The Use of Photo-Biological Parameters to Assess the Establishment Success of Posidonia oceanica Cuttings after Transplantation
Abstract
:1. Introduction
2. Material and Methods
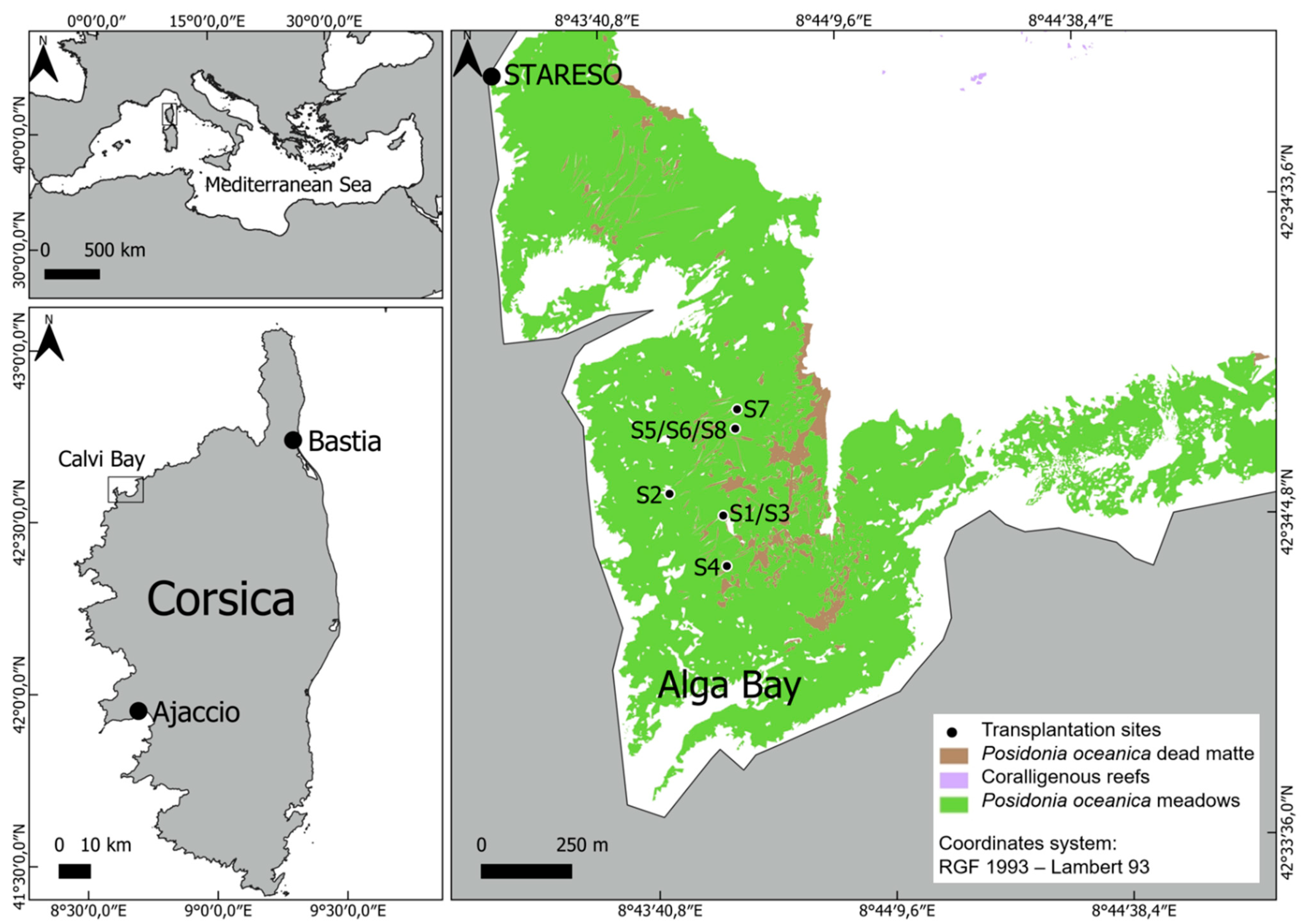
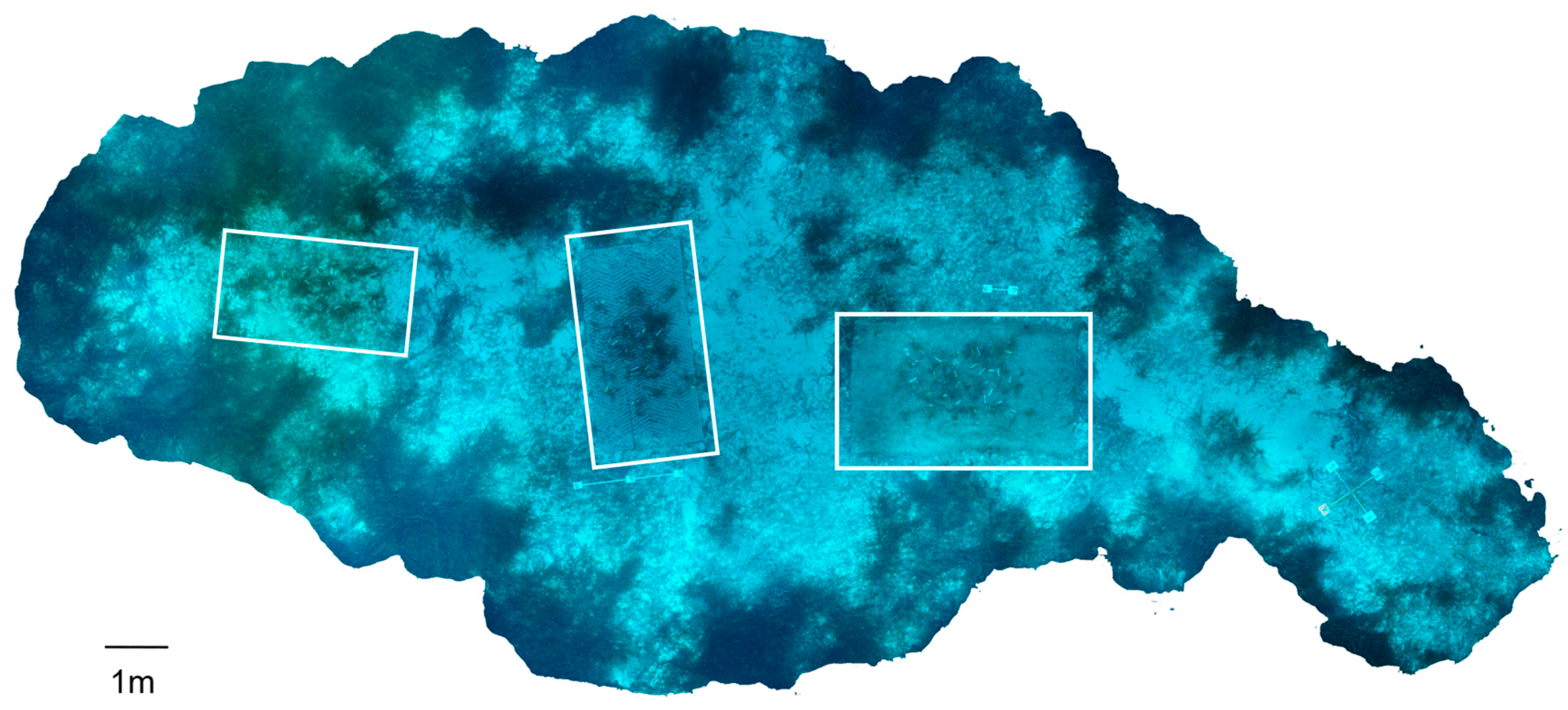
2.1. Study Area and Seagrass Transplantation
2.2. Sampling Campaigns and Biometric Measurements
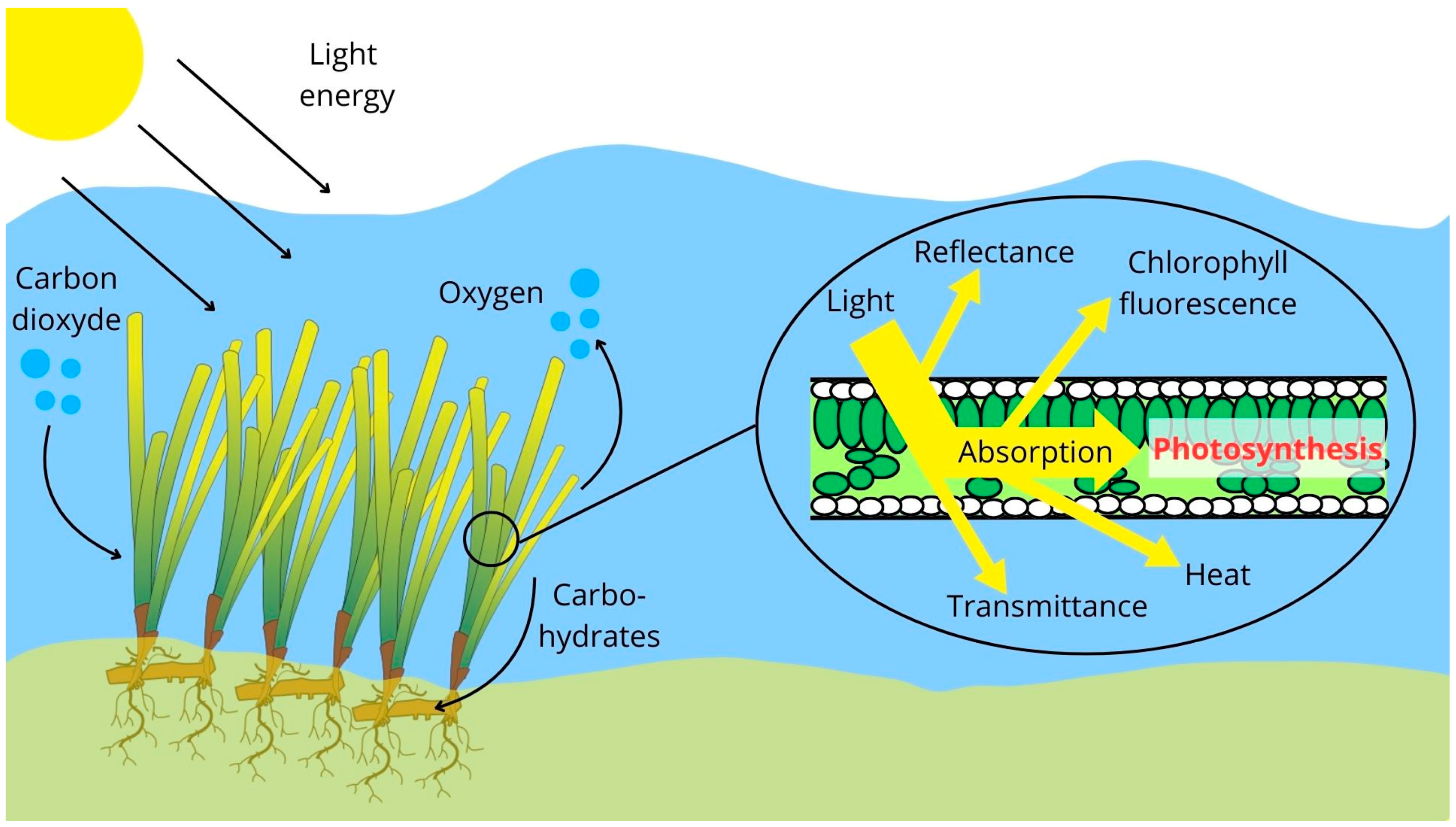
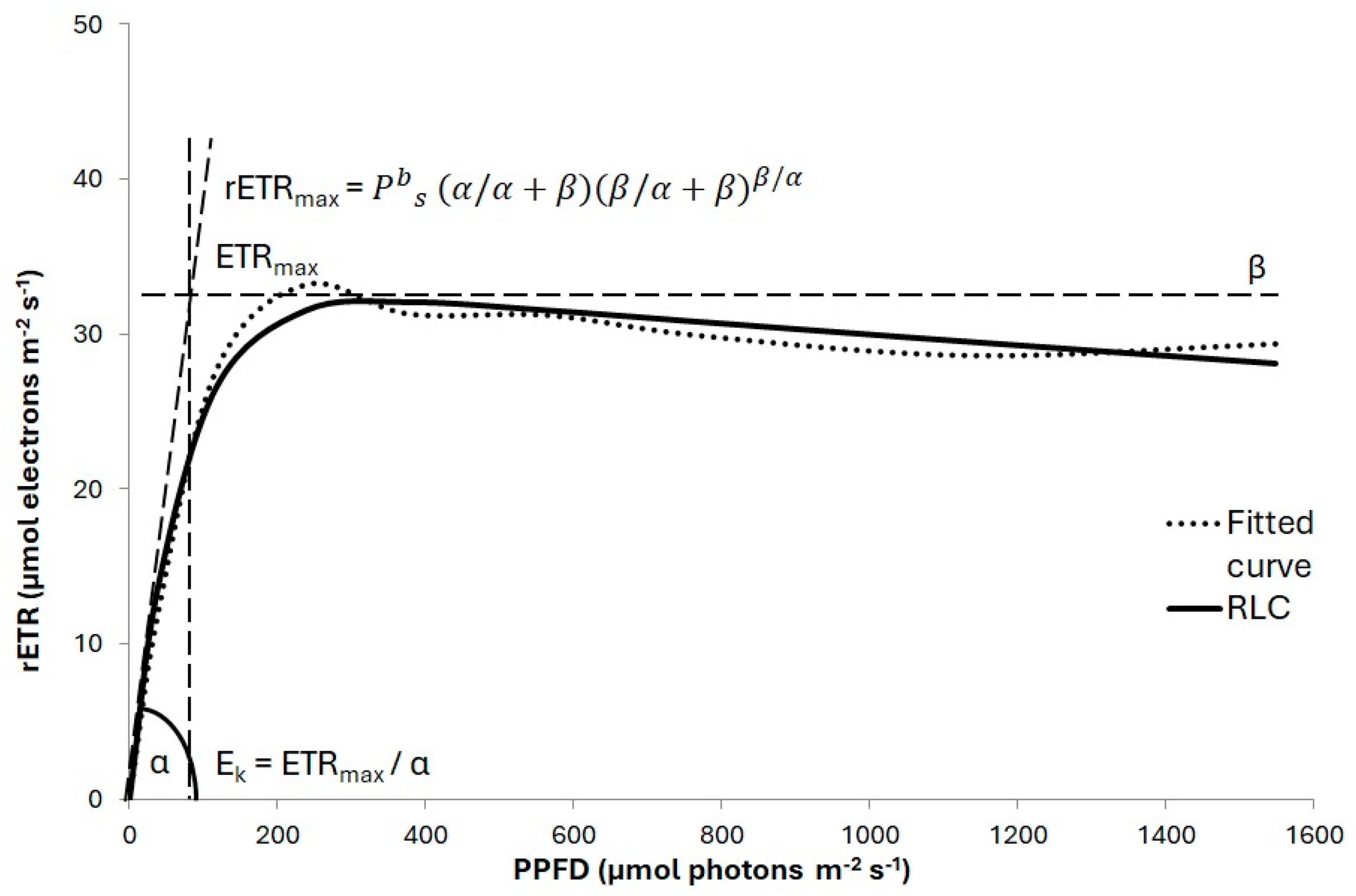
2.3. Photosynthetic Activity Measurements
2.4. Pigment Concentration and Analysis by HPLC
2.5. Carbohydrate Content in Rhizomes
2.6. Data Analysis
3. Results
3.1. Survival, Shoot Morphology, and Biomass
3.2. Photosynthetic Activity
3.3. Pigment Content
3.4. Carbohydrate Storage in Rhizomes
3.5. Correlation within and between Morphological and Photo-Biological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Turschwell, M.P.; Connolly, R.M.; Dunic, J.C.; Sievers, M.; Buelow, C.A.; Pearson, R.M.; Tulloch, V.J.D.; Côté, I.M.; Unsworth, R.K.F.; Collier, C.J.; et al. Anthropogenic Pressures and Life History Predict Trajectories of Seagrass Meadow Extent at a Global Scale. Proc. Natl. Acad. Sci. USA 2021, 118, e2110802118. [Google Scholar] [CrossRef] [PubMed]
- Cullen-Unsworth, L.C.; Unsworth, R.K.F. A Call for Seagrass Protection. Science 2018, 361, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.K.F.; McKenzie, L.J.; Nordlund, L.M.; Cullen-Unsworth, L.C. A Changing Climate for Seagrass Conservation? Curr. Biol. 2018, 28, R1229–R1232. [Google Scholar] [CrossRef] [PubMed]
- Rezek, R.J.; Furman, B.T.; Jung, R.P.; Hall, M.O.; Bell, S.S. Long-Term Performance of Seagrass Restoration Projects in Florida, USA. