Enhancing Oil–Water Separation Efficiency with WO3/MXene Composite Membrane
Abstract
:1. Introduction
- WO3/MXene composite: This is a novel approach for oil–water separation membranes. The existing research primarily focuses on pristine MXene membranes or composites with other materials.
- Enhanced separation efficiency: The introduction of WO3 is expected to improve the membrane’s underwater oleophobicity, leading to superior oil rejection compared to pristine MXene membranes.
- Photocatalytic self-cleaning: WO3 possesses photocatalytic properties that can degrade organic contaminants under light irradiation. This could significantly improve the membrane’s reusability and anti-fouling properties, addressing a major challenge for MXene membranes.
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Membrane
2.3. Instruments and Characterization
2.4. Oil-in-Water Filtration Test
3. Results and Discussion
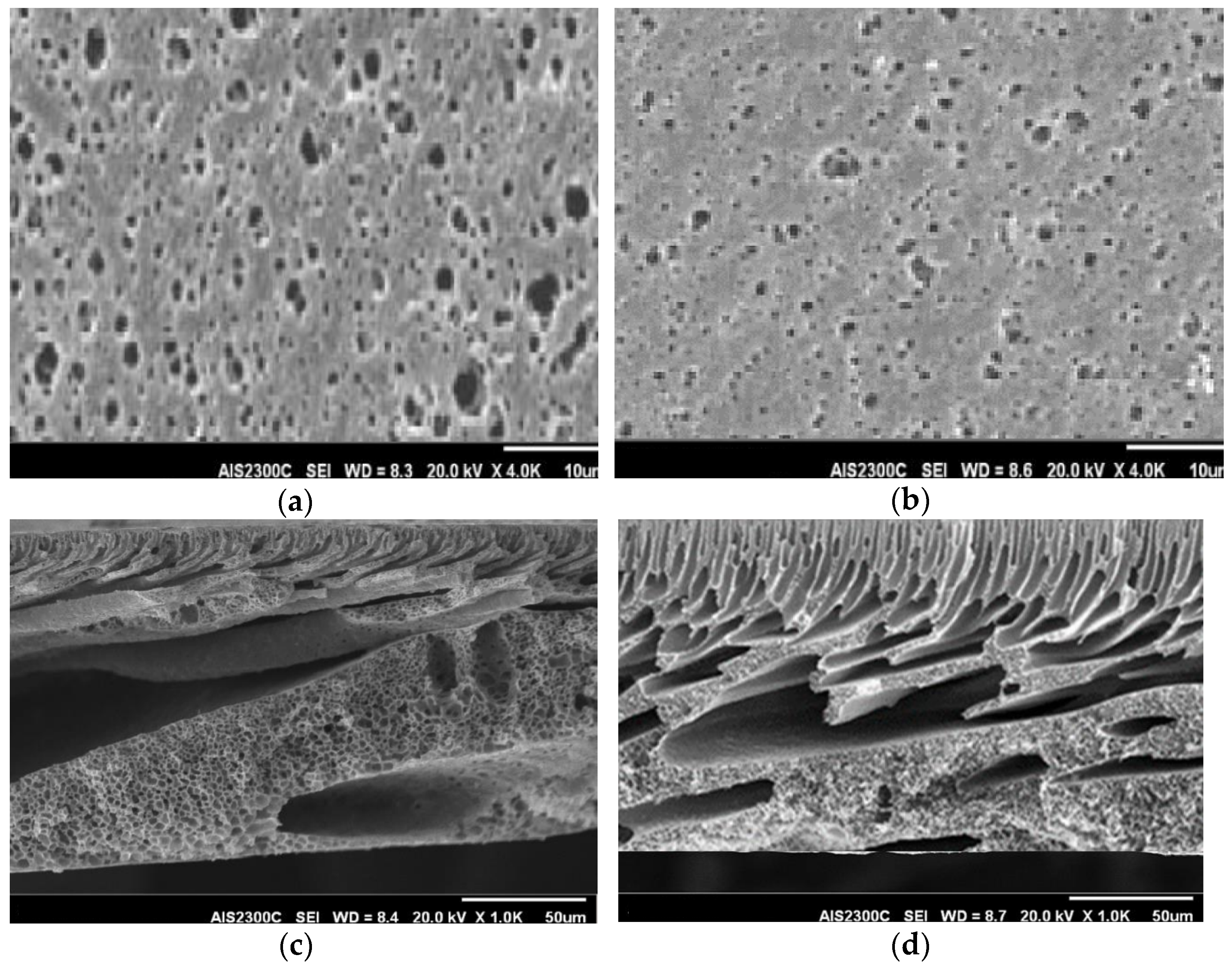
Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, S.; Bambrick, H.; Beggs, P.J.; Chen, L.; Hu, Y.; Ma, W.; Steffen, W.; Tan, J. Current and Future Threats to Human Health in the Anthropocene. Environ. Int. 2022, 158, 106892. [Google Scholar] [CrossRef]
- Hamta, A.; Mohammadi, A.; Dehghani, M.R.; Feyzi, F. Liquid–Liquid Equilibrium and Thermodynamic Modeling of Aqueous Two-Phase System Containing Polypropylene Glycol and NaClO4 at T = (288.15 and 298.15) K. J. Solut. Chem. 2018, 47, 1–25. [Google Scholar] [CrossRef]
