Novel Implications of the PARAFAC Model for Characterizing and Distributing DOM in Groundwater Networks by Using Spectroscopic Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Analytical Characterization
2.3. DOM Characterization
2.4. PARAFAC Modeling
2.5. Statistical Analysis
3. Results
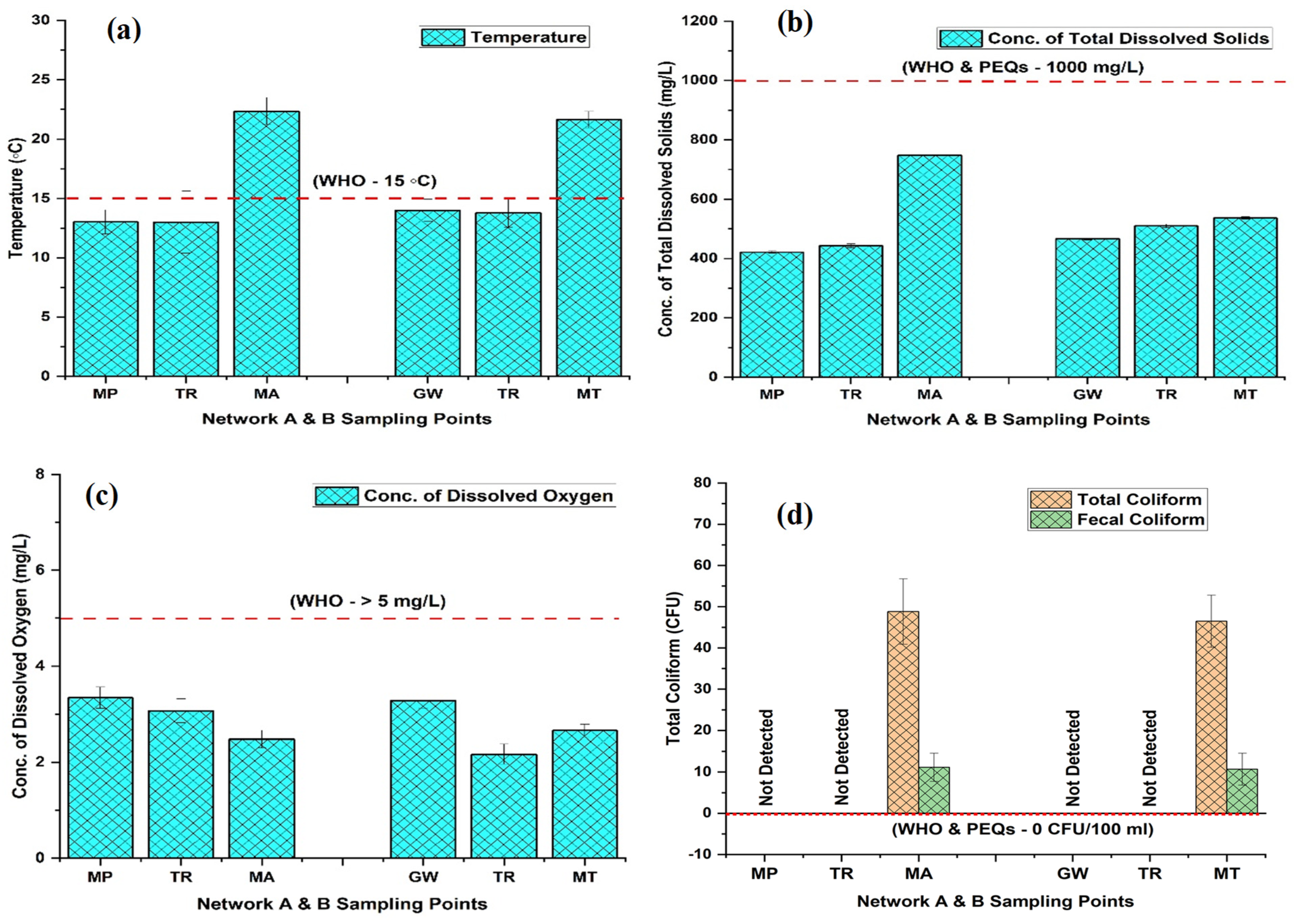
3.1. General Water Quality Parameters
3.2. Spatial Evaluation of Heavy Metals
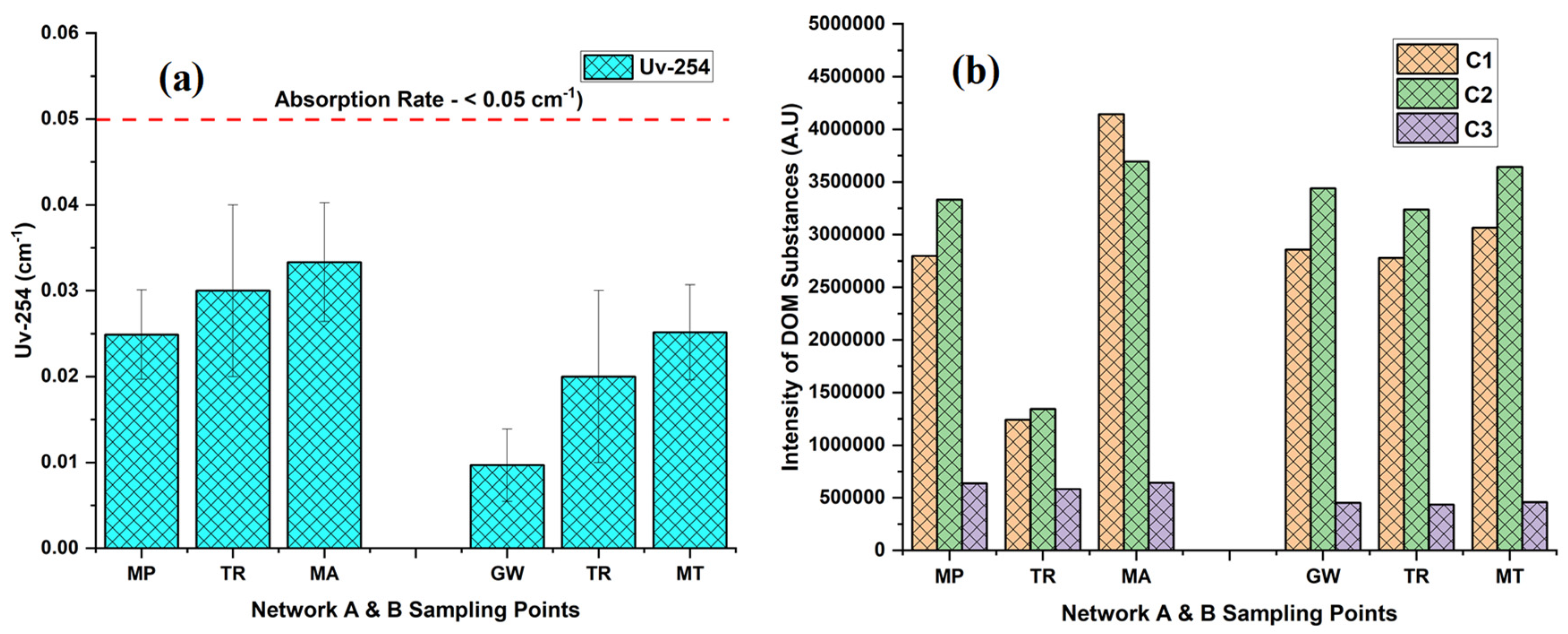
3.3. Fluorescence EEM-PARAFAC Analysis and Uv-254
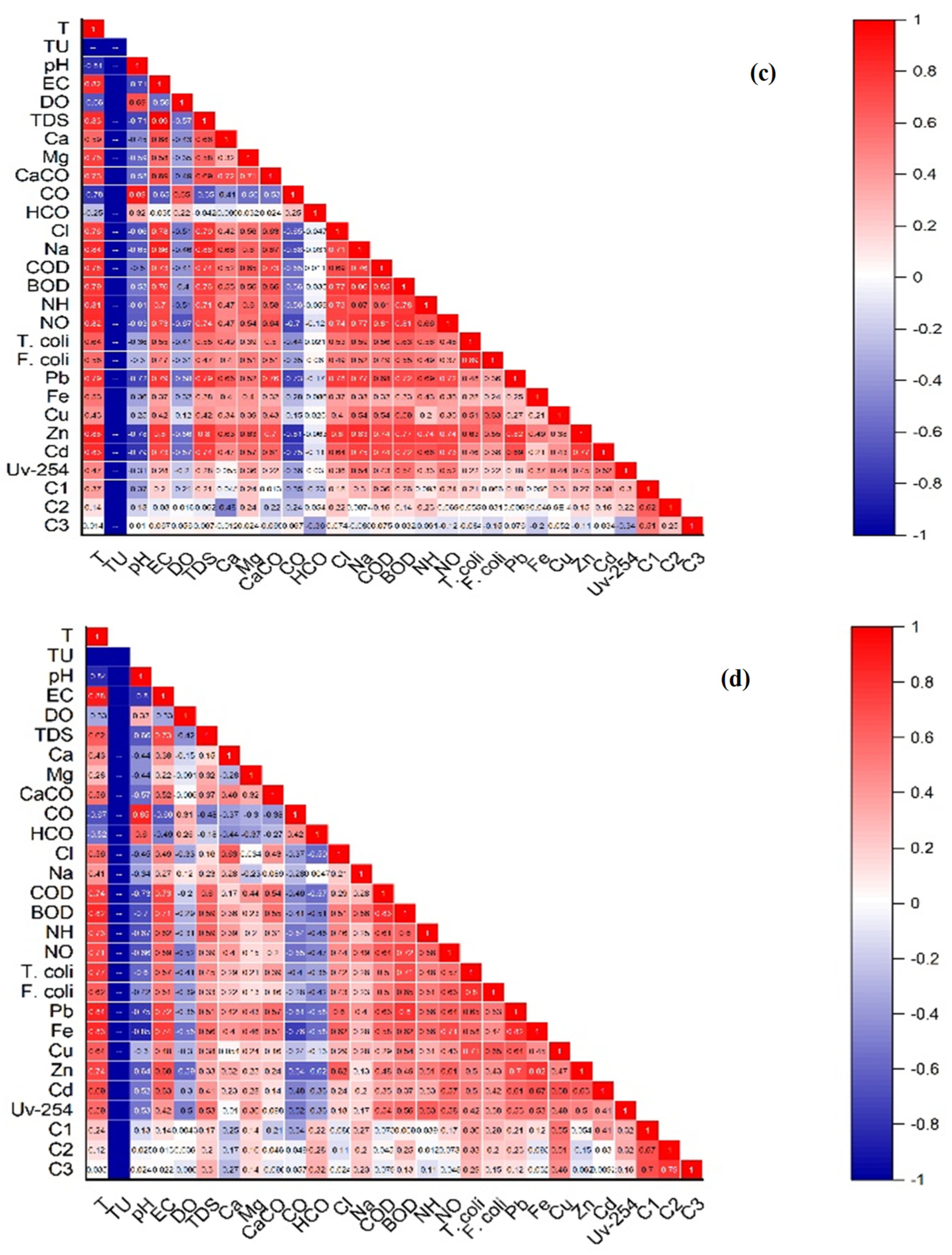
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Layani, G.; Bakhshoodeh, M.; Zibaei, M.; Viaggi, D. Sustainable Water Resources Management under Population Growth and Agricultural Development in the Kheirabad River Basin, Iran. Bio-Based Appl. Econ. 2022, 10, 305–323. [Google Scholar] [CrossRef]
- Ishaque, W.; Mukhtar, M.; Tanvir, R. Pakistan’s Water Resource Management: Ensuring Water Security for Sustainable Development. Front. Environ. Sci. 2023, 11, 1096747. [Google Scholar] [CrossRef]
- Xiao, Y.; Hao, Q.; Zhang, Y.; Zhu, Y.; Yin, S.; Qin, L.; Li, X. Investigating Sources, Driving Forces and Potential Health Risks of Nitrate and Fluoride in Groundwater of a Typical Alluvial Fan Plain. Sci. Total Environ. 2022, 802, 149909. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, A.; Mirjat, M.S.; Mangio, H.; Soomro, A. Assessment of Drinking Water Quality Status and Its Impact on Health in Tandojam City. J. Basic Appl. Sci. 2017, 13, 363–369. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Wania, F. Organic Contaminant Amplification during Snowmelt. Water Res. 2008, 42, 1847–1865. [Google Scholar] [CrossRef]
- Fabris, R.; Chow, C.W.K.; Drikas, M.; Eikebrokk, B. Comparison of NOM Character in Selected Australian and Norwegian Drinking Waters. Water Res. 2008, 42, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, H.; Kong, Q.; Li, M.; Wu, S.; Wu, H. Interactions of High-Rate Nitrate Reduction and Heavy Metal Mitigation in Iron-Carbon-Based Constructed Wetlands for Purifying Contaminated Groundwater. Water Res. 2020, 169, 115285. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, J.; Guo, Z.; Li, M.; Wu, H. Distributions and Interactions of Dissolved Organic Matter and Heavy Metals in Shallow Groundwater in Guanzhong Basin of China. Environ. Res. 2022, 207, 112099. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Ok, Y.S.; El-Naggar, A.; Kim, H.; Song, F.; Kang, S.; Tsang, Y.F. Dissolved Organic Matter Characterization of Biochars Produced from Different Feedstock Materials. J. Environ. Manag. 2019, 233, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chapelle, F.H.; Strom, E.W.; Benner, R. Origins and Bioavailability of Dissolved Organic Matter in Groundwater. Biogeochemistry 2014, 122, 61–78. [Google Scholar] [CrossRef]
- Schwalm, M.; Zeitz, J. Concentrations of Dissolved Organic Carbon in Peat Soils as Influenced by Land Use and Site Characteristics—A Lysimeter Study. Catena 2015, 127, 72–79. [Google Scholar] [CrossRef]
