Abstract
Struvite crystallization can recover nitrogen and phosphorus simultaneously from various kinds of wastewaters as a slow-release fertilizer. However, the enhancement of the removal efficiency of NH4-N is challenging because the molar concentration of NH4-N is higher than that of PO4-P in many types of sewage including digested sludge filtrate. In this study, phosphorus eluate was recovered from sewage sludge incineration ash (SSA) and applied to the struvite crystallization process to increase the removal efficiency of NH4-N for the digested sludge filtrate. Under acidic conditions, a maximum of 98.4% of phosphorus was eluted from SSA; in alkaline conditions, a maximum of 51.2% was eluted; and in sequential elution conditions with (NaOH+H2SO4), a maximum of 98.0% was eluted. Jar tests were performed by injecting three types of eluates (H2SO4 1 N_elulate, NaOH 1 N_elulate, and (NaOH+H2SO4)_eluate), and PO4-P was stably removed (>86%) under all tested conditions. When the NaOH 1 N_eluate was injected, the NH4-N removal efficiency was highest at 84.4%, followed by 78.4% with the (NaOH+H2SO4)_eluate, and 58.7% with the H2SO4 1 N_eluate at the molar ratio of Mg:P:N of 1.5:1.5:1. In addition, the sequential jar tests were conducted by injecting both the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate. In the pH range of 8.5–9.5, the PO4-P and NH4-N removal efficiencies reached 92.3–94.5% and 97.9–99.1%, respectively. X-ray diffraction analyses confirmed that the majority of the crystal phases were struvite forms. Therefore, the combined application of both the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate was adequate to enhance not only the phosphorus recovery but also the removal efficiencies of PO4-P and NH4-N. SSA recovering PO4-P could be utilized as a new phosphorus source in the struvite crystallization process.
1. Introduction
Struvite (MgNH4PO4∙6H2O) crystallization is a common method used to remove and recover ammonium and phosphate from wastewater [1]. Struvite consists of three components: Mg2+, NH4+, and PO43− in an equal molecular ratio (1:1:1) and crystallizes under alkaline conditions at pH 8.0–10.0. Moreover, it can be used as slow-release fertilizers for commercialization [2,3].
Several studies have been conducted to derive the optimal conditions for struvite crystallization from synthetic wastewater, digested sludge filtrate, and livestock manure on a laboratory scale, using K2HPO4 or KH2PO4 as phosphorus sources to compensate for insufficient phosphorus compared to ammonium [4,5,6,7,8,9]. In these studies, the Mg:P:N molar ratio and pH were considered the operating factors, and the PO4-P and NH4-N removal efficiencies exceeded 90%. However, most full-scale struvite crystallization plants use digested sludge filtrates, which have a molar concentration of NH4-N that is approximately several times higher than that of phosphate. Therefore, as a result of full-scale plant operations, because phosphorus and nitrogen react at the same molar ratio, the PO4-P removal efficiency reaches 85–95%; however, the NH4-N removal efficiency is as low as 20% [10,11,12,13,14,15]. To solve this problem, phosphorus must be additionally injected; however, the economic feasibility of such a procedure is challenging, owing to the high chemical costs. In this study, sewage sludge incineration ash (SSA) was considered as a new phosphorus source in the struvite crystallization process.
SSA is a good material for recovering phosphorus because its moisture content and organic matter are almost zero, meaning that its volume is significantly reduced compared to sludge [16,17]. Furthermore, it does not have an odor and it has a very high phosphorus content. The phosphorus content of SSA can reach ~10%, with phosphorus being combined with metals, such as Al-P, Fe-P, or Ca-P [18]. SSA can be used as a fertilizer or soil conditioner; however, the recovery of phosphorus compounds that can be used by plants, such as Ca-P or Mg-P, is necessary, excluding Al-P or Fe-P. Undesirably, SSA contains a large amount of heavy metals and their removal is essential for meeting fertilizer standards. The solubility of each phosphorus compound differs depending on the pH; this difference in solubility can be used in the recovery of phosphorus compounds and removal of heavy metals [19]. Phosphorus