Abstract
Constructed wetland systems employing submerged macrophytes are increasingly utilized for treating municipal and industrial wastewater, as well as odoriferous and eutrophic water bodies. However, the pollutant removal efficiency of these systems needs further enhancement. In this study, we examined the impact of the gas-to-water ratio on the treatment efficiency of the constructed wetland of Vallisneria. We also examined the extracellular polymeric substances (EPSs) of the floating biofilm and the structure of the microbial community in this system. Our findings showed that the gas-to-water ratio significantly affects the total nitrogen (TN) removal rate within the Vallisneria wetlands, with an optimum removal at a gas-to-water ratio of 15:1, while the removal efficiencies for chemical oxygen demand (COD), NH4+-N, and total phosphorus (TP) remain relatively unaffected. Increased gas-to-water ratios corresponded to a notable decrease in biofilm EPSs. High-throughput sequencing analysis demonstrated a shift in biofilm-denitrifying bacteria from anoxic heterotrophic to aerobic denitrifiers, alongside a significant rise in the abundance of denitrifying bacteria, whereas excessively high gas-to-water ratios inhibited the growth of these bacteria. A gas-to-water ratio of 15:1 constituted the optimal condition for ecological restoration of the water body within the Vallisneria wetland systems. These results could contribute to the optimization of submerged-macrophyte constructed wetland system design and the enhancement of treatment efficiency.
1. Introduction
High levels of nitrogen and phosphorus in water bodies can threaten the biodiversity of aquatic environments, disturb the stability of ecosystems, and lead to eutrophication [1]. The current denitrification methods used to solve the prevalent problem of water eutrophication can be classified as physicochemical or biological methods. Among them, biological methods (such as constructed wetlands) have attracted extensive attention due to their effective denitrification performance and non-toxic by-products and their potential application in wastewater treatment [2,3].
Constructed wetlands are extensively utilized in the treatment of domestic sewage [1], industrial wastewater [4], and black and malodorous water [5] due to their cost-effectiveness, ease of operation and maintenance, and environmentally friendly nature [6]. As a crucial component of constructed wetlands, plants, particularly submerged plants, are fully immersed beneath the water surface and come into direct contact with both sewage and microorganisms. The roots, stems, leaves, and attached biofilms of these plants play a significant role in the removal of nutrients from the water column [7,8]. However, previous studies have indicated that the impact of submerged plants on nutrient removal during water purification is limited, with the removal of nitrogen and phosphorus attributed to plant-attached microbial communities [9]. Submerged plants primarily serve as habitats and provide fixed substrates for the attached biota [10], while also releasing DO for other microorganisms [11,12]. This influences the abundance, activity, and reaction processes of the functional bacteria in the plants’ epiphytic biofilms, thereby affecting the efficacy of the ecosystem treatment.
However, plants have a limited secretory capacity, and their efficiency in reducing water pollution requires further improvement [13]. Studies have revealed that in constructed wetlands dominated by emergent aquatic plants, enhanced measures such as aeration are often employed to augment the removal capacity of constructed wetlands. Specifically, a higher gas-to-water ratio can lead to improved effectiveness in the removal of pollutants [14]. Currently, there is a greater emphasis on comprehensive investigations of constructed wetlands with submerged plants, both domestically and internationally [10]. Conversely, research on submerged-plant-based constructed wetlands primarily focuses on the removal mechanisms of pollutants by attached biofilms [15,16]; intramembrane microbial community structures [10]; external environmental conditions such as microcystins [17], microplastics [18], antibiotics [19], and harmful algal bloom harvests [20]; and other factors in the structure of biofilm microbial communities. However, limited attention has been paid to measures aimed at enhancing the removal efficiency of submerged-plant-based constructed wetlands, which hinders their widespread adoption and application.
The Vallisneria exhibits well-developed root tissue, strong pollution resistance, high reproductive capacity, low light tolerance, and effective removal of ammonia nitrogen [21]. Vallisneria natans is a common submerged macrophyte in most eutrophic lakes in China that can tolerate and purify polluted water [17]. Therefore, this study used the epiphytic biofilm on grass leaves as its research object, focusing on investigating the wastewater treatment efficiency of a constructed wetland with submerged plants and different air–water ratios. Additionally, the microbial community structure within the epiphytic biofilm was characterized to explore the influence mechanism of the air–water ratio on the submerged plant system.
