genet. 2022, 1–26. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bhatt, K.; Simsek, H. Microalgae-based removal of pollutants from wastewaters: Occurrence, toxicity and circular economy. Chemosphere 2022, 306, 135576. [Google Scholar] [CrossRef] [PubMed]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.-G.; Maqbool, F.; Hu, Y. Removal of antibiotics pollutants in wastewater by uv-based advanced oxidation processes: Influence of water matrix components, processes optimization and application: A review. J. Water Process. Eng. 2022, 45, 102496. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in aop degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Sajid, M. Multifunctional membranes with super-wetting characteristics for oil-water separation and removal of hazardous environmental pollutants from water: A review. Adv. Colloid Interface Sci. 2020, 285, 102276. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liao, Z.; Zhang, M.; Ni, L.; Qi, J.; Wang, C.; Sun, X.; Wang, L.; Wang, S.; Li, J. Sequential ultrafiltration-catalysis membrane for excellent removal of multiple pollutants in water. Environ. Sci. Technol. 2021, 55, 2652–2661. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Salman, M.S.; Hasan, M.N.; Hasan, M.M.; Kubra, K.T.; Sheikh, M.C.; Rehan, A.I.; Waliullah, R.M.; Rasee, A.I.; Awual, M.E.; Hossain, M.S.; et al. Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent. J. Mol. Struct. 2023, 1282, 135259. [Google Scholar] [CrossRef]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of functionalized magnetic biochar in environmental remediation: A review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Chen, H.; Ni, B.J. Recent advances in waste-derived functional materials for wastewater remediation. Eco-Environ. Health 2022, 1, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, H.; Wu, S.; Pan, S.; Cui, D.; Wu, D.; Xu, F.; Wang, Z. Production of biomass-based carbon materials in hydrothermal media: A review of process parameters, activation treatments and practical applications. J. Energy Inst. 2023, 110, 101357. [Google Scholar] [CrossRef]
- Li, S.; Deng, J.; Xiong, L.; Wang, J.; Chen, Y.; Jiao, Y.; Jiang, L.; Dan, Y. Design and construct CeO2-ZrO2-Al2O3 materials with controlled structures via co-precipitation method by using different precipitants. Ceram. Int. 2018, 44, 20929–20938. [Google Scholar] [CrossRef]
- Zhao, H.; Lang, Y. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J. Taiwan Inst. Chem. Eng. 2018, 88, 152–160. [Google Scholar] [CrossRef]
- Gong, L.; Qiu, X.; Tratnyek, P.G.; Liu, C.; He, F. FeNx(C)-coated microscale zero-valent iron for fast and stable trichloroethylene dechlorination in both acidic and basic pH conditions. Environ. Sci. Technol. 2021, 55, 5393–5402. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, J.; Wang, Y.; Liu, T. Enhanced elimination of V5+ in wastewater using zero-valent iron activated by ball milling: The overlooked crucial roles of energy input and sodium chloride. J. Hazard. Mater. 2022, 435, 129050. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, S.; Liang, T.; Yan, X.; Zhang, Y.; Zhou, Y.; Sarkar, B.; Ok, Y.S. Ball-milled magnetite for efficient arsenic decontamination: Insights into oxidation-adsorption mechanism. J. Hazard. Mater. 2022, 427, 128117. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, M.; Pulingam, T.; Lee, K.T.; Sudesh, K. Activated carbon from biomass waste precursors: Factors affecting production and adsorption mechanism. Chemosphere 2022, 294, 133764. [Google Scholar] [CrossRef]
- Jiang, B.; Lin, Y.; Mbog, J.C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Gonçalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of carbon nanotubes by ball-milling to be used as ozonation catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef]
- Wang, Z.S.; Wang, Y.; Liao, J.L.; Yang, Y.Y.; Liu, N.; Tang, J. Improving the adsorption ability of graphene sheets to uranium through chemical oxidation, electrolysis and ball-milling. J. Radioanal. Nucl. Chem. 2016, 308, 1095–1102. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Xue, B.; Huang, P.; Hao, Y.; Tang, J.; Maletić, S.P.; Rončević, S.D.; Sun, H. Preparation of graphite-like biochars derived from straw and newspaper based on ball-milling and tempo-mediated oxidation and their supersorption performances to imidacloprid and sulfadiazine. Chem. Eng. J. 2021, 411, 128502. [Google Scholar] [CrossRef]
- Wang, W.; Gao, P.; Yang, C.; Zhao, Z.; Zhen, S.; Zhou, Y.; Zhang, T. Separable and reactivated magnetic mZVAl/nFe3O4 composite induced by ball milling for efficient adsorption-reduction- sequestration of aqueous Cr(VI). Sep. Purif. Technol. 2022, 288, 120689. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar–based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process. Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Yu, C.; Fang, H.; Li, Z. Mechanochemical preparation of single atom catalysts for versatile catalytic applications: A perspective review. Mater. Today 2023, 63, 288–312. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Dong, X.L.; Lu, A.H. Mechanochemical synthesis of porous carbons and their applications in catalysis. ChemPlusChem 2020, 85, 866–875. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Q.; Li, S.; Cagnetta, G.; Huang, J.; Deng, S.; Yu, G. Mechanochemical synthesis of catalysts and reagents for water decontamination: Recent advances and perspective. Sci. Total Environ. 2022, 825, 153992. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Liu, T.; Han, G.; Ma, W.-M.; Li, J. New insights into ball-milled zero-valent iron composites for pollution remediation: An overview. J. Clean. Prod. 2023, 385, 135513. [Google Scholar] [CrossRef]