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van Katwijk, M.M.; Thorhaug, A.; Marbà, N.; Orth, R.J.; Duarte, C.M.; Kendrick, G.A.; Althuizen, I.H.J.; Balestri, E.; Bernard, G.; Cambridge, M.L.; et al. Global Analysis of Seagrass Restoration: The Importance of Large-Scale Planting. J. Appl. Ecol. 2016, 53, 567–578. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Blanfuné, A.; Pergent, G.; Thibaut, T. Restoration of Seagrass Meadows in the Mediterranean Sea: A Critical Review of Effectiveness and Ethical Issues. Water 2021, 13, 1034. [Google Scholar] [CrossRef]
- Calvo, S.; Calvo, R.; Luzzu, F.; Raimondi, V.; Assenzo, M.; Cassetti, F.P.; Tomasello, A. Performance Assessment of Posidonia oceanica (L.) Delile Restoration Experiment on Dead Matte Twelve Years after Planting—Structural and Functional Meadow Features. Water 2021, 13, 724. [Google Scholar] [CrossRef]
- Rende, S.F.; Bosman, A.; Menna, F.; Lagudi, A.; Bruno, F.; Severino, U.; Montefalcone, M.; Irving, A.D.; Raimondi, V.; Calvo, S.; et al. Assessing Seagrass Restoration Actions through a Micro-Bathymetry Survey Approach (Italy, Mediterranean Sea). Water 2022, 14, 1285. [Google Scholar] [CrossRef]
- Horn, L.E.; Paling, E.I.; van Keulen, M. Photosynthetic Recovery of Transplanted Posidonia sinuosa, Western Australia. Aquat. Bot. 2009, 90, 149–156. [Google Scholar] [CrossRef]
- Ralph, P.J.; Tomasko, D.; Moore, K.; Seddon, S.; Macinnis-Ng, C.M.O. Human Impacts on Seagrasses: Eutrophication, Sedimentation and Contamination. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 567–593. [Google Scholar]
- Li, W.-T.; Park, J.-I.; Park, S.-R.; Zhang, X.-M.; Lee, K.-S. Chlorophyll a Fluorescence as an Indicator of Establishment of Zostera marina Transplants on the Southern Coast of Korea. Algae 2010, 25, 89–97. [Google Scholar] [CrossRef]
- Belshe, E.F.; Durako, M.J.; Blum, J.E. Photosynthetic Rapid Light Curves (RLC) of Thalassia testudinum Exhibit Diurnal Variation. J. Exp. Mar. Biol. Ecol. 2007, 342, 253–268. [Google Scholar] [CrossRef]
