Experimental Study on the Combined Effect of Electromagnetic and Electrochemical Processes on Descaling and Anticorrosion
Abstract
:1. Introduction
2. Theoretical Analysis
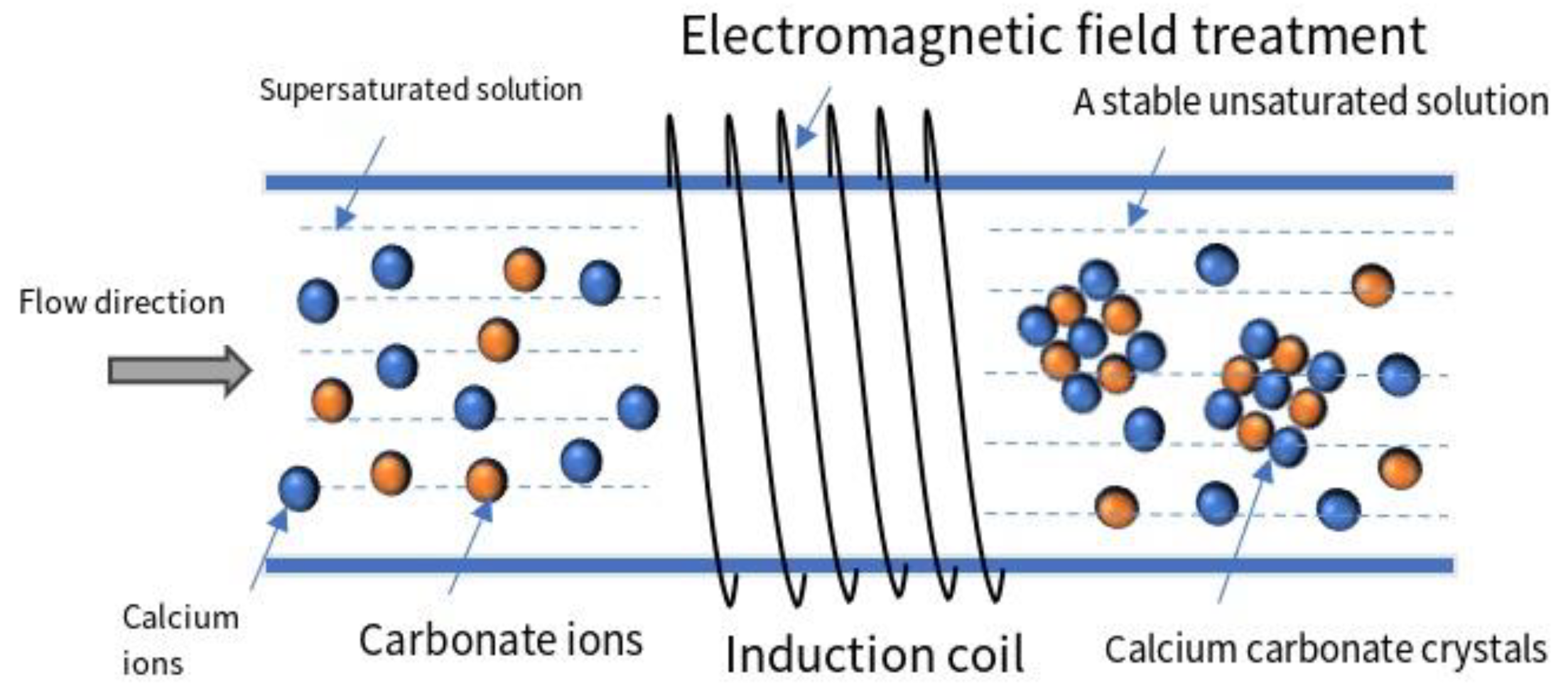
2.1. Electromagnetic Scale Inhibition and Corrosion Protection Principles
- Scale inhibition principle: During the process of circulating water experiencing electromagnetic fields, water molecules act as dipoles and undergo repeated polarization, leading to distortion, deformation, inversion, and vibration of water molecules. Under the influence of electromagnetic fields, the outer electrons of water molecules are activated, transitioning from lower energy level orbitals to higher energy level orbitals, causing the molecular lattice of scale samples to change from calcite structure to aragonite structure. With the increase in high-frequency vibration and molecular motion, various comprehensive chain like and cluster like macromolecules (H2O)n formed in water are decomposed into individual water molecules n (H2O), ultimately forming relatively stable diatomic water molecules connected by hydrogen bonds. This process enhances the activity and polarity of water, thereby weakening the electrostatic attraction between scaling ions and preventing their aggregation or chemical bonding. At the same time, various positive and negative particles are surrounded by water dipole clusters, no longer moving freely, but oriented along the direction of magnetic field lines, thereby disrupting the conditions for scale formation and achieving the goal of scale inhibition.