2. Materials and Methods
2.1. System Construction
Three PVC setups measuring 50 × 30 × 40 cm, with a 5 cm layer of river sand at their base and 15 L water, were constructed. Forty-eight Vallisneria plants of similar growth were selected from a laboratory-acclimated biofilm culture and planted in each sept using sixteen plants per setup. Three gas-to-water ratios of 10:1 (A, 2 h of aeration), 15:1 (B, 3 h of aeration), and 20:1 (C, 4 h of aeration) were investigated for their effects on the water quality, biofilm EPS content, and microbial community structure. A 3-day water renewal cycle and a 25-day experimental run were adopted. COD, TN, TP, NH4+-N, NO3−-N, NO2−-N, DO, and pH of the influent and effluent were measured. High-throughput sequencing analysis of the biofilm was performed at the end of the experiments using plant samples from each setup (Shanghai Meji Biomedical Technology Co., Ltd.; Shanghai, China).
2.2. Determination of EPS Protein and Polysaccharide in Biofilm
Polysaccharides and proteins in EPSs were extracted by thermal digestion: 3.0 g of leaves was first removed from the reactor and then placed in a 30 mL centrifuge tube and shaken with 20 mL of deionized water for 1 min to separate the leaves from the biofilm. We removed the leaves, placed the remaining solution in a 70 °C water bath for 30 min, and then centrifuged at 2500× g for 15 min [20] and the supernatant was used for EPS analysis. Phenol-sulfuric acid method and BCA assay (Beyotime, P0012; Shanghai Biyuntian Biotechnology Co., Ltd., Shanghai, China) were utilized for quantifying polysaccharides and proteins, respectively.
2.3. High-Throughput Sequencing
High-throughput sequencing was used to analyze microbial communities in leaf epiphytic biofilms. The collected plant leaf samples were transported on ice to the lab, where the biofilm was detached via repeated ultrasonication and vortexing in phosphate-buffered saline (pH = 8.0). The samples were then filtered through a 0.22 μm membrane. Membranes were flash-frozen in liquid nitrogen and stored at −80 °C. The biofilm was cut into 50 μm slices using a cryostat, and the DNA was extracted from the biofilm samples for subsequent gel electrophoresis and NanoDrop-2000 spectrophotometry to check the quality and quantity of the DNA. Amplification of the 16S V3-V4 region was performed on a Veriti FAST thermal cycler using 338F and 806R universal primers. After the sequencing data were spliced, quality-controlled, and de-spliced, the optimized sequences were obtained. Based on the optimized sequences, OUT clustering was performed to obtain the OUT abundance for subsequent biological information analysis. The whole sequencing experiment was completed by Shanghai Meiji Biomedical Technology Co., Ltd., Shanghai, China.
2.4. Data Processing and Analysis
Data processing and analyses were performed using SPSS 24.0 software (IBM, Armonk, NY, USA) for ANOVA or Student’s t-test to discern significant differences across treatment groups (p < 0.05), with plotting and further analyses conducted in Origin 2018b (OriginLab Corporation, Northampton, MA, USA).