- Chiu, T.P.; Huang, W.S.; Chen, T.C.; Yeh, Y.L. Fluorescence Characteristics of Dissolved Organic Matter (Dom) in Percolation Water and Lateral Seepage Affected by Soil Solution (s-s) in a Lysimeter Test. Sensors 2019, 19, 4016. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating Binding Characteristics of Cadmium and Copper to DOM Derived from Compost and Rice Straw Using EEM-PARAFAC Combined with Two-Dimensional FTIR Correlation Analyses. J. Hazard. Mater. 2018, 344, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.A.; Horwáth, W.R. Eliminating Interference from Iron(III) for Ultraviolet Absorbance Measurements of Dissolved Organic Matter. Chemosphere 2010, 78, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Jaouadi, M.; Jebri, S.; M’nif, A. Dissolved Organic Matter Extracted from Groundwater and Heavy Metals Behavior in Ain Senan-Kef, Tunisia. Groundw. Sustain. Dev. 2019, 9, 100254. [Google Scholar] [CrossRef]
- Williams, C.J.; Conrad, D.; Kothawala, D.N.; Baulch, H.M. Selective Removal of Dissolved Organic Matter Affects the Production and Speciation of Disinfection Byproducts. Sci. Total Environ. 2019, 652, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.-P.; Criquet, J. Natural Organic Matter-Cations Complexation and Its Impact on Water Treatment: A Critical Review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence Spectroscopy for Wastewater Monitoring: A Review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef]
- Maqbool, T.; Li, C.; Qin, Y.; Zhang, J.; Asif, M.B.; Zhang, Z. A Year-Long Cyclic Pattern of Dissolved Organic Matter in the Tap Water of a Metropolitan City Revealed by Fluorescence Spectroscopy. Sci. Total Environ. 2021, 771, 144850. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing Dissolved Organic Matter Fluorescence with Parallel Factor Analysis: A Tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Bieroza, M.; Baker, A.; Bridgeman, J. Relating Freshwater Organic Matter Fluorescence to Organic Carbon Removal Efficiency in Drinking Water Treatment. Sci. Total Environ. 2009, 407, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhuang, W.-E.; Hur, J.; Yang, L. Monitoring Dissolved Organic Matter in Wastewater and Drinking Water Treatments Using Spectroscopic Analysis and Ultra-High Resolution Mass Spectrometry. Water Res. 2021, 188, 116406. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, T.; Qin, Y.; Ly, Q.V.; Zhang, J.; Li, C.; Asif, M.B.; Zhang, Z. Exploring the Relative Changes in Dissolved Organic Matter for Assessing the Water Quality of Full-Scale Drinking Water Treatment Plants Using a Fluorescence Ratio Approach. Water Res. 2020, 183, 116125. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.; Thorn, R.; Anesio, A.; Cox, T.; Attridge, J.; Reynolds, D. Microbial Processing and Production of Aquatic Fluorescent Organic Matter in a Model Freshwater System. Water 2018, 11, 10. [Google Scholar] [CrossRef]
- Malik, A.; Parvaiz, A.; Mushtaq, N.; Hussain, I.; Javed, T.; Rehman, H.U.; Farooqi, A. Characterization and Role of Derived Dissolved Organic Matter on Arsenic Mobilization in Alluvial Aquifers of Punjab, Pakistan. Chemosphere 2020, 251, 126374. [Google Scholar] [CrossRef] [PubMed]
- Mahfooz, Y.; Yasar, A.; Sohail, M.T.; Tabinda, A.B.; Rasheed, R.; Irshad, S.; Yousaf, B. Investigating the Drinking and Surface Water Quality and Associated Health Risks in a Semi-Arid Multi-Industrial Metropolis (Faisalabad), Pakistan. Environ. Sci. Pollut. Res. 2019, 26, 20853–20865. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Natasha; Shah, A.H.; Saeed, F.; Ali, M.; Qaisrani, S.A.; Dumat, C. Heavy Metal Contamination and Exposure Risk Assessment via Drinking Groundwater in Vehari, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 39852–39864. [Google Scholar] [CrossRef]