- Gobert, S.; Lepoint, G.; Silva, J.; Santos, R.; Lejeune, P.; Dujardin, P.; Delvaux, B.; Cornelis, J.-T.; Richir, J. A Consensual Diving-PAM Protocol to Monitor Posidonia oceanica Photosynthesis. In Proceedings of the 4th Mediterranean Seagrass Workshop—MSW’15, Oristano, Italy, 18–22 May 2015. [Google Scholar] [CrossRef]
- Madonia, A.; Caporale, G.; Penna, M.; Bonamano, S.; Marcelli, M. Assessment of the Photosynthetic Response of Posidonia oceanica (Linneaus) Delile, 1813 along a Depth Gradient in the Northern Tyrrhenian Sea (Latium, Italy). Geosciences 2021, 11, 202. [Google Scholar] [CrossRef]
- Gera, A.; Alcoverro, T.; Mascaró, O.; Pérez, M.; Romero, J. Exploring the Utility of Posidonia oceanica Chlorophyll Fluorescence as an Indicator of Water Quality within the European Water Framework Directive. Environ. Monit. Assess. 2012, 184, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Larkum, A.W.; Drew, E.A.; Ralph, P.J. Photosynthesis and Metabolism in Seagrasses at the Cellular Level. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2007; pp. 323–345. [Google Scholar] [CrossRef]
- Léger-Daigle, R.; Noisette, F.; Bélanger, S.; Cusson, M.; Nozais, C. Photoacclimation and Light Thresholds for Cold Temperate Seagrasses. Front. Plant Sci. 2022, 13, 805065. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W., III; Ebbert, V.; Logan, B.A. Ecophysiology of the Xanthophyll Cycle. In The Photochemistry of Carotenoids; Frank, H.A., Young, A.J., Britton, G., Cogdell, R.J., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1999; pp. 245–269. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid Light Curves: A Powerful Tool to Assess Photosynthetic Activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Alcoverro, T.; Manzanera, M.; Romero, J. Annual Metabolic Carbon Balance of the Seagrass Posidonia oceanica: The Importance of Carbohydrate Reserves. Mar. Ecol. Prog. Ser. 2001, 211, 105–116. [Google Scholar] [CrossRef]