3. Results and Discussion
3.1. Effect of Different Gas-to-Water Ratios on Water Quality Treatment
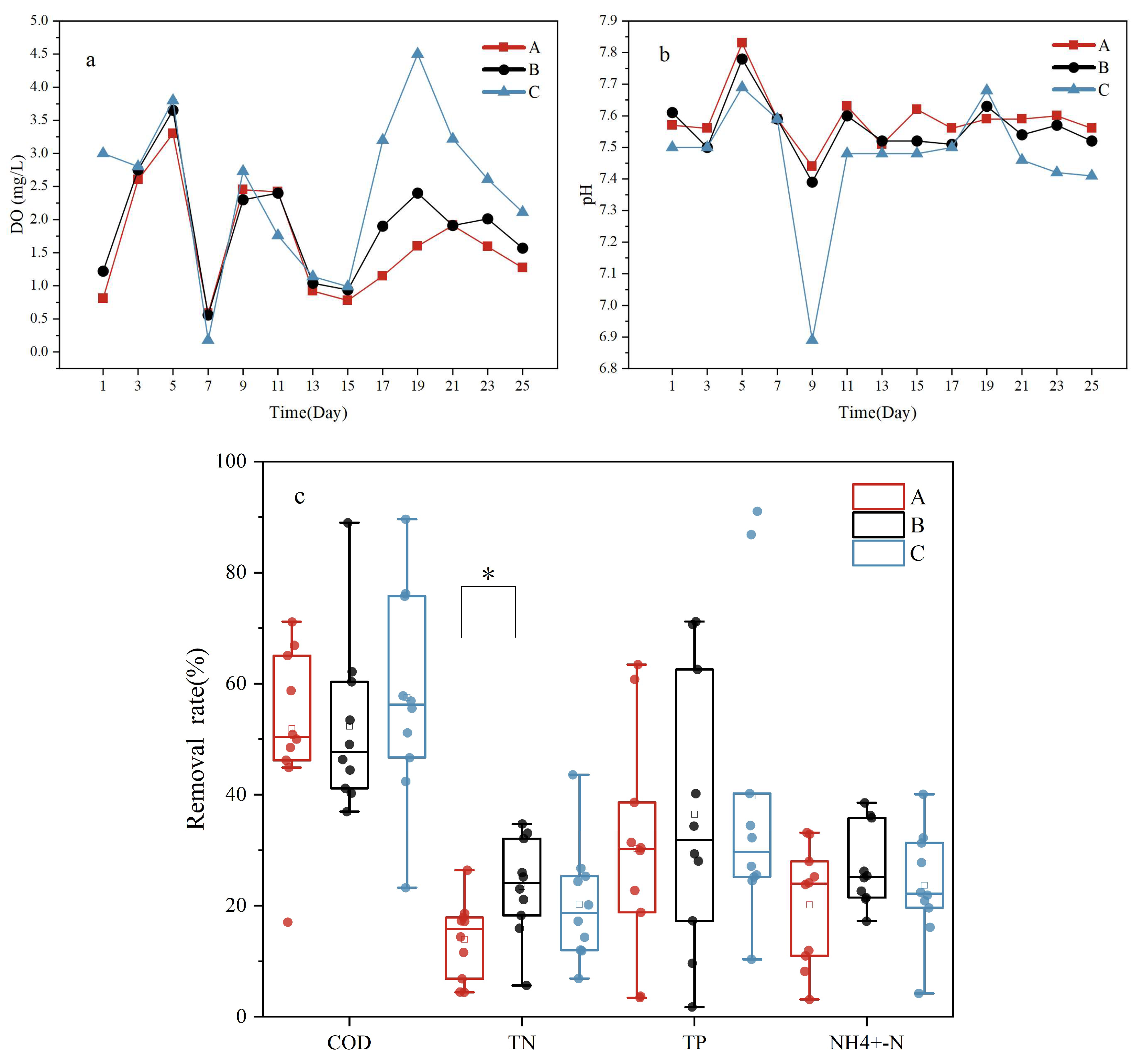
Different gas-to-water ratios were used to assess the effectiveness of the water treatment using Vallisneria. These gas-to-water ratios were 10:1 (A), 15:1 (B), and 20:1 (C). The removal rates for the various indicators were as follows: A—COD, 51.92 ± 15.37%; TN, 13.90 ± 7.08%; TP, 30.32 ± 6.42%, NH4+-N, 20.13 ± 10.71%; B—COD, 57.51 ± 19.08%%; TN, 23.49 ± 8.86%; TP, 36.49 ± 7.81%, NH4+-N, 26.97 ± 7.31; and C—COD, 57.51 ± 19.08%; TN, 20.24 ± 10.49%; TP, 20.51 ± 8.56%, NH4+-N, 23.64 ± 9.89%. Significant differences in TN removal between groups A and B were noted (p < 0.05), with no significant differences found for other parameters. The DO in the outflow water for groups A, B, and C averaged 1.64 ± 1.31 mg/L, 1.89 ± 1.20 mg/L, and 2.46 ± 0.90 mg/L, respectively, while the average pH values in these groups were 7.58 ± 0.08, 7.56 ± 0.90, and 7.46 ± 0.19, respectively. All three gas-to-water ratios resulted in an increase in the concentration of DO in the effluent (Figure 1a). The COD removal rates increased, whereas the pH decreased with rising gas-to-water ratios, with a significant difference in pH value between conditions A and C. Increases in COD removal rates and decreases in pH values upon increasing gas-to-water ratios could possibly be a result of a positive correlation between the DO and COD and a negative correlation between the DO and pH [22].