recovery via wet chemical methods is a technique for recovering metal-bound phosphorus into the desired form using the solubility differences under acidic or alkaline conditions [20,21]. Several studies have been conducted to recover phosphorus in the form of Al-P, Ca-P, and H3PO4 after eluting phosphorus under acidic or alkaline conditions. After eluting phosphorus from SSA using sulfuric acid or hydrochloric acid, the pH is raised at values between 3.5 and 4.0 and phosphorus is recovered in the form of Al-P [22]. To recover phosphorus in the form of Ca-P, CaO or CaCl2 is injected into the acid eluate, followed by filtration through an ion-exchange resin to remove metals, resulting in the recovery of H3PO4 [23]. Additionally, some research studies were conducted to recover phosphorus from SSA and produce the struvite. However, this process had a problem with low ammonia removal efficiency, and optimal operating conditions (Mg:P:N molar ratio and pH, etc.) were not presented [24,25]. Therefore the recovery of phosphorus from SSA is challenging; additional heavy metal removal processes should be included to improve the phosphorus recovery rate. In addition, to recover the desired form of phosphorus with high purity, it is necessary to examine the behavior of phosphorus and the mechanism of each process.
In this study, the applicability of SSA as a new phosphorus source for struvite crystallization was investigated. First, three types of phosphorus eluates (H2SO4 1 N_elulate, NaOH 1 N_eluate, and (NaOH+H2SO4)_eluate) were prepared, and the concentrations of phosphorus and heavy metals were analyzed. Subsequently, the phosphorus eluates were injected into the struvite crystallization process at a molar concentration of Mg:P:N = (1–1.5):(1–1.5):1. Depending on the type of eluates, the phosphate and ammonium removal efficiencies were examined together with the removal mechanism of each stage. Finally, the struvite crystal content was confirmed via an X-ray diffraction (XRD) analysis of the recovered precipitates.
2. Materials and Methods
2.1. Elution of Phosphorus from SSA in Acid and Alkaline Conditions
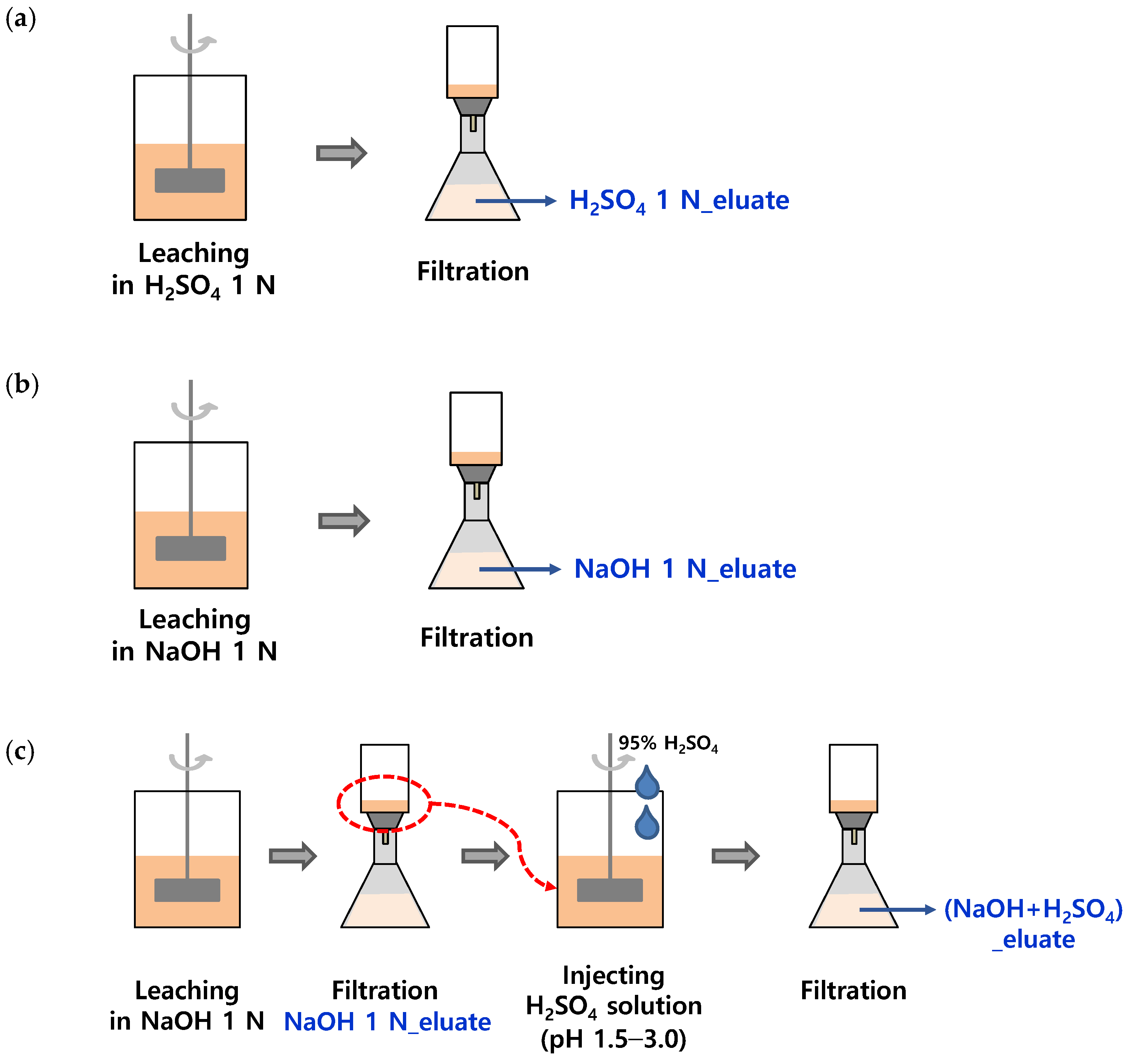
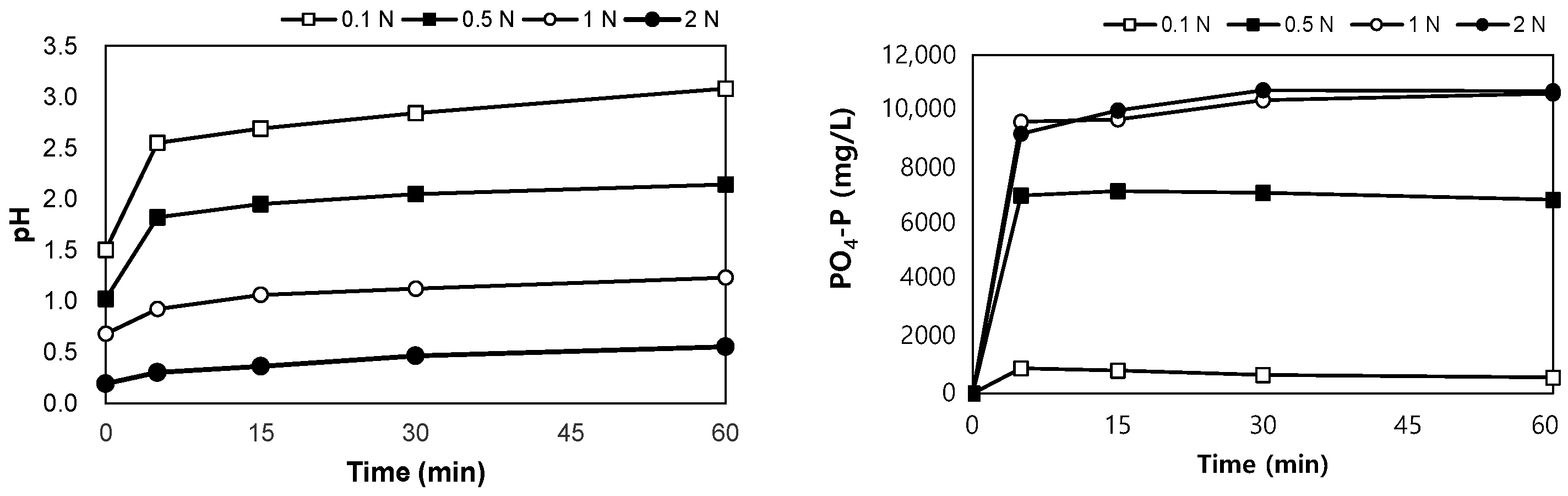
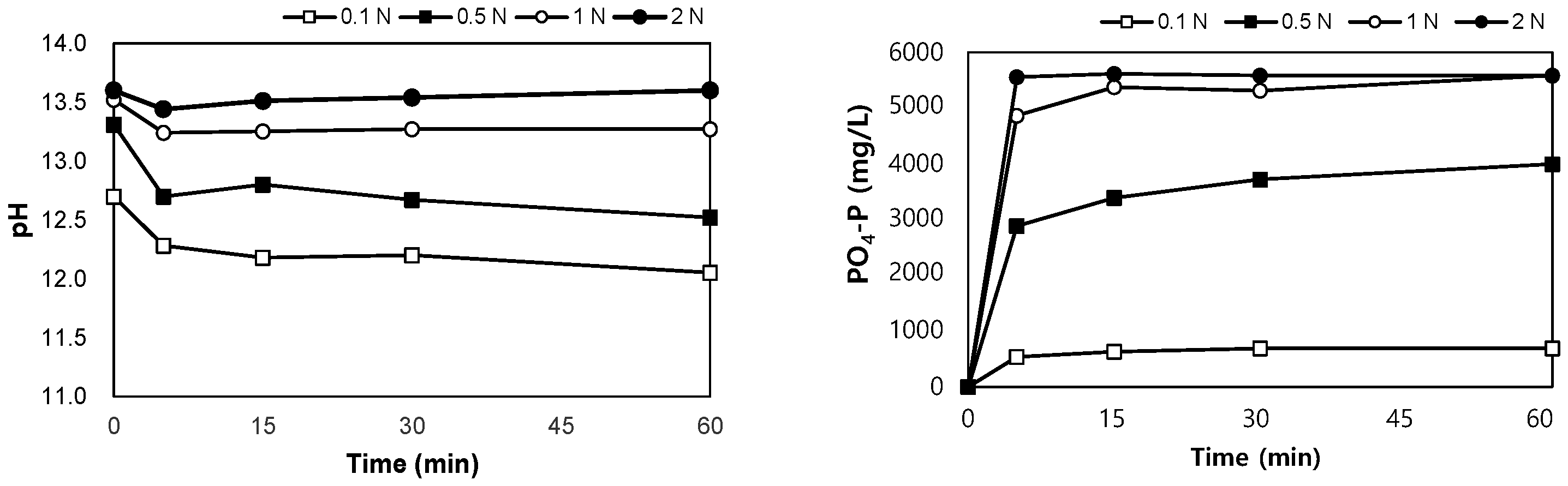
SSA was collected from an ‘N’ wastewater treatment plant in Korea, and it was dried at 110 °C for more than 6 h before the experiment. The ratio of the eluate to SSA was defined as the liquid/solid (L/S) ratio and was adjusted to 10 mL/g. SSA (50 g) was added to 500 mL of an acidic or alkaline solution. H2SO4 solution (95%, Samchun, Seoul, Republic of Korea) and NaOH powder (98%, Samchun) were used to adjust the concentrations of the solutions to 0.1, 0.5, 1.0, and 2.0 N, respectively, as shown in Figure 1a,b. The solutions were stirred at 120 rpm for 60 min, and 10 mL of each sample was collected at 0, 5, 15, 30, and 60 min of stirring to measure the pH and PO4-P concentrations.
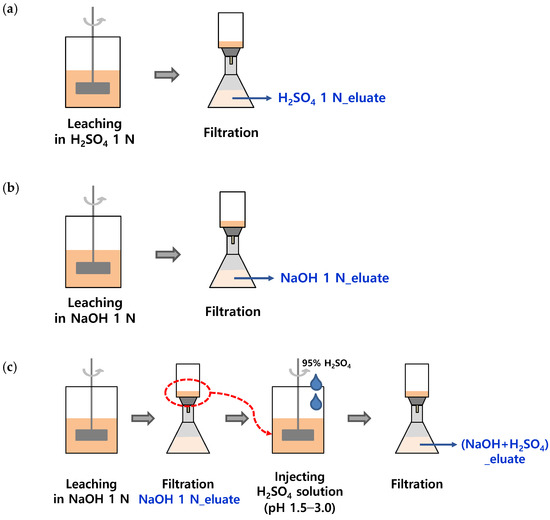
Figure 1.
Preparation of three types of phosphorus eluates; (a) H2SO4 1 N_eluate, (b) NaOH 1 N_eluate, and (c) (NaOH+H2SO4)_eluate.
2.2. Re-Elution of Phosphorus from Residue of Alkaline Treatment
Sequential wet chemical treatment of SSA for phosphorus recovery was performed to simultaneously maximize the leaching of phosphorus and reduce heavy metals. The sequential phosphorus elution procedure is shown in Figure 1c. In the first step, 50 g of SSA was eluted with 500 mL of a NaOH 1 N solution (L/S ratio of 10) for 30 min in a jar tester, followed by filtration of the alkaline eluate through a GF/C filter. In the second step, the alkaline-treated residue was recovered and re-eluted with 500 mL of a H2SO4 solution. The H2SO4 solution (95%) was injected into the eluate to adjust the pH to 1.5, 1.75, 2.0, and 3.0. Each mixture was stirred for 30 min in a jar tester and then filtered through a GF/C filter. The concentrations of PO4-P and heavy metals were compared with those of the acidic and alkaline eluates.
2.3. Application of Phosphorus Eluates to Struvite Crystallization
2.3.1. Single Injection of Three Types of Eluates
Jar tests were performed by injecting three types of eluates into the digested sludge filtrate to examine the ammonium removal efficiencies. The digested sludge filtrate was obtained from an ‘I’ wastewater treatment plant in Korea.
