High-Efficiency Hydrogen Recovery from Corn Straw Hydrolysate Using Functional Bacteria and Negative Pressure with Microbial Electrolysis Cells
Abstract
1. Introduction
2. Materials and Methods
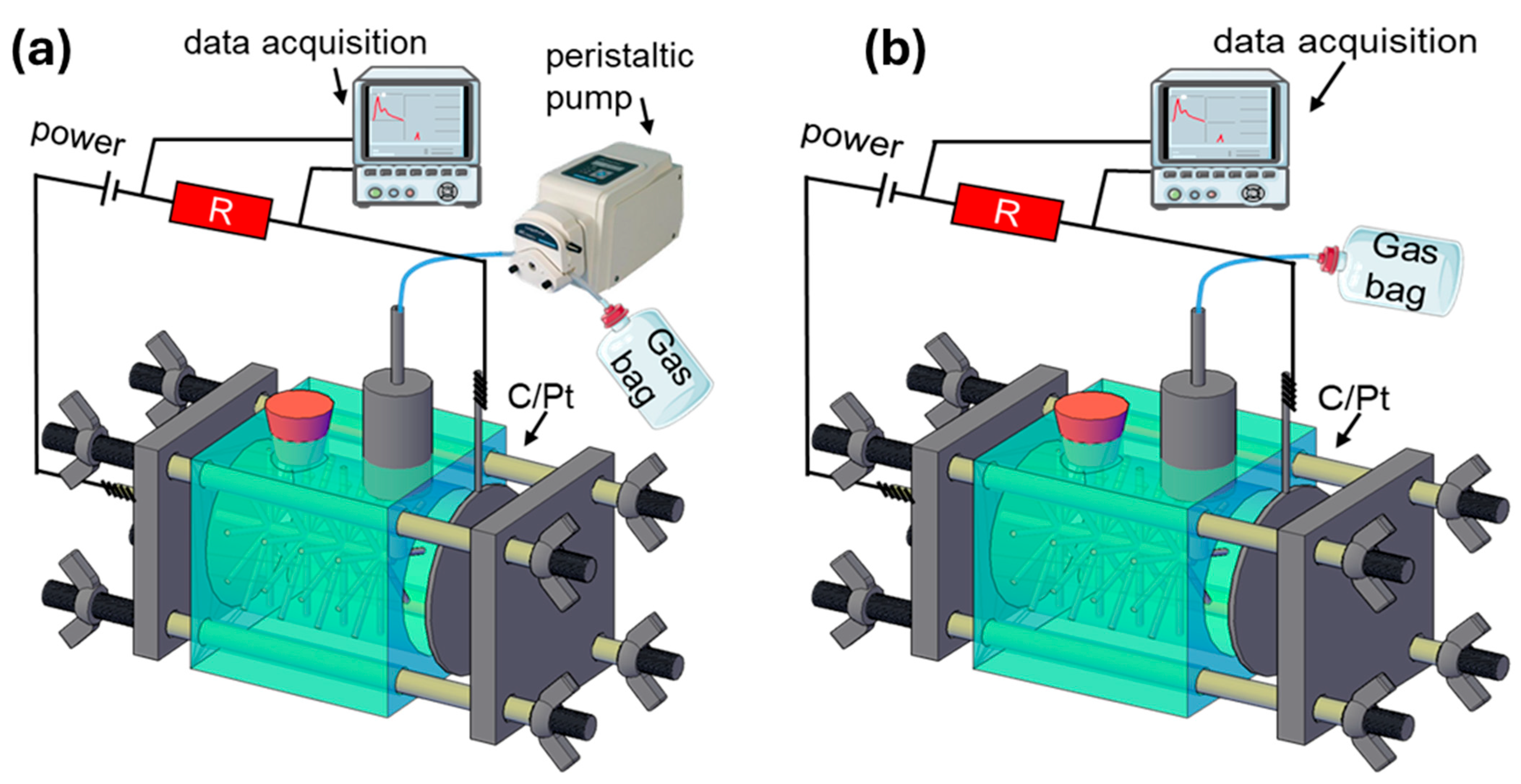
2.1. Reactor Configuration and Operation
2.2. Bioelectrochemical Characteristics and Wastewater Analyses
2.3. Biofilm Community Analysis
2.4. Calculations
3. Results
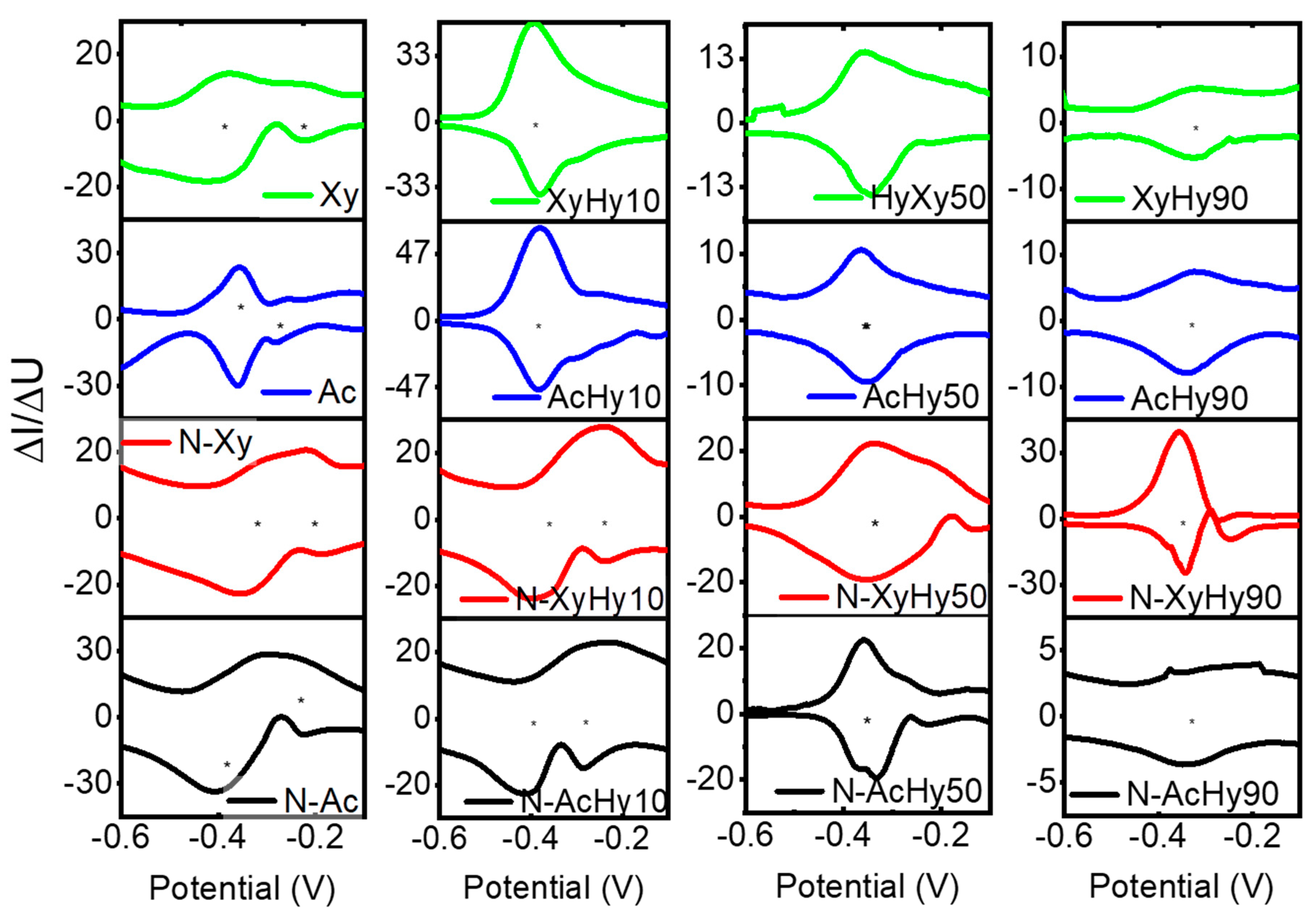
3.1. MEC Performance with Different Enrichment Substrates in Passive Mode
3.2. MEC Performance under Negative Pressure
3.3. Microbial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Reynolds, L.J.; Sala-Comorera, L.; Khan, M.F.; Martin, N.A.; Whitty, M.; Stephens, J.H.; Nolan, T.M.; Joyce, E.; Fletcher, N.F.; Murphy, C.D. Coprostanol as a population biomarker for SARS-CoV-2 wastewater surveillance studies. Water 2022, 14, 225. [Google Scholar] [CrossRef]
- Gautam, R.; Nayak, J.K.; Ress, N.V.; Steinberger-Wilckens, R.; Ghosh, U.K. Bio-hydrogen production through microbial electrolysis cell: Structural components and influencing factors. Chem. Eng. J. 2023, 455, 140535. [Google Scholar] [CrossRef]
- Lee, H.-S.; Vermaas, W.F.; Rittmann, B.E. Biological hydrogen production: Prospects and challenges. Trends Biotechnol. 2010, 28, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Younesi, H.; Pontié, M.; Rahimpour, A.; Rahimnejad, M.; Zinatizadeh, A.A. A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery. J. Clean. Prod. 2020, 264, 121446. [Google Scholar] [CrossRef]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.-L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; He, W.; Li, C.; Liu, J.; Liu, S.; Lee, H.-S.; Feng, Y. Efficient hydrogen recovery with CoP-NF as cathode in microbial electrolysis cells. Appl. Energy 2020, 264, 114700. [Google Scholar] [CrossRef]