- Wagner, M.; Lin, K.-Y.A.; Oh, W.-D.; Lisak, G. Metal-Organic Frameworks for Pesticidal Persistent Organic Pollutants Detection and Adsorption–A Mini Review. J. Hazard. Mater. 2021, 413, 125325. [Google Scholar] [CrossRef] [PubMed]
- Sadek, A.H.; Fahmy, O.M.; Nasr, M.; Mostafa, M.K. Predicting Cu(II) Adsorption from Aqueous Solutions onto Nano Zero-Valent Aluminum (NZVAl) by Machine Learning and Artificial Intelligence Techniques. Sustainability 2023, 15, 2081. [Google Scholar] [CrossRef]
- Hamta, A.; Dehghani, M.R. Application of Polyethylene Glycol Based Aqueous Two-Phase Systems for Extraction of Heavy Metals. J. Mol. Liq. 2017, 231, 20–24. [Google Scholar] [CrossRef]
- Assegide, E.; Alamirew, T.; Dile, Y.T.; Bayabil, H.; Tessema, B.; Zeleke, G. A Synthesis of Surface Water Quality in Awash Basin, Ethiopia. Front. Water 2022, 4, 782124. [Google Scholar] [CrossRef]
- Nawaz, S.; Tabassum, A.; Muslim, S.; Nasreen, T.; Baradoke, A.; Kim, T.; Boczkaj, G.; Jesionowski, T.; Bilal, M. Effective Assessment of Biopolymer-Based Multifunctional Sorbents for the Remediation of Environmentally Hazardous Contaminants from Aqueous Solutions. Chemosphere 2023, 329, 138552. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Nasr, M.; Abdel-Gaber, A.; Fadly, S. Biosorption of Cr(VI) from Aqueous Solution Using Agricultural Wastes, with Artificial Intelligence Approach. Sep. Sci. Technol. 2016, 51, 416–426. [Google Scholar] [CrossRef]
- Hamta, A.; Ashtiani, F.Z.; Karimi, M.; Moayedfard, S. Asymmetric Block Copolymer Membrane Fabrication Mechanism through Self-Assembly and Non-Solvent Induced Phase Separation (SNIPS) Process. Sci. Rep. 2022, 12, 771. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Xu, X.; Zhang, X.; Shi, Y. A Deep Koopman Operator-based Modelling Approach for Long-term Prediction of Dynamics with Pixel-level Measurements. CAAI Trans. Intell. Technol. 2024, 9, 178–196. [Google Scholar] [CrossRef]
