Construction of Genetically Engineered Escherichia coli Cell Factory for Enhanced Cadmium Bioaccumulation in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Strain and Plasmid
2.1.2. Main Reagent
2.1.3. Culture Medium
2.2. Method
2.2.1. Construction of Fusion Protein Gene Expression Vector
2.2.2. Expression and Purification of Recombinant Fusion Protein
2.2.3. Morphological Characterization of Recombinant Fusion Protein
2.2.4. Adsorption of Recombinant Protein rHF-EC20 to Cadmium
2.2.5. Adsorption of Recombinant Strain to Cadmium
Influence of Induction Time on Cadmium Adsorption by Recombinant Strain
Optimal Adsorption pH and Temperature
- (1)
- Optimal adsorption temperature: The recombinant strain BL21 (FLE), auto-induced for expression, was collected after centrifugation and resuspended in Tris-HCl (pH 7.0). The bacterium with a density of 3 g/L was added to cadmium chloride with a final concentration of 50 mg/L. The strains were separately placed on a shaker at 16, 25, 37, and 45 °C and incubated for two hours at 150 rpm. Samples were collected into nitric acid-soaked centrifuge tubes and centrifuged at 12,000 rpm for two minutes. The bacterial cells were dried, digested with nitric acid, and then quantitatively analyzed for their cadmium content using cadmium detection kits.
- (2)
- Optimal adsorption pH: The recombinant strain BL21 (FLE) were collected after centrifugation and resuspended in Tris-HCl buffer at pH values of 5.0, 6.0, 7.0, 8.0, and 9.0 at the optimal temperature. The bacterial density was 3 g/L. After adding cadmium chloride with a final concentration of 50 mg/L, the strains were separately incubated at 150 r/min for two hours. The bacterial cells were collected, and a quantitative analysis was performed on