- Genot, I.; Caye, G.; Meinesz, A.; Orlandini, M. Role of Chlorophyll and Carbohydrate Contents in Survival of Posidonia oceanica Cuttings Transplanted to Different Depths. Mar. Biol. 1994, 119, 23–29. [Google Scholar] [CrossRef]
- Pirc, H. Seasonal Changes in Soluble Carbohydrates, Starch, and Energy Content in Mediterranean Seagrasses. Mar. Ecol. 1989, 10, 97–105. [Google Scholar] [CrossRef]
- Horn, L. The Measurement of Seagrass Photosynthesis Using Pulse Amplitude Modulated (PAM) Fluorometry and Its Practical Applications, Specifically in Regard to Transplantation. Ph.D. Thesis, Murdoch University, Perth, Australia, 2006. [Google Scholar]
- Abadie, A.; Lejeune, P.; Pergent, G.; Gobert, S. From Mechanical to Chemical Impact of Anchoring in Seagrasses: The Premises of Anthropogenic Patch Generation in Posidonia oceanica Meadows. Mar. Pollut. Bull. 2016, 109, 61–71. [Google Scholar] [CrossRef]
- Fullgrabe, L.; Fontaine, Q.; Marengo, M.; Donnay, A.; Sirjacobs, D.; Iborra, L.; Cnudde, S.; Mellet, N.; Seveno, J.; Boulenger, A.; et al. STARECAPMED (STAtion of Reference and Research on Change of Local and Global Anthropogenic Pressures on Mediterranean Ecosystems Drifts)—Year 2021; Research Report; STARESO: Calvi, France, 2022; 123p. [Google Scholar]
- Gobert, S.; Lepoint, G.; Pelaprat, C.; Remy, F.; Lejeune, P.; Richir, J.; Abadie, A. Temporal Evolution of Sand Corridors in a Posidonia oceanica Seascape: A 15-Year Study. Mediterr. Mar. Sci. 2016, 17, 777–784. [Google Scholar] [CrossRef]
- DONIA EXPERT. Available online: https://plateforme.medtrix.fr (accessed on 2 February 2024).