- Under the action of electromagnetic fields, water molecules undergo changes. Electromagnetic fields can affect the hydrogen bonding structure of water molecules, leading to the rearrangement of hydrogen bonds between water molecules, thereby changing the physical and chemical properties of water. At the same time, the solubility of oxygen also increases. As the solubility of oxygen increases, reaction occurs between iron rust, active water, and oxygen, forming a dense black oxide film, thereby preventing further corrosion of the pipe wall and achieving the effects of rust removal and rust prevention [10]. During the action of the electromagnetic field, a magnetic field gradient force is generated in pitting corrosion pits, with the maximum magnetic flux density produced at the edge of the pits. The gradient force acts on magnetic materials, attracting erosive ions to move outward, thereby slowing down the pitting corrosion process [11].
- Disinfection and algae elimination principle: Microorganisms generally can only adapt and survive in an electric field strength of 130 eV/m. The electromagnetic field can cause the loss of metabolic function of “repair enzymes” in microbial cells, inhibiting cell activity, thereby disrupting the living biological field of microorganisms and causing the leakage of protoplasm from the cells, leading to death. Simultaneously, a certain amount of reactive oxygen species (·OH, H2O2, etc.) can be generated in the water. Reactive oxygen can destroy ion channels of biological cells and accelerate the aging of cell membranes, thus exhibiting strong bactericidal and algaecidal effects.
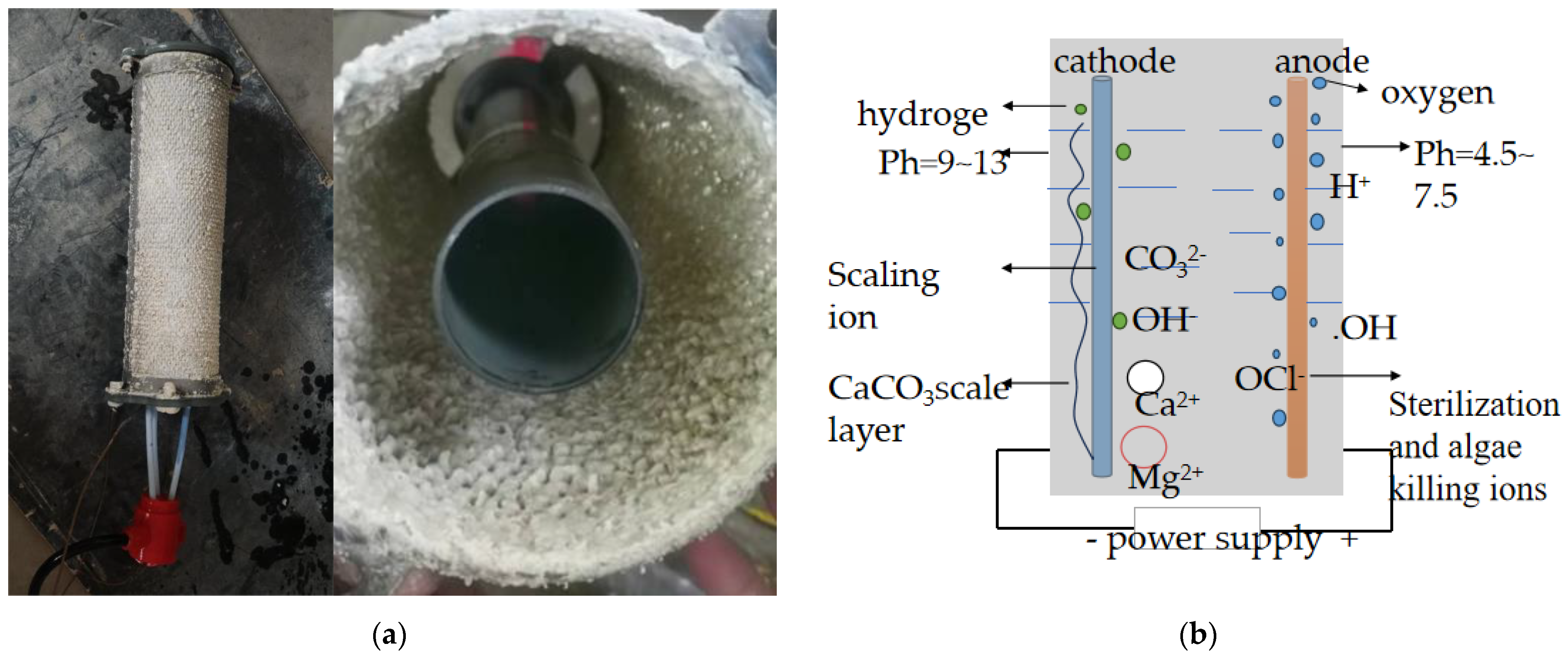
2.2. Principles of Electrochemical Water Treatment Technology
3. Experimental Research Design
3.1. Construction of Industrial Circulating Water Composite Scale Removal Experimental Platform
3.2. Experimental Design
- Experiment 1—Control Experiment: Under the conditions of a circulating water volume of 200 L, temperature of (30 ± 1) °C, and flow rate of 1000 L/h (0.6 m/s), the scaling and corrosion of circulating water were studied without the use of electromagnetic and electrochemical water treatment equipment, serving as a contrast to the experimental results of the electromagnetic group, electrochemical group, and their combined action.