- Son, H.; Seo, H.; Han, S.; Kim, S.M.; Khan, M.F.; Sung, H.J.; Kang, S.-H.; Kim, K.-J.; Kim, Y.H. Extra disulfide and ionic salt bridge improves the thermostability of lignin peroxidase H8 under acidic condition. Enzym. Microb. Technol. 2021, 148, 109803. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Jiang, Z.; Jin, Y.; Wu, W. Lignocellulose pretreatment by deep eutectic solvents and related technologies: A review. J. Bioresour. Bioprod. 2023, 8, 33–44. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Sun, J.; Li, D.; Huang, Y.; Feng, Y. Effect of Extractives on Digestibility of Cellulose in Corn Stover with Liquid Hot Water Pretreatment. BioResources 2015, 11, 54–70. [Google Scholar] [CrossRef][Green Version]
- Ullery, M.L.; Logan, B.E. Anode acclimation methods and their impact on microbial electrolysis cells treating fermentation effluent. Int. J. Hydrogen Energy 2015, 40, 6782–6791. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, N.K.; Bibra, M.; Salem, D.R.; Sani, R.K. Thermophiles for biohydrogen production in microbial electrolytic cells. Bioresour. Technol. 2019, 277, 171–178. [Google Scholar] [CrossRef]
- Yan, D.; Yang, X.; Yuan, W. Electricity and H2 generation from hemicellulose by sequential fermentation and microbial fuel/electrolysis cell. J. Power Sources 2015, 289, 26–33. [Google Scholar] [CrossRef]