the adsorbed cadmium following the above procedure. The adsorption rate A was calculated using the formula A = Ce/C0 × 100%, where C0 represents the initial concentration of cadmium chloride (in mg), and Ce denotes the cadmium ions adsorbed by the recombinant strains (in mg).
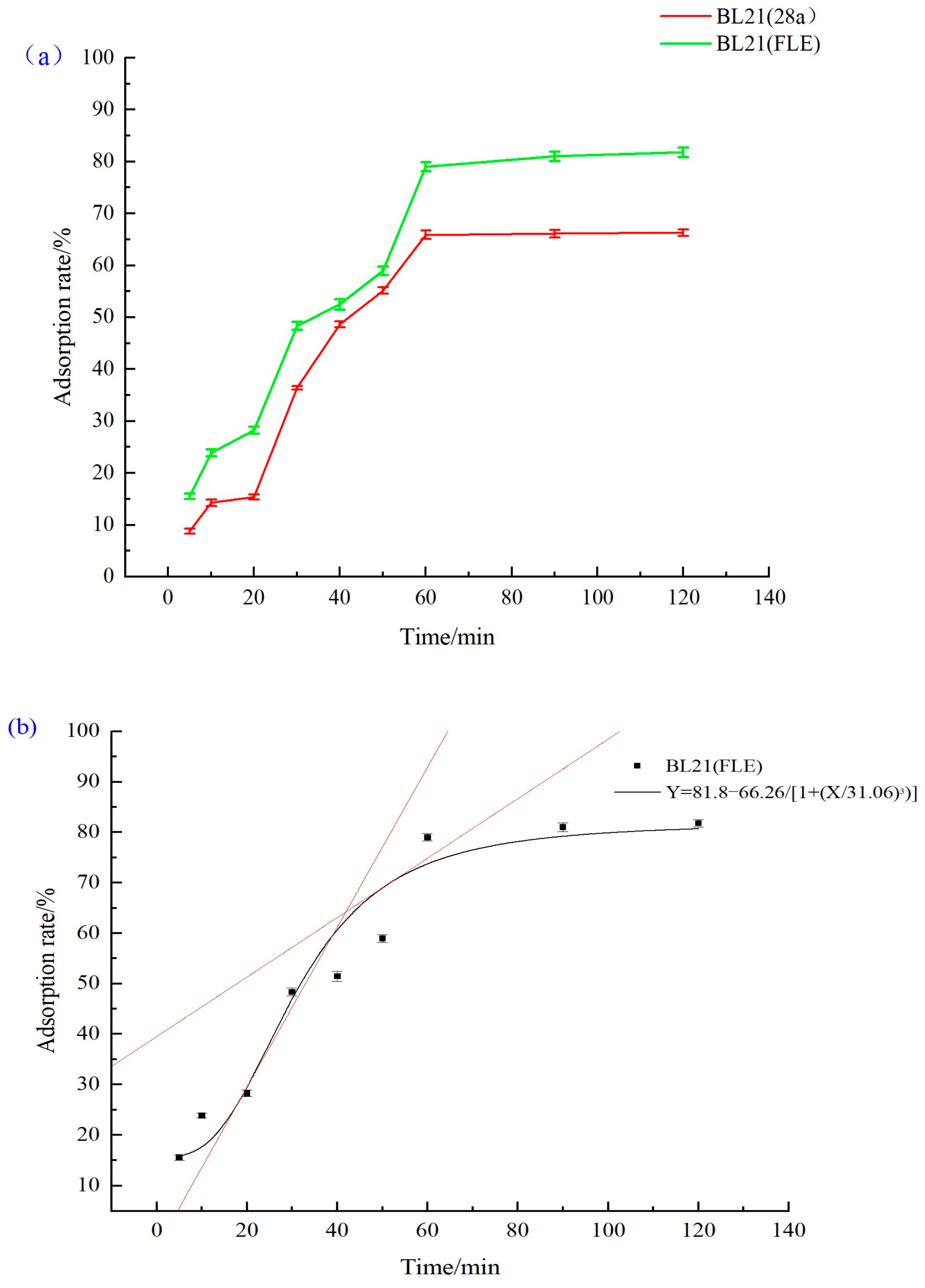
Time Adsorption Curve of Recombinant Strain
Equilibrium Adsorption Capacity of Recombinant Strain to Cadmium
2.3. Statistical Analyses
3. Result and Analysis
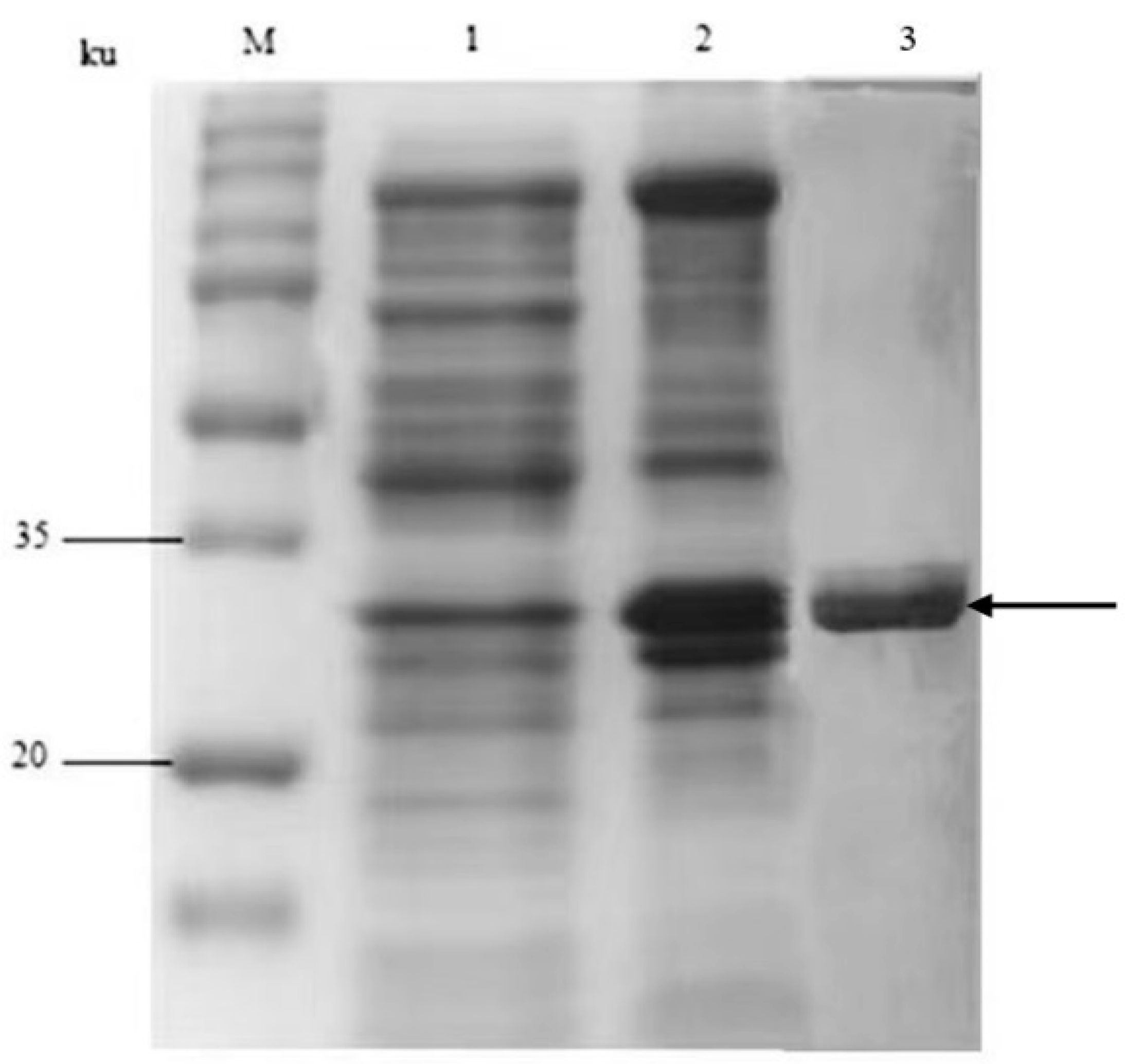
3.1. Construction of Fusion Gene Expression Vector and Analysis of Fusion Protein Expression
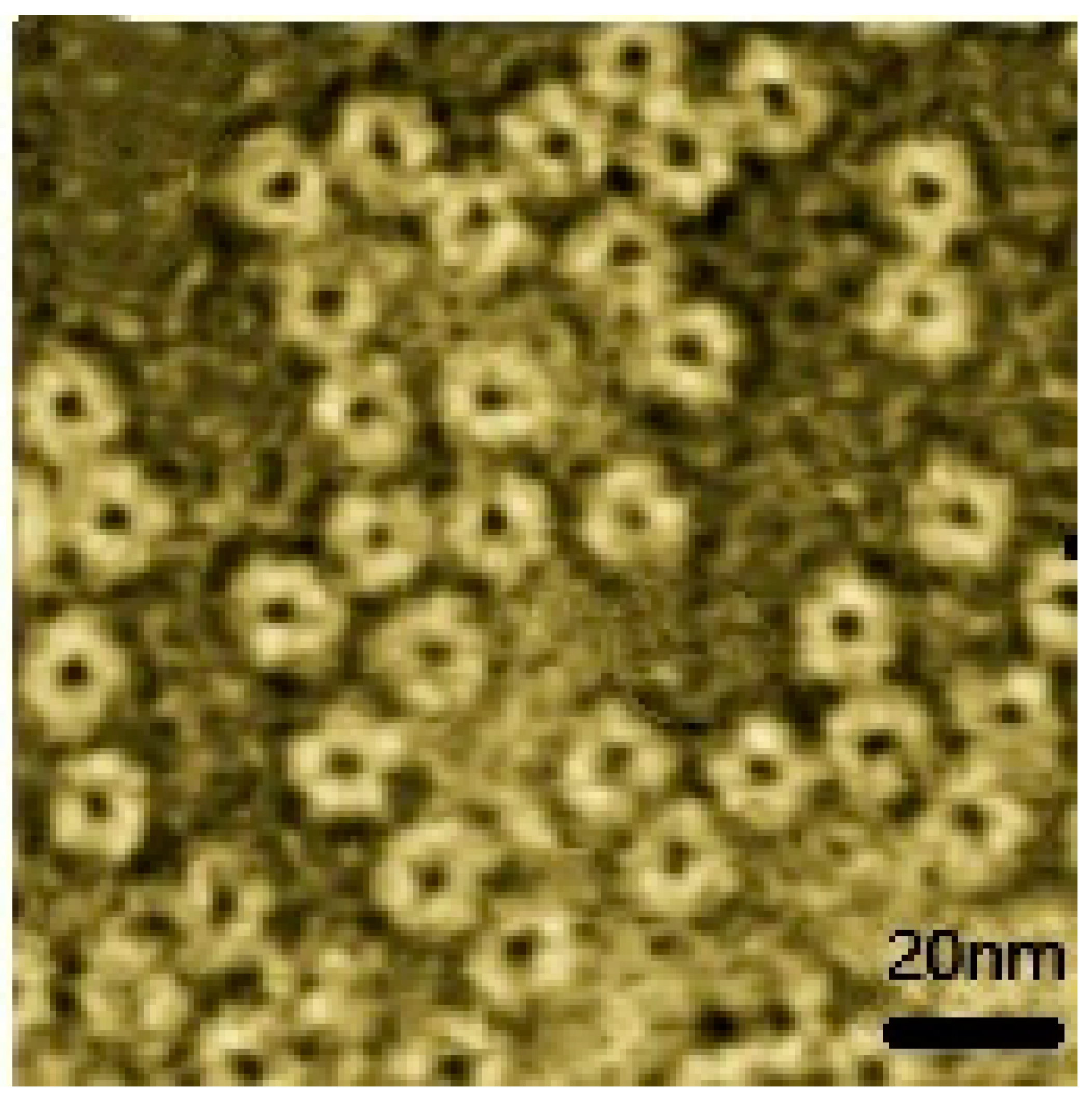
3.2. Morphological Analysis of Fusion Protein
3.3. Adsorption Capacity of Recombinant Fusion Protein rHF-EC20 to Cadmium
3.4. Adsorption Characteristics of Engineered Bacterium E. coli BL21 (FLE) to Cadmium
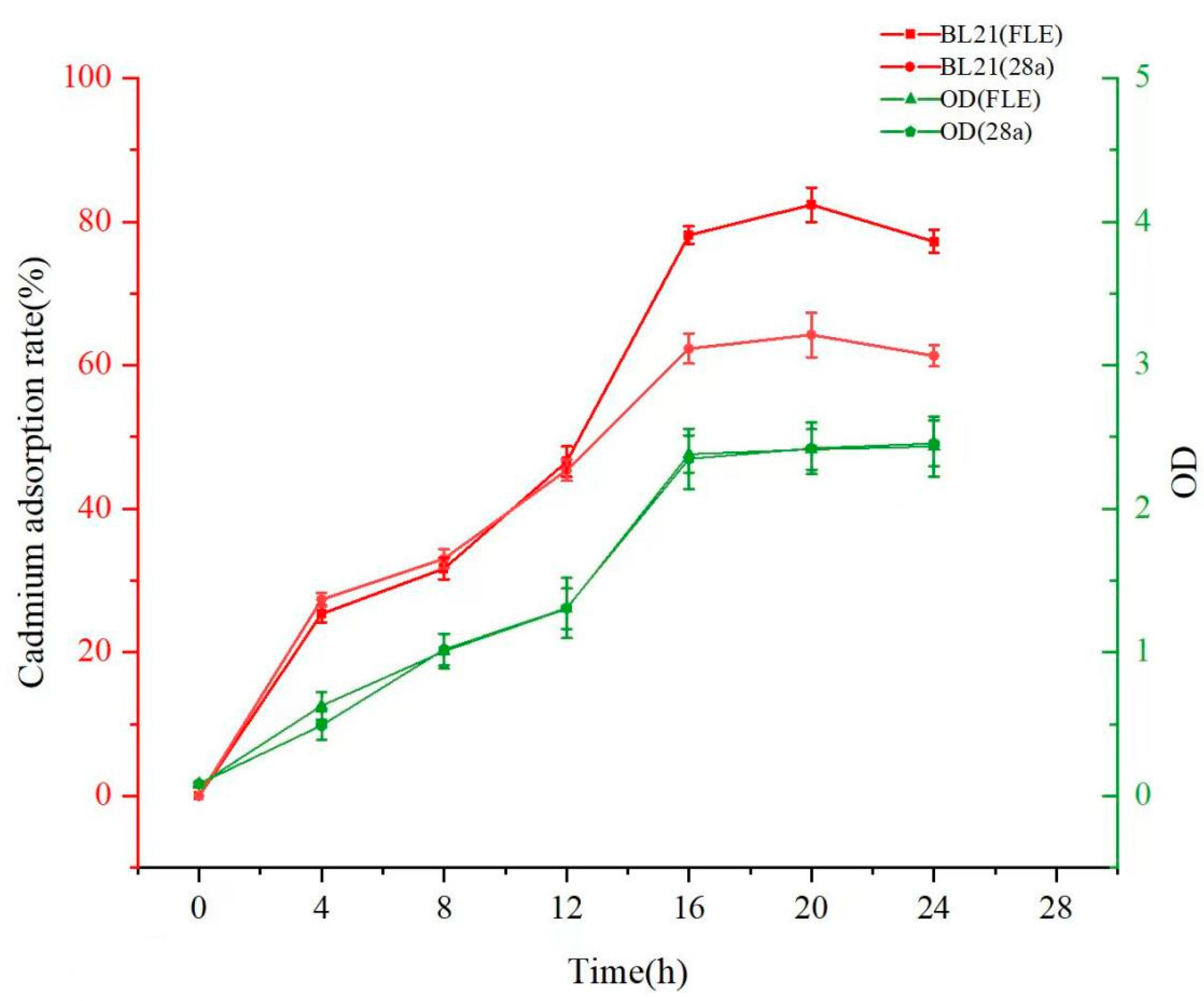
3.4.1. Impact of Induction Time on Cadmium Adsorption by Engineered Strain
3.4.2. Optimal Adsorption Temperature of Engineered Bacterium BL21 (FLE)
3.4.3. Optimal Adsorption pH of Engineered Bacterium E. coli BL21 (FLE)
3.4.4. Evaluation of Adsorption Efficiency of Recombinant Strain to Cd2+
3.4.5. Equilibrium Adsorption Capacity of Recombinant Strain to Cd2+
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pratush, A.; Kumar, A.; Hu, Z. Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: A review. Int. Microbiol. 2018, 21, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Sun, J.; Sun, M.; Shi, X.; He, Z.; Gong, Z.; Yao, M.; Sun, Y.; Xu, X.; Sui, H. Research progress on cadmium toxicity and prevention measures. J. Toxicol. 2022, 36, 517–520. [Google Scholar]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nano-filtration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Priyanka, R.; Sharma, A.C.; Sunil, K.S.; Geng, L.; Nasim, A.; Darren, M.; Benjamin, S.H. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium (II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar]