- Experiment 2—Electromagnetic Group Experiment (EM Group): Keeping the circulating water volume, temperature, and flow rate constant, only the electromagnetic water treatment equipment was activated, with the frequency set to 1 kHz. The changes in water quality parameters and the scaling and corrosion of hanging plates after electromagnetic treatment were observed to study the effects of electromagnetic treatment on scale formation, corrosion, and algae inhibition in circulating water.
- Experiment 3—Electrochemical Group Experiment (EC Group): Keeping the circulating water volume, temperature, and flow rate constant, only the electrochemical water treatment equipment was activated, with the voltage set to 24 V and current to 10 A. The changes in water quality parameters and the scaling and corrosion of hanging plates after electrochemical treatment were observed to study the effects of electrochemical treatment on scale formation, corrosion, and algae inhibition in circulating water.
- Experiment 4—Electromagnetic + Electrochemical Group Experiment (EM + EC Group): Maintaining the circulating water volume at 200 L, temperature at (30 ± 1) °C, and flow rate at 1000 L/h (0.6 m/s), both electromagnetic and electrochemical equipment were activated. The frequency of the electromagnetic water treatment equipment was set to 1 kHz, and the voltage of the electrochemical water treatment equipment was set to 24 V with a current of 10 A. The changes in water quality parameters and the scaling and corrosion of hanging plates after the combined action of electromagnetic and electrochemical treatment were observed to study their effects on scale formation, corrosion, and algae inhibition in circulating water.
3.3. Experimental Analysis Methods
4. Discussion of Experimental Results
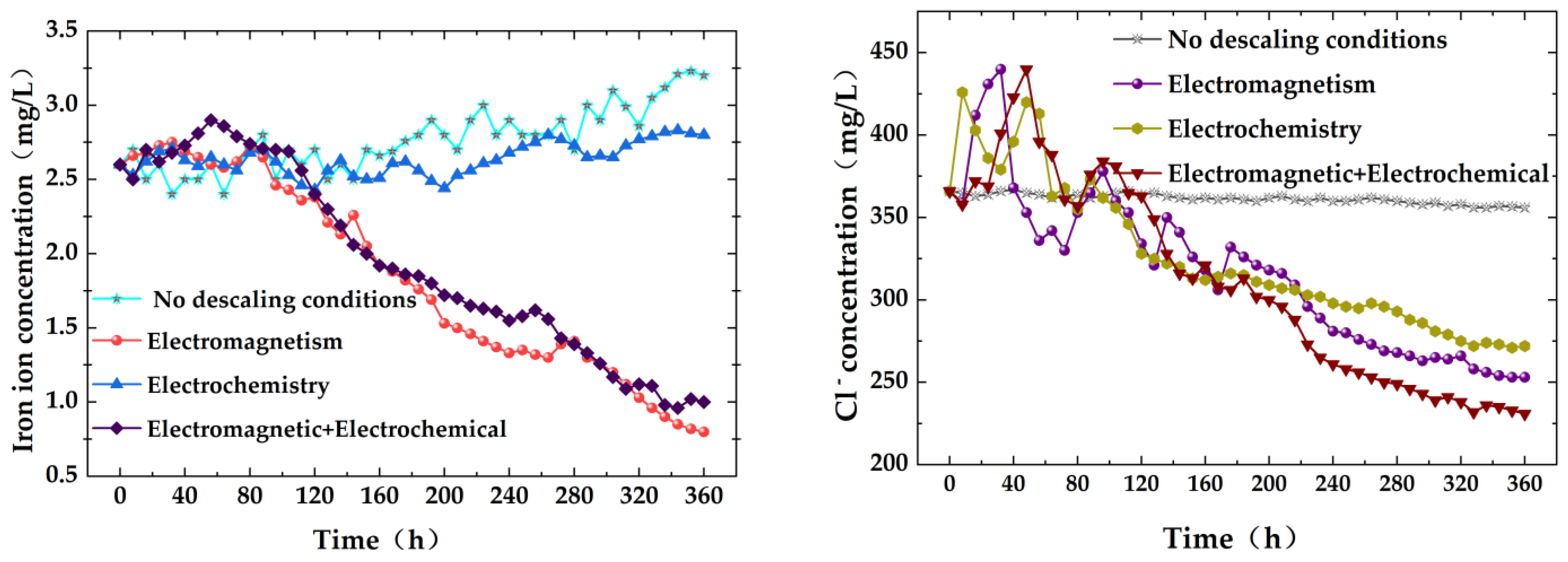
4.1. Change Rate of Water Quality Parameters under Different Descaling Conditions
4.2. Changes in the Quality of Stainless Steel Hanging Plates inside Heat Exchangers
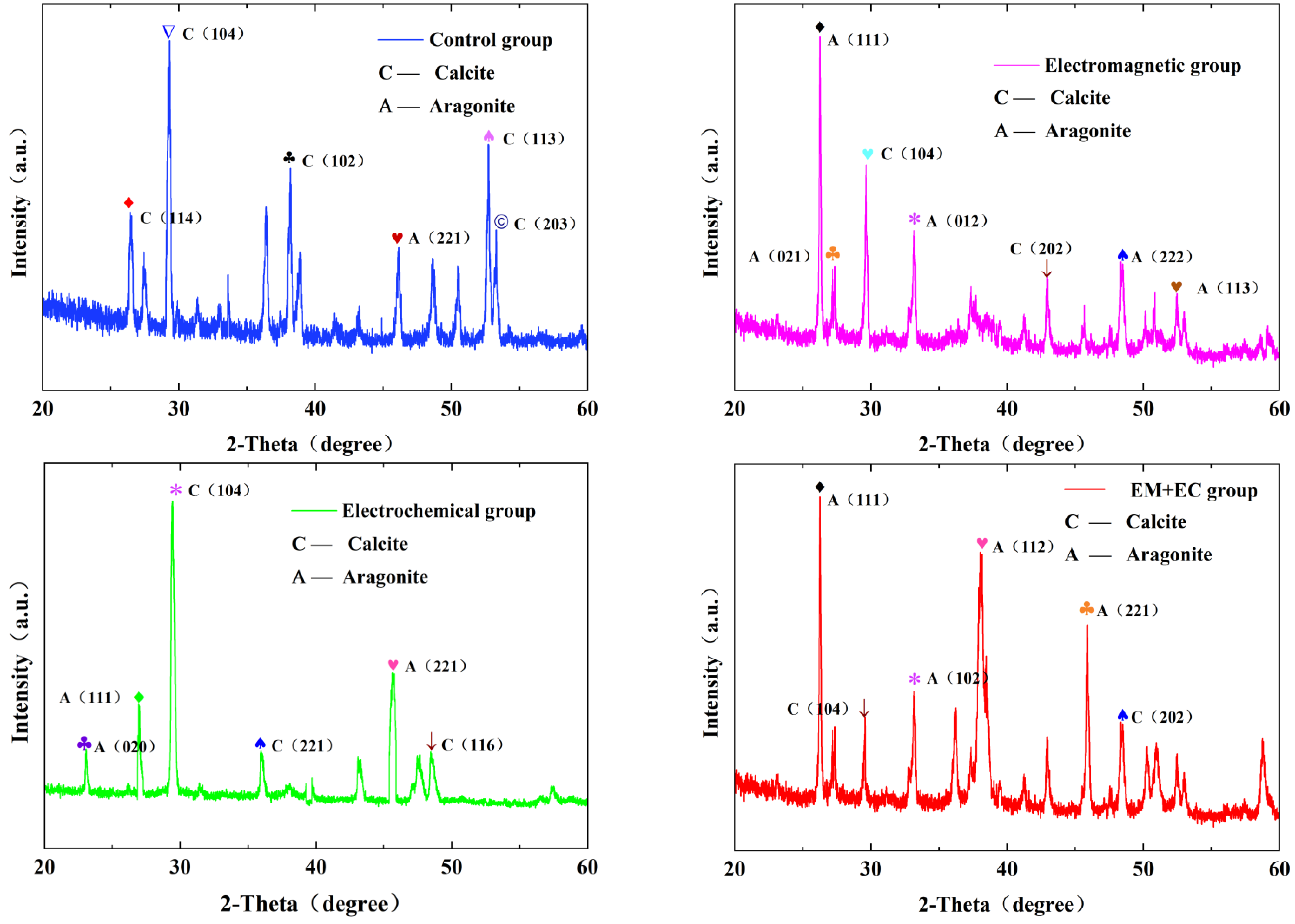
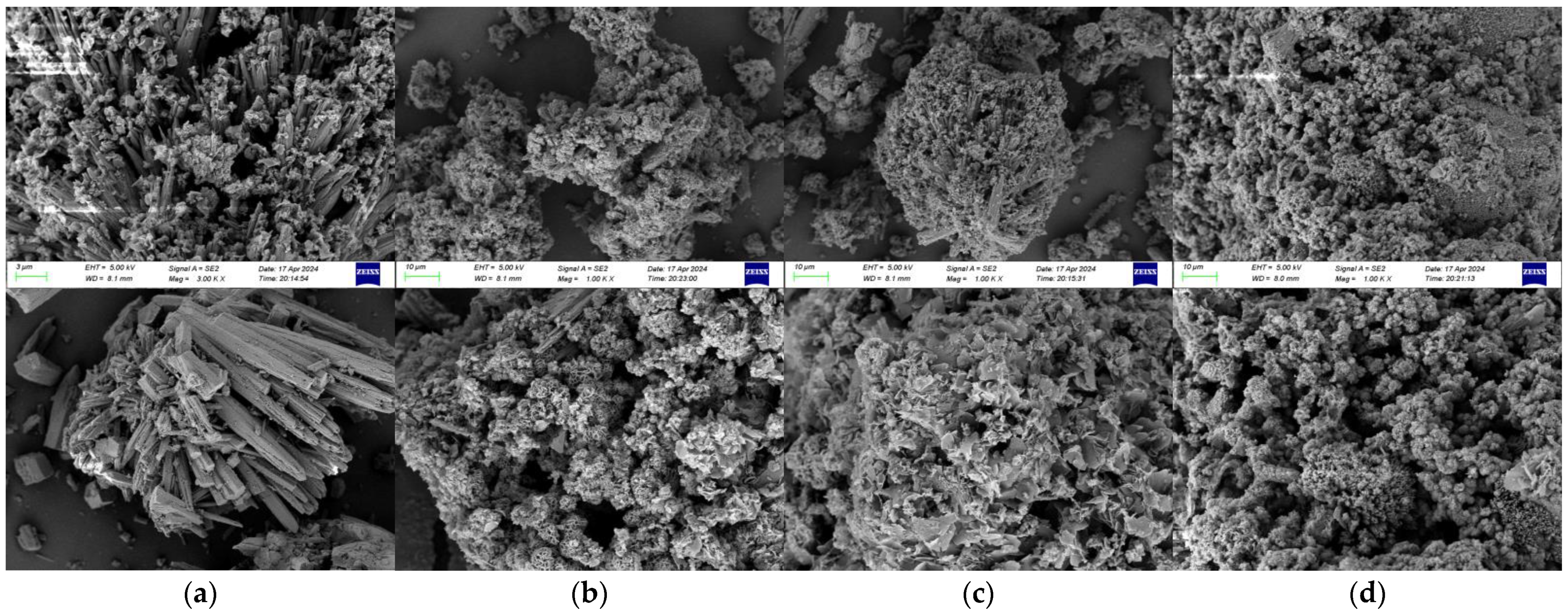
4.3. Effects of Different Descaling Conditions on Calcium Carbonate Crystals
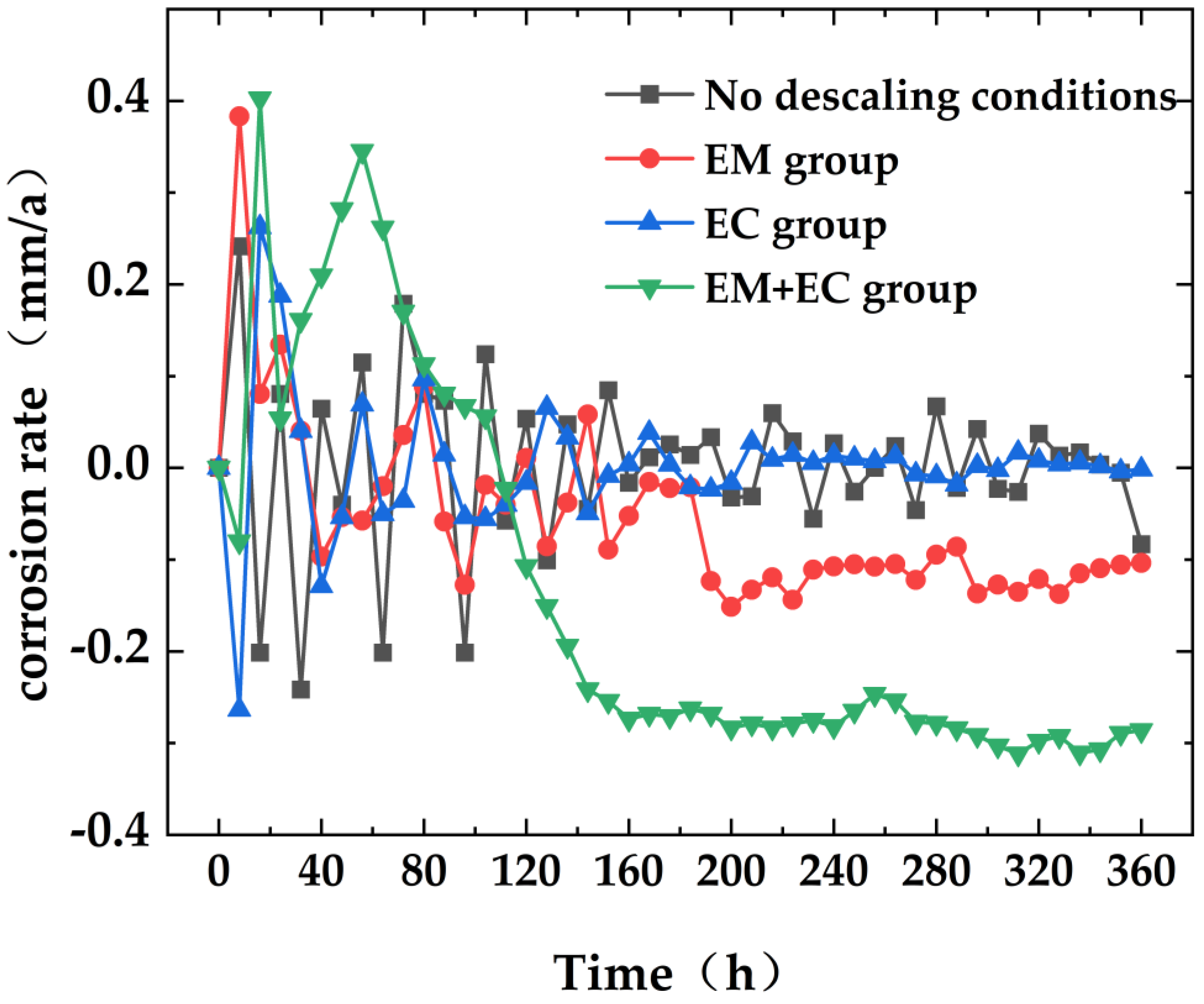