- Wei, M.; Wang, B.; Chen, M.; Lyu, H.; Lee, X.; Wang, S.; Yu, Z.; Zhang, X. Recent advances in the treatment of contaminated soils by ball milling technology: Classification, mechanisms, and applications. J. Clean. Prod. 2022, 340, 130821. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Ditenberg, I.A.; Osipov, D.A.; Smirnov, I.V.; Grinyaev, K.V.; Esikov, M.A. Effect of preliminary high-energy ball milling on the structural-phase state and microhardness of ni3al samples obtained by spark plasma sintering. Adv. Powder Technol. 2023, 34, 130919. [Google Scholar] [CrossRef]
- Chuev, I.I.; Kovalev, D.Y. Effects of titanium high energy ball milling on the solid-phase reaction Ti+C. Mater. Chem. Phys. 2022, 283, 126025. [Google Scholar] [CrossRef]
- Delogu, F.; Gorrasi, G.; Sorrentino, A. Fabrication of polymer nanocomposites via ball milling: Present status and future perspectives. Prog. Mater. Sci. 2017, 86, 75–126. [Google Scholar] [CrossRef]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of iron oxide nanoparticles by mechanical milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Qian, L.; Li, H.; Wei, Z.; Liang, C.; Dong, X.; Lin, D.; Chen, M. Enhanced removal of cis-1,2-dichloroethene and vinyl chloride in groundwater using ball-milled sulfur- and biochar-modified zero-valent iron: From the laboratory to the field. Environ. Pollut. 2023, 336, 122424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qian, L.; Liang, C.; Zheng, T.; Dong, X.; Chen, M. Enhanced Cr(VI) reduction by zero-valent iron and ferroferric oxide wet ball milling: Synergy of electron storage and electron transfer. Chem. Eng. J. 2023, 457, 141254. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Yu, X.; Hu, C.; Hu, J.; Li, R.; Zhang, Y. Functional metal powders: Design, properties, applications, and prospects. Mater. Sci. Eng. B 2022, 280, 115708. [Google Scholar] [CrossRef]
- Li, H.N.; Zhang, H.M.; Huang, K.K.; Liang, D.; Zhao, D.D.; Jiang, Z.Y. Effect of ball milling speed on the quality of Al2O3 stripped graphene in a wet milling medium. Ceram. Int. 2022, 48, 17171–17177. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Yang, S.; Fan, D.; Fu, P. Graphite-modified zero-valent aluminum prepared by mechanical ball milling for selective removal of hydrophobic carbon tetrachloride. Chem. Eng. J. 2023, 474, 145591. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, T.; Li, J.; Zhu, X. Enhanced gaseous acetone adsorption on montmorillonite by ball milling generated Si–OH and interlayer under synergistic modification with H2O2 and tetramethylammonium bromide. Chemosphere 2023, 321, 138114. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Shao, X.; Tong, J.; Zhou, J.; Feng, Y.; Chen, R.; Yang, Q.; Han, Y.; Yang, X.; et al. Characteristics and aqueous dye removal ability of novel biosorbents derived from acidic and alkaline one-step ball milling of hickory wood. Chemosphere 2022, 309, 136610. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Tong, J.; Shao, X.; Feng, Y.; Zhou, J.; Han, Y.; Yang, X.; Ding, F.; Zhang, J.; et al. Alkaline ball-milled peanut-hull biosorbent effectively removes aqueous organic dyes. Chemosphere 2023, 313, 137410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Wang, L.; Lyu, H.; Xia, S.; Tang, J. Effective removal of Hg(II) and MeHg from aqueous environment by ball milling aided thiol-modification of biochars: Effect of different pyrolysis temperatures. Chemosphere 2022, 294, 133820. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Khan, A.S.; Bhat, A.H.; Din, I.U.; Inayat, A.; Muhammad, N.; Bakhsh, E.M.; Khan, S.B. Effect of short time ball milling on physicochemical and adsorption performance of activated carbon prepared from mangosteen peel waste. Renew. Energy 2021, 168, 723–733. [Google Scholar] [CrossRef]
- Lee, S.H.; Annamalai, S.; Shin, W.S. Engineered ball-milled colloidal activated carbon material for advanced oxidation process of ibuprofen: Influencing factors and insights into the mechanism. Environ. Pollut. 2023, 322, 121023. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, N.; Goswami, R.; Kumar, M.; Kataki, R. Biosorption of Co(II) from aqueous solution using algal biochar: Kinetics and isotherm studies. Bioresour. Technol. 2017, 244, 1465–1469. [Google Scholar] [CrossRef]
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef]
- Wei, L.K.; Abd Rahim, S.Z.; Al Bakri Abdullah, M.M.; Yin, A.T.M.; Ghazali, M.F.; Omar, M.F.; Nemes, O.; Sandu, A.V.; Vizureanu, P.; Abdellah, A.E. Producing metal powder from machining chips using ball milling process: A review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef]
- Zhang, F.; Han, F.; Lu, Y.; Wen, G.; Gu, S.; Wang, Z.; Tang, P. Experimental study and numerical simulation of the influence of ball milling parameters on granule sizes of mold powder. Powder Technol. 2023, 413, 118037. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Saito, F. A review on mechanochemical syntheses of functional materials. Adv. Powder Technol. 2012, 23, 523–531. [Google Scholar] [CrossRef]
- Tucho, W.M.; Mauroy, H.; Walmsley, J.C.; Deledda, S.; Holmestad, R.; Hauback, B.C. The effects of ball milling intensity on morphology of multiwall carbon nanotubes. Scr. Mater. 2010, 63, 637–640. [Google Scholar] [CrossRef]
- Pierard, N.; Fonseca, A.; Colomer, J.F.; Bossuot, C.; Benoit, J.M.; Van Tendeloo, G.; Pirard, J.P.; Nagy, J.B. Ball milling effect on the structure of single-wall carbon nanotubes. Carbon 2004, 42, 1691–1697. [Google Scholar] [CrossRef]