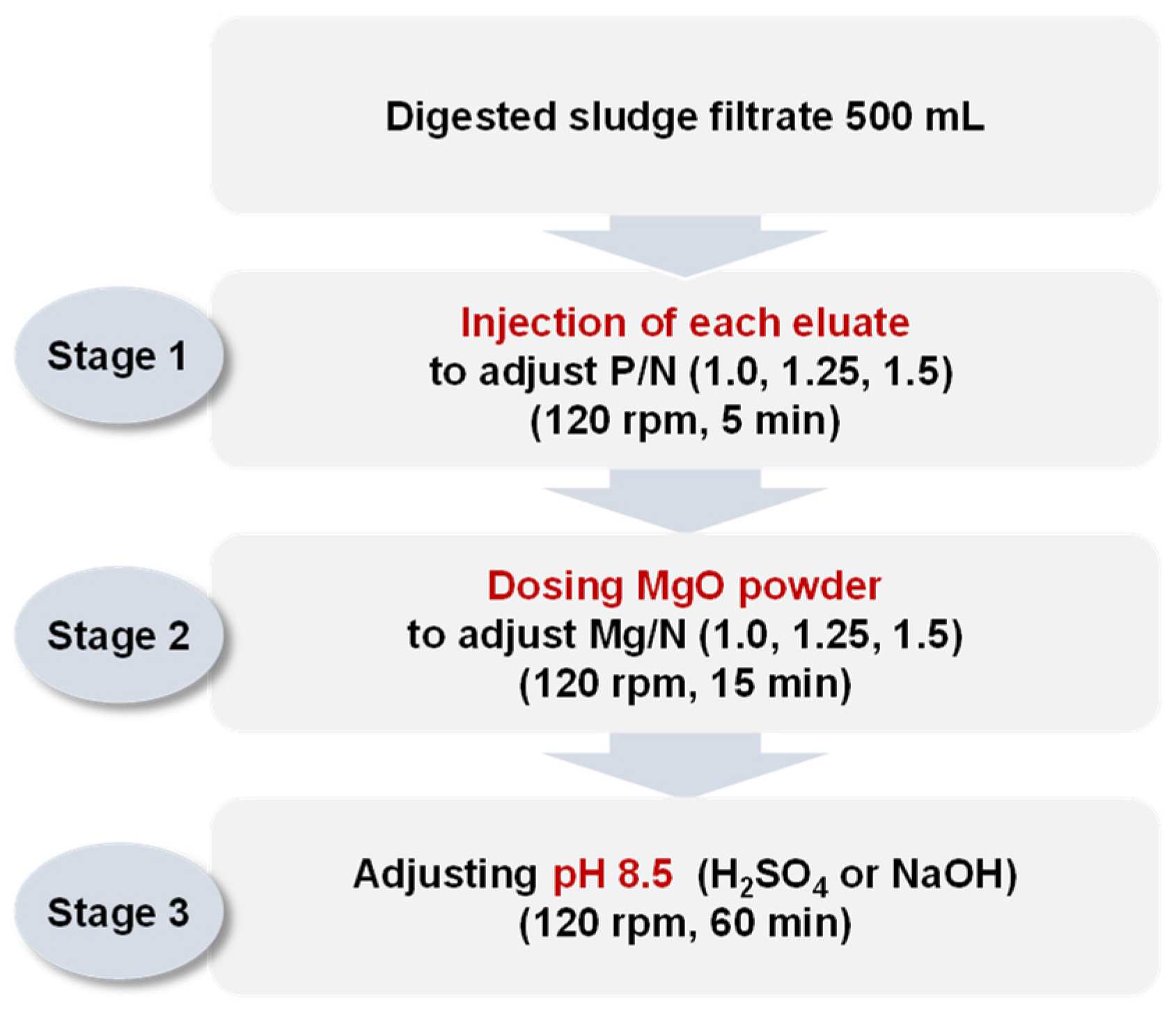
Each eluate was injected into 500 mL of the digested sludge filtrate to adjust the P/N molar ratios to 1.0, 1.25, and 1.5. The mixture was then stirred at 120 rpm for 5 min. MgO (98%, KONOSHIMA chemical Co., Ltd., Osaka, Japan) was injected as the magnesium source to adjust the Mg/N molar ratios to 1.0, 1.25, and 1.5. The mixture was stirred at 120 rpm for 15 min to dissolve MgO in the solution, and then the pH was set to 8.5 using H2SO4 95% or NaOH 1 N. Finally, the mixture was stirred at 120 rpm for 60 min and precipitated for 30 min. The experimental setup is shown in Figure 2. The ion concentrations (Mg2+, NH4-N, and PO4-P) in the supernatants were analyzed. The precipitates were dried for 2 h at 105 °C and the crystalline phases and compositions were analyzed via XRD.
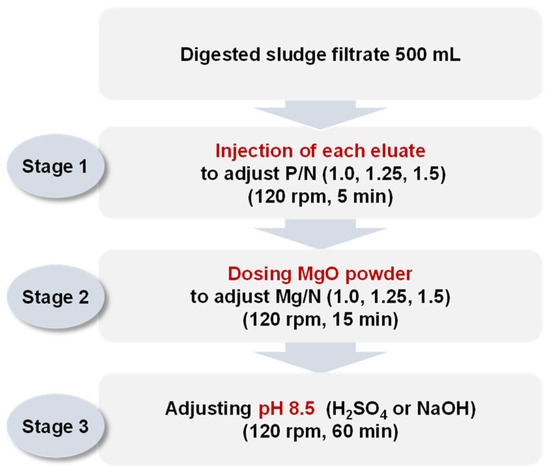
Figure 2.
Procedure of jar tests simulating struvite crystallization by injecting each eluate (H2SO4 1 N_eluate, NaOH 1 N_eluate, and (NaOH+H2SO4)_eluate).
2.3.2. Sequential Injection of Two Types of Eluates
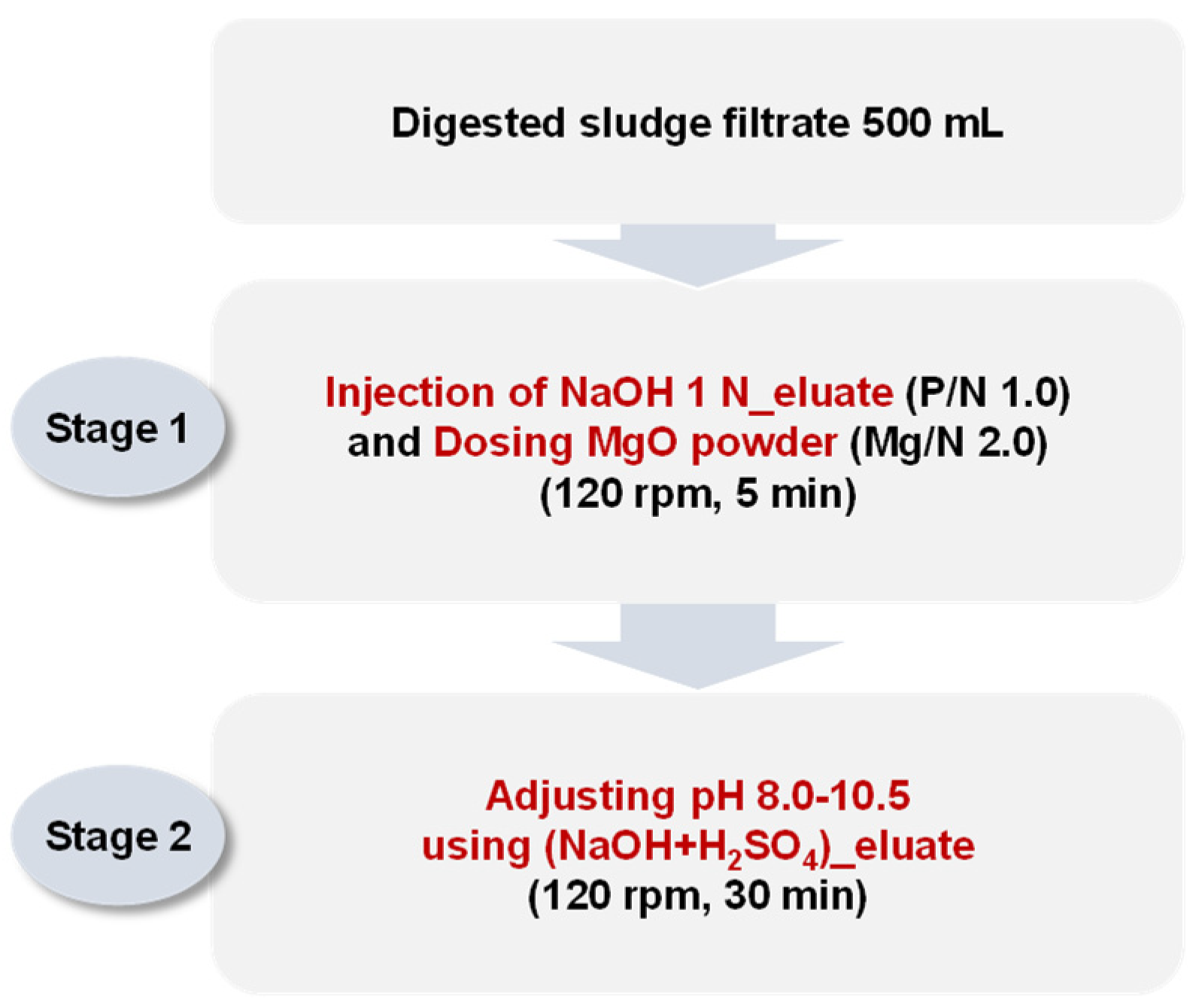
Jar tests were performed by sequentially injecting both the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate. The (NaOH+H2SO4)_eluate was injected for pH adjustments between 8.0 and 10.5, with an additional phosphorus supply. The detailed experimental method is shown in Figure 3. The NaOH 1 N_eluate was injected into 500 mL of the digested sludge filtrate to adjust the P/N molar ratio to 1.0. MgO was then injected into the mixture to adjust the Mg/N molar ratio to 2.0. The mixture was stirred at 120 rpm for 5 min.
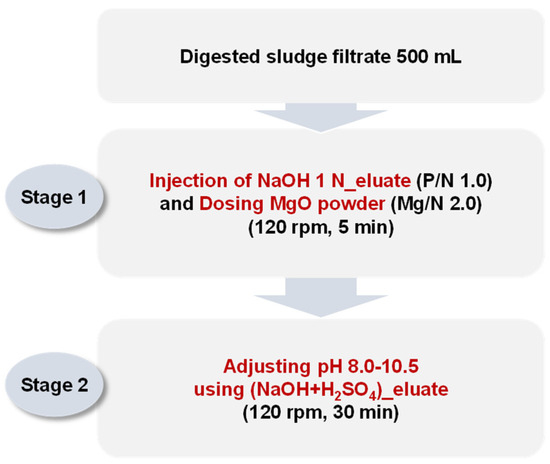
Figure 3.