- Hussain, R.; Khattak, S.A.; Ali, L.; Sattar, S.; Zeb, M.; Hussain, M.L. Correction to: Impacts of the Linear Flowing Industrial Wastewater on the Groundwater Quality and Human Health in Swabi, Pakistan (Environmental Science and Pollution Research, (2021), 28, 40, (56741-56757), 10.1007/S11356-021-13842-5). Environ. Sci. Pollut. Res. 2021, 28, 56758. [Google Scholar] [CrossRef]
- Saleem, A.; Mahmood, S. Spatio-Temporal Assessment of Urban Growth Using Multi-Stage Satellite Imageries in Faisalabad, Pakistan. Adv. Remote Sens. 2023, 3, 10–18. [Google Scholar]
- Jamal, S. Situational Analysis of Water Resources in Faisalabad City (Establishing a Case for Water Stewardship). WWF Pakistan Report. WWF Situational Analysis of Water Resources of Karachi. Wwf. 2019. Available online: https://wwfasia.awsassets.panda.org/downloads/situational_analysis_of_water_resources_of_faisalabad_city__1_.pdf (accessed on 14 June 2024).
- Zsolnay, Á. Dissolved Organic Matter: Artefacts, Definitions, and Functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- American Public Health Association; Eaton, A.D.; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Pepper, I.L.; Gerba, C.P.; Brendecke, J.W. Environmental Microbiology: A Laboratory Manual; Academic Press: Cambridge, MA, USA, 1995; ISBN 0125506554. [Google Scholar]
- Simmons, G.M. Standard Methods for the Examination of Water and Waste Water. A. E. Greenberg, L.S. Clesceri, A.D. Eaton. J. N. Am. Benthol. Soc. 1993, 12, 308–309. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013; pp. 1–243. [Google Scholar]
- Aftab, B.; Hur, J. Fast Tracking the Molecular Weight Changes of Humic Substances in Coagulation/Flocculation Processes via Fluorescence EEM-PARAFAC. Chemosphere 2017, 178, 317–324. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; pp. 1–614. [Google Scholar]
- Tsegaye, H.; Thillaigovindan, N.; Alemayehu, G. An Efficient Method for Solving Intuitionistic Fuzzy Multi-objective Optimization Problems. Punjab Univ. J. Math. 2021, 53, 631–664. [Google Scholar] [CrossRef]
- McIntyre, A.M.; Guéguen, C. Binding Interactions of Algal-Derived Dissolved Organic Matter with Metal Ions. Chemosphere 2013, 90, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yao, X.; Sun, Z.; Sang, D.; Liu, L.; Deng, H.; Zhang, Y. Properties and Metal Binding Behaviors of Sediment Dissolved Organic Matter (SDOM) in Lakes with Different Trophic States along the Yangtze River Basin: A Comparison and Summary. Water Res. 2023, 231, 119605. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zheng, X.; Li, B.; Chen, S.; Zhang, B.; Hu, S.; Qiao, H.; Liu, T.; Wang, Q. Trace Metal Complexation with Dissolved Organic Matter Stresses Microbial Metabolisms and Triggers Community Shifts: The Intercorrelations. Environ. Pollut. 2022, 314, 120221. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Li, J.; Li, B.; Liu, S.; Ma, R.; Yang, S.; Cai, H.; Xue, Z.; Wang, T. Roles of Dissolved Organic Matter (DOM) in Shaping the Distribution Pattern of Heavy Metal in the Yangtze River. J. Hazard. Mater. 2023, 460, 132410. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing Dissolved Organic Matter in Aquatic Environments Using a New Approach to Fluorescence Spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Makki, Z.F.; Zuhaira, A.A.; Al-Jubouri, S.M.; Al-Hamd, R.K.S.; Cunningham, L.S. GIS-Based Assessment of Groundwater Quality for Drinking and Irrigation Purposes in Central Iraq. Environ. Monit. Assess. 2021, 107, 193. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.K.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R.A.; Shakoor, M.B.; Arshad, M.U.; Chatha, S.A.S.; Deeba, F.; Murad, W.; et al. Drinking Water Quality Status and Contamination in Pakistan. Biomed Res. Int. 2017, 2017, 7908183. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Aziz, T.; Noor-Ul-Ain, A.K.; Ahmed, I.; Nida, A. Drinking Water Quality in 13 Different Districts of Sindh, Pakistan. Health Care Curr. Rev. 2018, 6, 4. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A Meta-Analysis of the Distribution, Sources and Health Risks of Arsenic-Contaminated Groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Altaf, F.; Anwaar, K.; Ashraf, M. Drinking Water Quality in Pakistan: Current Status and Challenges; Pakistan Council of Research in Water Resources (PCRWR): Islamabad, Pakistan, 2021; p. 141. [Google Scholar]
- Ahmad, M.N.; Sultana, R.; Yoshida, M.; Salahuddin, M. Groundwater Contamination Issues in Chiniot Area, Punjab, Pakistan. Int. J. Environ. Sci. Dev. 2020, 11, 123–127. [Google Scholar] [CrossRef]
- Aleem, M.; Shun, C.; Li, C.; Aslam, A.; Yang, W.; Nawaz, M.; Ahmed, W.; Buttar, N. Evaluation of Groundwater Quality in the Vicinity of Khurrianwala Industrial Zone, Pakistan. Water 2018, 10, 1321. [Google Scholar] [CrossRef]
- Din, I.U.; Muhammad, S.; Rehman, I.U. Groundwater Quality Assessment for Drinking and Irrigation Purposes in the Hangu District, Pakistan. J. Food Compos. Anal. 2023, 115, 104919. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H.; Tiwari, D.M. Groundwater Chemistry, Distribution and Potential Health Risk Appraisal of Nitrate Enriched Groundwater: A Case Study from the Semi-Urban Region of South India. Ecotoxicol. Environ. Saf. 2021, 207, 111277. [Google Scholar] [CrossRef]
- Kothari, V.; Vij, S.; Sharma, S.; Gupta, N. Correlation of Various Water Quality Parameters and Water Quality Index of Districts of Uttarakhand. Environ. Sustain. Indic. 2021, 9, 100093. [Google Scholar] [CrossRef]
- Duressa, G.; Assefa, F.; Jida, M. Assessment of Bacteriological and Physicochemical Quality of Drinking Water from Source to Household Tap Connection in Nekemte, Oromia, Ethiopia. J. Environ. Public Health 2019, 2019, 2129792. [Google Scholar] [CrossRef]
- Jacob, A.A.; Ndukwe, G.I.; Gimba, C.E.; Yilleng, M.T.; Stephen, D.; Fasanya, O.; Samuel, N.Y.; Madaki, L.A. Physicochemical Assessment of Ground Water Quality from Borehole and Hand Dug Wells around Obajana Community, Lokoja, Kogi State, Nigeria. J. Appl. Sci. Environ. Manag. 1970, 26, 505–511. [Google Scholar] [CrossRef]
- An, H.T.; Bich, T.T.N.; Yi-Ching, C.; Hien, T.T.T. Assessment of Groundwater Quality for Drinking and Domestic Purposes through Local Survey and Water Quality Index in Vietnam. J. Southwest Jiaotong Univ. 2021, 56, 83–93. [Google Scholar] [CrossRef]