- Cheng, Z.; Lyu, H.; Shen, B.; Tian, J.; Sun, Y.; Wu, C. Removal of antimonite Sb(III) from aqueous solution using a magnetic iron-modified carbon nanotubes (CNTs) composite: Experimental observations and governing mechanisms. Chemosphere 2022, 288, 132581. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of metal hydrides: Recent advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Toraman, O.Y.; Katırcıoglu, D. A study on the effect of process parameters in stirred ball mill. Adv. Powder Technol. 2011, 22, 26–30. [Google Scholar] [CrossRef]
- Bulgakov, V.; Pascuzzi, S.; Ivanovs, S.; Kaletnik, G.; Yanovich, V. Angular oscillation model to predict the performance of a vibratory ball mill for the fine grinding of grain. Biosyst. Eng. 2018, 171, 155–164. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Wijesekera, R. Ball milling—A green and sustainable technique for the preparation of titanium based materials from ilmenite. Curr. Res. Green Sustain. Chem. 2022, 5, 100236. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Feng, G. Electromagnetic design and thermal analysis of module combined permanent magnet motor with wrapped type for mine ball mill. IET Electr. Power App. 2021, 16, 139–157. [Google Scholar] [CrossRef]
- Fu, H.; Li, X.; Wang, J.; Lin, P.; Chen, C.; Zhang, X.; Suffet, I.H. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, Y.; Kim, S.; Chung, C.M.; Cho, K. Enhanced sulfate ion adsorption selectivity in capacitive deionization with ball-milled activated carbon. Desalination 2022, 540, 116014. [Google Scholar] [CrossRef]
- Chen, T.; Gu, W.; Li, G.; Wang, Q.; Liang, P.; Zhang, X.; Huang, X. Significant enhancement in catalytic ozonation efficacy: From granular to super-fine powdered activated carbon. Front. Environ. Sci. Eng. 2017, 12, 6. [Google Scholar] [CrossRef]
- Yuan, R.; Dong, Y.; Hou, R.; Shang, L.; Zhang, J.; Zhang, S.; Chen, X.; Song, H. Structural transformation of porous and disordered carbon during ball-milling. Chem. Eng. J. 2023, 454, 140418. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Lu, Y.; Liu, C.; Yang, W. Carbon quantum dots displaying dual-wavelength photoluminescence and electrochemiluminescence prepared by high-energy ball milling. Carbon 2015, 94, 472–478. [Google Scholar] [CrossRef]
- Gohr, M.; Abd-Elhamid, A.I.; El-Shanshory, A.A.; Soliman, H.M.A. Adsorption of cationic dyes onto chemically modified activated carbon: Kinetics and thermodynamic study. J. Mol. Liq. 2022, 346, 118227. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, F.; Beesley, L.; Trakal, L.; Ma, Y.; Sun, Y.; Zhang, Z.; Ding, Y. Removal of cadmium in aqueous solutions using a ball milling-assisted one-pot pyrolyzed iron-biochar composite derived from cotton husk. Environ. Sci. Pollut. Res. 2023, 30, 12571–12583. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling-optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption recovery of phosphate from aqueous solution by cao-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Z.; Jiang, Y.; Li, F.; Xue, B.; Dong, Z.; Ding, M.; Chen, R.; Yang, Q.; An, T.; et al. Micro-structure, surface properties and adsorption capacity of ball-milled cellulosic biomass derived biochar based mineral composites synthesized via carbon-bed pyrolysis. Appl. Clay Sci. 2020, 199, 105877. [Google Scholar] [CrossRef]
- Xiang, W.; Wan, Y.; Zhang, X.; Tan, Z.; Xia, T.; Zheng, Y.; Gao, B. Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere 2020, 255, 127057. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, Y.; Deng, Z.; Tang, J.; Liu, Y.; Ma, J.; Zhang, Z. Ball milling and phosphoric acid hydrothermally co-functionalized sludge biochar for efficiently adsorptive removal of environmental concentration sulfamethoxazole: Experimental, characterization and dft study. Sep. Purif. Technol. 2024, 328, 125051. [Google Scholar] [CrossRef]
- Zou, H.; Zhao, J.; He, F.; Zhong, Z.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Yu, F.; Bashir, M.A.; et al. Ball milling biochar iron oxide composites for the removal of chromium Cr(VI) from water: Performance and mechanisms. J. Hazard. Mater. 2021, 413, 125252. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, Z.; Huang, J.; Gao, B.; Zhao, L.; Qiu, H.; Cao, X. Sorption of reactive red by biochars ball milled in different atmospheres: Co-effect of surface morphology and functional groups. Chem. Eng. J. 2021, 413, 127468. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Miao, X.; Xiang, W.; Gao, B. Effect of ball milling with hydrogen peroxide or ammonia hydroxide on sorption performance of volatile organic compounds by biochar from different pyrolysis temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, J.; Hu, R.; Wang, T.; Shao, X.; Chen, G.; Chen, L.; Tian, X. Engineered phosphorous-functionalized biochar with enhanced porosity using phytic acid-assisted ball milling for efficient and selective uptake of aquatic uranium. J. Mol. Liq. 2020, 303, 112659. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Y.; Zhang, X.; Sun, M.; Tao, Y.; Zhang, X.; Zhang, G.; Ge, C.; Zhang, Y. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism. Appl. Catal. B Environ. Energy 2022, 316, 121639. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Liu, Y.; Tang, W.; Geng, S.; Chen, J. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar. Chemosphere 2022, 303, 135264. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Xia, S.; Tang, J.; Zhang, Y.; Gao, B.; Shen, B. Thiol-modified biochar synthesized by a facile ball-milling method for enhanced sorption of inorganic Hg2+ and organicCH3Hg. J. Hazard. Mater. 2020, 384, 121357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Chen, J.; Chen, H.; Gao, B. Mgo modified biochar produced through ball milling: A dual-functional adsorbent for removal of different contaminants. Chemosphere 2020, 243, 125344. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile ball-milling synthesis of cuo/biochar nanocomposites for efficient removal of reactive red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X.; et al. Zno/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.; Sui, F.; Wang, M.; Cui, L.; Wang, H.; Xiang, W.; Li, G.; Yan, J. Mechanochemical modification of biochar-attapulgite nanocomposites for cadmium removal: Performance and mechanisms. Biochem. Eng. J. 2022, 179, 108332. [Google Scholar] [CrossRef]