- Gobert, S.; Lefebvre, L.; Boissery, P.; Richir, J. A Non-Destructive Method to Assess the Status of Posidonia oceanica Meadows. Ecol. Indic. 2020, 119, 106838. [Google Scholar] [CrossRef]
- Dauby, P.; Poulicek, M. Methods for Removing Epiphytes from Seagrasses: SEM Observations on Treated Leaves. Aquat. Bot. 1995, 52, 217–228. [Google Scholar] [CrossRef]
- Buia, M.C.; Zupo, V.; Mazzella, L. Primary Production and Growth Dynamics in Posidonia oceanica. Mar. Ecol. 1992, 13, 2–16. [Google Scholar] [CrossRef]
- Lassauque, J. Ecophysiological Early Bio-Indicators of Anthropic-Induced Stress on Posidonia oceanica Meadows. Ph.D. Thesis, Université de Nice Sophia Antipolis, Nice, France, 2009. [Google Scholar]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of Photosynthesis in Natural Assemblages of Marine Phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Marín-Guirao, L.; Sandoval-Gil, J.M.; Bernardeau-Esteller, J.; Ruíz, J.M.; Sánchez-Lizaso, J.L. Responses of the Mediterranean Seagrass Posidonia oceanica to Hypersaline Stress Duration and Recovery. Mar. Environ. Res. 2013, 84, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Roberty, S.; Vega de Luna, F.; Pierangelini, M.; Bomhals, J.; Plumier, J.C.; Levy, O.; Cardol, P. Shallow and mesophotic colonies of the coral Stylophora pistillata share similar regulatory strategies of photosynthetic electron transport but differ in their sensitivity to light. Coral Reefs 2023, 42, 645–659. [Google Scholar] [CrossRef]
- Zimmerman, R.C.; Smith, R.D.; Alberte, R.S. Thermal acclimation and whole-plant carbon balance in Zostera marina L. (eelgrass). J. Exp. Mar. Biol. Ecol. 1989, 130, 93–109. [Google Scholar] [CrossRef]
- Gera, A.; Pagès, J.F.; Romero, J.; Alcoverro, T. Combined effects of fragmentation and herbivory on Posidonia oceanica seagrass ecosystems. J. Ecol. 2013, 101, 1053–1061. [Google Scholar] [CrossRef]
- Huber, S.C.; Israel, D.W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982, 69, 691–696. [Google Scholar] [CrossRef]
- Yemn, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.K. Exploring process data. J. Process Control 2001, 11, 179–194. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.; Cohen, L.; Jarvis, P. Practical Statistics for Field Biology; Wiley: Chichester, UK, 1998. [Google Scholar]
- Pansini, A.; Bosch-Belmar, M.; Berlino, M.; Sarà, G.; Ceccherelli, G. Collating Evidence on the Restoration Efforts of the Seagrass Posidonia oceanica: Current Knowledge and Gaps. Sci. Total Environ. 2022, 851, 158320. [Google Scholar] [CrossRef] [PubMed]
- Lepoint, G.; Vangeluwe, D.; Eisinger, M.; Paster, M.; Van Treeck, P.; Bouquegneau, J.M.; Gobert, S. Nitrogen Dynamics in Posidonia oceanica Cuttings: Implications for Transplantation Experiments. Mar. Pollut. Bull. 2004, 48, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Gobert, S.; Lepoint, G.; Bouquegneau, J.M.; Vangeluwe, D.; Eisinger, M.; Paster, M.; Schuhmaker, H.; Van Treeck, P. Restoration of Seagrass Meadows: Means and Limitations. In Proceedings of the 7th International Conference on the Mediterranean Coastal Environment, MEDCOAST, Kusadasi, Turkey, 25–29 October 2005; Volume 1, pp. 461–472. [Google Scholar]