- Gallagher, C.D.; Dietrich, A.M. TDS and Temperature Affect Consumer Taste Preferences. Opflow 2010, 36, 20–22. [Google Scholar] [CrossRef]
- Burlingame, G.A.; Doty, R.L. Important Considerations for Estimating Odor Threshold Concentrations of Contaminants Found in Water Supplies. J. AWWA 2018, 110, 1–12. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, T.; Qu, Z.; Li, H.; Yang, Z. Contribution of Filamentous Fungi to the Musty Odorant 2,4,6-Trichloroanisole in Water Supply Reservoirs and Associated Drinking Water Treatment Plants. Chemosphere 2017, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Liu, Y.Q.; Li, Y.Y.; Lin, L.A.; Zheng, B.H.; Ji, M.F.; Li, B.L.; Han, X.M. The Seasonal Patterns, Ecological Function and Assembly Processes of Bacterioplankton Communities in the Danjiangkou Reservoir, China. Front. Microbiol. 2022, 13, 884765. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, T.; Sabarathinam, C.; Panda, B.; Elumalai, V. Influence of Dissolved Oxygen, Water Level and Temperature on Dissolved Organic Carbon in Coastal Groundwater. Hydrology 2023, 10, 85. [Google Scholar] [CrossRef]
- Kalyani, D.S.; Rajesh, V.; Reddi, E.U.B.; Kumar, K.C.; Rao, S.S. Correlation between Corrosion Indices and Corrosiveness of Groundwater: A Study with Reference to Selected Areas of Krishna District, Andhra Pradesh, India. Environ. Earth Sci. 2017, 76, 568. [Google Scholar] [CrossRef]
- Fakioğlu, M.; Karpuzcu, M.E.; Öztürk, İ. İçme Sularında Alg Kaynaklı Tat Ve Koku Sorununun Değerlendirilmesi. Pamukkale Univ. Muh. Bilim. Derg. 2018, 24, 1141–1156. [Google Scholar]
- Zhang, Y.L.; Yang, L.Y.; Qin, B.Q.; Gao, G.; Luo, L.C.; Zhu, G.W.; Liu, M.L. Spatial Distribution of COD and the Correlations with Other Parameters in the Northern Region of Lake Taihu. Huanjing Kexue/Environ. Sci. 2008, 29, 1457–1462. [Google Scholar]
- Du, Y.; Deng, Y.; Ma, T.; Xu, Y.; Tao, Y.; Huang, Y.; Liu, R.; Wang, Y. Enrichment of Geogenic Ammonium in Quaternary Alluvial–Lacustrine Aquifer Systems: Evidence from Carbon Isotopes and DOM Characteristics. Environ. Sci. Technol. 2020, 54, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Rusydi, A.F.; Onodera, S.I.; Saito, M.; Hyodo, F.; Maeda, M.; Sugianti, K.; Wibawa, S. Potential Sources of Ammonium-Nitrogen in the Coastal Groundwater Determined from a Combined Analysis of Nitrogen Isotope, Biological and Geological Parameters, and Land Use. Water 2021, 13, 25. [Google Scholar] [CrossRef]
- Ghssein, G.; Awada, R.; Salami, A.; Bahmad, H.F.; Awad, A.; Joumaa, W.H.; El Roz, A. Prevalence, Laboratory Findings and Clinical Characteristics of Campylobacteriosis Agents among Hospitalized Children with Acute Gastroenteritis in Lebanon. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 346–356. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Wu, N.; Gao, M.; Tan, Y. Combined Toxicity of Pyrethroid Insecticides and Heavy Metals: A Review. Environ. Chem. Lett. 2019, 17, 1693–1706. [Google Scholar] [CrossRef]
- Lazhar, B.; Ammar, T.; Lotfi, M. Assessment of Heavy Metals Contamination in Groundwater: A Case Study of the South of Setif Area, East Algeria. In Achievements and Challenges of Integrated River Basin Management; Komatina, D., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Oyeleke, P.; Okparaocha, F. Assessment of Some Heavy Metals in Groundwater in the Vicinity of an Oil Depot in Nigeria. Am. Chem. Sci. J. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Nsdwq, U. Nigerian Standard for Drinking Water Quality. Niger. Ind. Stand. NIS 2007, 554, 13–14. [Google Scholar]