- Li, F.; Wan, Y.; Chen, J.; Hu, X.; Tsang, D.C.W.; Wang, H.; Gao, B. Novel ball-milled biochar-vermiculite nanocomposites effectively adsorb aqueous As(Ⅴ). Chemosphere 2020, 260, 127566. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, B.; Lyu, H.; Li, D. Development of a novel pyrite/biochar composite (BM-FeS2@BC) by ball milling for aqueous Cr(VI) removal and its mechanisms. J. Hazard. Mater. 2021, 413, 125415. [Google Scholar] [CrossRef]
- Zhao, B.; Tang, J.; Lyu, H.; Liu, F.; Wang, L. Low molecular weight organic acids strengthen the electron transfer of natural fes2/biochar composite for Cr(VI) reduction: Experimental observations and governing mechanisms. J. Environ. Chem. Eng. 2022, 10, 107181. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Zhao, B.B.; Lyu, H.H. Activation of peroxydisulfate by ball-milled α-feooh/biochar composite for phenol removal: Component contribution and internal mechanisms. Environ. Pollut. 2022, 293, 118596. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Guo, S.; Tang, J.; Lyu, H.; Ri, C.; Sun, H. Enhanced removal of aged and differently functionalized polystyrene nanoplastics using ball-milled magnetic pinewood biochars. Environ. Pollut. 2023, 316, 120696. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci. Total Environ. 2020, 722, 137972. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, K.; Pi, Z.; Shen, P.; Zhou, P.; He, L.; Li, X.; Yang, Q. Insight to the mechanism of tetracycline removal by ball-milled nanocomposite CeO2/Fe3O4/biochar: Overlooked degradation behavior. Sep. Purif. Technol. 2023, 307, 122703. [Google Scholar] [CrossRef]
- Ai, D.; Wei, T.; Meng, Y.; Chen, X.; Wang, B. Ball milling sulfur-doped nano zero-valent iron @biochar composite for the efficient removal of phosphorus from water: Performance and mechanisms. Bioresour. Technol. 2022, 357, 127316. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, J.; Diao, Y.; Zhou, X.; Luo, T.; Wang, H.; Quan, G.; Sun, X.; Wang, J. Ball milled Mg/Al hydroxides modified nitrogen-rich biochar for arsenic removal: Performance and governing mechanism. Carbon Res. 2023, 2, 30. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, R.; Peng, Y.; Gao, X.; Li, Z.; Fan, B.; Guan, C.Y.; Beiyuan, J.; Zhou, Y.; Liu, J.; et al. New insights into ball milling effects on mgal-ldhs exfoliation on biochar support: A case study for cadmium adsorption. J. Hazard. Mater. 2021, 416, 126258. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Tong, J.; Shao, X.; Chen, R.; Yang, Q.; Li, F.; Xue, B.; Li, G.; Han, Y.; et al. Synthesis of hickory biochar via one-step acidic ball milling: Characteristics and titan yellow adsorption. J. Clean. Prod. 2022, 338, 130575. [Google Scholar] [CrossRef]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon nanotube assembly and integration for applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef]
- Huang, J.Y.; Yasuda, H.; Mori, H. Highly curved carbon nanostructures produced by ball-milling. Chem. Phys. Lett. 1999, 303, 130–134. [Google Scholar] [CrossRef]
- Oh, Y.; Choi, J.; Kim, Y.; Kim, K.; Baik, S. The effects of ball milling process on the diameter dependent fracture of single walled carbon nanotubes. Scr. Mater. 2007, 56, 741–744. [Google Scholar] [CrossRef]
- Liu, Z.T.; Fei, Z.H.; Wang, J.P.; Li, Z.X.; Chen, J.; Wu, X.H. Adsorption of nitrobenzene using short open-ended carbon nanotubes as adsorbent. Asian J. Chem. 2014, 26, 903–905. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, H.S.; Kim, Y.J.; Chung, H. Structural modification of carbon nanotubes by various ball milling. J. Alloys Compd. 2007, 434, 428–432. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Coa, F.; Strauss, M.; Clemente, Z.; Rodrigues Neto, L.L.; Lopes, J.R.; Alencar, R.S.; Souza Filho, A.G.; Alves, O.L.; Castro, V.; Barbieri, E.; et al. Coating carbon nanotubes with humic acid using an eco-friendly mechanochemical method: Application for Cu(II) ions removal from water and aquatic ecotoxicity. Sci. Total Environ. 2017, 607–608, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Bor, A.; Ichinkhorloo, B.; Uyanga, B.; Lee, J.; Choi, H. Cu/CNT nanocomposite fabrication with different raw material properties using a planetary ball milling process. Powder Technol. 2018, 323, 563–573. [Google Scholar] [CrossRef]
- Jargalsaikhan, B.; Bor, A.; Lee, J.; Choi, H. Al/CNT nanocomposite fabrication on the different property of raw material using a planetary ball mill. Adv. Powder Technol. 2020, 31, 1957–1962. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zhang, X.M.; Deng, J.H.; Liu, Y. A novel CNTs-Fe3O4 synthetized via a ball-milling strategy as efficient fenton-like catalyst for degradation of sulfonamides. Chemosphere 2021, 277, 130305. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Cao, T.M.; Nguyen, T.T.; Van Pham, V. Improving photocatalytic oxidation of semiconductor (TiO2, SnO2, ZnO)/CNTs for nox removal. J. Ind. Eng. Chem. 2023, 127, 321–330. [Google Scholar] [CrossRef]