Procedure of sequential jar tests simulating struvite crystallization by injecting both the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate.
Finally, the (NaOH+H2SO4)_eluate was injected to adjust the pH to 8.0, 8.5, 9.0, 9.5, 10.0, and 10.5. The mixture was stirred at 120 rpm for 30 min and then precipitated for 30 min. The ion concentrations (Mg2+, NH4-N, and PO4-P) in the supernatants were analyzed. The precipitates were dried for 2 h at 105 °C, and the crystalline phases and composition were analyzed via XRD.
2.4. Analytical Methods
The pH was measured using a HI 2210 pH meter (HANNA instruments, Smithfield, RI, USA). The concentrations of Mg2+, NH4-N, and PO4-P were measured using an AQUION (Thermo Fisher Scientific, Waltham, MA, USA). The constituent elements of SSA were analyzed quantitatively using an S8 TIGER (Bruker, Billerica, MA, USA) X-ray fluorescence (XRF) instrument. The concentrations of heavy metals in the eluates were measured using a Varian 820-MS (Agilent, Santa Clara, CA, USA) inductively coupled plasma (ICP) mass spectrometer. The crystal structures of the samples were analyzed using an EMPYREAN (Malvern Panalytical, Worcestershire, UK) X-ray diffractometer.
3. Results and Discussion
3.1. Characterization of Phosphorus Leaching from SSA by Acid and Base
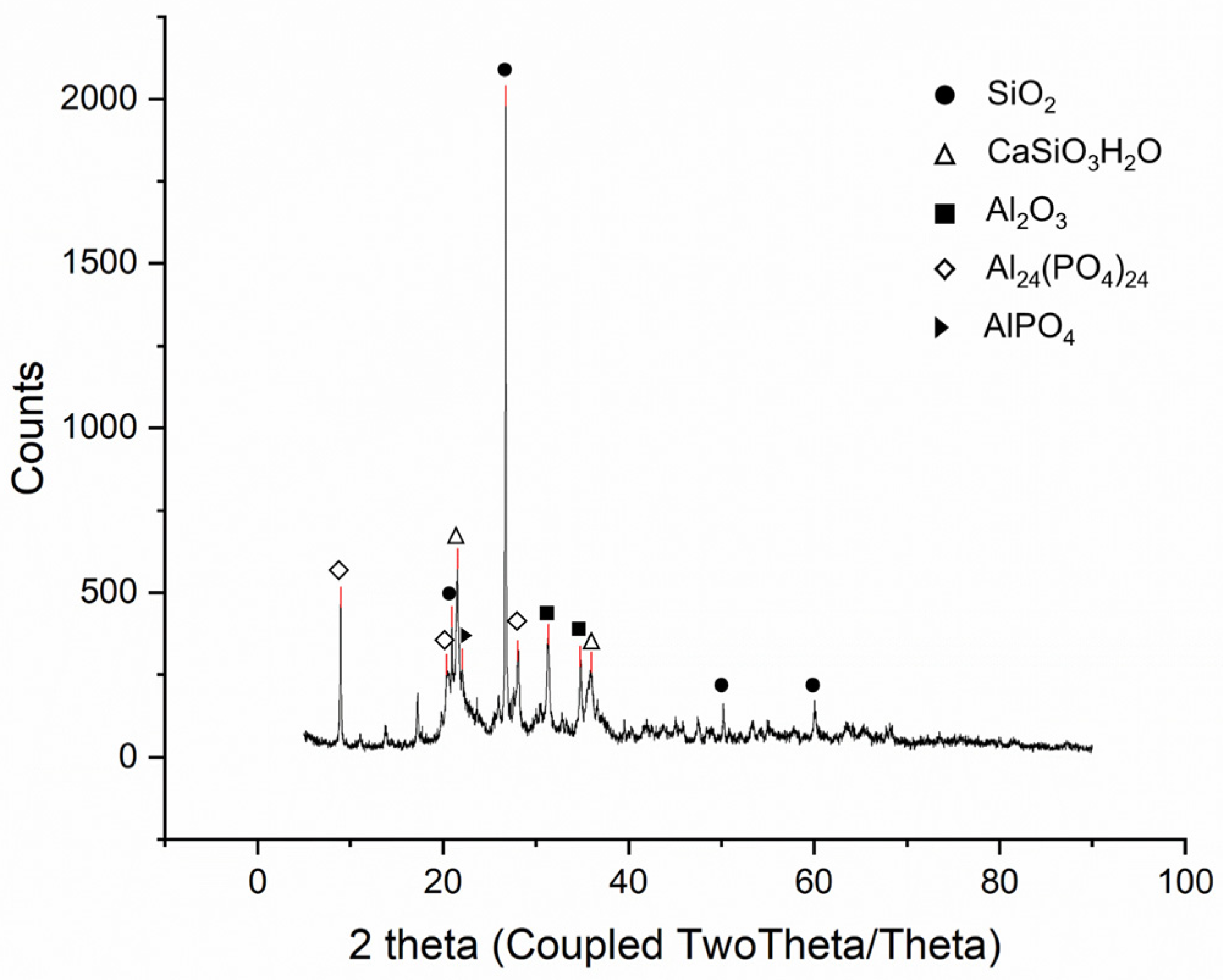
SSA from the ‘N’ wastewater treatment plant (Seoul, Republic of Korea) was analyzed via XRF and XRD to examine its properties; the results are presented in Table 1 and Figure 4. The XRF analysis revealed that the main components of SSA were Al2O3, P2O5, and SiO2, whereas the secondary components were CaO, Fe2O3, and MgO. The phosphorus content of SSA was approximately 10.9% (P2O5 24.9%).

Table 1.
XRF analysis results of SSA.
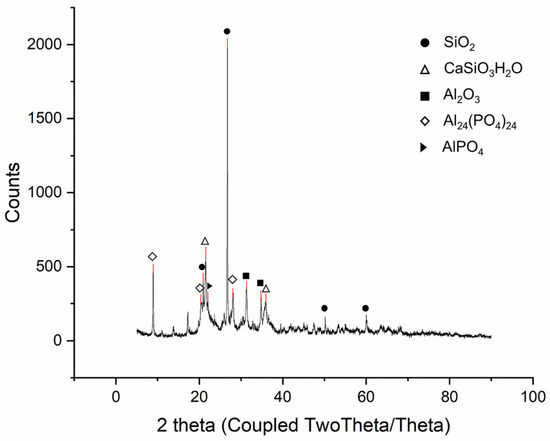
Figure 4.
XRD analysis results of SSA.
The results of the leaching tests using H2SO4 are shown in Figure 5. After mixing the SSA in H2SO4 0.1–2.0 N for 60 min, the pH of the mixture was between 0.6 and 3.1. PO4-P was eluted in the range of 570–10,720 mg/L, and when the concentration of H2SO4 was higher than 1 N, the same leaching phosphorus concentration was observed after 30 min, regardless of the concentration of H2SO4. The eluted phosphorus reached 5.2–98.4% of the total phosphorus content in SSA.
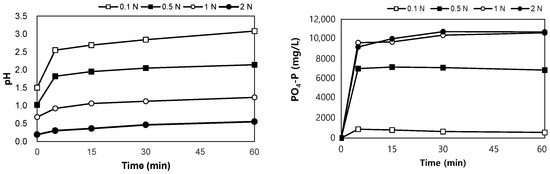
Figure 5.
Variations in pH and PO4-P with elution time in acidic conditions.
The results of the leaching tests using NaOH are shown in Figure 6. After mixing the SSA in NaOH 0.1–2.0 N for 60 min, the pH of the mixture was between 12.1 and 13.6. PO4-P was eluted in the range of 690–5580 mg/L, and when the concentration of NaOH was higher than 1 N, the same leaching phosphorus concentration was observed after 30 min, regardless of the concentration of NaOH. The eluted phosphorus corresponded to 6.3–51.2% of the total phosphorus content in SSA.
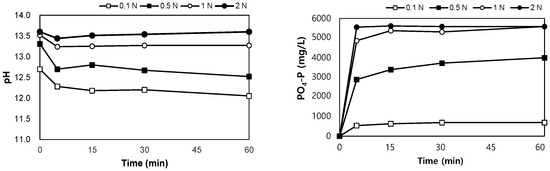
Figure 6.
Variations in pH and PO4-P with elution time in alkaline conditions.
The concentrations of heavy metals in the eluates of H2SO4 1 N and NaOH 1 N are shown in Table 2, expressed as the amount of heavy metals eluted per 1 kg of SSA (mg/kg SSA). Excessive amounts of Cu, Zn, Mn, and Fe were eluted in H2SO4 1 N compared to NaOH 1 N. In the case of aluminum, it was eluted at high concentrations in both acidic and alkaline solutions, possibly attributed to the aluminum-based coagulant used in the advanced phosphorus removal process after the conventional biological treatments.

Table 2.
Concentrations of heavy metals in H2SO4 1 N_eluate and NaOH 1 N_eluate (ND: non-detected).
In the case of the acidic eluate, phosphorus was eluted at high concentrations; however, heavy metals were also eluted at high concentrations. In the case of the alkaline eluate, heavy metals were hardly eluted; however, only 50% of the phosphorus could be eluted compared to that of the acid.