- Guo, Z.; Zhang, X.; Kang, Y.; Zhang, J. Biomass-Derived Carbon Sorbents for Cd (II) Removal: Activation and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 4103–4109. [Google Scholar] [CrossRef]
- Khan, S.A.; Siddiqui, M.F.; Khan, T.A. Ultrasonic-assisted synthesis of polyacrylamide/bentonite hydrogel nanocomposite for the sequestration of lead and cadmium from aqueous phase: Equilibrium, kinetics and thermodynamic studies. Ultrason. Sonochem. 2020, 60, 104761. [Google Scholar] [CrossRef] [PubMed]
- Muya, F.N.; Sunday, C.E.; Baker, P.; Iwuoha, E. Environmental remediation of heavy metal ions from aqueous solution through hydrogel adsorption: A critical review. Water Sci. Technol. 2016, 73, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, C.; Chi, R. Remediation of soil cadmium pollution by biomineralization using microbial-induced precipitation: A review. World J. Microbiol. Biotechnol. 2021, 37, 208–213. [Google Scholar] [CrossRef]
- Saumya, A.; Ankur, S.; Vipin, K. Recent advancements in cadmium-microbe interactive relations and their application for environmental remediation: A mechanistic overview. Environ. Sci. Pollut. Res. Int. 2023, 30, 17009–17038. [Google Scholar]
- Alabssawy, A.N.; Hashem, A.H. Bioremediation of hazardous heavy metals by marine microorganisms: A recent review. Arch. Microbiol. 2024, 206, 103. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Samanta, S.; Pandit, S.; Naaz, T.; Banerjee, S.; Rawat, J.M.; Chaubey, K.K.; Saha, R.P. An Overview of Bacteria-Mediated Heavy Metal Bioremediation Strategies. Appl. Biochem. Biotechnol. 