- Olszewski, R.; Nadolska, M.; Lapinski, M.; Przesniak-Welenc, M.; Cieslik, B.M.; Zelechowska, K. Solvent-free synthesis of phosphonic graphene derivative and its application in mercury ions adsorption. Nanomaterials 2019, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ji, X.; Wang, W.; Chen, X.; Wang, P.; Wang, L.; Bai, J. Highly flexible, thermally stable, and static dissipative nanocomposite with reduced functionalized graphene oxide processed through 3d printing. Compos. Part B Eng. 2021, 208, 108598. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-based materials for effective adsorption of organic and inorganic pollutants: A critical and comprehensive review. Sci. Total Environ. 2023, 863, 160871. [Google Scholar] [CrossRef]
- Yu, W.G.; Gao, X.F.; Yuan, Z.C.; Liu, H.H.; Wang, X.C.; Zhang, X.X. Facial fabrication of few-layer functionalized graphene with sole functional group through diels-alder reaction by ball milling. RSC Adv. 2022, 12, 17990–18003. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Yadav, S.; Hung, W.S.; Lai, J.Y. One-pot eco-friendly synthesis of edge-carboxylate graphene via dry ball milling for enhanced removal of acid and basic dyes from single or mixed aqueous solution. J. Clean. Prod. 2020, 263, 121498. [Google Scholar] [CrossRef]
- Chandran, S.V.; Narayanan, B.N. Copper oxide incorporated ball-mill produced less-defective graphene for hybrid supercapacitors. Diam. Relat. Mater. 2024, 143, 110842. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Binitha, N.N. Graphene preparation by jaggery assisted ball-milling of graphite for the adsorption of Cr(VI). Mater. Today Proc. 2020, 25, 236–240. [Google Scholar] [CrossRef]
- Pan, P.F.; Wang, X.; Ji, Y.Q.; Dong, W.M.; Zhang, L.; Wang, L.; Zhang, M. One-step synthesis of ZrO2 nanopowders dispersed with graphene by ball milling. Ceram. Int. 2020, 46, 24799–24804. [Google Scholar] [CrossRef]
- Caicedo, F.M.C.; López, E.V.; Agarwal, A.; Drozd, V.; Durygin, A.; Hernandez, A.F.; Wang, C.L. Synthesis of graphene oxide from graphite by ball milling. Diam. Relat. Mater. 2020, 109, 108064. [Google Scholar] [CrossRef]
- Zhuang, S.; Lee, E.S.; Lei, L.; Nunna, B.B.; Kuang, L.; Zhang, W. Synthesis of nitrogen-doped graphene catalyst by high-energy wet ball milling for electrochemical systems. Int. J. Energy Res. 2016, 40, 2136–2149. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Chen, D.; Wei, F.H.; Chen, N.N.; Liang, Z.; Luo, Y. Removal of congo red dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason. Sonochem. 2017, 39, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chi, Q.Q.; Zhou, H.; Gao, P. Ball-milling preparation of titanium/graphene composites and its enhanced hydrogen storage ability. Int. J. Hydrogen Energy 2018, 43, 19164–19173. [Google Scholar] [CrossRef]
- Wang, W.G.; Babu, D.D.; Huang, Y.Y.; Lv, J.Q.; Wang, Y.B.; Wu, M.X. Atomic dispersion of Fe/Co/N on graphene by ball-milling for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 10351–10358. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Increase in the dehydrogenation rates and hydrogen-storage capacity of mg-graphene composites by adding nickel via reactive ball milling. Mater. Res. Bull. 2020, 130, 110938. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.Q.; Song, J.H.; Zhang, Y.J.; Shi, Q.; Wang, J.X.; Tian, F.H.; Yuan, S.; Su, Z.; Zhou, C.; et al. Functionalization-assistant ball milling towards si/graphene anodes in high performance li-ion batteries. Carbon 2021, 181, 300–309. [Google Scholar] [CrossRef]
- Kahimbi, H.; Hong, S.B.; Yang, M.H.; Choi, B.G. Simultaneous synthesis of nio/reduced graphene oxide composites by ball milling using bulk ni and graphite oxide for supercapacitor applications. J. Electroanal. Chem. 2017, 786, 14–19. [Google Scholar] [CrossRef]
- Tan, M.A.; Qin, Y.; Luo, J.X.; Wang, Y.P.; Zhang, F.Z.; Zhao, X.H.; Lei, X.D. Cus loaded on reduced graphene oxide prepared by ball milling method as cathode material for high- power aqueous Cu-Al hybrid-ion batteries. Electrochim. Acta 2024, 476, 143734. [Google Scholar] [CrossRef]
- Ahmad, J.; Sofi, F.A.; Mehraj, O.; Majid, K. Fabrication of highly photocatalytic active anatase TiO2-graphene oxide heterostructures via solid phase ball milling for environmental remediation. Surf. Interfaces 2018, 13, 186–195. [Google Scholar] [CrossRef]
- Cheng, J.P.; Xiong, D.P.; Jiang, W.Q.; Ye, W.B.; Song, P.; Feng, Z.Y.; He, M. SnO2-MoO2 nanoparticles coated on graphene oxide as a high-capacity, high-speed, long-life lithium-ion battery anode. Chem. Phys. Lett. 2024, 835, 140994. [Google Scholar] [CrossRef]
- Ravichandran, S.; Sahadevan, J.; Sivaprakash, P.; Sagadevan, S.; Kim, I.; Tighezza, A.M.; Ali, A.; Muthu, S.E. Synthesis and physicochemical properties of graphene incorporated indium tin oxide nanocomposites for optoelectronic device applications. Mat. Sci. Eng. B-Adv. 2024, 301, 117199. [Google Scholar] [CrossRef]
- Mondal, O.; Mitra, S.; Pal, M.; Datta, A.; Dhara, S.; Chakravorty, D. Reduced graphene oxide synthesis by high energy ball milling. Mater. Chem. Phys. 2015, 161, 123–129. [Google Scholar] [CrossRef]