- Collier, C.J.; Lavery, P.S.; Ralph, P.J.; Masini, R.J. Shade-Induced Response and Recovery of the Seagrass Posidonia sinuosa. J. Exp. Mar. Biol. Ecol. 2009, 370, 89–103. [Google Scholar] [CrossRef]
- Dattolo, E.; Marín-Guirao, L.; Ruiz, J.M.; Procaccini, G. Long-term acclimation to reciprocal light conditions suggests depth-related selection in the marine foundation species Posidonia oceanica. Ecol. Evol. 2017, 7, 1148–1164. [Google Scholar] [CrossRef] [PubMed]
- Stipcich, P.; Resaikos, V.; Ceccherelli, G. Experimental thermocline deepening highlights the resilience of the seagrass Posidonia oceanica: An opportunity to investigate shoot adaptability. Mar. Pollut. Bull. 2023, 189, 114824. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Romero, J. Effects of disturbances caused by coastal constructions on spatial structure, growth dynamics and photosynthesis of the seagrass Posidonia oceanica. Mar. Pollut. Bull. 2003, 46, 1523–1533. [Google Scholar] [CrossRef]
- Dattolo, E.; Ruocco, M.; Brunet, C.; Lorenti, M.; Lauritano, C.; D’Esposito, D.; de Luca, P.; Sanges, R.; Mazzuca, S.; Procaccini, G. Response of the seagrass Posidonia oceanica to different light environments: Insights from a combined molecular and photo-physiological study. Mar. Environ. Res. 2014, 101, 225–236. [Google Scholar] [CrossRef]
- Shafer, D.J.; Kaldy, J.E. Comparison of photosynthetic characteristics of the seagrass congeners Zostera marina L. and Zostera japonica Ascher. & Graeb. Aquat. Bot. 2014, 112, 91–97. [Google Scholar] [CrossRef]
- Campbell, S.; Miller, C.; Steven, A.; Stephens, A. Photosynthetic responses of two temperate seagrasses across a water quality gradient using chlorophyll fluorescence. J. Exp. Mar. Biol. Ecol. 2003, 291, 57–78. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Jiménez, C.; Viñegla, B.; Pérez-Rodríguez, E.; Aguilera, J.; Flores-Moya, A.; Altamirano, M.; Lebert, M.; Häder, D.P. Effects of solar UV radiation on photosynthesis of the marine angiosperm Posidonia oceanica from southern Spain. Mar. Ecol. Prog. Ser. 2002, 230, 59–70. [Google Scholar] [CrossRef]
- Major, K.M.; Dunton, K.H. Variations in light-harvesting characteristics of the seagrass, Thalassia testudinum: Evidence for photoacclimation. J. Exp. Mar. Biol. Ecol. 2002, 275, 173–189. [Google Scholar] [CrossRef]
- Collier, C.J.; Lavery, P.S.; Ralph, P.J.; Masini, R.J. Physiological characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability. Mar. Ecol. Prog. Ser. 2008, 353, 65–79. [Google Scholar] [CrossRef]
- Dawes, C.J. Biomass and photosynthetic responses to irradiance by a shallow and a deep water population of Thalassia testudinum on the west coast of Florida. Bull. Mar. Sci. 1998, 62, 89–96. [Google Scholar]
- Durako, M.J.; Kunzelman, J.I.; Kenworthy, W.J.; Hammerstrom, K.K. Depth-related variability in the photobiology of two populations of Halophila johnsonii and Halophila decipiens. Mar. Biol. 2003, 142, 1219–1228. [Google Scholar] [CrossRef]