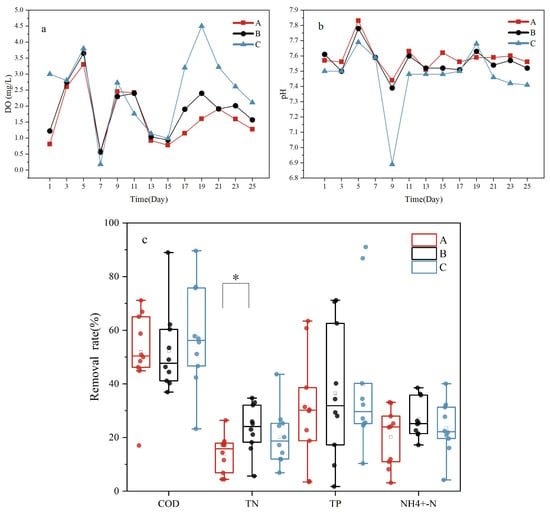
Figure 1.
Removal rate of pollutants from the system at different gas-to-water ratios. (a) changes in DO over time; (b) changes in pH over time; (c) changes in removal rates for COD, TN, TP, and NH4+-N. “*” represents significant differences in pollutant treatment effects between samples under this indicator.
3.2. Effects of Different Gas-to-Water Ratios on EPS
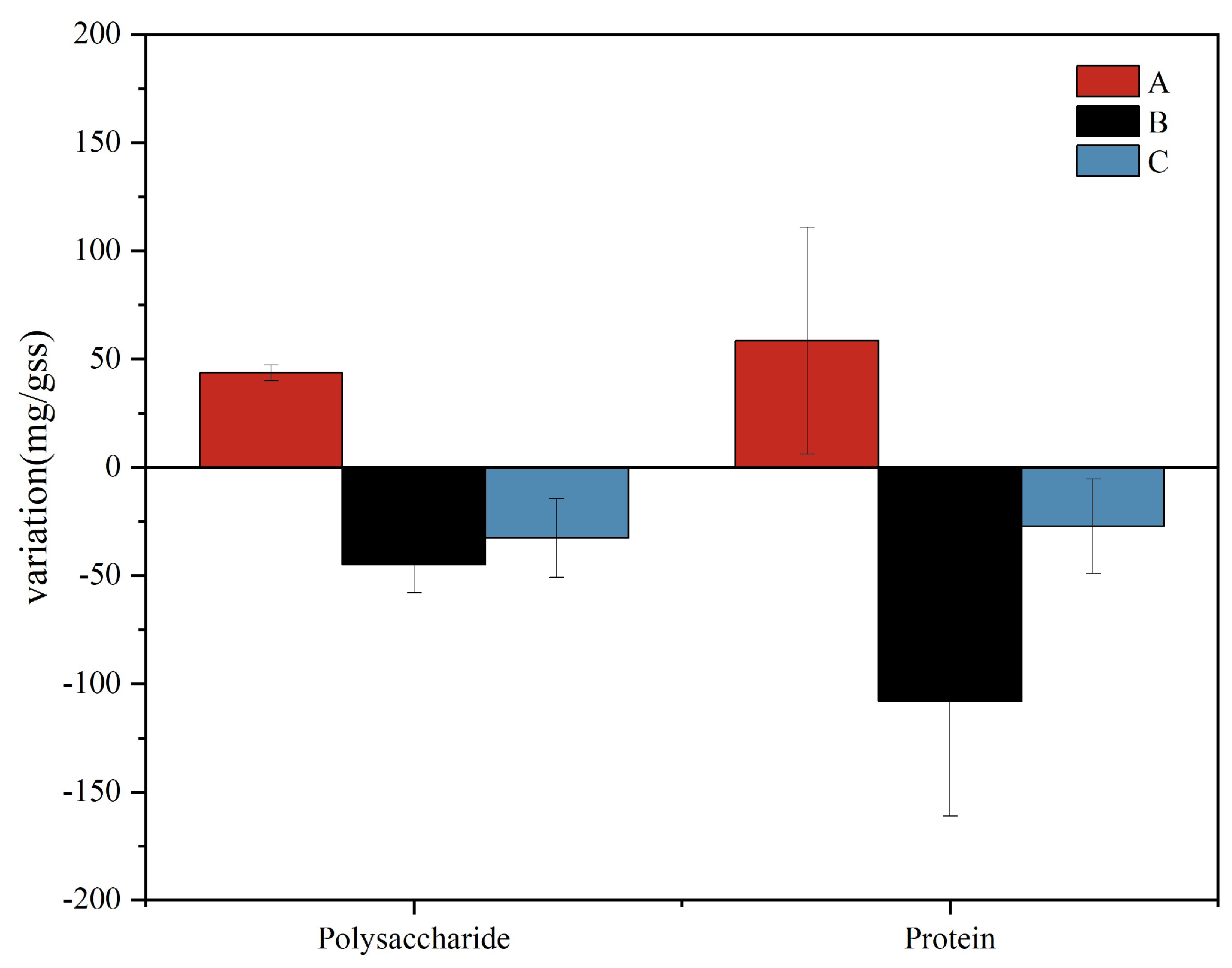
As shown in Figure 2, the contents of proteins and polysaccharides in group A increased with time, while the contents of polysaccharides and proteins in supernatants from group B and group C decreased with treatment time. On the one hand, the proteins and polysaccharides in the EPSs were biodegradable, and the increase in gas-to-water ratio increased the concentration of DO in the system. As the microorganisms in the system adapted to the environment, their activities became more intense. The accumulated EPS in the system is often used as a substrate for microbial consumption and decomposition [23]. On the other hand, the decrease in the amount of proteins and polysaccharides in groups B and C may be due to the increase in the gas-to-water ratio, oxygen deoxygenation, and oxygen transfer rate in the system, so that the microbial membrane has a higher level of metabolic activity, and the polysaccharide production rate is less than the consumption rate [24]. As polysaccharide is the main source of EPS stickiness [25], a decrease in polysaccharide content and EPS stickiness may lead to a decrease in the shedding of the attached biofilm on the leaf surface, consequently leading to a decrease in the removal rate of ammonium nitrogen. At the same time, the pH value was higher under the condition of a low gas-to-water ratio (Figure 1c), and the alkaline environment would cause the aggregation of polysaccharide sticky substances [26], which may be the reason for the higher EPS content in group A. Although the content of polysaccharides and protein in group A increased, in the case of a gas-to-water ratio of 15:1, the system has the best nitrogen removal effect (Figure 1a). A low aeration rate may affect the nitrification efficiency and fail to provide sufficient electron acceptors for denitrification, resulting in poor TN removal, while a high gas-to-water ratio may lead to excessive oxidation of carbon sources and affect the denitrification effect, resulting in a poor TN removal effect.
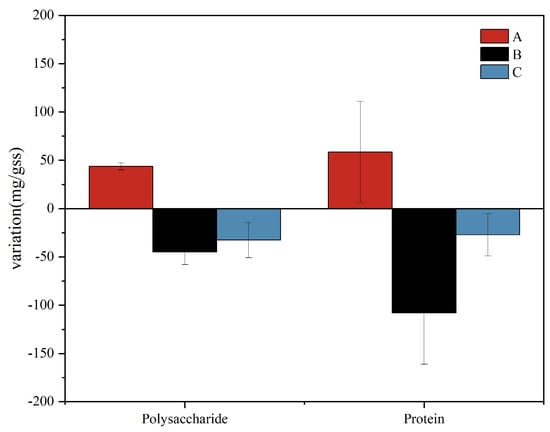
Figure 2.
Polysaccharide and protein mass variation chart.
3.3. Alpha Diversity Analysis and Microbial Community Composition
As depicted in Table 1, the alpha diversity analysis identified an average of 1042, 789 and 1608 observed OTU sequences in groups A, B, and C. This indicates that group C bacteria have a higher abundance of microbial species. The coverage indices for all samples exceeded 0.96, indicating a sufficient sequencing depth. The Shannon and Chao indexes are closely related to bacterial community diversity, while the Simpson index reflects the most prevalent species within communities, and the Ace indicator can be used to measure the sample coverage. No statistical differences in the Simpson index were found among the three groups (p = 0.0509), whereas significant differences were observed among the three groups for the following three indices: Shannon (p = 0.0273), Chao indices (p = 0.0273), and Ace indices (p = 0.0273) (Table 1). Based on these findings, it was anticipated that the gas-to-water ratio could significantly influence microbial community diversity. However, it does not affect the uniformity of the species, supporting the results of significant differences between groups.

Table 1.
Statistical table of microbial diversity analysis.