4.4. Corrosion of Iron Hanging Pieces in the Cooling Tower
5. Conclusions
- The experiments above verify that the combined action of electromagnetic and electrochemical methods has a good anti-scaling and descaling effect. Qualitative and quantitative analysis of CaCO3 fouling samples was conducted under different descaling conditions using XRD, and the morphology of fouling samples was observed by SEM electron microscopy. It was found that the combined action of electromagnetic and electrochemical methods reduced the calcite content in CaCO3 fouling samples from 92.8% to 16.2%, while increasing the aragonite content from 7.2% to 83.8%, resulting in a growth of 76.6%.
- Considering the changes in water quality parameters, hanging piece quality, and corrosion conditions, we conclude that the combined anti-scaling and anti-corrosion effect is significantly superior to individual applications. Under specific conditions, such as a water temperature of 30 °C, electromagnetic field frequency of 1 kHz, electrochemical descaling equipment voltage of 24 V, current of 10 A, and water flow rate of 0.6 m/s, the combined action of electromagnetic and electrochemical methods resulted in a decrease in solution conductivity by 11.6%, hardness by 42.0%, COD by 59.7%, turbidity by 48.1%, chloride ion concentration by 36.6%, and iron ion concentration by 63.1%. The composite anti-scaling efficiency reached 53.8%, demonstrating excellent anti-scaling, descaling, bactericidal, and algal inhibition effects.
- The synergistic effect of electromagnetism and electrochemistry not only solves the problem of poor scale inhibition effect caused by long-term operation of electromagnetic scale inhibition technology, but also solves the problem of poor scale removal effect of electrochemical scale removal technology on pipelines and heat exchange equipment.
6. Future Work Suggestions
- Considering that scaling and corrosion processes require a considerable amount of time, we extended the experimental duration and conducted data checks every 8 h. However, this may lead to the inability to detect short-term fluctuations in the data. Therefore, future work will involve conducting more experiments to shorten the measurement time and improve data stability.