- Hussain, R.; Khattak, S.A.; Sattar, S.; Ali, L.; Ullah, Z.; Muhammad, N. Evaluation of the Contaminated Soil and Its Impacts on Tobacco (Nicotiana tabacum L.) Crops in Swabi, Pakistan. J. Himal. Earth Sci. 2020, 53, 34–48. [Google Scholar]
- Gimba, C.E.; Ndukwe, G.I.; Paul, E.D.; Habila, J.D.; Madaki, L.A. Heavy Metals (Cd, Cu, Fe, Mn and Zn,) Assessment of Groundwater, in Kaltungo LGA, Gombe State, Nigeria. Int. J. Sci. Technol. 2015, 4, 49–56. [Google Scholar]
- Lwimbo, Z.D.; Komakech, H.C.; Muzuka, A.N.N. Impacts of Emerging Agricultural Practices on Groundwater Quality in Kahe Catchment, Tanzania. Water 2019, 11, 2263. [Google Scholar] [CrossRef]
- Ren, Y.S.; Ilyas, M.; Xu, R.Z.; Ahmad, W.; Wang, R. Concentrations of Lead in Groundwater and Human Blood in the Population of Palosai, a Rural Area in Pakistan: Human Exposure and Risk Assessment. Adsorpt. Sci. Technol. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Reckhow, D.A.; Singer, P.C.; Malcolm, R.L. Chlorination of Humic Materials: Byproduct Formation and Chemical Interpretations. Environ. Sci. Technol. 1990, 24, 1655–1664. [Google Scholar] [CrossRef]
- White, M.C.; Thompson, J.D.; Harrington, G.W.; Singer, P.C. Evaluating Criteria for Enhanced Coagulation Compliance. J. AWWA 1997, 89, 64–77. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Jensen, J.N. THM and TOX Formation: Routes, Rates, and Precursors. J. AWWA 1986, 78, 156–162. [Google Scholar] [CrossRef]
- Vo-Minh Nguyen, H.; Hur, J.; Shin, H.-S. Humic Acids and Fulvic Acids: Characteristics, Sorption of Hydrophobic Organic Contaminants, and Formation of Disinfection by-Products during Chlorination. In Humus and Humic Substances–Recent Advances; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Murphy, K.R.; Ruiz, G.M.; Dunsmuir, W.T.M.; Waite, T.D. Optimized Parameters for Fluorescence-Based Verification of Ballast Water Exchange by Ships. Environ. Sci. Technol. 2006, 40, 2357–2362. [Google Scholar] [CrossRef]
- Huang, S.B.; Wang, Y.X.; Ma, T.; Tong, L.; Wang, Y.Y.; Liu, C.R.; Zhao, L. Linking Groundwater Dissolved Organic Matter to Sedimentary Organic Matter from a Fluvio-Lacustrine Aquifer at Jianghan Plain, China by EEM-PARAFAC and Hydrochemical Analyses. Sci. Total Environ. 2015, 529, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.K.L.; Boyer, T.H. Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef]
- Pisani, O.; Bosch, D.D.; Coffin, A.W.; Endale, D.M.; Liebert, D.; Strickland, T.C. Riparian Land Cover and Hydrology Influence Stream Dissolved Organic Matter Composition in an Agricultural Watershed. Sci. Total Environ. 2020, 717, 137165. [Google Scholar] [CrossRef]
- Baghoth, S.A.; Sharma, S.K.; Amy, G.L. Tracking Natural Organic Matter (NOM) in a Drinking Water Treatment Plant Using Fluorescence Excitation–Emission Matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef]
- Jiang, T.; Bravo, A.G.; Skyllberg, U.; Björn, E.; Wang, D.; Yan, H.; Green, N.W. Influence of Dissolved Organic Matter (DOM) Characteristics on Dissolved Mercury (Hg) Species Composition in Sediment Porewater of Lakes from Southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef]