- Hamta, A.; Zokaee Ashtiani, F.; Karimi, M.; Safikhani, A. Manipulating of Polyacrylonitrile Membrane Porosity via SiO2 and TiO2 Nanoparticles: Thermodynamic and Experimental Point of View. Polym. Adv. Technol. 2020, 32, 872–885. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, W.; Zhang, D.; Zhang, W. Two Kinds of Average Approximation Accuracy. CAAI Trans. Intell. Technol. 2024, 9, 481–490. [Google Scholar] [CrossRef]
- Hamta, A.; Ashtiani, F.Z.; Karimi, M.; Sadeghi, Y.; MoayedFard, S.; Ghorabi, S. Copolymer Membrane Fabrication for Highly Efficient Oil-in-Water Emulsion Separation. Chem. Eng. Technol. 2021, 44, 1321–1326. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, W.; Qian, Q.; Kumar, A.; Sun, W.; Zhou, Y. TCM in Milling Processes Based on Attention Mechanism-Combined Long Short-Term Memory Using a Sound Sensor under Different Working Conditions. Int. J. Hydromechatron. 2022, 5, 243–259. [Google Scholar] [CrossRef]
- Li, J.; Shang, H.; Yan, J.; Qiao, M.; Yu, J.; Zhu, D.; He, C. Micro Drilling of PMMA with Double-Pulse Femtosecond Laser. Int. J. Hydromechatron. 2023, 6, 34–44. [Google Scholar] [CrossRef]
- Hamta, A.; Dehghani, M.R.; Gholami, M. Novel Experimental Data on Aqueous Two–Phase System Containing PEG–6000 and Na2CO3 at T = (293.15, 303.15 and 313.15) K. J. Mol. Liq. 2017, 241, 144–149. [Google Scholar] [CrossRef]