2024, 196, 1712–1751. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Jun, B.; Flora, J.; Park, C.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, G.; Niu, Q.; Liu, Y.; Zhou, L.; Jiang, L.; Tan, X.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Znad, H.; Awual, M.R.; Martini, S. The Utilization of Algae and Seaweed Biomass for Bioremediation of Heavy Metal-Contaminated Wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.S.; Roh, H.S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B.H. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.; Othman, N.; Nasri, N.S. Treatment of Wastewater Using Seaweed: A Review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tao, H. Cell surface engineering of microorganisms towards adsorption of heavy metals. Crit. Rev. Microbiol. 2015, 41, 140–149. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V.; Anshumali, A. comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Ramesh, B.; Srinivasan, S. Removal of toxic heavy metals using genetically engineered microbes: Molecular tools, risk assessment and management strategies. Chemosphere 2022, 298, 134341. [Google Scholar] [CrossRef]
- Thévenod, F.; Fels, J.; Lee, W.K.; Zarbock, R. Channels.transporters and receptors for cadmium and cadmium complexes in eukaryotic cells: Myths and facts. Biometals 2019, 32, 469–489. [Google Scholar] [CrossRef]
- Chang, J.D.; Huang, S.; Konishi, N. Overexpression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain. J. Exp. Bot. 2020, 71, 5705–5715. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Esser-Kahn, A.P.; Iavarone, A.T.; Francis, M.B. Metallothionein-cross-linked hydrogels for the selective removal of heavy metals from water. J. Am. Chem. Soc. 2008, 130, 15820–15822. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.; Abdel-Motleb, A.; Abdel-Wareth, M.T.A. Metallothionein expression in Aspergillus exposed to environmentally relevant concentrations of heavy metals at different pH levels. Environ. Sci. Pollut. Res. Int. 2021, 28, 49936–49948. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, X.; Deng, X. Bioaccumulation of heavy metal cadmium in wastewater by genetically engineered bacteria. Technol. Water Treat. 2006, 32, 26–29. [Google Scholar]
- Bae, W.; Chen, W.; Mulchandania, A.; Mehra, R.K. Enhanced bioaccu-mulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 2000, 70, 518–524. [Google Scholar] [CrossRef]
- Bae, W.; Mulchandania, A.; Chen, W. Cell surface display of synthetic phytochelatins using ice nucleation protein for enhanced heavy metal bioaccumulation. J. Org. Chem. 2002, 88, 223–227. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, K.; Li, X.; Luo, X.; Wang, M.; Li, L.; Wang, G.; Li, M. Reducing cadmium in rice using metallothionein surface-engineered bacteria WH16-1-MT. Environ. Res. 2022, 203, 111801. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, B.; Yu, Q. Genetic engineering-facilitated co-assembly of synthetic bacterial cells and magnetic nanoparticles for efficient heavy metal removal. ACS Appl. Mater. Interfaces 2020, 12, 22948–22957. [Google Scholar] [CrossRef] [PubMed]
- Theil, E.C. Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorg. Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Chakrabarti, P. Self-Assembly of Ferritin: Structure, Biological Function and Potential Applications in Nanotechnology. Adv. Exp. Med. Biol. 2019, 1174, 313–329. [Google Scholar] [PubMed]