- Given the potential harm of chloride ions to metal equipment and pipelines, and the limited effectiveness of electromagnetic anti-scaling and electrochemical descaling technologies in removing chloride ions, along with restrictions on fan usage, we will further research methods for removing chloride ions from industrial circulating water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fischer, L.; Hartmann, S.S.; Maljusch, A.; Däschlein, C.; Prymak, O.; Ulbricht, M. The influence of anion-exchange membrane nanostructure onto ion transport: Adjusting membrane performance through fabrication conditions. J. Membr. Sci. 2023, 669, 121306. [Google Scholar] [CrossRef]
- Khan, Z.U.; Moronshing, M.; Shestakova, M.; Al-Othman, A.; Sillanpää, M.; Zhan, Z.; Song, B.; Lei, Y. Electro-deionization (EDI) technology for enhanced water treatment and desalination: A review. Desalination 2023, 548, 116254. [Google Scholar] [CrossRef]
- Kamar, N.; Mostefa, M.; Muhr, H. Scaling control of gasketed plate heat exchanger by using very low frequency electromagnetic resonance fields. Chem. Eng. Sci. 2022, 262, 118040. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Zhao, X.; Liu, J.; Gao, M. Research on Descaling Characteristics and Simulation Calculation of a Coaxial High-Frequency Electronic Descaling Device. Water 2021, 13, 789. [Google Scholar] [CrossRef]
- Martínez Moya, S.; Boluda Botella, N. Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in Water Treatment by Magnetism and Electromagnetism. Water 2021, 13, 2365. [Google Scholar] [CrossRef]
- Zhang, C.X. Research on Key Technologies of Alternating Electromagnetic and Ultrasonic Anti-Scaling; Yanshan University: Qinhuangdao, China, 2019. [Google Scholar] [CrossRef]
- He, F.; Meng, X.H.; Wu, Y.S.; Zhou, C. Pilot-scale comparison of variable frequency electromagnetic and chemical agents for reverse osmosis membrane fouling control. Chem. Eng. 2023, 51, 62–65. [Google Scholar] [CrossRef]
- Kong, D.H.; Liu, Z.A.; Zhao, J.D.; Jia, J.J.; Ma, W.B.; Niu, H.J.; Yang, X.; Shao, X.J.; Liu, Q.W. Influence of combined action of electrostatic field and ultrasonic on CaCO3 crystallization behavior. Chem. Eng. 2016, 44, 28–31+36. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Li, G.; Yu, H.; Dong, X.; Jiang, H. Research on the descaling characteristics of a new electrochemical water treatment device. Water Sci. Technol. 2023, 88, 2566–2580. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.C.; Cao, S.X.; Lv, C.Q. Influence of alternating electromagnetic field on corrosion behavior of several metals. Corros. Prot. 2018, 39, 213–217. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Zhao, Y.; Sun, X.; Zhang, H.; Piao, H.-G.; Zhang, Y.; Huang, Y. The effect of magnetic field pretreatment on the corrosion behavior of carbon steel in static seawater. RSC Adv. 2020, 10, 2060–2066. [Google Scholar] [CrossRef]
- Sanjuán, I.; Benavente, D.; García-García, V.; Expósito, E.; Montiel, V. Paired electrolysis for simultaneous electrochemical water softening and production of weak acid solutions. Electrochem. Commun. 2019, 101, 88–92. [Google Scholar] [CrossRef]
- Xu, H.; Xu, Z.; Guo, Y.; Guo, S.; Xu, X.; Gao, X.; Wang, L.; Yan, W. Research and application progress of electrochemical water quality stabilization technology for recirculating cooling water in China: A short review. J. Water Process Eng. 2020, 37, 101433. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Y.; Chen, X. Membrane-based electrochemical precipitation for water softening. J. Membr. Sci. 2020, 597, 117639. [Google Scholar] [CrossRef]
- Jiani, L.; Zhicheng, X.; Hao, X.; Dan, Q.; Zhengwei, L.; Wei, Y.; Yu, W. Pulsed electrochemical oxidation of acid Red G and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P.; Singh, N. Amoxicillin electro-catalytic oxidation using Ti/RuO2 anode: Mechanism, oxidation products and degradation pathway. Electrochim. Acta 2019, 296, 856–866. [Google Scholar] [CrossRef]
- Liu, B.C.; Sun, H.; Liu, N.; Li, C.; Zhang, M. The effect of pulse frequency of wound pulse electromagnetic field on scale inhibition. Ind. Water Wastewater 2018, 49, 57–60. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.Z. Influence of direct current and pulse current on calcium carbonate electrodeposition. Ind. Water Treat. 2024, 44, 126–131. [Google Scholar] [CrossRef]