3.2. Re-Elution of Phosphorus from Residue of Alkaline Treatment
To maximize phosphorus elution and reduce heavy metal elution, SSA was leached via a two-step procedure performed under alkaline and acidic conditions. In the second step after the elution with NaOH, the pH was adjusted to 1.5, 1.75, 2.0, and 3.0. The concentrations of PO4-P and heavy metals were determined based on the pH. The results are presented in Table 3. In the first step, the PO4-P concentrations were in the range of 5640–5660 mg/L in the NaOH 1 N_eluate. When the pH was adjusted between 1.5 and 3.0 in the second step, the PO4-P concentrations ranged from 240 to 5040 mg/L. The total amount of eluted PO4-P was 5840–10,680 mg/L, which was 53.6–98.0% of the total phosphorus content in SSA. Referring to the XRF analysis results of SSA (Table 1), most of the phosphorus of SSA was combined in the form of Al-P, Ca-P, Fe-P, and Mg-P. According to the solubility curves as a function of pH for different phosphorus compounds, all these compounds could be dissolved readily as the pH level was decreased [26]. In other words, a portion of P compounds not eluted at a high pH (>11) were dissolved under acidic conditions below pH 2, resulting in increased leaching efficiencies of phosphate.

Table 3.
Comparison of phosphorus concentrations in each eluate and re-eluate.
The concentrations of heavy metals are presented in Table 4 after adjusting the pH to values between 1.5 and 3.0. As the pH decreased, the concentrations of heavy metals increased. Among the heavy metals, Cu, Zn, and Mn were eluted at high concentrations. In particular, the concentrations of heavy metals increased significantly at a pH ≤ 2.0. In the re-eluate at pH 1.5, the Cu, Zn, and Mn concentrations were reduced by approximately 10% compared to the H2SO4 1 N_eluate. Specifically, both the heavy metals and phosphorus remaining in SSA were re-eluted during the additional elution step using H2SO4.

Table 4.
Comparison of heavy metal concentrations in each eluate (ND: non-detected).
3.3. Application of Each Phosphorus Eluate to Struvite Crystallization
Three types of phosphorus eluates were prepared under acidic and alkaline conditions: H2SO4 1 N_eluate, NaOH 1 N_eluate, and (NaOH+H2SO4)_eluate at a pH of 1.5. Jar tests were performed by injecting each phosphorus eluate into the digested sludge filtrate. The concentrations of Mg2+, PO4-P, and NH4-N were analyzed in the raw water and phosphorus eluates, and the results are presented in Table 5. The molar concentrations of PO4-P in the digested sludge filtrate were analyzed to be 2.8 to 4.8 mmol/L (average 3.5 mmol/L), and those of NH4-N were analyzed to be 15.9 to 24.7 mmol/L (average 19.4 mmol/L). Based on the average concentration, it was found that molar concentration of NH4-N was approximately 5.5 times higher than that of PO4-P.

Table 5.
Characteristics of raw water and each eluate.
3.3.1. Application of a H2SO4 1 N_eluate
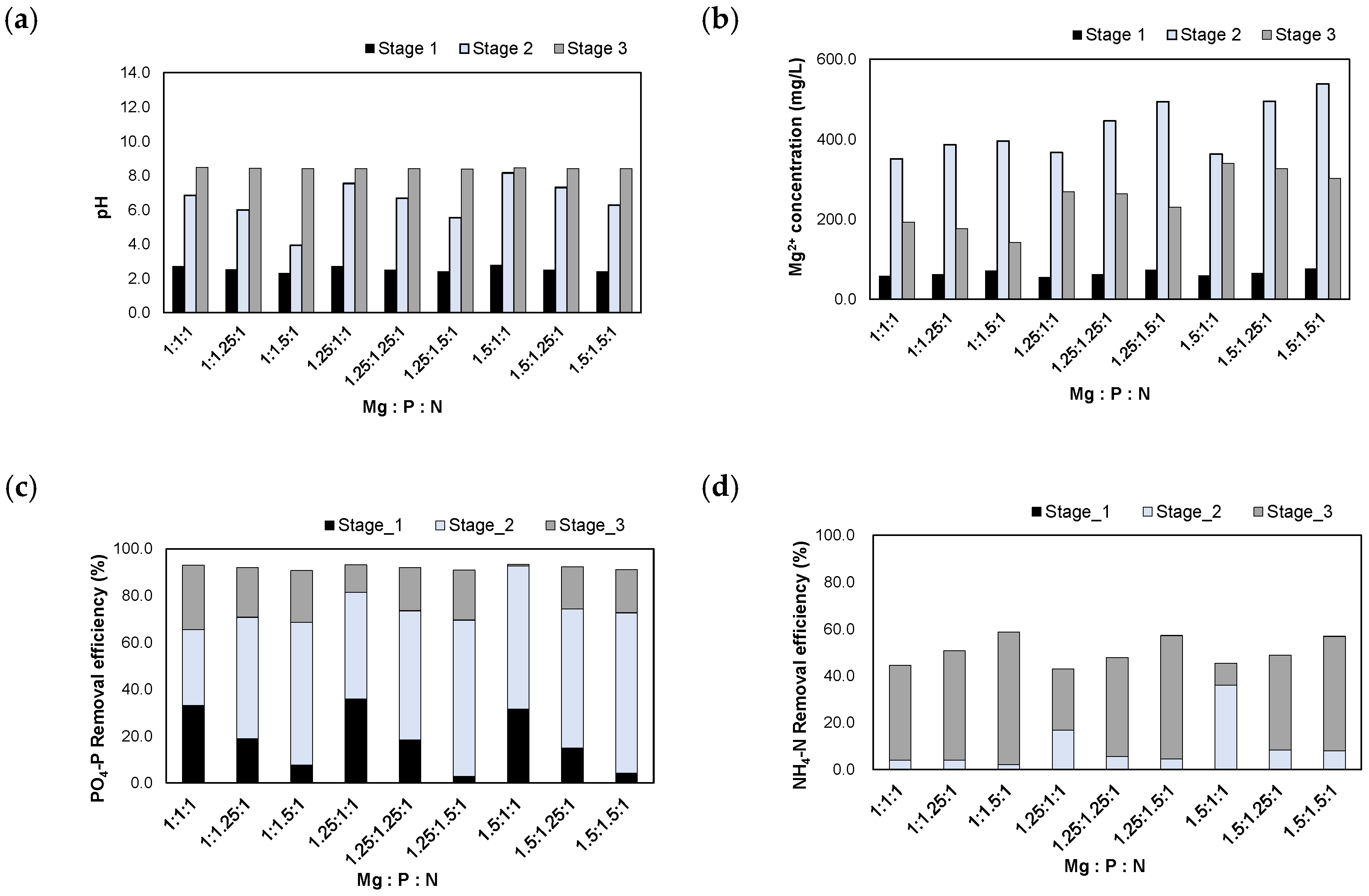
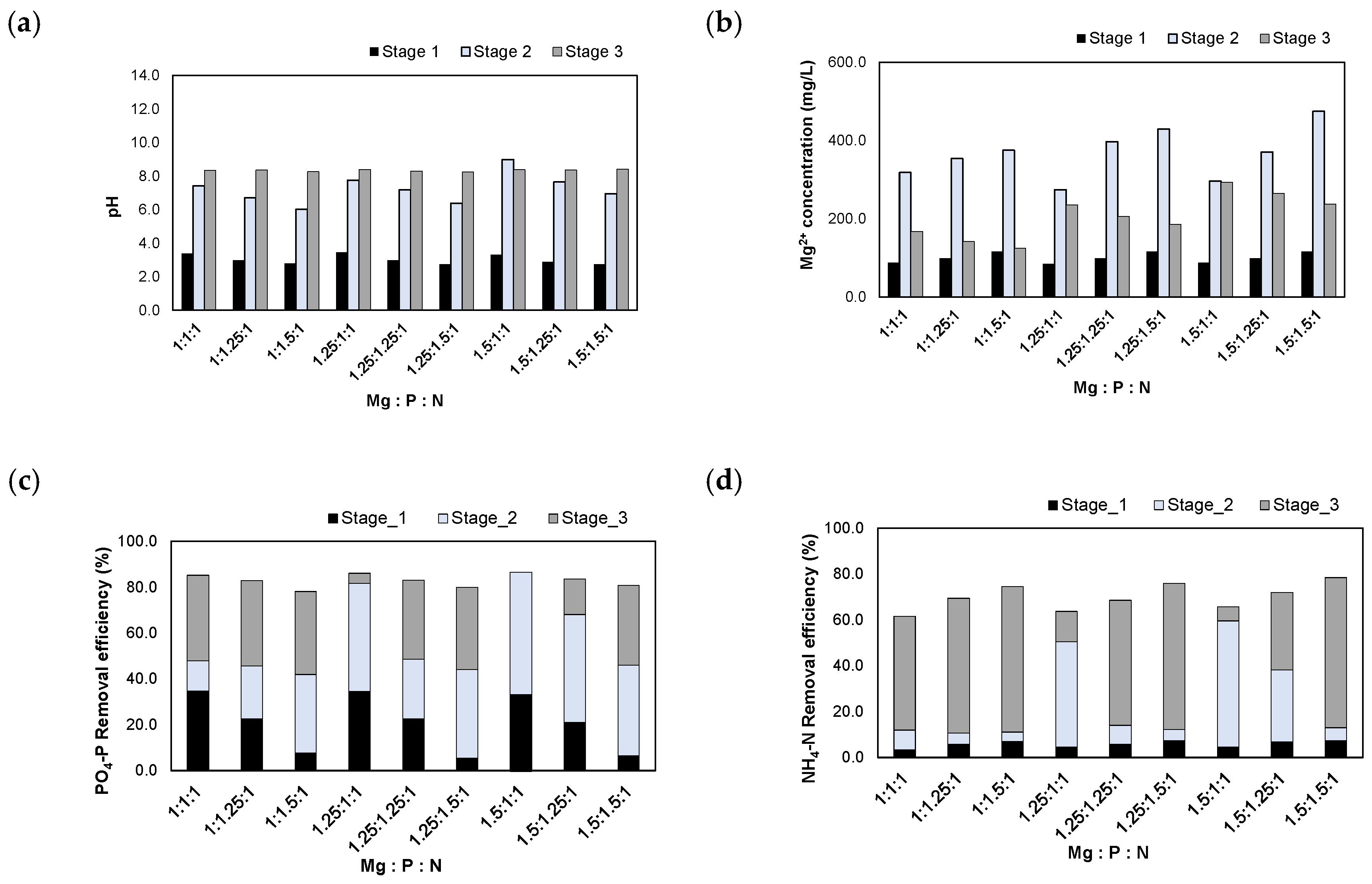
The experimental results after injecting the H2SO4 1 N_eluate are presented in Figure 7. When the H2SO4 1 N_eluate was injected, the pH was adjusted between 2.3 and 2.8. After injecting MgO according to the Mg/N molar ratios, the pH increased to 3.9–8.2. Finally, the pH was adjusted to 8.5 using NaOH powder. The Mg2+ concentrations increased to 351–538 mg/L after the injection of MgO. When the pH was adjusted to 8.5, the Mg2+ concentrations decreased to 142–340 mg/L due to the formation of struvite crystals.