- Wu, J.; Li, Y.; Wu, H.; Zhang, H.; Sha, X.; Ma, J.; Yang, R. The application of ferritin in transporting and binding diverse metal ions. Food Chem. 2024, 439, 138132. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wu, Z.; Yuan, X. Overexpression of human-derived soluble transferrin receptor sTfR antigen and preparation and application of polyclonal antibodies. J. Biol. 2023, 40, 111–115. [Google Scholar]
- Zhao, X.; Zhou, Y.; Zhang, Y.; Zhang, Y. Ferritin: Significance in viral infections. Rev. Med. Virol. 2024, 34, e2531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Malviya, R.; Chaudhary, R. Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. 2006, 137, 267–276. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Leaching behavior and immobilization of heavy metals in solidified/stabilized products. J. Hazard. Mater. 2006, 137, 207–217. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Xu, Z.; Bae, W.; Mulchandani, A.; Mehra, R.K.; Chen, W. Heavy metal removal by novel CBD-EC20 sorbents immobilized on cellulose. Biomacromolecules 2002, 3, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Drees, S.L.; Beyer, D.F.; Lenders-Lomscher, C.; Lübben, M. Distinct functions of serial metal-binding domains in the Escherichia coli P1B-ATPase CopA. Mol. Microbiol. 2015, 97, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Kolaj-Robin, O.; Russell, D.; Hayes, K.A.; Pembroke, J.T.; Soulimane, T. Cation Diffusion Facilitator family: Structure and function. FEBS Lett. 2015, 589, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Montanini, B.; Blaudez, D.; Jeandroz, S.; Sanders, D.; Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genom. 2007, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Avanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, Y. Response to temperature shifts of expression of the amp gene on pBR322 in Escherichia coli K-12. J. Bacteriol. 1987, 169, 2294–2297. [Google Scholar] [CrossRef] [PubMed]