- Kaufman, J.A.; Wright, J.M.; Evans, A.; Rivera-Núñez, Z.; Meyer, A.; Narotsky, M.G. Disinfection By-Product Exposures and the Risk of Musculoskeletal Birth Defects. Environ. Epidemiol. 2020, 4, e081. [Google Scholar] [CrossRef] [PubMed]
- Costet, N.; Villanueva, C.M.; Jaakkola, J.J.K.; Kogevinas, M.; Cantor, K.P.; King, W.D.; Lynch, C.F.; Nieuwenhuijsen, M.J.; Cordier, S. Water Disinfection By-Products and Bladder Cancer: Is There a European Specificity? A Pooled and Meta-Analysis of European Case-Control Studies. Occup. Environ. Med. 2011, 68, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G. Methods of Soil, Plant and Water Analysis. FRI Bull. N. Z. For. Serv. For. Res. Inst. 1984, 70, 65–119. [Google Scholar]




| Sr. No. | Parameters | Network A | Network B | PEQS | WHO | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tube Wells | TR | Taps | Tube Wells | TR | Taps | ||||
| 1 | Color (TCU) | U.O | U.O | U.O | U.O | U.O | U.O | <15 | <15 |
| 2 | Taste | U.O | U.O | O | U.O | U.O | O | U.O | U.O |
| 3 | Odor | U.O | U.O | O | U.O | U.O | O | U.O | U.O |
| 4 | Temperature(°C) | 13.02 ± 1.02 | 13.00 ± 2.61 | 22.36 ± 1.15 | 13.99 ± 0.94 | 13.80 ± 1.23 | 21.64 ± 0.70 | - | 15 |
| 5 | Turbidity (NTU) | n.d | n.d | n.d | n.d | n.d | n.d | <5 | <5 |
| 6 | pH | 7.45 ± 0.07 | 7.30 ± 0.36 | 6.72 ± 0.25 | 7.72 ± 0.11 | 7.50 ± 0.59 | 6.81 ± 0.16 | 6.5–8.5 | 6.5–8.5 |
| 7 | EC (µS/cm) | 285.51 ± 6.09 | 365.67 ± 4.93 | 504.31 ± 3.71 | 334.50 ± 5.08 | 347.33 ± 4.51 | 459.91 ± 6.75 | 1500 | 1000 |
| 8 | DO (mg/L) | 3.34 ± 0.22 | 3.07 ± 0.25 | 2.48 ± 0.18 | 3.28 ± 0.15 | 2.17 ± 0.21 | 2.66 ± 0.13 | - | >5 |
| 9 | TDS (mg/L) | 421.24 ± 3.57 | 443.00 ± 6.24 | 748.96 ± 3.61 | 466.60 ± 4.36 | 510.00 ± 6 | 536.64 ± 3.99 | 1000 | 1000 |
| 10 | Ca2+ (mg/L) | 51.24 ± 3.34 | 62.00 ± 2.65 | 65.00 ± 3.67 | 52.77 ± 2.06 | 51.33 ± 4.16 | 61.88 ± 2.73 | - | 200 |
| 11 | Mg2+ (mg/L) | 11.86 ± 1.06 | 12.27 ± 1.55 | 19.62 ± 1.48 | 14.88 ± 1.12 | 15.07 ± 1.04 | 16.77 ± 1.06 | - | 150 |
| 12 | CO32− (mg/L) | 15.74 ± 1.18 | 14.33 ± 2.31 | 3.66 ± 1.65 | 20.40 ± 0.99 | 18.00 ± 2.00 | 6.65 ± 1.54 | 60 | 60 |
| 13 | HCO3− (mg/L) | 144.21 ± 4.07 | 141.33 ± 6.66 | 129.04 ± 4.84 | 180.83 ± 4.52 | 172.33 ± 5.13 | 141.21 ± 7.15 | - | 250 |
| 14 | Cl− (mg/L) | 50.02 ± 2.88 | 58.97 ± 3.50 | 81.85 ± 2.40 | 58.10 ± 3.05 | 65.57 ± 7.84 | 72.80 ± 2.55 | 250 | 250 |
| 15 | Na+ (mg/L) | 46.18 ± 4.59 | 57.17 ± 3.34 | 97.70 ± 3.41 | 82.33 ± 5.17 | 79.97 ± 4.37 | 99.62 ± 3.67 | 200 | 200 |
| 16 | CaCO3 (mg/L) | 178.96 ± 5.53 | 191.00 ± 6.76 | 228.29 ± 3.88 | 190.65 ± 3.94 | 192.33 ± 6.81 | 209.35 ± 4.25 | 500 | 300 |
| 17 | COD (mg/L) | 13.41 ± 1.66 | 12.77 ± 2.41 | 19.45 ± 2.47 | 11.74 ± 1.24 | 13.07 ± 3.00 | 17.85 ± 2.09 | - | 10 |
| 18 | BOD (mg/L) | 3.49 ± 0.66 | 4.10 ± 1.10 | 6.90 ± 1.06 | 3.25 ± 0.32 | 4.47 ± 0.40 | 6.11 ± 0.35 | - | <5 |
| 19 | NO3− (mg/L) | 9.08 ± 1.21 | 9.25 ± 0.85 | 13.73 ± 0.61 | 6.76 ± 0.15 | 9.83 ± 1.10 | 12.77 ± 0.51 | 50 | 50 |
| 20 | NH4+ (mg/L) | 1.34 ± 0.07 | 1.11 ± 0.09 | 3.47 ± 0.41 | 1.06 ± 0.09 | 0.94 ± 0.06 | 1.80 ± 0.09 | - | 1.5 |
| 21 | T. coli (CFU) | n.d | n.d | 48.81 ± 7.95 | n.d | n.d | 46.45 ± 6.32 | 0/100 mL | 0/100 mL |
| 22 | F. coli (CFU) | n.d | n.d | 11.14 ± 3.40 | n.d | n.d | 10.70 ± 3.88 | 0/100 mL | 0/100 mL |