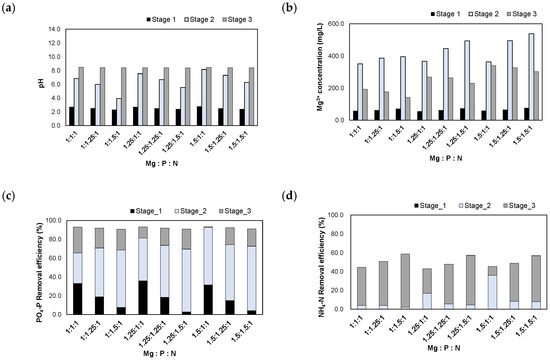
Figure 7.
Results of injecting the H2SO4 1 N_eluate in accordance with the Mg:P:N molar ratios: (a) pH; (b) Mg2+ concentration; (c) removal efficiencies of PO4-P; (d) removal efficiencies of NH4-N.
The PO4-P removal efficiencies were 90.8–93.3% at these molar ratios. During stage 1, 2.8–36.0% of PO4-P was removed in the form of Al-P and Fe-P in the low pH range as the acidic eluate was injected. During stage 2, 32.4–68.4% of PO4-P was removed. Therefore, large amounts of Al-P, Fe-P, and Mg-P compounds were formed during the process of increasing the pH via MgO injection. Finally, when the pH was adjusted to 8.5, 0.6–27.5% of PO4-P was removed at stage 3. Struvite crystals were formed by removing both PO4-P and NH4-N during this step.
The NH4-N removal efficiencies were 42.9–58.7% at these molar ratios. Before the formation of struvite crystals, Al-P, Fe-P, and Mg-P compounds were formed during the process of increasing the pH; therefore, the removal efficiencies of NH4-N were lower than those of PO4-P.
3.3.2. Application of a NaOH 1 N_eluate
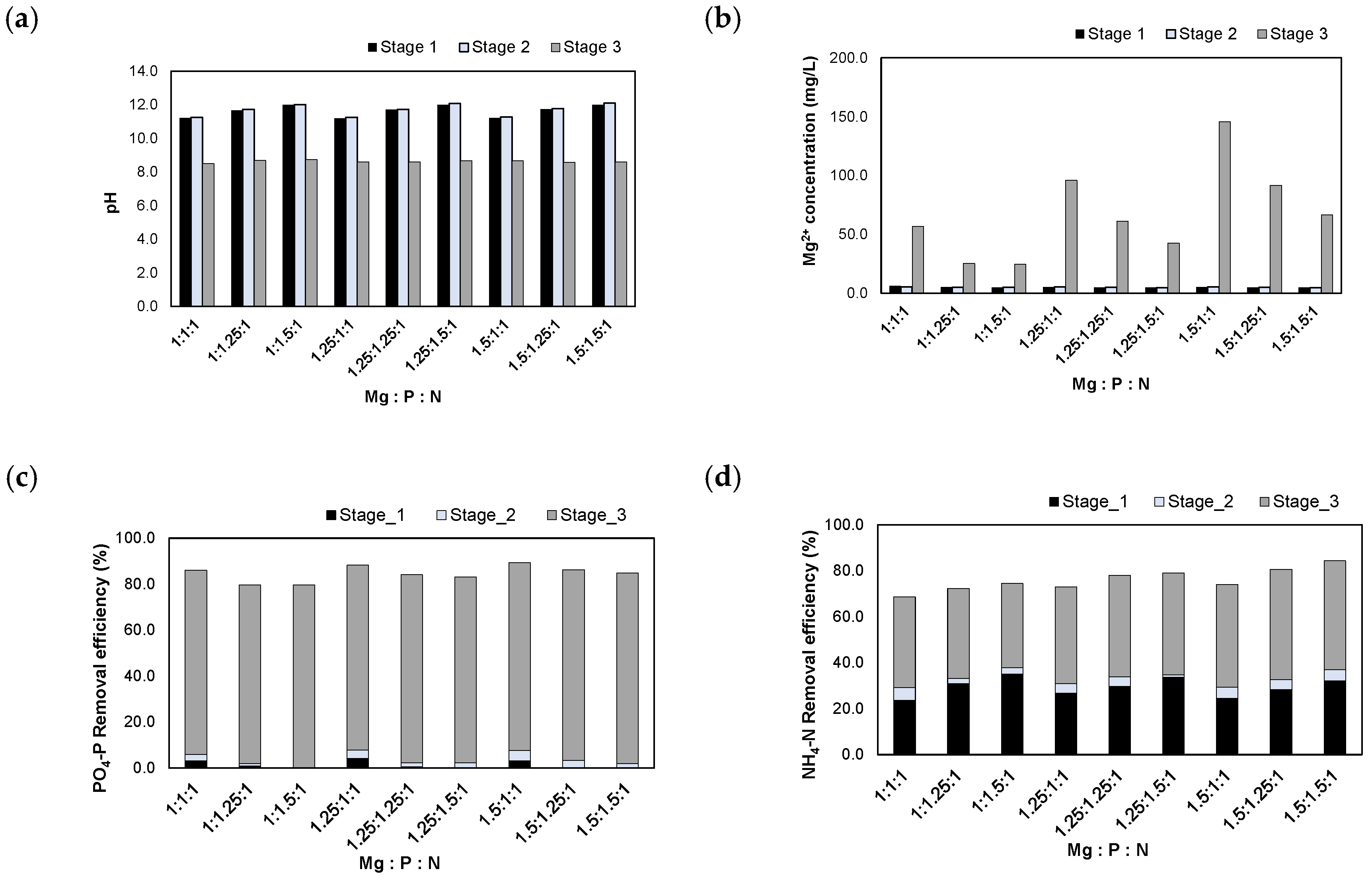
The experimental results obtained after injection of the NaOH 1 N_eluate are shown in Figure 8. When the NaOH 1 N_eluate was injected, the pH was adjusted to 11.0–12.0. On the other hand, MgO is sparingly soluble in water but readily soluble in acidic conditions. MgO solubility was determined by donated [H+] ions in the solution [27]. Because MgO did not dissolve favorably after injecting into NaOH 1 N_eluate, the pH remained constant in the range of pH 11.1–12.1. Finally, the pH was adjusted to 8.5 by the injection of H2SO4 95% solution. The residual Mg2+ concentrations increased to 24.7–145.5 mg/L during the process of pH adjustment.
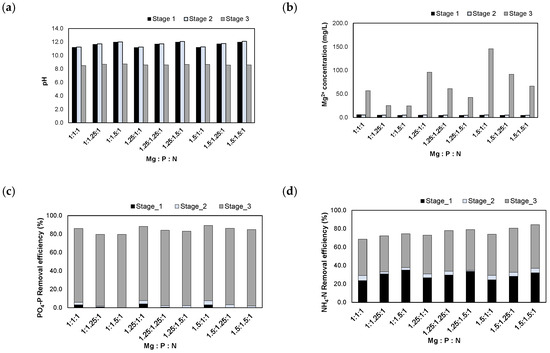
Figure 8.
Results of injecting the NaOH 1 N_eluate in accordance with the Mg:P:N molar ratios: (a) pH; (b) Mg2+ concentration; (c) removal efficiencies of PO4-P; (d) removal efficiencies of NH4-N.
The PO4-P removal efficiencies were 79.6–89.4% at these molar ratios. Most PO4-P was removed during stage 3. In this step, MgO was dissolved as the pH was adjusted to 8.5, and PO4-P reacted in the form of struvite crystals, Al-P, and Mg-P compounds.
The NH4-N removal efficiencies were 68.6–84.4% at these molar ratios. At stage 1, parts of NH4-N were removed by stripping in a high pH range (pH 11.0–12.0). Additionally, 36.6–47.9% of NH4-N was removed by the struvite crystallization reaction in stage 3. Therefore, the NH4-N removal efficiencies were improved compared to those of the previous experiments using the H2SO4 1 N_eluate.
3.3.3. Application of a (NaOH+H2SO4)_eluate
Figure 9 shows the experimental results obtained after injecting the (NaOH+H2SO4)_eluate. When the (NaOH+H2SO4)_eluate was injected, the pH was adjusted to 2.7–3.4. After injecting MgO according to the Mg/N molar ratios, the pH increased to 6.0–9.0. Finally, the pH was adjusted to 8.5 with NaOH powder or H2SO4 solution. The Mg2+ concentrations increased to 296–474 mg/L after the injection of MgO. When the pH was adjusted to 8.5, the Mg2+ concentrations decreased to 125–294 mg/L due to the formation of struvite crystals.