- Solyali, D.; Mollaei, A. A Simulation Model Based on Experimental Data to Determine the Optimal Tilt Angle for a Fixed Photovoltaic Panel. Arch. Adv. Eng. Sci. 2023, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, P.; Dai, J.; Li, D. A Cloud Bayesian Network Approach to Situation Assessment of Scouting Underwater Targets with Fixed-wing Patrol Aircraft. CAAI Trans. Intell. Technol. 2023, 8, 532–545. [Google Scholar] [CrossRef]
- Koo, D.C.H.; Tan, N.N.; Ng, Q.H.; Rahim, S.K.E.A.; Low, S.C.; Yeo, R.Y.Z. Integrating Advanced Keggin-Structure Polyoxometalate into Polymeric Membrane to Enhance Photocatalytic Self-Cleaning and Antifouling Functionalities. Korean J. Chem. Eng. 2022, 39, 1045–1052. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Yi, G.; Zhang, D.; Zhang, Y.; Lin, X.; Liu, J.; Shi, Z.; Lin, Q. Fabrication of a Ag/CNQDs/g-C3N4-PVDF Photocatalytic Composite Membrane with Excellent Photocatalytic and Self-Cleaning Properties. J. Environ. Chem. Eng. 2022, 10, 108488. [Google Scholar] [CrossRef]
- Asmael, M.; Memarzadeh, A. A Review on Recent Achievements and Challenges in Electrochemical Machining of Tungsten Carbide. Arch. Adv. Eng. Sci. 2024, 2, 1–23. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Chandrasekaran, A. Recent Advances in TiO2/ZnS-Based Binary and Ternary Photocatalysts for the Degradation of Organic Pollutants. Sci. Total Environ. 2023, 868, 161525. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Lumkes, J.; Zhang, S.; Yu, K.; Chen, L. Parameter Optimisation Design of Mixing and Distributing System of Vertical Biaxial Bladed Mixer. Int. J. Hydromechatron. 