- Olesen, B.; Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Depth-acclimation of photosynthesis, morphology and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Mar. Ecol. Prog. Ser. 2002, 236, 89–97. [Google Scholar] [CrossRef]
- Pirc, H. Seasonal aspects of photosynthesis in Posidonia oceanica: Influence of depth, temperature and light intensity. Aquat. Bot. 1986, 26, 203–212. [Google Scholar] [CrossRef]
- Lorenti, M.; Gera, A.; Lassauque, J.; Mattera, F.; Buia, M.C. Comparative in situ estimates of the photosynthetic activity of Posidonia oceanica: RLC and maximum quantum yield measurements. Biol. Mar. Mediterr. 2006, 13, 56–59. [Google Scholar]
- Molenaar, H.; Meinesz, A. Vegetative Reproduction in Posidonia oceanica II. Effects of Depth Changes on Transplanted Orthotopic Shoots. Mar. Ecol. 1992, 13, 175–185. [Google Scholar] [CrossRef]
- Augier, H.; Maudinas, B. Variations de la croissance et de la teneur en pigments de la phanérogame marine Posidonia oceanica dans le parc national de Port-Cros en fonction de la profondeur et du degré de pollution. Etude préliminaire des paramètres physiologiques et biochimiques susceptibles de caractériser le degré d’impact de la pollution sur l’herbier de posidonies. Trav. Sci. Parc Natl. Port-Cros 1977, 3, 36–56. [Google Scholar]
- Caye, G. Sur la morphogenèse et le cycle végétatif de Posidonia oceanica (L.) Delile. Ph.D. Thesis, Université d’Aix-Marseille II, Marseille, France, 1980. [Google Scholar]
- Caye, G.; Rossignol, M. Etude des variations saisonnières de la croissance des feuilles et des racines de Posidonia oceanica. Mar. Biol. 1983, 75, 79–88. [Google Scholar] [CrossRef]
- Dennison, W.C.; Alberte, R.S. Photoadaptation and growth of Zostera marina L. (eelgrass) transplants along a depth gradient. J. Exp. Mar. Biol. Ecol. 1986, 98, 265–282. [Google Scholar] [CrossRef]
- Duarte, C.M. Seagrass nutrient content. Mar. Ecol. Prog. Ser. 1990, 67, 201–207. [Google Scholar] [CrossRef]
- Bhagooli, R.; Mattan-Moorgawa, S.; Kaullysing, D.; Louis, Y.D.; Gopeechund, A.; Ramah, S.; Soondur, M.; Pilly, S.S.; Beesoo, R.; Wijayanti, D.P.; et al. Chlorophyll fluorescence—A tool to assess photosynthetic performance and stress photophysiology in symbiotic marine invertebrates and seaplants. Mar. Pollut. Bull. 2021, 165, 112059. [Google Scholar] [CrossRef] [PubMed]
- de Kock, W.; Hasler-Sheetal, H.; Holmer, M.; Tsapakis, M.; Apostolaki, E.T. Metabolomics and Traditional Indicators Unveil Stress of a Seagrass (Cymodocea Nodosa) Meadow at Intermediate Distance from a Fish Farm. Ecol. Indic. 2020, 109, 105765. [Google Scholar] [CrossRef]
- Jung, E.M.U.; Abdul Majeed, N.A.B.; Booth, M.W.; Austin, R.; Sinclair, E.A.; Fraser, M.W.; Martin, B.C.; Oppermann, L.M.F.; Bollen, M.; Kendrick, G.A. Marine Heatwave and Reduced Light Scenarios Cause Species-Specific Metabolomic Changes in Seagrasses under Ocean Warming. New Phytol. 2023, 239, 1692–1706. [Google Scholar] [CrossRef]
- Griffiths, L.L.; Melvin, S.D.; Connolly, R.M.; Pearson, R.M.; Brown, C.J. Metabolomic Indicators for Low-Light Stress in Seagrass. Ecol. Indic. 2020, 114, 106316. [Google Scholar] [CrossRef]