- Lee, H.S.; Torres, C.I.; Rittmann, B.E. Effects of Substrate Diffusion and Anode Potential on Kinetic Parameters for Anode-Respiring Bacteria. Environ. Sci. Technol. 2009, 43, 7571–7577. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Ren, N.; Logan, B.E. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells. Bioresour. Technol. 2012, 124, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Kalil, M.S.; Chandrasekhar, K.; Mohanakrishna, G.; Saratale, G.D.; Saratale, R.G.; Kumar, G.; Pugazhendhi, A.; Sivagurunathan, P. Surpassing the current limitations of high purity H2 production in microbial electrolysis cell (MECs): Strategies for inhibiting growth of methanogens. Bioelectrochemistry 2018, 119, 211–219. [Google Scholar] [CrossRef]
- Lu, L.; Hou, D.; Wang, X.; Jassby, D.; Ren, Z.J. Active H2 Harvesting Prevents Methanogenesis in Microbial Electrolysis Cells. Environ. Sci. Technol. Lett. 2016, 3, 286–290. [Google Scholar] [CrossRef]
- Feng, H.J.; Huang, L.J.; Wang, M.Z.; Xu, Y.F.; Shen, D.S.; Li, N.; Chen, T.; Guo, K. An effective method for hydrogen production in a single-chamber microbial electrolysis by negative pressure control. Int. J. Hydrogen Energy 2018, 43, 17556–17561. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, H.; Qu, Y.; Feng, Y. Effect of long-term operation on stability and electrochemical response under water pressure for activated carbon cathodes in microbial fuel cells. Chem. Eng. J. 2016, 299, 314–319. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Middaugh, J.; Cheng, S.; Liu, W.; Wagner, R. How to Make Cathodes with a Diffusion Layer for Single-Chamber Microbial Fuel Cells. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=23.%09Middaugh%2C+J.%3B+Cheng%2C+S.%3B+Liu%2C+W.%3B+Wagner%2C+R.+How+to+make+cathodes+with+a+diffusion+layer+for+single-chamber+microbial+fuel+cells.+Journal+2006.&btnG= (accessed on 20 August 2024).