2023, 6, 133–158. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Quang, H.H.P.; Khan, A.A.P.; Bajpai, A.; Van Le, Q.; Thakur, V.K.; Thakur, S.; Nguyen, V.-H.; Raizada, P. Emerging Architecture Titanium Carbide (Ti3C2Tx) MXene Based Photocatalyst toward Degradation of Hazardous Pollutants: Recent Progress and Perspectives. Chemosphere 2022, 293, 133541. [Google Scholar] [CrossRef] [PubMed]
- Shekoofa, O.; Wang, J.; Li, D. Fabrication of N-Type Nanocrystalline Silicon Thin-Film by Magnetron Sputtering and Antimony Induced Crystallization. Arch. Adv. Eng. Sci. 2023, 2, 71–78. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L.; Jiang, S.; Chen, L.; Sang, S.; Zhang, H. A Photocatalytic Degradation Self-Cleaning Composite Membrane for Oil-Water Separation Inspired by Light-Trapping Effect of Moth-Eye. J. Memb. Sci. 2023, 669, 121337. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Yan, G.; Luo, J.; Cheng, X.; Zhao, Z.; Li, H. Self-Cleaning Photocatalytic MXene Composite Membrane for Synergistically Enhanced Water Treatment: Oil/Water Separation and Dyes Removal. Chem. Eng. J. 2022, 427, 131668. [Google Scholar] [CrossRef]
- He, H.; Li, Z.; Ouyang, L.; Liang, Y.; Yuan, S. Hierarchical WO3@ Cu (OH)2 Nanorod Arrays Grown on Copper Mesh with Superwetting and Self-Cleaning Properties for High-Performance Oil/Water Separation. J. Alloys Compd. 2021, 855, 157421. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Yulfarida, M.; Kumoro, A.C.; Sumardiono, S.; Djaeni, M.; Kurniawan, T.A.; Othman, M.H.D.; Budiyono, B. A Highly Durable and Hydrophilic PVDF-MoS2/WO3-PVA Membrane with Visible Light Driven Self-Cleaning Performance for Pollutant-Burdened Natural Rubber Wastewater Treatment. J. Environ. Chem. Eng. 2023, 11, 109583. [Google Scholar] [CrossRef]