- Li, T.L.; Teng, Y.X.; Li, X.; Luo, S.J.; Xiu, Z.M.; Wang, H.T.; Sun, H.W. Sulfidated microscale zero-valent iron/reduced graphene oxide composite (S-mZVI/rGO) for enhanced degradation of trichloroethylene: The role of hydrogen spillover. J. Hazard. Mater. 2023, 446, 130657. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Devasena, M.; Nambi, I.M. Synthesis, characterization and performance of high energy ball milled meso-scale zero valent iron in fenton reaction. J. Environ. Manag. 2016, 181, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; She, L.; Guo, F.; Jia, F.; Zhang, L.; Ai, Z.; Liu, X. Ball-milled zero-valent iron with formic acid for effectively removing Cu(II)-edta accomplished by edta ligands oxidative degradation and Cu(II) removal. J. Hazard. Mater. 2024, 465, 133009. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zheng, Z.; Zhao, L.; Qiu, Z.; Zeng, D. Oxalic acid modification enables high efficiency and proton conductive of Fe-base amorphous toward acid orange II in wastewater removal. Sep. Purif. Technol. 2024, 332, 125768. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Gao, Y.; Yan, S.; Peng, X.; Shen, W. Efficient Cu(II) removal with boronated zero-valent iron via inhibition of oxygen adsorption and enhanced electron transfer. Appl. Surf. Sci. 2024, 654, 159466. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, Y.; Jiao, X.; Ding, N.; Jiao, Y.; Pan, Y.; Xue, J.; Zhang, Y. Polyphenol-modified zero-valent iron prepared using ball milling technology for hexavalent chromium removal: Kinetics and mechanisms. Sep. Purif. Technol. 2023, 326, 124874. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, J.; Xiao, M.; Zhang, X.; Li, J.; Jiang, W.; Yan, J.; Qin, Z.; Zhang, S.; He, W.; et al. Ethylenediaminetetraacetic acid induces surface erosion of zero-valent iron for enhanced hexavalent chromium removal. Appl. Surf. Sci. 2020, 525, 146593. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, L.; Zhang, Y.; Zhou, S.; Yu, Z.; Liu, X.; Chen, C. Enhanced removal of organic pollutants by ball-milled Fes/ZVl activated persulfate process: Characterization, performance, and mechanisms. Surf. Interfaces 2022, 29, 101697. [Google Scholar] [CrossRef]
- Du, M.; Kuang, H.; Zhang, Y.; Zeng, X.; Yi, C.; Hussain, I.; Huang, S.; Zhao, S. Enhancement of ball-milling on pyrite/zero-valent iron for persulfate activation on imidacloprid removal in aqueous solution: A mechanistic study. J. Environ. Chem. Eng. 2021, 9, 105647. [Google Scholar] [CrossRef]
- Song, X.; Tian, J.; Ma, J.; Ni, J.; Liu, D.; Wang, W.; Shi, W.; Yuan, Y.; Cui, F.; Chen, Z. Peroxydisulfate activation by a versatile ball-milled nZVl@MoS2 composite: Performance and potential activation mechanism. Chem. Eng. J. 2023, 453, 139830. [Google Scholar] [CrossRef]
- Wu, S.; Deng, S.; Ma, Z.; Liu, Y.; Yang, Y.; Jiang, Y. Ferrous oxalate covered zvi through ball-milling for enhanced catalytic oxidation of organic contaminants with persulfate. Chemosphere 2022, 287, 132421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ding, G.; Yan, L.; Wang, R.; Zhang, W.; Wang, X.; Rao, P. Ball-milled sulfide iron-copper bimetals based composite permeable materials for Cr(VI) removal: Effects of preparation parameters and kinetics study. Chemosphere 2023, 338, 139388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.; Kang, S.; Bai, H.; Gu, W.; Fang, D.; Komarneni, S.; Zhang, Q. Mechanical activation of zero-valent iron (ZVl) in the presence of CaCo3: Improved reactivity of zvi for enhancing As(III) removal from water. J. Clean. Prod. 2021, 286, 124926. [Google Scholar] [CrossRef]
- Zhao, J.; Su, A.; Tian, P.; Tang, X.; Collins, R.N.; He, F. Arsenic (III) removal by mechanochemically sulfidated microscale zero valent iron under anoxic and oxic conditions. Water Res. 2021, 198, 117132. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, S.; Liu, Y.; Cao, Z.; Yang, L.; He, F. Enhanced removal of hexavalent chromium by lignosulfonate modified zero valent iron: Reaction kinetic, performance and mechanism. Sci. Total Environ. 2023, 857, 159397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liu, Q.; Qin, Y.; Liu, W.; Zhang, L. Efficient Fe(III)/Fe(II) cycling mediated by l-cysteine functionalized zero-valent iron for enhancing Cr(VI) removal. J. Hazard. Mater. 2023, 456, 131717. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, D.; Gao, F.; Liu, L.; Liu, Q.; Wang, L.; Tang, J. Mechanochemical nitridation of micron zero-valent iron for enhanced dechlorination of trichloroethylene: Mechanistic insights into nitrogen sources and ball milling conditions. Sep. Purif. Technol. 2024, 337, 126381. [Google Scholar] [CrossRef]
- Hu, Y.; Ke, K.; Sun, H.; Wang, Z.; Zhang, X.; Shen, W.; Huang, S.; Lu, W.; Wang, X. Coffee grounds modified zero-valent iron for efficient heavy metal removal. J. Water Process. Eng. 2023, 56, 104397. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Fan, J.; Wang, W.; Li, L.; Liu, J. Spinel ferrite-enhanced Cr(VI) removal performance of micro-scale zero-valent aluminum: Synergistic effects of oxide film destruction and lattice spacing expansion. Sep. Purif. Technol. 2022, 294, 121110. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, Y.; Liu, J.; Zhang, Y.; Yang, S. Ethanol-assisted mechanical activation of zero-valent aluminum for fast and highly efficient removal of Cr(VI). Appl. Surf. Sci. 2020, 533, 147543. [Google Scholar] [CrossRef]
- Wu, S.; Yang, S.Y.; Liu, S.J.; Zhang, Y.X.; Ren, T.F.; Zhang, Y.Q. Enhanced reactivity of zero-valent aluminum with ball milling for phenol oxidative degradation. J. Colloid Interface Sci. 2020, 560, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.T.; Yang, S.Y.; Wang, M.Q.; Xue, Y.C.; Liu, J.Q.; Li, Y.; Zhao, D.Y. A novel ball-milled aluminum-carbon composite for enhanced adsorption and degradation of hexabromocyclododecane. Chemosphere 2021, 279, 130520. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, Q.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K. Insights into mathematical characteristics of adsorption models and physical meaning of corresponding parameters. J. Mol. Liq. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: Adsorption behavior and process study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Ye, H.Y.; Yu, K.; Li, B.; Guo, J.Z. Study on adsorption properties and mechanism of sodium hydroxide-modified ball-milled biochar to dislodge lead(II) and mb from water. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, P.; Wang, X.; Cao, Y.; Han, L. Qualitative and quantitative correlation of physicochemical characteristics and lead sorption behaviors of crop residue-derived chars. Bioresour. Technol. 2018, 270, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, R.; Chen, G. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Deng, H.; Zhang, Z.; Wang, J.J.; Shaheen, S.M.; Xiao, R.; Rinklebe, J.; Xi, B.; He, X.; et al. Removing tetracycline and Hg(II) with ball-milled magnetic nanobiochar and its potential on polluted irrigation water reclamation. J. Hazard. Mater. 2020, 384, 121095. [Google Scholar] [CrossRef]