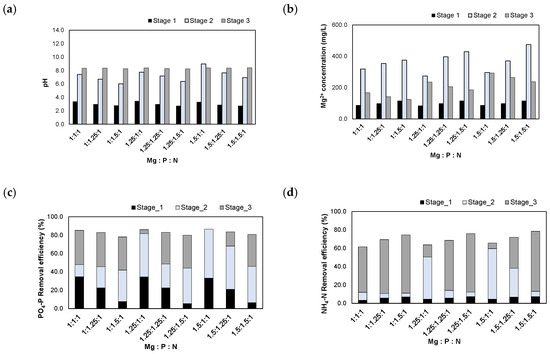
Figure 9.
Results of injecting the (NaOH+H2SO4)_eluate in accordance with the Mg:P:N molar ratios: (a) pH; (b) Mg2+ concentration; (c) removal efficiencies of PO4-P; (d) removal efficiencies of NH4-N.
The removal efficiencies of PO4-P and NH4-N were 78.2–86.3% and 61.5–78.4%, respectively. The removal characteristics of PO4-P and NH4-N were similar to those observed in the experiments using the H2SO4 1 N_eluate. In stage 1, Al-P and Fe-P compounds were formed in a low pH range. Additionally, Al-P, Fe-P, and Mg-P were formed as MgO was dissolved, and the pH increased in stage 2. Finally, when the pH was adjusted to 8.5 at stage 3, MAP crystals were formed as the removal of PO4-P and NH4-N occurred.
In this experiment, the removal efficiencies of NH4-N were slightly increased, although those of PO4-P decreased, and these results were compared to the removal efficiencies observed in the previous experiment using the H2SO4 1 N_eluate. This is because the (NaOH+H2SO4)_eluate contained lower concentrations of Al, Fe, and other heavy metals compared to the H2SO4 1 N_eluate; therefore, the amount of produced struvite crystals increased, and the NH4-N removal efficiency improved.
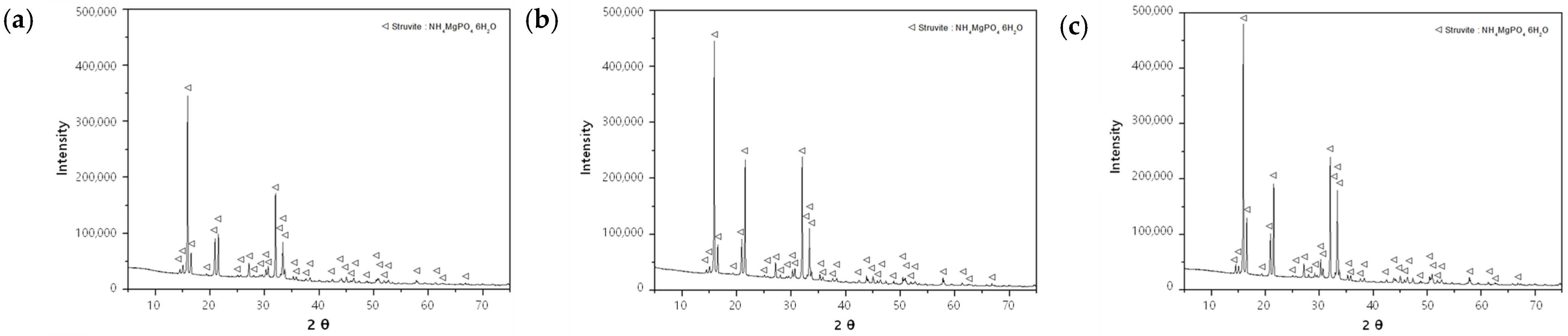
The precipitates of each experiment (Mg:P:N = (1.0–1.5):1.0:1.0) were analyzed via XRD for the qualitative and quantitative analyses of crystals. Only struvite crystals were detected in all the samples (Figure 10). The crystalline contents of each sample are listed in Table 6. The sample using the (NaOH+H2SO4)_eluate exhibited the highest crystal contents in the range of 43.4–49.6%. The crystal contents of the samples using the H2SO4 1 N_eluate and NaOH 1 N_eluates were similar at 33.9–35.4% and 36.2–39.6%, respectively. Meanwhile, SEM-EDS data were not presented in this paper, since they overlapped with previous research results. Please refer to reference [4].
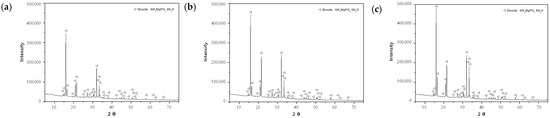
Figure 10.
Results of XRD analyses at the (Mg:P:N) molar ratios of (1.5:1.5:1): (a) H2SO4 1 N_eluate; (b) NaOH 1 N_eluate; (c) (NaOH+H2SO4)_eluate.

Table 6.
Crystalline contents of each sample using the H2SO4 1 N_eluate, NaOH 1 N_eluate, and (NaOH+H2SO4)_eluate.
3.4. Combined Application of NaOH 1 N_eluate and (NaOH+H2SO4)_eluate to Struvite Crystallization
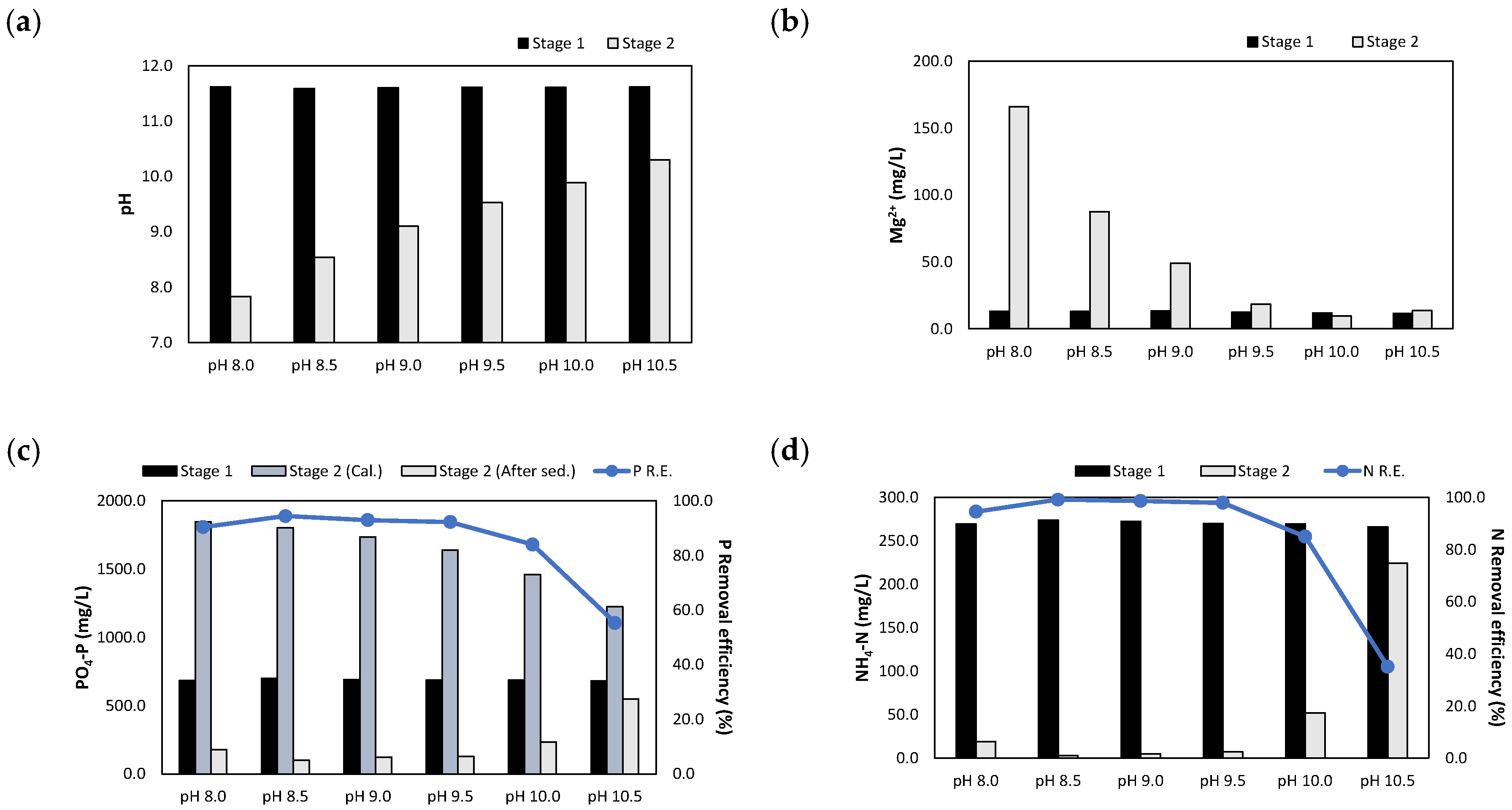
The sequential injection of the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate afforded the experimental results presented in Figure 11. When the NaOH eluate and MgO were injected at a P/N molar ratio of 1.0, the pH was adjusted to 11.6. The pH was then adjusted between 8.0 and 10.5 by injecting the (NaOH+H2SO4)_eluate at an injection volume of 65–185 mL. As the injection volume increased, the Mg2+ concentrations increased to 13.6–165.9 mg/L.