- Harrison, W.L. Synthesis and Characterization of Sulfonated Poly (Arylene Ether Sulfone) Copolymers via Direct Copolymerization: Candidates for Proton Exchange Membrane Fuel Cells. Ph.D. Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2002. [Google Scholar]
- Najafi-Ashtiani, H.; Bahari, A.; Gholipour, S.; Hoseinzadeh, S. Structural, Optical and Electrical Properties of WO3–Ag Nanocomposites for the Electro-Optical Devices. Appl. Phys. A 2018, 124, 24. [Google Scholar] [CrossRef]
- Pandurangarao, K.; Kumar, V.R. Preparation and Characterization of Nanocrystalline Tungsten Oxide Thin Films for Electrochromic Devices: Effect of Deposition Parameters. Mater. Today Proc. 2019, 19, 2596–2603. [Google Scholar] [CrossRef]
- Kanan, S.M.; Lu, Z.; Cox, J.K.; Bernhardt, G.; Tripp, C.P. Identification of Surface Sites on Monoclinic WO3 Powders by Infrared Spectroscopy. Langmuir 2002, 18, 1707–1712. [Google Scholar] [CrossRef]
- Du, Q.; Men, Q.; Li, R.; Cheng, Y.; Zhao, B.; Che, R. Electrostatic Adsorption Enables Layer Stacking Thickness-Dependent Hollow Ti3C2Tx MXene Bowls for Superior Electromagnetic Wave Absorption. Small 2022, 18, 2203609. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Zhang, X.; Hu, J.; Zhao, X.; Li, J.; Ren, Q. Quaternary Ammonium-Functionalized Crosslinked Poly (Aryl Ether Sulfone) s Anion Exchange Membranes with Enhanced Alkaline Stability for Water Electrolysis. J. Memb. Sci. 2023, 685, 121946. [Google Scholar] [CrossRef]