- Costa, M.M.; Silva, J.; Barrote, I.; Santos, R. Heatwave Effects on the Photosynthesis and Antioxidant Activity of the Seagrass Cymodocea Nodosa under Contrasting Light Regimes. Oceans 2021, 2, 448–460. [Google Scholar] [CrossRef]

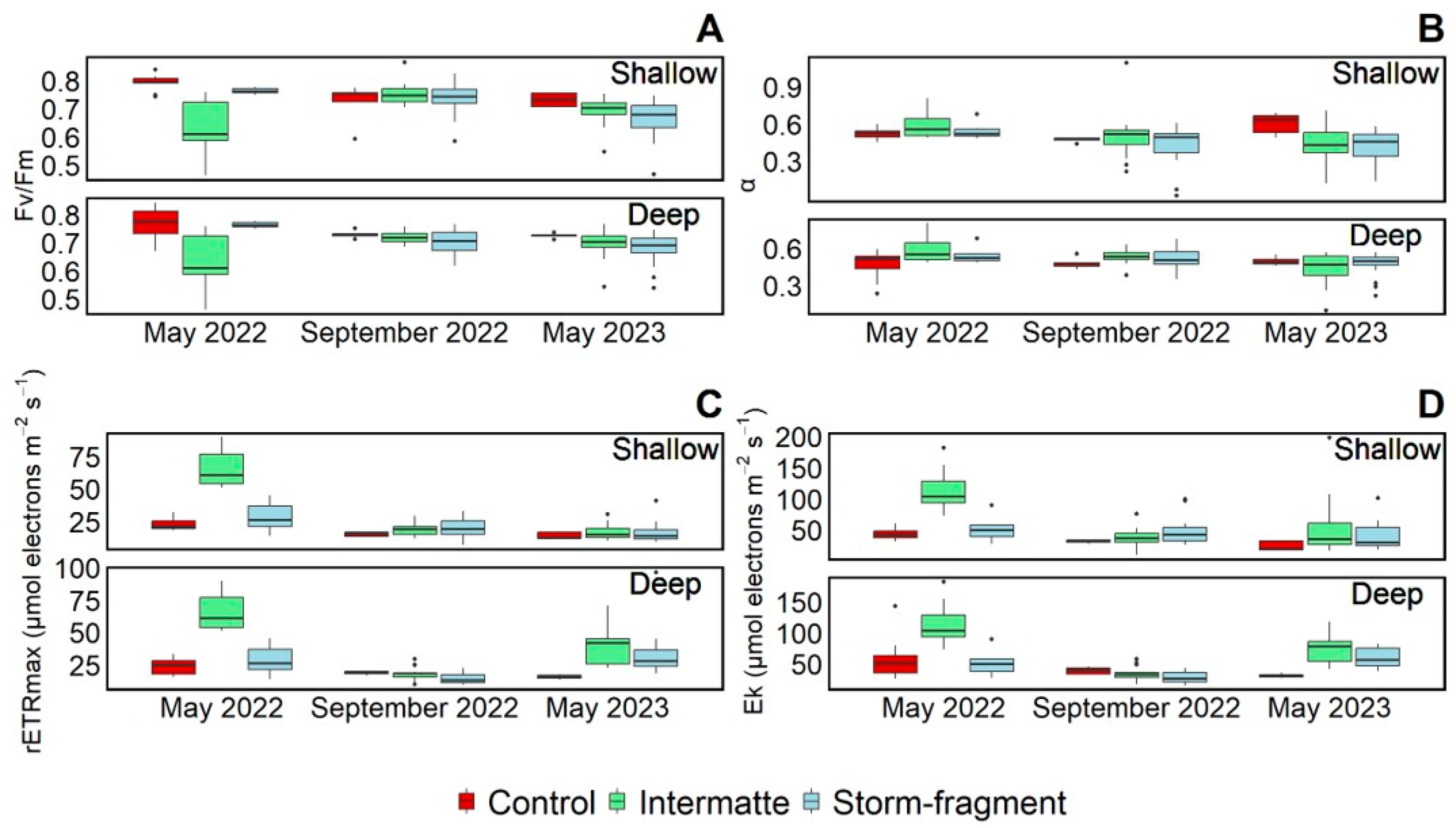
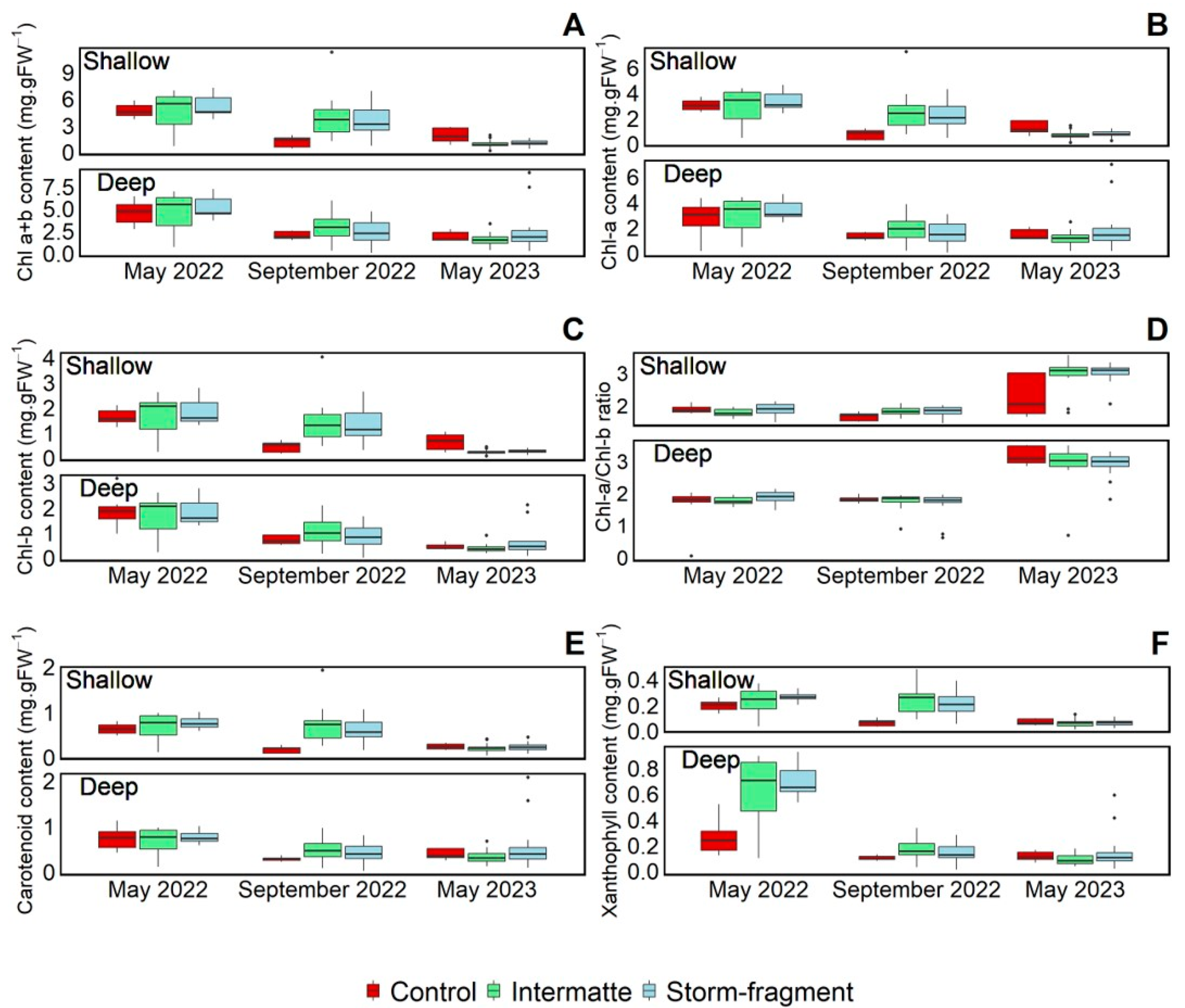

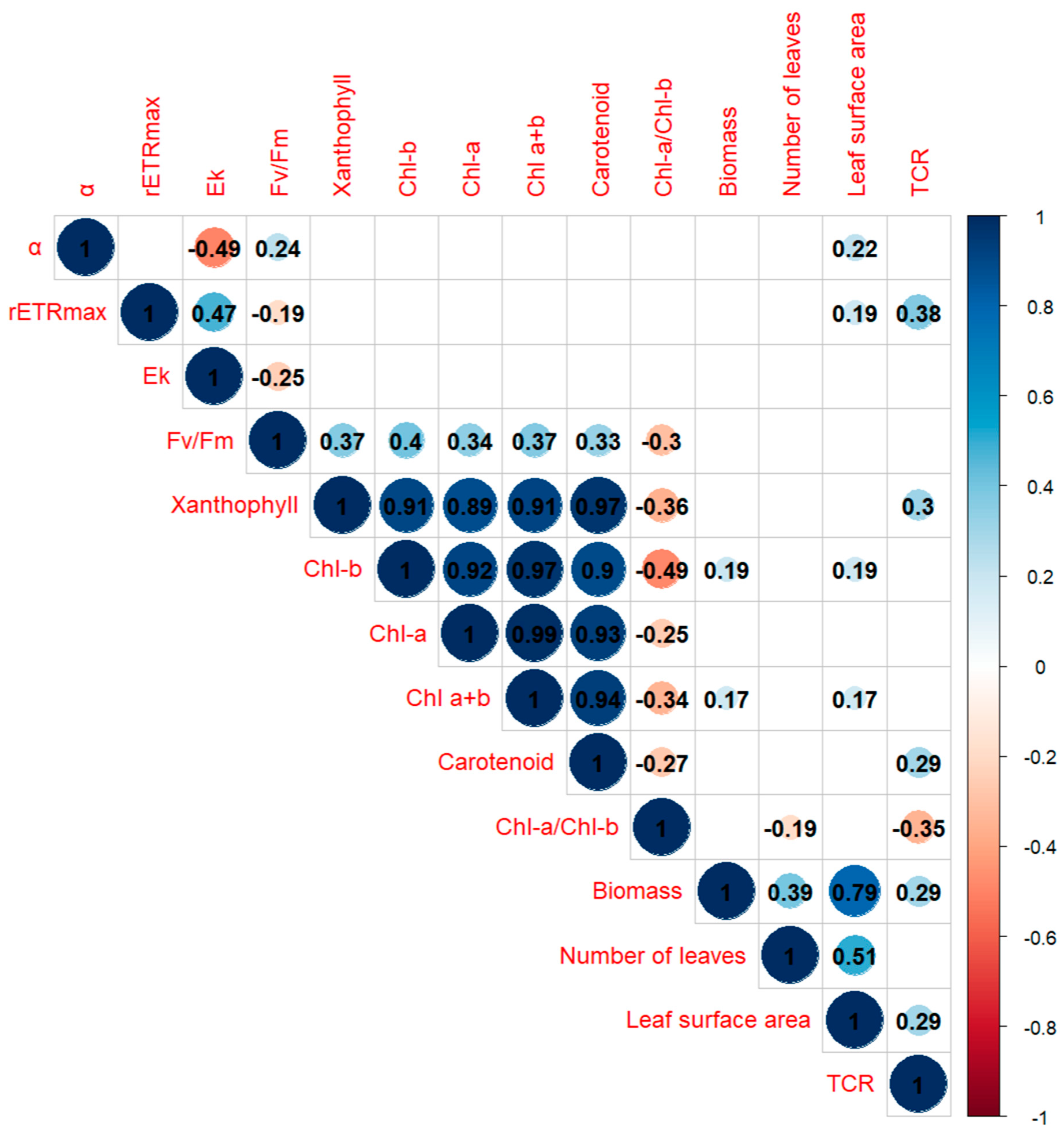

| Effect Type | May 2022 | September 2022 | May 2023 | ||||
| Origin | Origin | Bathymetry | OxB | Origin | Bathymetry | OxB | |
| Morphology | |||||||
| Survival | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Number of leaves | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Leaf surface area | n.s. | * | n.s. | n.s. | * | n.s. | n.s. |
| Biomass | n.s. | * | n.s. | n.s. | * | n.s. | n.s. |
| Photo-biology | |||||||
| Fv/Fm | * | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| α | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| rETRmax | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Ek | * | n.s. | * | n.s. | n.s. | * | n.s. |
| Chl-a | n.s. | * | * | n.s. | n.s. | * | n.s. |
| Chl-b | n.s. | * | * | n.s. | * | * | * |
| Chl a+b | n.s. | * | * | n.s. | n.s. | * | n.s. |
| Chl-a/Chl-b | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Xanthophyll | n.s. | * | * | n.s. | n.s. | * | n.s. |
| Carotenoid | n.s. | * | * | n.s. | n.s. | * | n.s. |
| TCR | n.s. | NA | NA | NA | n.s. | * | n.s. |
| Pairwise test | May 2022 | September 2022 | May 2023 | ||||
| Origin | Origin | OxB | Origin | OxB | |||
| Leaf surface area | C ≠ I C ≠ E | C ≠ I C ≠ E | |||||
| Biomass | C ≠ I C ≠ E | C ≠ I C ≠ E | |||||
| Fv/Fm | C ≠ I | C ≠ E | |||||
| rETRmax | C ≠ I I ≠ E | ||||||
| Ek | C ≠ I | ||||||
| Chl-a | C ≠ I | ||||||
| Chl-b | C ≠ I | C ≠ I | SI ≠ DE SE ≠ DE | ||||
| Chl a+b | C ≠ I | ||||||
| Xanthophyll | C ≠ I C ≠ E | ||||||
| Carotenoid | C ≠ I C ≠ E | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulenger, A.; Roberty, S.; Lopez Velosa, M.M.; Marengo, M.; Gobert, S. The Use of Photo-Biological Parameters to Assess the Establishment Success of Posidonia oceanica Cuttings after Transplantation. Water 2024, 16, 1702. https://doi.org/10.3390/w16121702
Boulenger A, Roberty S, Lopez Velosa MM, Marengo M, Gobert S. The Use of Photo-Biological Parameters to Assess the Establishment Success of Posidonia oceanica Cuttings after Transplantation. Water. 2024; 16(12):1702. https://doi.org/10.3390/w16121702
Chicago/Turabian StyleBoulenger, Arnaud, Stéphane Roberty, Maria Margarita Lopez Velosa, Michel Marengo, and Sylvie Gobert. 2024. "The Use of Photo-Biological Parameters to Assess the Establishment Success of Posidonia oceanica Cuttings after Transplantation" Water 16, no. 12: 1702. https://doi.org/10.3390/w16121702
APA StyleBoulenger, A., Roberty, S., Lopez Velosa, M. M., Marengo, M., & Gobert, S. (2024). The Use of Photo-Biological Parameters to Assess the Establishment Success of Posidonia oceanica Cuttings after Transplantation. Water, 16(12), 1702. https://doi.org/10.3390/w16121702