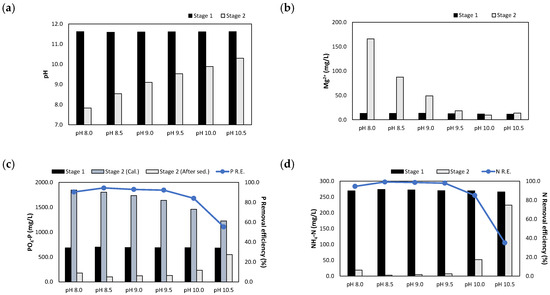
Figure 11.
Results of sequentially injecting both the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate in accordance with the pH: (a) pH; (b) Mg2+ concentration; (c) concentrations and removal efficiencies of PO4-P; (d) concentrations and removal efficiencies of NH4-N.
When the pH was adjusted to 10.5, the removal efficiencies of PO4-P and NH4-N were 55.3% and 35.1%, respectively. In this case, the injection volume of the (NaOH+H2SO4)_eluate was low, and the MgO was not sufficiently dissolved. In the pH range of 8.5–9.5, the PO4-P and NH4-N removal efficiencies reached 92.3–94.5% and 97.9–99.1%, respectively. At pH 8.0, the PO4-P and NH4-N removal efficiencies decreased slightly to 90.4% and 94.5%, respectively.
Interestingly, the XRD patterns of the precipitates revealed the absence of any crystalline phase at pH 10.5; however, struvite and some Na2SO4 crystals were observed at pH values between 8.0 and 10.0. Analyses of the crystalline content revealed that the struvite content was 17.5–23.0%, which was slightly lower than that observed in the previous experiments. It is judged that a large amount of Al(OH)3 was produced at pH 10.5, and the struvite peak was not detected; and then, the content of struvite was lowered because AlPO4 was also precipitated together with struvite in the pH range of 8–10. In addition, it was observed that the Na2SO4 content was 2.5–4.4% in pH 8.0–9.5 since the NaOH and H2SO4 were reacted in eluates.
4. Conclusions
In this study, high concentrations of phosphorus eluates were recovered from sewage sludge incineration ash (SSA) and used as a new phosphorus substitute during the struvite crystallization process to improve the NH4-N removal efficiency. The H2SO4 1 N_eluate was inadequate for the struvite crystallization process since NH4-N removal efficiency was relatively low, although it had a high recovery rate of phosphorus in the SSA (>98%). On the other hand, the NaOH 1 N_eluate was suitable in terms of the removal efficiency of NH4-N; however, it revealed a disadvantage of low phosphorus recovery in the SSA (<51%). Finally, the (NaOH+H2SO4)_eluate was adequate in terms of both the removal efficiency of NH4-N and phosphorus recovery (>98%). Additionally, a combined application of the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate was carried out; the PO4-P and NH4-N removal efficiencies reached 94.5% and 99.1%, respectively; therefore, it was concluded that the NaOH 1 N_eluate and (NaOH+H2SO4)_eluate are preferable for the enhancement of the NH4-N removal efficiency.
In conclusion, the NH4-N removal efficiency can be improved to > 97% using the high phosphorus concentration of the eluates recovered from SSA. However, because the eluates contained high aluminum concentrations, compounds in the form of Al-P and Al(OH)3 were also formed along with struvite crystals, which could cause a decrease in the purity of the struvite crystals. Therefore, to improve purity, further studies are required to overcome the high aluminum issues in these eluates.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, N.P.; visualization, resource, M.K.; validation, project administration, J.J.; resource, investigation, S.J.; conceptualization, validation, investigation, data curation, supervision, W.K. All authors have read and agreed to the published version of the manuscript.
Funding
Research for this paper was carried out under the KICT Research Program (project no. 20240125-001, Research on Next-Generation Environmental Technology for Carbon Neutrality) funded by the Ministry of Science and ICT.
Data Availability Statement
The relevant data can be found in this article.
Conflicts of Interest
All authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Zhang, Y.; Huang, J.; Yang, Y.; Tsang, D.C.; Wang, H.; Chen, H.; Gao, B. Recovery of phosphorus from wastewater: A review based on current phosphorous removal technologies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1148–1172. [Google Scholar] [CrossRef] [PubMed]
- Park, N.R.; Chang, H.Y.; Jang, Y.J.; Lim, H.M.; Jung, J.H.; Kim, W.J. Critical conditions of struvite growth and recovery using MgO in pilot scale crystallization plant. Water Sci. Technol. 2020, 81, 2511–2521. [Google Scholar] [CrossRef]
- Liu, X.; Wen, G.; Hu, Z.; Wang, J. Coupling effects of pH and Mg/P ratio on P recovery from anaerobic digester supernatant by struvite formation. J. Clean. Prod. 2018, 198, 633–641. [Google Scholar] [CrossRef]
- Shih, Y.J.; Abarca, R.R.M.; de Luna, M.D.G.; Huang, Y.H.; Lu, M.C. Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: Effects of pH, phosphate concentration and coexisting ions. Chemosphere 2017, 173, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, R.; Yu, X.; Zhao, Y.; Sang, Q.; Wang, F.; Chen, Y. Investigation of anaerobic side-stream phosphorus recovery and its effect on the performance of mainstream EBPR subjected to low-consumption. Water Sci. Technol. 2019, 80, 1944–1955. [Google Scholar]
- Forrest, A.L.; Fattah, K.P.; Mavinic, D.S.; Koch, F.A. Optimizing Struvite Production for Phosphate Recovery in WWTP. J. Environ. Eng. 2008, 134, 395–402. [Google Scholar] [CrossRef]
- Taddeo, R.; Lepistö, R. Struvite precipitation in raw and co-digested swine slurries for nutrients recovery in batch reactors. Water Sci. Technol. 2015, 71, 892–897. [Google Scholar] [CrossRef]
- Nieminen, J. Phosphorus Recovery and Recycling from Municipal Wastewater Sludge. Master’s Thesis, Aalto University, Espoo, Finland, 2010. [Google Scholar]
- Ueno, Y.; Fujii, M. Three years experience of operating and selling recovered struvite from full-scale plant. Environ. Technol. 2001, 22, 1373–1381. [Google Scholar] [CrossRef]
- Cullen, N.; Baur, R.; Schauer, P. Three years of operation of North America’s first nutrient recovery facility. Water Sci. Technol. 2013, 68, 763–768. [Google Scholar] [CrossRef]
- Marchi, A.; Geerts, S.; Weemaes, M.; Wim, S.; Christine, V. Full-scale phosphorus recovery from digested waste water sludge in Belgium–part I: Technical achievements and challenges. Water Sci. Technol. 2015, 71, 487–494. [Google Scholar] [CrossRef]
- Moerman, W.; Carballa, M.; Vanderkerckhove, A.; Derycke, D.; Verstraete, W. Phosphate removal in agro-industry: Pilot and full-scale operational considerations of struvite crystallization. Water Res. 2009, 43, 1887–1892. [Google Scholar] [CrossRef]
- Remy, M.; Driessen, W.; Hendrickx, T.; Haarhuis, R. Recovery of Phosphorus by Formation of Struvite with the PHOSPAQ™ Process. In Proceedings of the 18th European Biosolids and Organic Resources Conference, Manchester, UK, 18–20 November 2013. [Google Scholar]
- Krüger, O.; Adam, C. Recovery potential of German sewage sludge ash. Waste Manag. 2015, 45, 400–406. [Google Scholar] [CrossRef]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage sludge ash—A promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef]
- Petzet, S.; Peplinski, B.; Cornel, P. On wet chemical phosphorus recovery from sewage sludge ash by acidic or alkaline leaching and an optimized combination of both. Water Res. 2012, 46, 3769–3780. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Mwamila, L.B.; Kergoat, K. The pH dependence of phosphate sorption and desorption in Swedish agricultural soils. Geoderma 2012, 189, 304–311. [Google Scholar] [CrossRef]
- Jama-Rodzeńska, A.; Sowiński, J.; Koziel, J.A.; Białowiec, A. Phosphorus recovery from sewage sludge ash based on cradle-to-cradle approach—Mini-review. Minerals 2021, 11, 985. [Google Scholar] [CrossRef]
- Ma, P.; Rosen, C. Land application of sewage sludge incinerator ash for phosphorus recovery: A review. Chemosphere 2021, 274, 129609. [Google Scholar] [CrossRef]
- Takahashi, M.; Kato, S.; Shima, H.; Sarai, E.; Ichioka, T.; Hatyakawa, S.; Miyajiri, H. Technology for recovering phosphorus from incinerated wastewater treatment sludge. Chemosphere 2001, 44, 23–29. [Google Scholar] [CrossRef]
- Franz, M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 2008, 28, 1809–1818. [Google Scholar] [CrossRef]
- Zin, M.M.T.; Kim, D.J. Simultaneous recovery of phosphorus and nitrogen from sewage sludge ash and food wastewater as struvite by Mg-biochar. J. Hazard. Mater. 2021, 403, 123704. [Google Scholar]
- Li, S.; Zeng, W.; Ren, Z.; Jia, Z.; Peng, X.; Peng, Y. A novel strategy to capture phosphate as high-quality struvite from the sewage sludge ash: Process, mechanism and application. J. Clean. Prod. 2021, 322, 129162. [Google Scholar] [CrossRef]
- Luyckx, L.; Sousa Correia, D.S.; Van Caneghem, J. Linking phosphorus extraction from different types of biomass incineration ash to ash mineralogy, ash composition and chemical characteristics of various types of extraction liquids. Waste Biomass Valorization 2021, 12, 5235–5248. [Google Scholar] [CrossRef]
- Park, N.R.; Chang, H.Y.; Jang, Y.J.; Lim, H.M.; Jung, J.H.; Kim, W.J. Prediction of adequate pH and Mg2+ dosage using an empirical MgO solubility model for struvite crystallization. Environ. Technol. Innov. 2021, 21, 101347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).