- Ling, M.; Yu, K.; Wang, J.; Wang, H.; Nie, H.; Wang, Z.; Zhou, G. Synthesis and Pyrolysis Mechanism of Phenolphthalein Poly (Aryl Ether Sulfone) Containing Isopropyl Groups. Thermochim. Acta 2022, 714, 179253. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Q.; Wu, D.; Jiang, Y.; Liu, Z.; Chen, B.; Zhu, M.; Schmidt, O.G. Flexible MXene Films for Batteries and Beyond. Carbon Energy 2022, 4, 598–620. [Google Scholar] [CrossRef]
- Iakunkov, A.; Nordenström, A.; Boulanger, N.; Hennig, C.; Baburin, I.; Talyzin, A.V. Temperature-Dependent Swelling Transitions in MXene Ti3C2Tx. Nanoscale 2022, 14, 10940–10949. [Google Scholar] [CrossRef] [PubMed]
- Melnik, A.; Bogoslovtseva, A.; Petrova, A.; Safonov, A.; Markides, C.N. Oil–Water Separation on Hydrophobic and Superhydrophobic Membranes Made of Stainless Steel Meshes with Fluoropolymer Coatings. Water 2023, 15, 1346. [Google Scholar] [CrossRef]
- Baig, U.; Dastageer, M.A. Fabrication of Photo-Responsive Mesh Membrane with Surface-Engineered Wettability for Oil–Water Separation and Photocatalytic Degradation of Organic Pollutants. Membranes 2023, 13, 302. [Google Scholar] [CrossRef]
- Havelka, O.; Yalcinkaya, F.; Wacławek, S.; Padil, V.V.T.; Amendola, V.; Cernik, M.; Torres-Mendieta, R. Sustainable and Scalable Development of PVDF-OH Ag/TiOx Nanocomposites for Simultaneous Oil/Water Separation and Pollutant Degradation. Environ. Sci. Nano 2023, 10, 2359–2373. [Google Scholar] [CrossRef]