- Qu, J.; Wu, Z.; Liu, Y.; Li, R.; Wang, D.; Wang, S.; Wei, S.; Zhang, J.; Tao, Y.; Jiang, Z.; et al. Ball milling potassium ferrate activated biochar for efficient chromium and tetracycline decontamination: Insights into activation and adsorption mechanisms. Bioresour. Technol. 2022, 360, 127407. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, J.; Xiao, X.; Liao, X.; Zhang, S.; Shi, B. Pyrolysis combined ball-milling for the preparation of biochar from chinese baijiu distillers’ grains for the adsorption of heavy metal ions. Ind. Crops Prod. 2023, 203, 117234. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Alhogbi, B.G. Preparation and characterization of SiO2@C nanocomposites from rice husk for removal of heavy metals from aqueous solution. Ceram. Int. 2021, 47, 23240–23248. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, B.; Tong, H.; Liu, Y.; Wang, S.; Wei, S.; Wang, L.; Wang, Y.; Zhang, Y. High-efficiency decontamination of Pb(II) and tetracycline in contaminated water using ball-milled magnetic bone derived biochar. J. Clean. Prod. 2023, 385, 135683. [Google Scholar] [CrossRef]
- Qu, J.; Che, N.J.; Niu, G.L.; Liu, L.F.; Li, C.L.; Liu, Y.L. Iron/manganese binary metal oxide-biochar nano-composites with high adsorption capacities of Cd2+: Preparation and adsorption mechanisms. J. Water Process. Eng. 2023, 51, 103332. [Google Scholar] [CrossRef]
- Di, Z.C.; Ding, J.; Peng, X.J.; Li, Y.H.; Luan, Z.K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hu, E.; Yang, S.; Gong, L.; He, F. Chromium(VI) removal by mechanochemically sulfidated zero valent iron and its effect on dechlorination of trichloroethene as a co-contaminant. Sci. Total Environ. 2019, 650, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiao, W.; Shen, G.; Ji, G.; Zhang, Y.; Gao, C.; Han, L. Carbonization and ball milling on the enhancement of Pb(II) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour. Technol. 2019, 273, 70–76. [Google Scholar] [CrossRef]
- Du, H.; Xi, C.; Tang, B.; Chen, W.; Deng, W.; Cao, S.; Jiang, G. Performance and mechanisms of naoh and ball-milling co-modified biochar for enhanced the removal of Cd2+ in synthetic water: A combined experimental and dft study. Arab. J. Chem. 2022, 15, 103817. [Google Scholar] [CrossRef]
- Meng, P.; Fang, X.; Maimaiti, A.; Yu, G.; Deng, S. Efficient removal of perfluorinated compounds from water using a regenerable magnetic activated carbon. Chemosphere 2019, 224, 187–194. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Pulicharla, R.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Pine-wood derived nanobiochar for removal of carbamazepine from aqueous media: Adsorption behavior and influential parameters. Arab. J. Chem. 2019, 12, 5292–5301. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zeng, C.; Ding, Y.; Tang, J.; Mašek, O.; Deng, Z.; Mu, R.; Zhang, Z. Facile synthesis of ball milling and magnetization co-modified sludge-derived biochar for efficient adsorbing environmental concentration sulfamethoxazole from various waters: Performance and mechanism. Sep. Purif. Technol. 2024, 331, 125584. [Google Scholar] [CrossRef]
- Tang, J.; Ma, Y.; Cui, S.; Ding, Y.; Zhu, J.; Chen, X.; Zhang, Z. Insights on ball milling enhanced iron magnesium layered double oxides bagasse biochar composite for ciprofloxacin adsorptive removal from water. Bioresour. Technol. 2022, 359, 127468. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yi, Y.; Zhang, N.; Tsang, P.E.; Fang, Z. Removal of fluconazole from aqueous solution by magnetic biochar treated by ball milling: Adsorption performance and mechanism. Environ. Sci. Pollut. R 2022, 29, 33335–33344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Zimmerman, A.R.; Chen, H.; Hu, X.; Gao, B. Mechanisms and adsorption capacities of hydrogen peroxide modified ball milled biochar for the removal of methylene blue from aqueous solutions. Bioresour. Technol. 2021, 337, 125432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wei, C.; Yu, Z.; Ji, L.; Liu, M.; Cao, Q.; Wu, L.; Li, F. Fabrication of iron-containing biochar by one-step ball milling for Cr(VI) and tetracycline removal from wastewater. Langmuir 2023, 39, 18958–18970. [Google Scholar] [CrossRef]
- Yan, N.; Hu, B.; Zheng, Z.; Lu, H.; Chen, J.; Zhang, X.; Jiang, X.; Wu, Y.; Dolfing, J.; Xu, L. Twice-milled magnetic biochar: A recyclable material for efficient removal of methylene blue from wastewater. Bioresour. Technol. 2023, 372, 128663. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhang, A.M.; Yin, Y.; Dong, Y.M.; Cui, Y.C.; Zhang, X.; Hong, J.M. The investigation of adsorptive performance on modified multi-walled carbon nanotubes by mechanical ball milling. Mater. Chem. Phys. 2007, 101, 30–34. [Google Scholar] [CrossRef]
- Araga, R.; Kali, S.; Sharma, C.S. Coconut-shell-derived carbon/carbon nanotube composite for fluoride adsorption from aqueous solution. CLEAN–Soil Air Water 2019, 47, 1800286. [Google Scholar] [CrossRef]