- Borgwardt, L.; Højgaard, L.; Carstensen, H.; Laursen, H.; Nowak, M.; Thomsen, C.; Schmiegelow, K. Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (nafion and PTFE) in single chamber microbial fuel cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, H.; Yin, X.; Wang, S. H2SO4 assisted hydrothermal conversion of biomass with solid acid catalysis to produce aviation fuel precursors. iScience 2023, 26, 108249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Guo, W.; Ying, X.; Zhao, N.; Yu, S.; Zhang, X.; Feng, H.; Zhang, Y.; Yu, H. Interspecies electron transfer between Geobacter and denitrifying bacteria for nitrogen removal in bioelectrochemical system. Chem. Eng. J. 2023, 455, 139821. [Google Scholar] [CrossRef]
- He, P.; Han, W.; Shao, L.; Lü, F. One-step production of C6–C8 carboxylates by mixed culture solely grown on CO. Biotechnol. Biofuels 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Long, F.; Liang, D.; Xiao, X.; Liu, H. Hydrogen production from lignocellulosic hydrolysate in an up-scaled microbial electrolysis cell with stacked bio-electrodes. Bioresour. Technol. 2021, 320, 124314. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Carracedo, B.; Morán, A.; Gómez, X. Performance of a semi-pilot tubular microbial electrolysis cell (MEC) under several hydraulic retention times and applied voltages. Bioresour. Technol. 2013, 146, 63–69. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Cotterill, S.; Dolfing, J.; Jones, C.; Curtis, T.; Heidrich, E. Low temperature domestic wastewater treatment in a microbial electrolysis cell with 1 m2 anodes: Towards system scale-up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Liang, D.; He, W.; Li, C.; Yu, Y.; Zhang, Z.; Ren, N.; Feng, Y. Bidirectional electron transfer biofilm assisted complete bioelectrochemical denitrification process. Chem. Eng. J. 2019, 375, 121960. [Google Scholar] [CrossRef]
- Deng, L.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Pandey, A.; Varjani, S.; Hoang, N.B. Recent advances in circular bioeconomy based clean technologies for sustainable environment. J. Water Process Eng. 2022, 46, 102534. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Muthukumar, K. Biohydrogen Production from Enzymatically Digested Cotton Stalks Using Citrobacter freundii. J. Inst. Eng. (India) Ser. E 2023, 104, 11–18. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Application of microbial biofilms in biocatalysis and biodegradation. In Enzymes for Pollutant Degradation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 93–118. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Logan, B.E. Electricity generation of single-chamber microbial fuel cells at low temperatures. Biosens. Bioelectron. 2011, 26, 1913–1917. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.S.; He, W.; Liang, D.; Li, C.; Yu, Y.; Ayaz, K.; Feng, Y. High-Efficiency Hydrogen Recovery from Corn Straw Hydrolysate Using Functional Bacteria and Negative Pressure with Microbial Electrolysis Cells. Water 2024, 16, 2423. https://doi.org/10.3390/w16172423
Yadav RS, He W, Liang D, Li C, Yu Y, Ayaz K, Feng Y. High-Efficiency Hydrogen Recovery from Corn Straw Hydrolysate Using Functional Bacteria and Negative Pressure with Microbial Electrolysis Cells. Water. 2024; 16(17):2423. https://doi.org/10.3390/w16172423
Chicago/Turabian StyleYadav, Ravi Shankar, Weihua He, Dandan Liang, Chao Li, Yanling Yu, Kamran Ayaz, and Yujie Feng. 2024. "High-Efficiency Hydrogen Recovery from Corn Straw Hydrolysate Using Functional Bacteria and Negative Pressure with Microbial Electrolysis Cells" Water 16, no. 17: 2423. https://doi.org/10.3390/w16172423
APA StyleYadav, R. S., He, W., Liang, D., Li, C., Yu, Y., Ayaz, K., & Feng, Y. (2024). High-Efficiency Hydrogen Recovery from Corn Straw Hydrolysate Using Functional Bacteria and Negative Pressure with Microbial Electrolysis Cells. Water, 16(17), 2423. https://doi.org/10.3390/w16172423