- Chen, W.; Liu, M.; Ding, M.; Zhang, L.; Dai, S. Advanced Thin-Film Composite Polyamide Membrane for Precise Trace Short-Chain PFAS Sieving: Solution, Environment and Fouling Effects. Process Saf. Environ. Prot. 2023, 169, 493–503. [Google Scholar] [CrossRef]



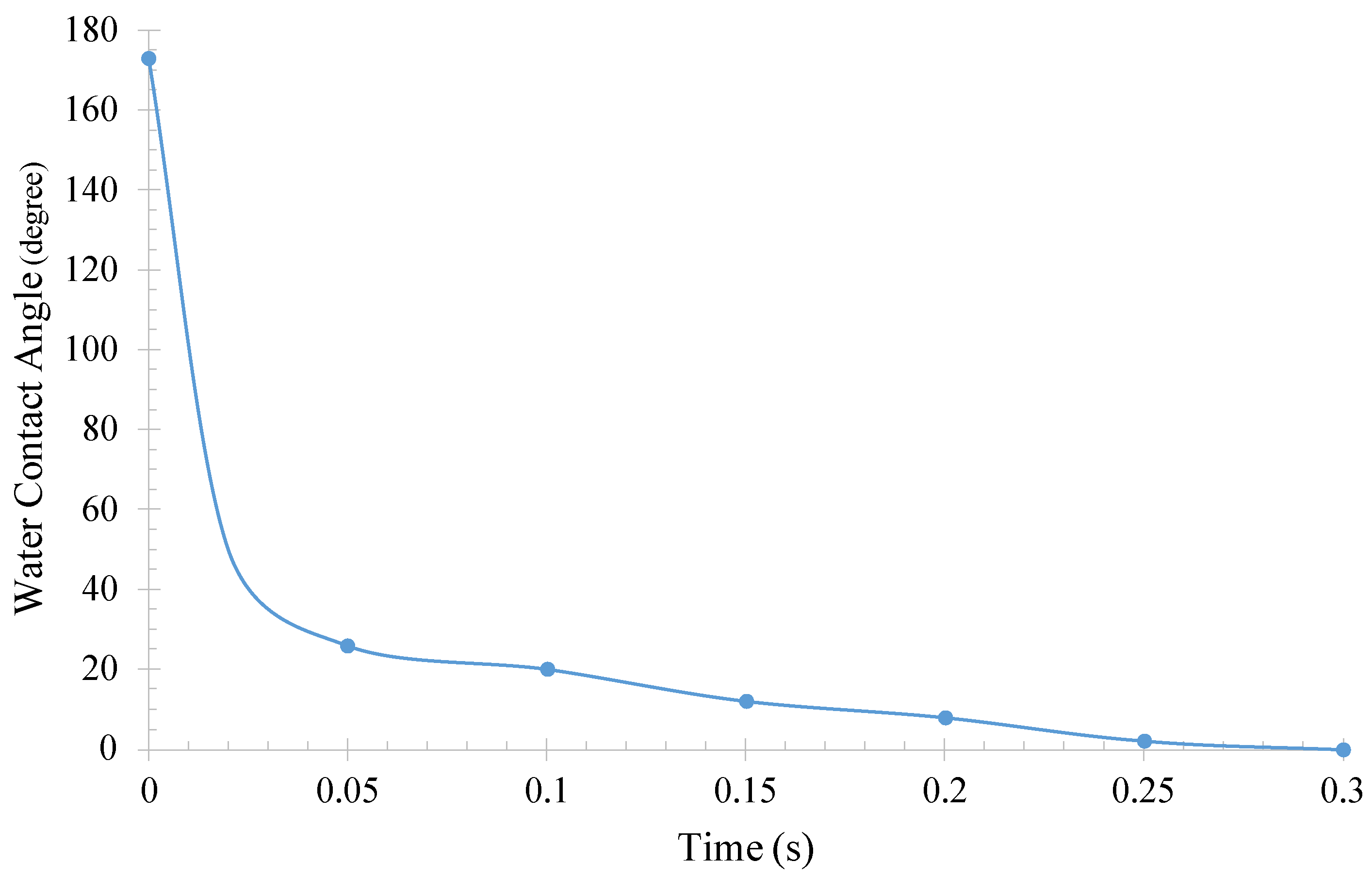

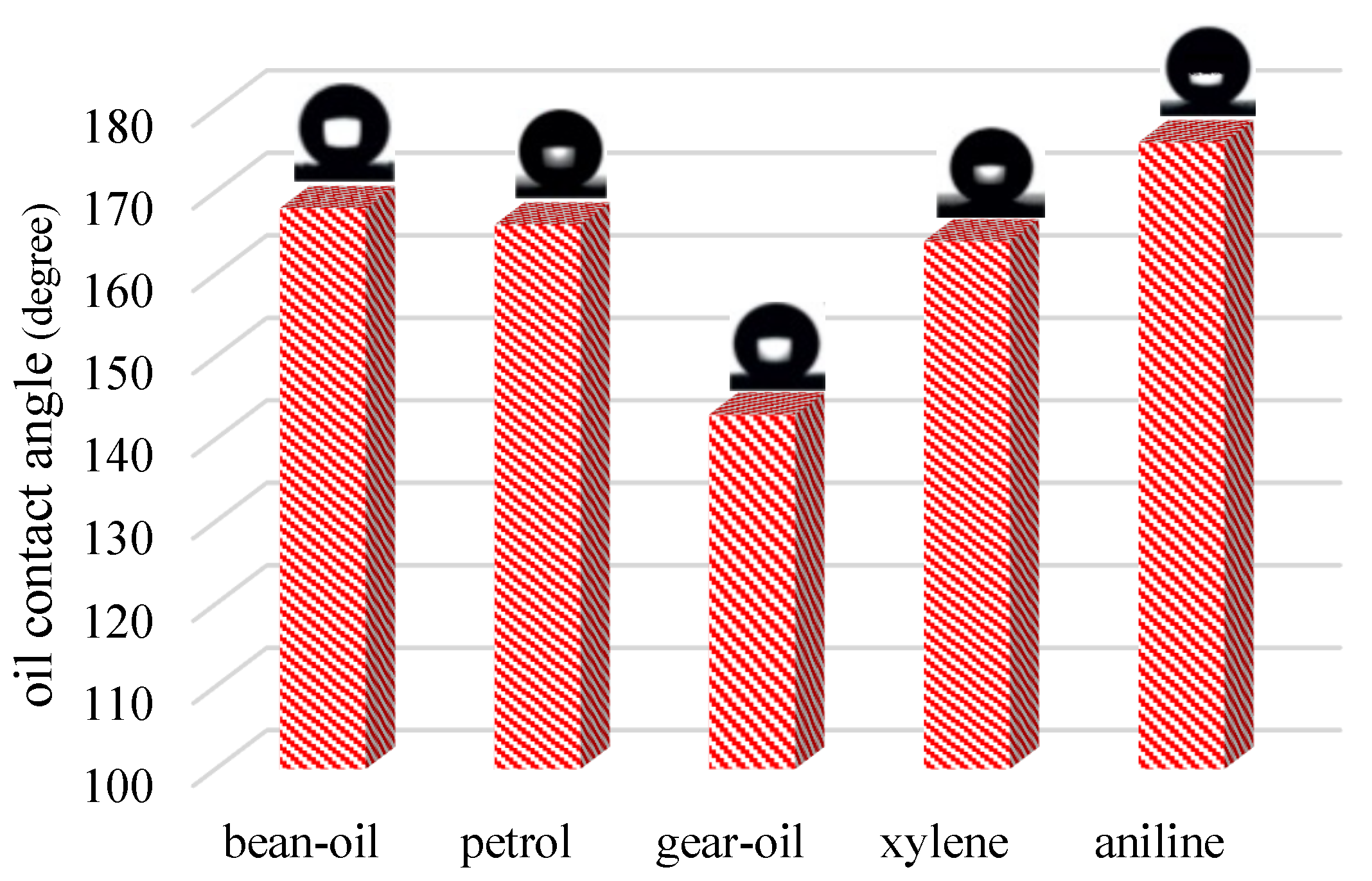
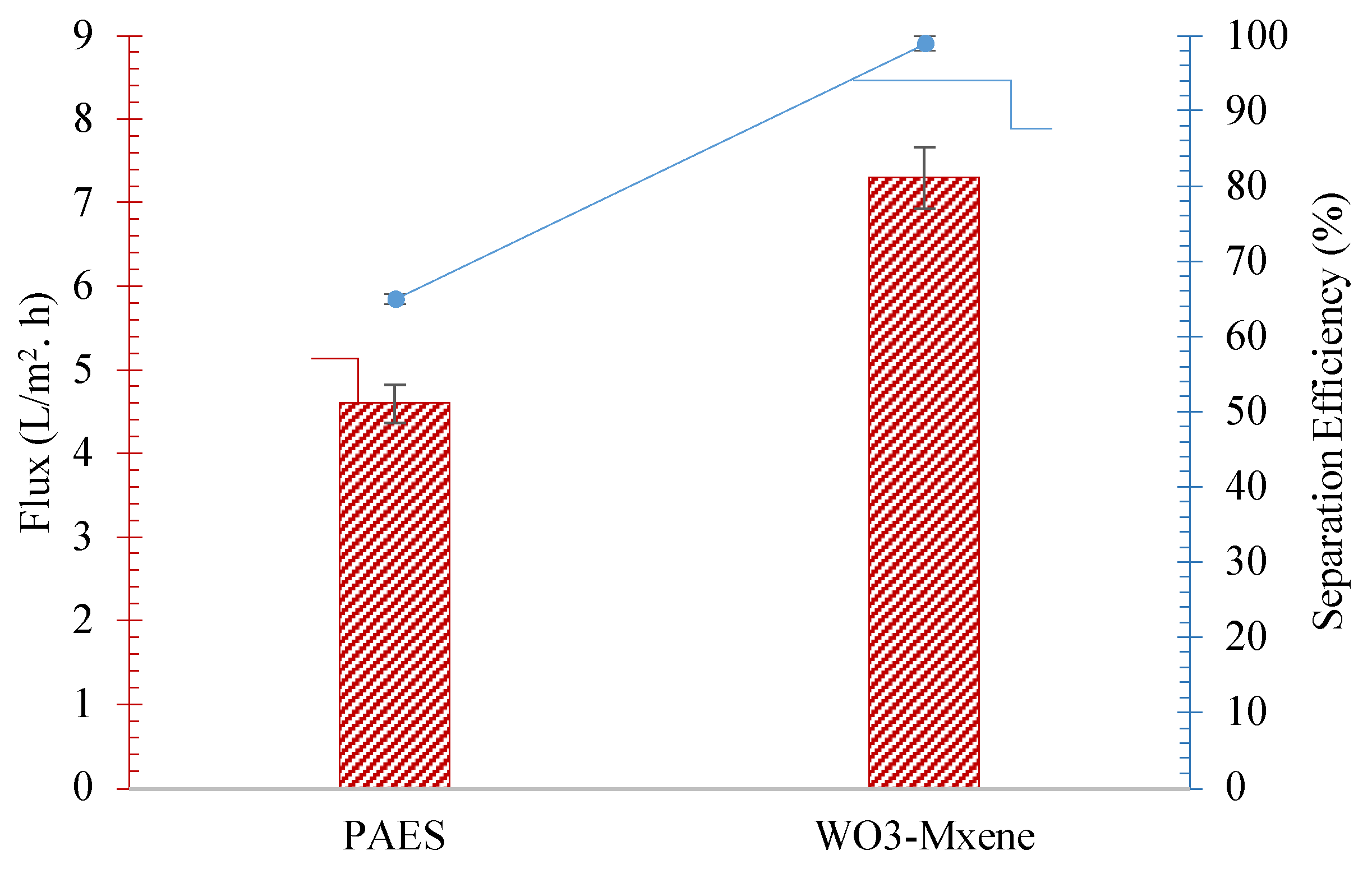
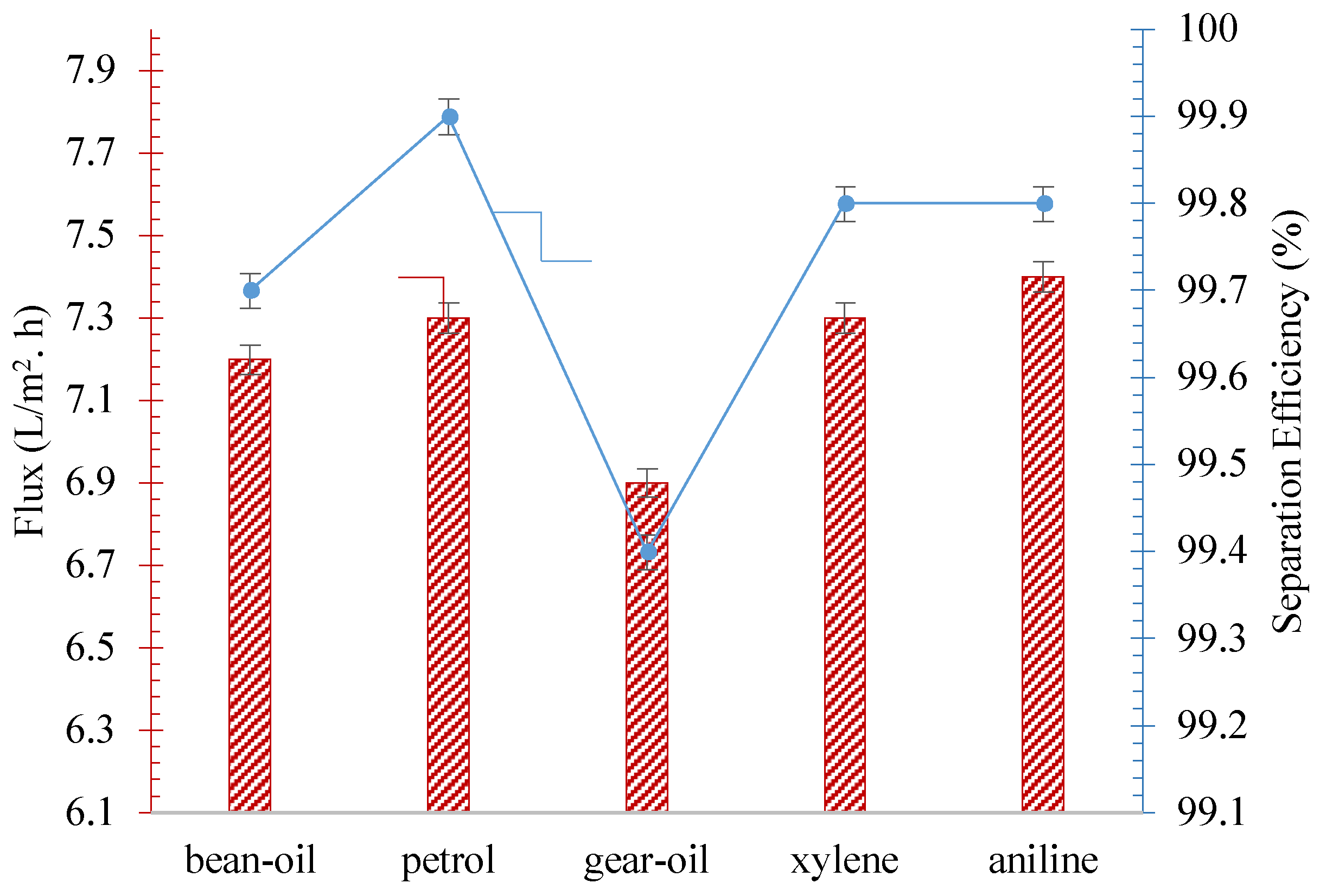
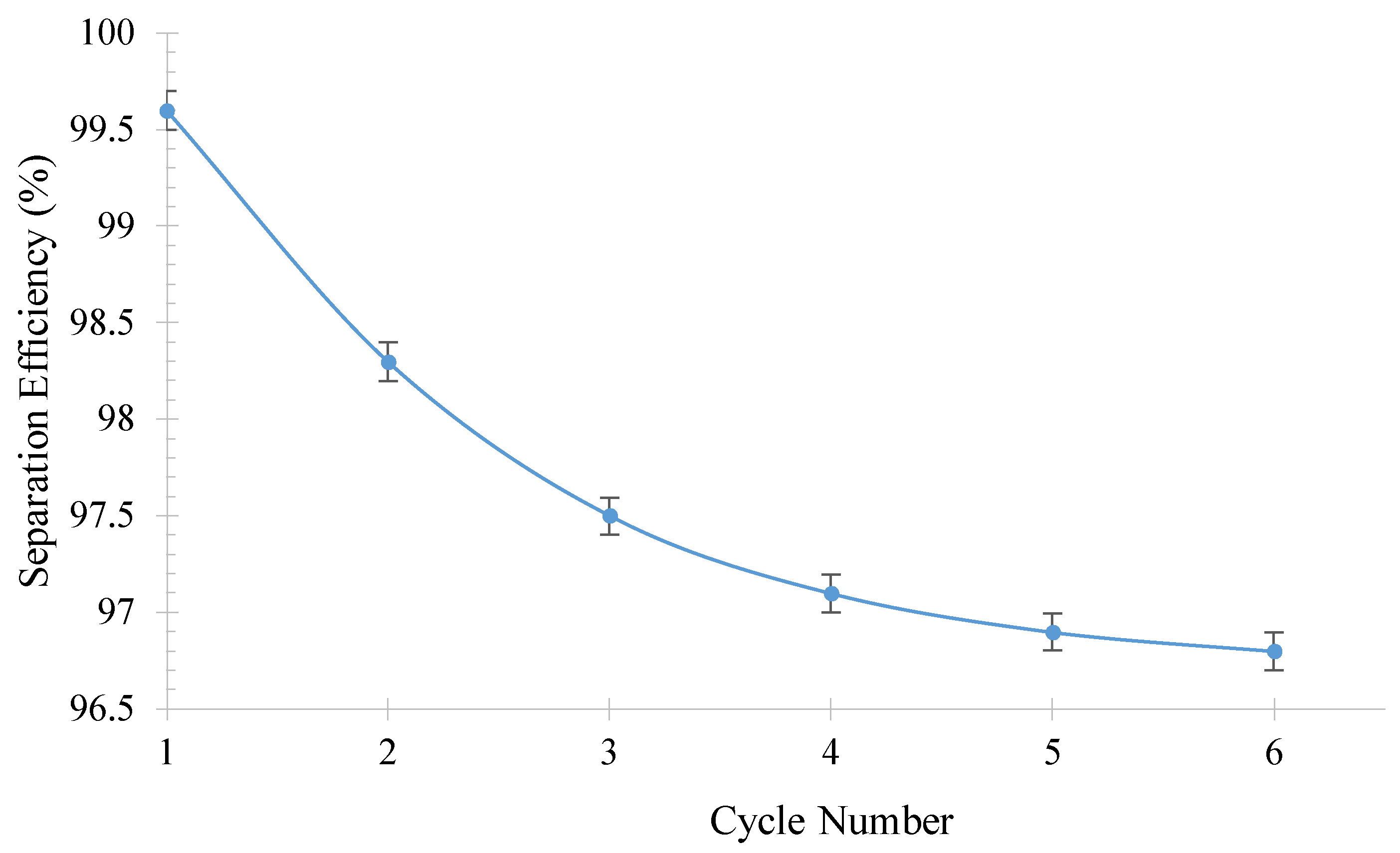



| Irradiated Time (h) | Oil Contact Angle | Separation Efficiency (%) | Flux (L/m2.h) |
|---|---|---|---|
| 0 | 73 ± 3 | 96.31 ± 0.01 | 0.7 ± 0.1 |
| 0.25 | 155 ± 4 | 99.22 ± 0.01 | 5.1 ± 0.1 |
| 0.5 | 159 ± 4 | 99.40 ± 0.01 | 6.2 ± 0.1 |
| 1 | 161 ± 3 | 99.97 ± 0.01 | 6.3 ± 0.1 |
| 1.5 | 164 ± 5 | 99.97 ± 0.01 | 6.3 ± 0.1 |
| 2 | 163 ± 4 | 99.98 ± 0.01 | 6.4 ± 0.1 |
| 3 | 165 ± 3 | 99.98 ± 0.01 | 6.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amari, A.; Osman, H.; Boujelbene, M.; Abdulameer, M.K.; Scholz, M.; Sammen, S.S. Enhancing Oil–Water Separation Efficiency with WO3/MXene Composite Membrane. Water 2024, 16, 1767. https://doi.org/10.3390/w16131767
Amari A, Osman H, Boujelbene M, Abdulameer MK, Scholz M, Sammen SS. Enhancing Oil–Water Separation Efficiency with WO3/MXene Composite Membrane. Water. 2024; 16(13):1767. https://doi.org/10.3390/w16131767
Chicago/Turabian StyleAmari, Abdelfattah, Haitham Osman, Mohamed Boujelbene, Maha Khalid Abdulameer, Miklas Scholz, and Saad Sh. Sammen. 2024. "Enhancing Oil–Water Separation Efficiency with WO3/MXene Composite Membrane" Water 16, no. 13: 1767. https://doi.org/10.3390/w16131767