| Sr. No. | Parameters | Network A | Network B | PEQS | WHO | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tube Wells | TR | Taps | Tube Wells | TR | Taps | ||||
| 1 | Fe (mg/L) | 0.08 ± 0.02 | 0.10 ± 0.02 | 0.13 ± 0.01 | 0.07 ± 0.01 | 0.11 ± 0.02 | 0.16 ± 0.01 | - | 0.3 |
| 2 | Pb (mg/L) | 0.015 ± 0.001 | 0.015 ± 0.004 | 0.028 ± 0.001 | 0.013 ± 0.001 | 0.017 ± 0.002 | 0.027 ± 0.002 | 0.05 | 0.01 |
| 3 | Cd (mg/L) | 0.004 ± 0.001 | 0.003 ± 0.002 | 0.006 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.002 | 0.008 ± 0.001 | 0.01 | 0.003 |
| 4 | Cu (mg/L) | 0.12 ± 0.02 | 0.14 ± 0.02 | 0.30 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.02 | 0.41 ± 0.02 | 2 | 2 |
| 5 | Zn (mg/L) | 1.10 ± 0.03 | 1.14 ± 0.10 | 1.35 ± 0.05 | 1.43 ± 0.05 | 1.46 ± 0.05 | 1.56 ± 0.03 | 5 | 3 |
| 6 | Uv-254 (cm−1) | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.01 | - | - |
| 7 | C1 (A.U) | 2,796,848.25 ± 46.71 | 1,241,836.38 ± 91.95 | 4,145,083.89 ± 52.08 | 2,856,364.44 ± 54.87 | 2,777,733.81 ± 44.82 | 3,067,002.09 ± 59.31 | - | - |
| 8 | C2 (A.U) | 3,333,520.09 ± 45.05 | 1,343,680.92 ± 57.21 | 3,693,985.98 ± 51.18 | 3,440,604.59 ± 42.89 | 3,237,889.35 ± 60.47 | 3,643,204.79 ± 44.98 | - | - |
| 9 | C3 (A.U) | 638,012.18 ± 36.63 | 582,705.01 ± 122.14 | 643,968.05 ± 59.32 | 453,994.01 ± 36.80 | 437,370.46 ± 59.57 | 459,525.57 ± 41.58 | - | - |
| Compounds | EEM Contour | EEM Location | Fluorescence Compounds | References |
|---|---|---|---|---|
| C1 |  | Ex: 255 (362) nm Em: 452 nm | UVA-humic-like component | [10,41,45] |
| C2 |  | Ex: 315 nm Em: 370 nm | UVA-humic-like component | [10,42,45] |
| C3 |  | Ex: 290 (452) nm Em: 510 nm | UVC-humic-like component | [43,44,45] |
| Network A | Network B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Fe | Zn | Cu | Pb | Cd | Fe | Zn | Cu | |
| C1 | +0.18 NS | +0.38 * | +0.05 NS | +0.27 NS | +0.3 NS | +0.2 NS | +0.4 NS | +0.12 NS | +0.05 NS | +0.55 * |
| C2 | −0.006 NS | +0.16 * | −0.04 NS | +0.15 NS | −0.09 NS | +0.23 NS | +0.03 NS | −0.09 NS | −0.15 NS | +0.51 NS |
| C3 | +0.07 NS | −0.03 NS | −0.2 NS | −0.11 NS | +0.05 NS | +0.12 NS | +0.009 NS | −0.03 NS | −0.09 NS | +0.46 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaj Hamoud, Y.; Maqsood, A.; Zia-ur-Rehman, M.; Shaghaleh, H.; Sahar, A.; Usman, M.; Rizwan, M.; Alharby, H.F.; Abohassan, R.A.; Abdulmajeed, A.M. Novel Implications of the PARAFAC Model for Characterizing and Distributing DOM in Groundwater Networks by Using Spectroscopic Techniques. Water 2024, 16, 1768. https://doi.org/10.3390/w16131768
Alhaj Hamoud Y, Maqsood A, Zia-ur-Rehman M, Shaghaleh H, Sahar A, Usman M, Rizwan M, Alharby HF, Abohassan RA, Abdulmajeed AM. Novel Implications of the PARAFAC Model for Characterizing and Distributing DOM in Groundwater Networks by Using Spectroscopic Techniques. Water. 2024; 16(13):1768. https://doi.org/10.3390/w16131768
Chicago/Turabian StyleAlhaj Hamoud, Yousef, Abdullah Maqsood, Muhammad Zia-ur-Rehman, Hiba Shaghaleh, Amna Sahar, Muhammad Usman, Muhammad Rizwan, Hesham F. Alharby, Refaat A. Abohassan, and Awatif M. Abdulmajeed. 2024. "Novel Implications of the PARAFAC Model for Characterizing and Distributing DOM in Groundwater Networks by Using Spectroscopic Techniques" Water 16, no. 13: 1768. https://doi.org/10.3390/w16131768