- Feng, K.; Xu, Z.; Gao, B.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Mesoporous ball-milling iron-loaded biochar for enhanced sorption of reactive red: Performance and mechanisms. Environ. Pollut. 2021, 290, 117992. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ma, Y.; Zeng, C.; Yang, L.; Cui, S.; Zhi, S.; Yang, F.; Ding, Y.; Zhang, K.; Zhang, Z. Fe-al bimetallic oxides functionalized-biochar via ball milling for enhanced adsorption of tetracycline in water. Bioresour. Technol. 2023, 369, 128385. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process. Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
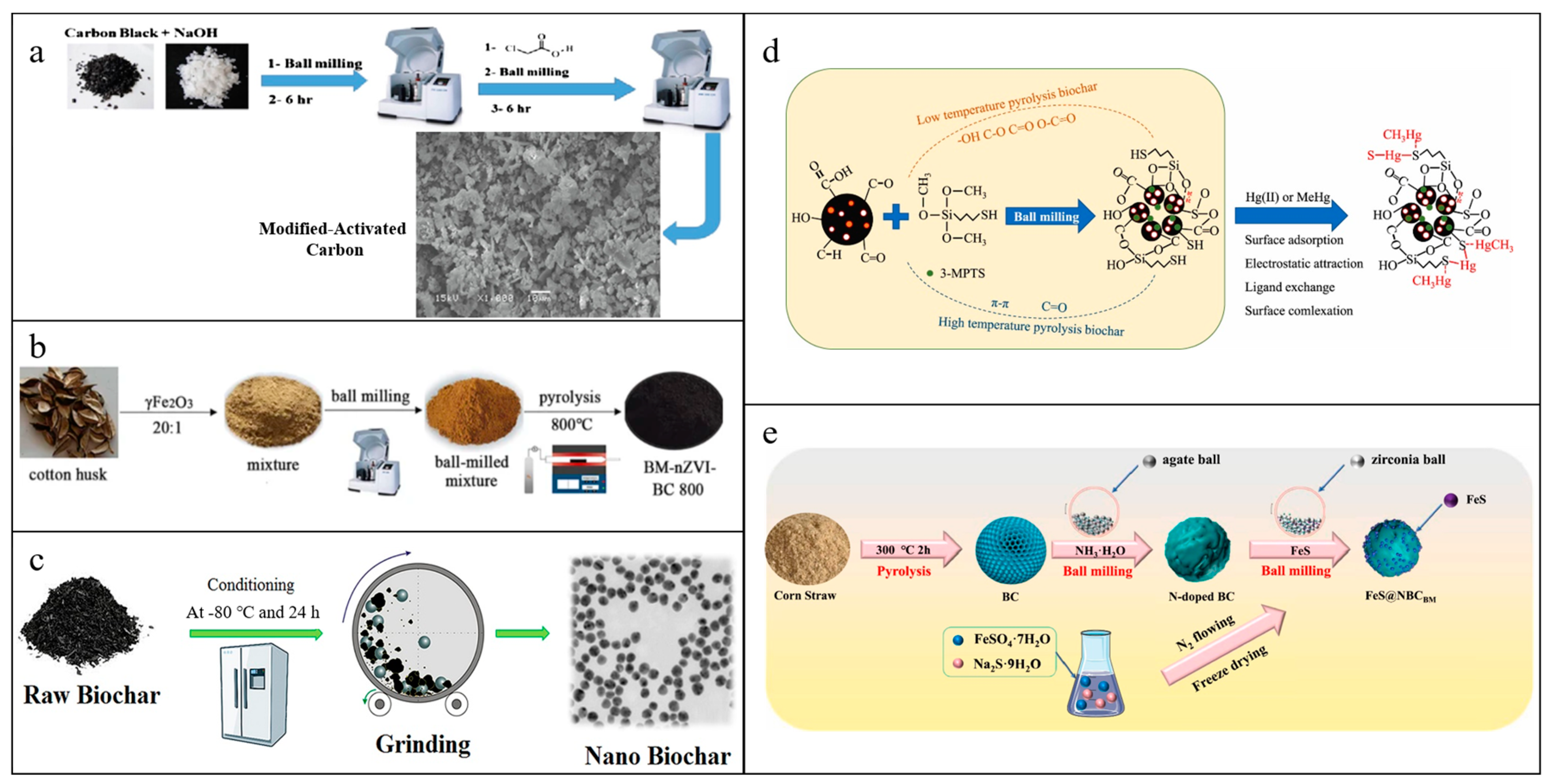
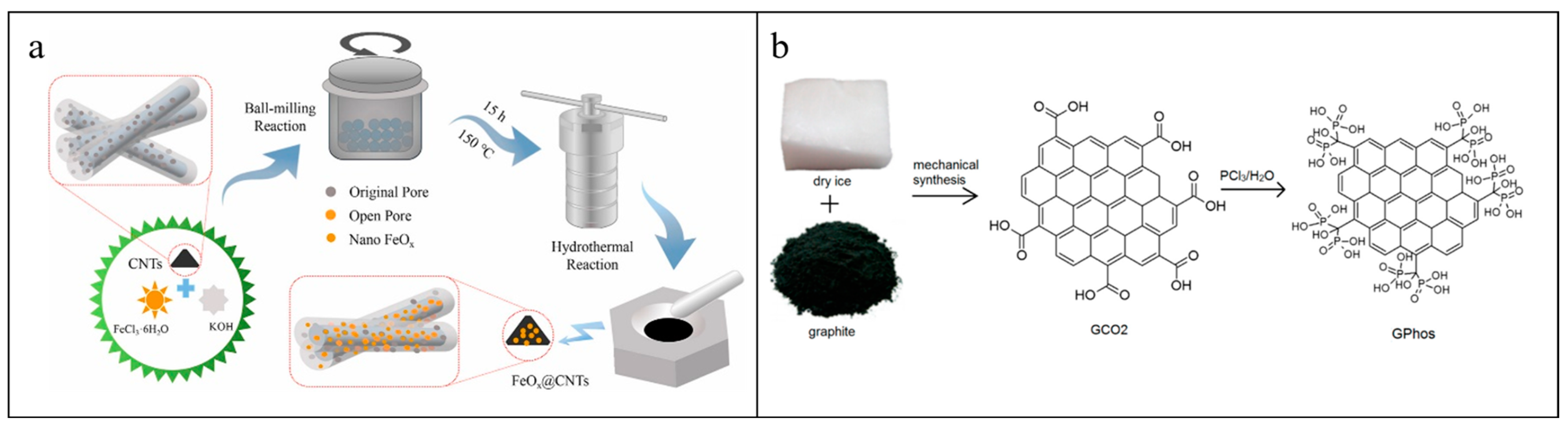
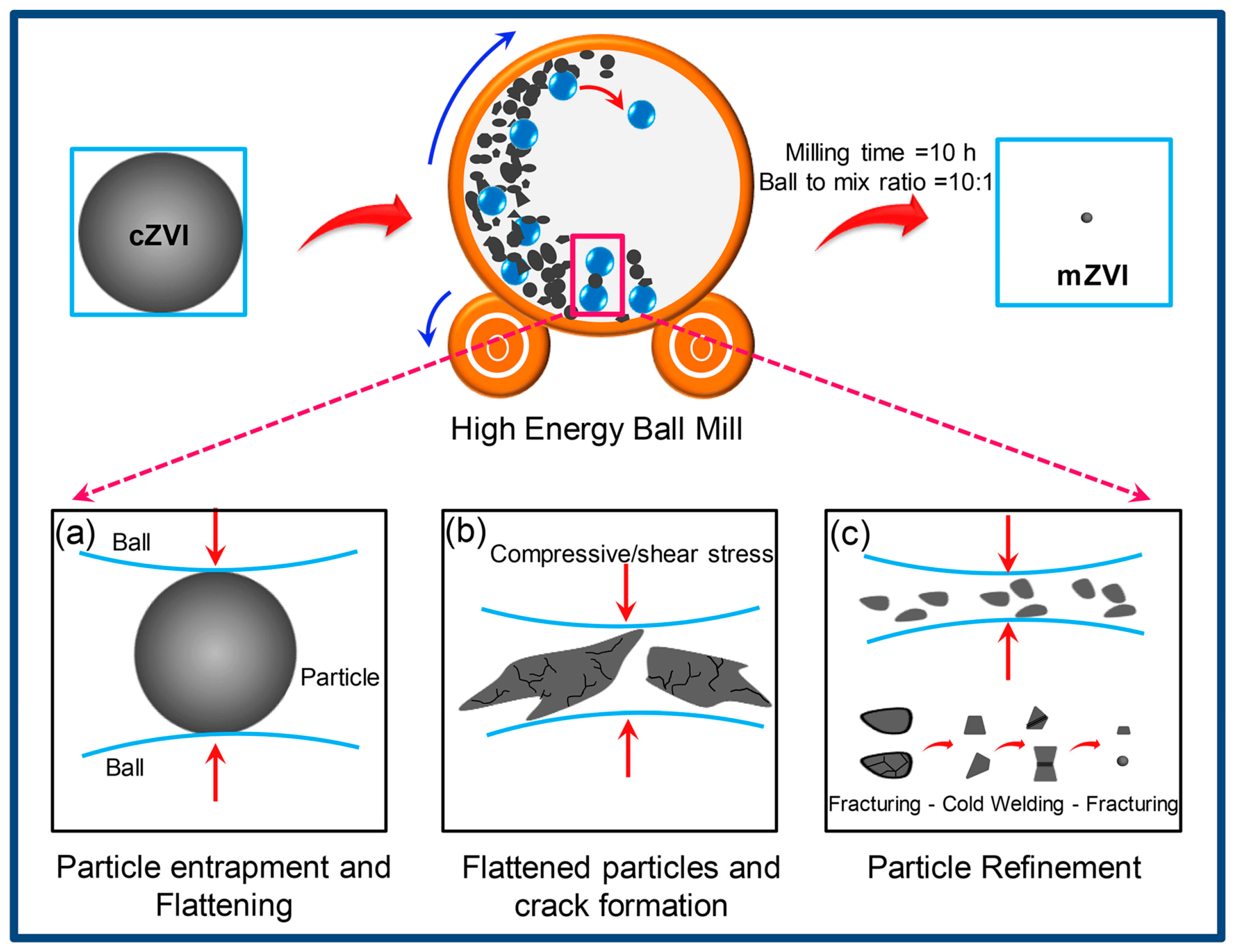
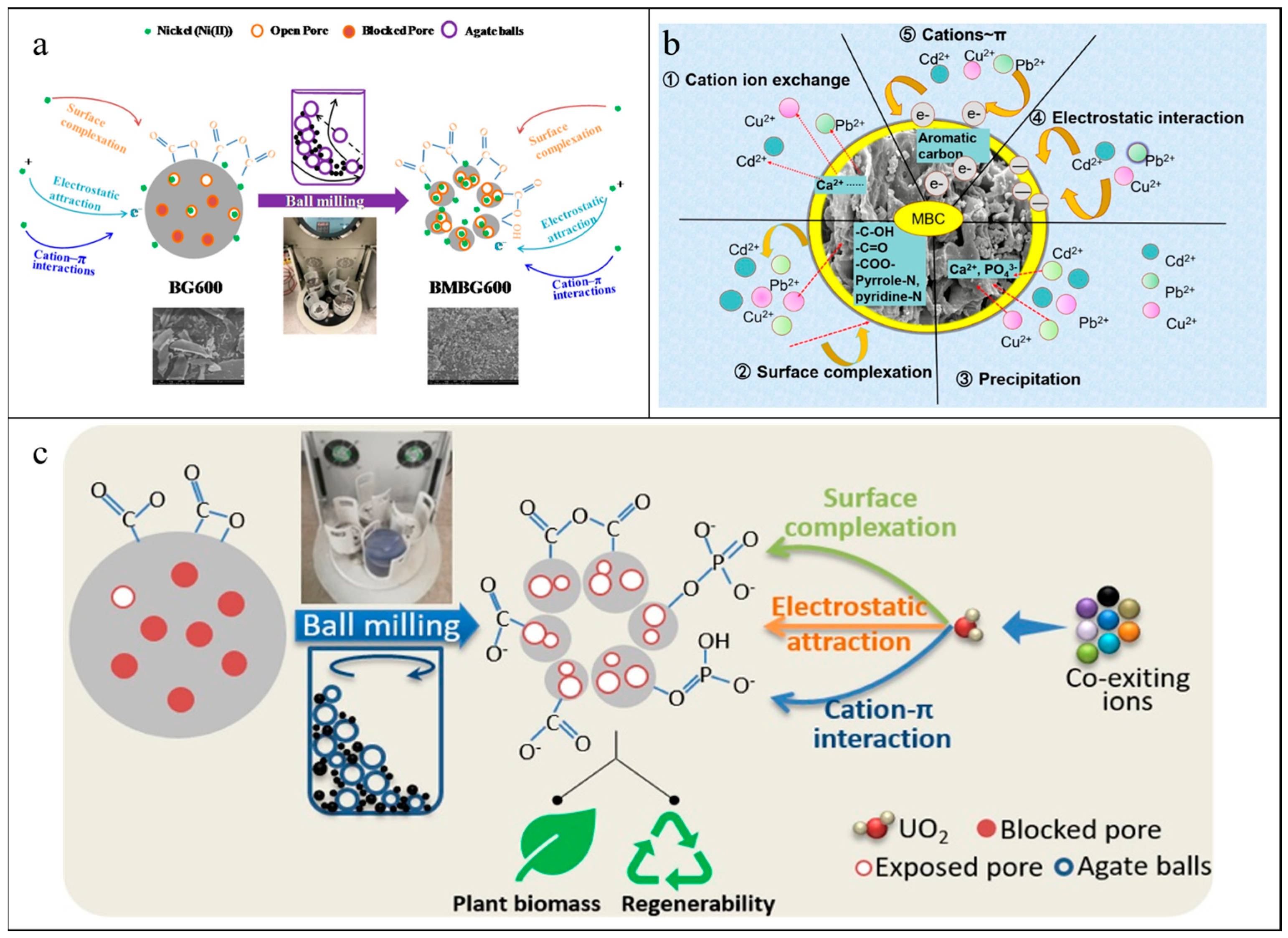
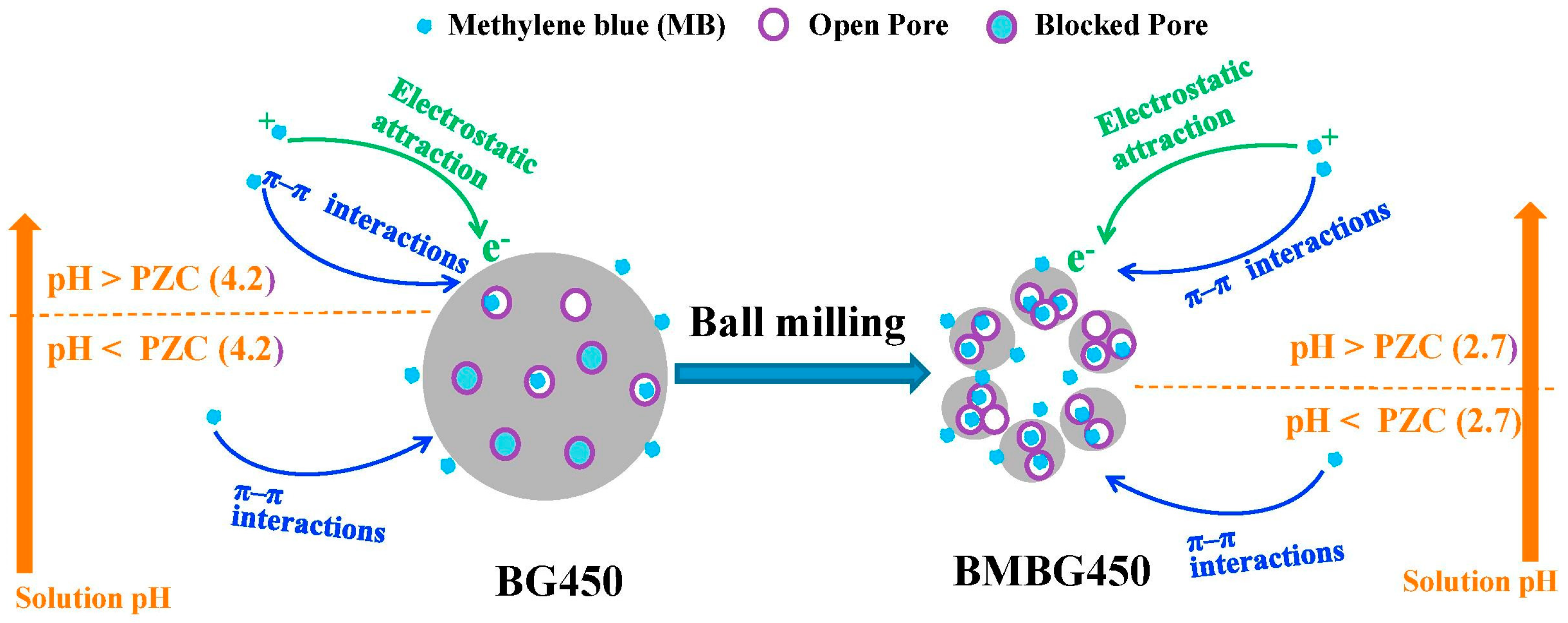
| Materials | Pollutants | Regeneration Method | Reusability | Ref. | |
|---|---|---|---|---|---|
| BM-SnZVI@BC | Phosphorus | Ball milling regeneration | Ball milling adsorbents again | The removal rate of phosphate was 91.6% by ball milling adsorbents again, and the removal rate still reached 80.4% after five cycles. | [106] |
| Mechanically activated ZVAl particles | Cr(VI) | Ball milling adsorbents again | The Cr(VI) removal was restored to 100% by ball milling ZVAls again. | [162] | |
| ZVAls/NiFe2O4 | Cr(VI) | Ball milling adsorbents again with additional amount of NiFe2O4 | The Cr(VI) removal was restored to 100% after the second ball milling of materials. | [161] | |
| ZVAls/Fe3O4 | Cr(VI) | Ball milling adsorbents again with additional amount of magnetic powders. | The removal efficiency of Cr(VI) was restored by ball milling ZVAl/Fe3O4 with magnetic powders again. | [34] | |
| ZVIs/AC | Cr(VI) | Ball milling adsorbents again with additional amount of ACs. | The efficiency of Cr(VI) removal could almost be recovered completely by ball milling ZVI/AC with a small amount of ACs. | [8] | |
| Graphite-like biochars | IMI SUL | Thermal regeneration | Pyrolysis at 500 °C for 2 h under N2 atmosphere | The adsorption capacities of IMI and SUL decreased to 85.6–88.3% and 86.7–89.7% of the initial adsorption capacities, respectively, after the fifth cycle. | [33] |
| HGO | Cr(VI) | Thermal regeneration combined with solvents | 1.0 M NaOH and calcination at 400 °C for 1 h | There was 100% retention of its initial performance in further adsorption studies. | [127] |
| HACs | Cr(VI) | Solvent regeneration | 0.1 M H2SO4 | The removal efficiency of Cr(VI) increased from 92.2% to 96.3% after acid regeneration. | [9] |
| PFBCs | U(VI) | 0.6 M HCl | The adsorption capacity of U(VI) decreased by about 33.25% within six cycles. | [89] | |
| 0.1 M Na2CO3 | The adsorption capacity of U(VI) decreased by about 21.6%, respectively, within six cycles. | ||||
| Fe/Mn-BCs | Cd(II) | 0.1 M HCl | The adsorption capacity maintained 41–70% of the first adsorption capacity after five cycles. | [175] | |
| BM-Fe3O4-BC | Pb(II) | 0.5 M NaOH | The adsorption capacity decreased by only 21.14% after three cycles. | [174] | |
| TC | Anhydrous ethanol | The desorption rate was still above 45.41% after three cycles. | |||
| MgO-biochars | Ni(II) | 0.01 M EDTA-2Na | The adsorption capacity remained at ∼80% in the 4th cycle and remained stable after that. | [172] | |
| Fe@MBC | TC | 0.1 M NaOH | The adsorption rate decreased from 84.56% to 78.43% after the third cycle. | [189] | |
| Mg/Al-BCs | TC | 0.1 M NaOH | The adsorption amount was 54.5 mg/g after the fifth cycle, which was 91.4% of the original adsorption capacity. | [194] | |
| BM-LDOs-BC | CIP | NaOH | The adsorption capacity was still about 50 mg/g after five cycles, which accounted for 83% of the original adsorption capacity. | [186] | |
| Ball-milling iron-loaded biochars | RR | 1.0 M NaOH | The adsorption capacities were 47.9 and 54.6 mg/g at pH of 3 and 7.5, respectively, after the third cycle. | [193] | |
| BM-PASBCs | SMX | 0.1 M NaOH | The adsorption capacities were 95.3% of the initial adsorption capacities after five cycles. | [84] | |
| BC-SBCs | SMX | 0.1M NaOH | The adsorption ability could reach 98.5% of the original amount after five cycles. | [185] | |
| BM-PBCs | Cr(VI) TC | 1.0 M NaOH | The desorption efficiencies for Cr (VI) and TC were above 72.6–73.5% and 58.8–65.0% after four cycles. | [171] | |
| BM-BCs | TC Hg(II) | 0.2 M NaOH 0.5 M Na2S | The adsorption amounts of TC and Hg(II) were approximately 90.55 and 87.36 mg/g, respectively, after five cycles. | [170] | |
| BM-BCs | Pb(II) | 0.1 M HNO3 | The adsorption efficiency of Pb(II) and MB remained about 85% after five cycles. | [167] | |
| MB | 0.1 M HCl | ||||
| AC-COOH | MB CV | 1.0 M HCl, 1.0 M NaOH, and water (1/1/1, v/v/v) | The adsorption capabilities of MB and CV decreased after each cycle. | [77] | |
| Magnetic ACs | PFCs | Methanol | In the first three adsorption cycles, the adsorption amount decreased slightly (from 0.66 mmol/g to 0.53 mmol/g) and then remained stable. | [180] | |
| MBCs | MB | Anhydrous ethanol | The adsorption rates were 90.1%, 86.7%, 84.8%, 82.3%, and 81.9%, respectively, in the five cycles. | [104] | |
| Twice-milled magnetic biochars | MB | Ethanol | There was only a slight drop in the adsorption capacity after four cycles. | [190] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Fan, X.; Sun, D.; Zeng, G.; Wang, Q.; Wang, Q. Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants. Water 2024, 16, 1639. https://doi.org/10.3390/w16121639
Gao P, Fan X, Sun D, Zeng G, Wang Q, Wang Q. Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants. Water. 2024; 16(12):1639. https://doi.org/10.3390/w16121639
Chicago/Turabian StyleGao, Pei, Xuanhao Fan, Da Sun, Guoming Zeng, Quanfeng Wang, and Qihui Wang. 2024. "Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants" Water 16, no. 12: 1639. https://doi.org/10.3390/w16121639