- Li, J.; Ge, H.H.; Zhang, J.L.; Yu, H.Q. Research progress on scale and corrosion control of electromagnetic water treatment. Corros. Prot. 2023, 44, 42–48. [Google Scholar] [CrossRef]
- Su, Q.; Tang, Y.Z.; Jiang, B. Study on the fouling performance of electrochemical-microfiltration coupling process in complex water environment. J. Qingdao Univ. Sci. Technol. 2022, 43, 108–113+151. [Google Scholar]
- Yu, Y.; Jin, H.C.; Meng, P.J.; Guan, Y.F.; Shao, S.Q.; Chen, X.M. Electrochemical water softening using air-scoured washing for scale detachment. Sep. Purif. Technol. 2018, 191, 216–224. [Google Scholar] [CrossRef]
- Kang, W.; Li, L.; Yan, L.; Mao, W.; Wang, X.; Yu, H.; Ma, C. Spatial and temporal regulation of homogeneous nucleation and crystal growth for high-flux electrochemical water softening. Water Res. 2023, 232, 119694. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, F.Y.; Cao, S.X. Effect of electromagnetic field on microbiological fouling and water quality parameters of circulating cooling water. Ind. Water Treat. 2017, 37, 27–30. [Google Scholar] [CrossRef]
- Liang, Y.D.; Xu, Y.; Xu, X.F.; Wang, J.G. Experimental study on scale inhibition characteristics of alternating magnetic field on heat transfer surface CaCO3 fouling. Proc. CSEE 2022, 42, 7913–7922. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, G.M.; Shen, Q.X.; Yan, B.H.; Wu, T.T.; Li, B. Study on corrosion of Q235 pipeline in circulating water of steel plant. J. Mater. Metall. 2019, 18, 132–140. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Li, W.Y.; Xiao, L.T.; Feng, L.M. Research progress on technology and mechanism of chloride ion removal in water. J. Water Purif. Technol. 2023, 42, 17–26. [Google Scholar] [CrossRef]



| Parameter | Value | Emission Standard | Percentage Deviation | After Reatment | Unit |
|---|---|---|---|---|---|
| Conductivity | 2008 ± 2 | None | 0% | 1776 | μS/cm |
| Hardness | 286.5 ± 1 | ≤250 | 14.6% | 166 | mg/L |
| Total alkalinity | 351 ± 0.5 | ≤200 | 75.5% | To be measured | mg/L |
| Total phosphorus | 0.9 ± 0.1 | ≤1.0 | 10% | To be measured | mg/L |
| COD | 62.1 ± 0.1 | ≤60 | 3.5% | 26 | mg/L |
| Turbidity | 20.8 ± 0.1 | ≤5 | 316% | 10.9 | NTU |
| Iron Ion | 2.60 ± 0.01 | ≤1 | 420% | 1 | mg/L |
| Chlorine Ion | 365 ± 1 | ≤250 | 66% | 221 | mg/L |
| Sulfate Ion | 105 ± 4.3 | None | 0% | To be measured | mg/L |
| No. | Descaling Condition | Conductivity | Hardness | COD | Turbidity | Chlorine Ion | Iron Ion |
|---|---|---|---|---|---|---|---|
| 1 | None | 10.6% | 29.1% | 19.6% | 35.7% | 2.7% | 7.6% |
| 2 | EM | 8.8% | 39.5% | 47.4% | 31.0% | 30.9% | 69.2% |
| 3 | EC | 12.8% | 38.8% | 43.5% | 44.8% | 25.8% | 6.5% |
| 4 | EM + EC | 11.6% | 42.0% | 59.7% | 48.1% | 36.6% | 63.1% |
| No. | Descaling Condition | Initial Weight (g) | Final Weight (g) | Change in Weight (g) | Scale Inhibition Rate |
|---|---|---|---|---|---|
| 1 | Control Group | 41.526 | 41.539 | 0.013 | 0 |
| 2 | EM | 36.528 | 36.536 | 0.008 | 38.5% |
| 3 | EC | 40.366 | 40.463 | 0.010 | 23.1% |
| 4 | EM + EC | 39.231 | 39.273 | 0.006 | 53.8% |
| No. | Descaling Condition | Calcite Mass Fraction | Dolomite Mass Fraction (%) |
|---|---|---|---|
| 1 | Control Group | 92.8% | 7.2% |
| 2 | EM | 30.7% | 69.3% |
| 3 | EC | 46.2% | 53.8% |
| 4 | EM + EC | 16.2% | 83.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, D.; Li, G.; Dong, X.; Jiang, H. Experimental Study on the Combined Effect of Electromagnetic and Electrochemical Processes on Descaling and Anticorrosion. Water 2024, 16, 1644. https://doi.org/10.3390/w16121644
Zhang S, Wang D, Li G, Dong X, Jiang H. Experimental Study on the Combined Effect of Electromagnetic and Electrochemical Processes on Descaling and Anticorrosion. Water. 2024; 16(12):1644. https://doi.org/10.3390/w16121644
Chicago/Turabian StyleZhang, Saiwei, Dongqiang Wang, Gangsheng Li, Xuewu Dong, and Haiqin Jiang. 2024. "Experimental Study on the Combined Effect of Electromagnetic and Electrochemical Processes on Descaling and Anticorrosion" Water 16, no. 12: 1644. https://doi.org/10.3390/w16121644
APA StyleZhang, S., Wang, D., Li, G., Dong, X., & Jiang, H. (2024). Experimental Study on the Combined Effect of Electromagnetic and Electrochemical Processes on Descaling and Anticorrosion. Water, 16(12), 1644. https://doi.org/10.3390/w16121644