- Vortuba, J.; Pazlarova, J.; Dvorakova, M.; Vachova, L.; Strnadova, M.; Kucerova, H.; Vinter, V.; Zourabian, R.; Chaloupka, J. External factors involved in the regulation of synthesis of an extracellular proteinase in Bacillus megaterium: Effect of temperature. Appl. Microbiol. Biotechnol. 1991, 35, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Effects of temperature on cell membranes. Symp. Soc. Exp. Biol. 1988, 42, 237–258. [Google Scholar]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Wang, D.; Liu, Y.; Wei, M.; Han, X.; Sun, X.; Yin, L.; Luo, G. Construction of Genetically Engineered Escherichia coli Cell Factory for Enhanced Cadmium Bioaccumulation in Wastewater. Water 2024, 16, 1759. https://doi.org/10.3390/w16131759
Tian L, Wang D, Liu Y, Wei M, Han X, Sun X, Yin L, Luo G. Construction of Genetically Engineered Escherichia coli Cell Factory for Enhanced Cadmium Bioaccumulation in Wastewater. Water. 2024; 16(13):1759. https://doi.org/10.3390/w16131759
Chicago/Turabian StyleTian, Lingna, Daiwei Wang, Yueying Liu, Mingjie Wei, Xuexue Han, Xiaomei Sun, Liang Yin, and Guanghong Luo. 2024. "Construction of Genetically Engineered Escherichia coli Cell Factory for Enhanced Cadmium Bioaccumulation in Wastewater" Water 16, no. 13: 1759. https://doi.org/10.3390/w16131759
APA StyleTian, L., Wang, D., Liu, Y., Wei, M., Han, X., Sun, X., Yin, L., & Luo, G. (2024). Construction of Genetically Engineered Escherichia coli Cell Factory for Enhanced Cadmium Bioaccumulation in Wastewater. Water, 16(13), 1759. https://doi.org/10.3390/w16131759