3.4. Microbial Community Diversity
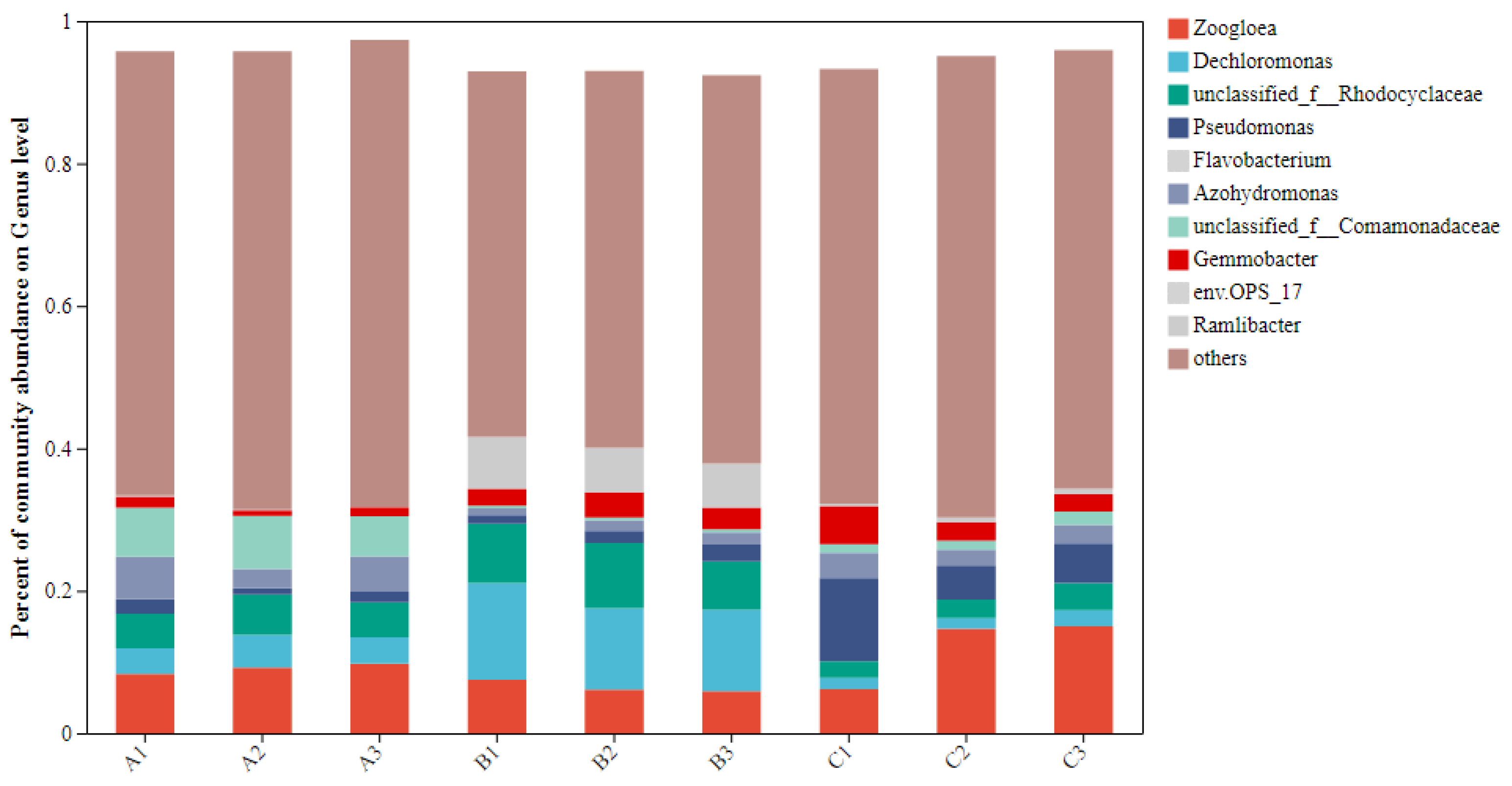
At the genus level, the top 10 abundant bacteria accounted for 35.92%, 47.09%, and 37.54% of the total bacteria in groups A, B, and C, respectively, with the dominant genera being Dechloromonas, unclassified_f__Rhodocyclaceae, unclassified_f__Comamonadaceae, Zoogloea, Gemmobacter, Azohydromonas, env.OPS_17, and Ramlibacter (Figure 3).
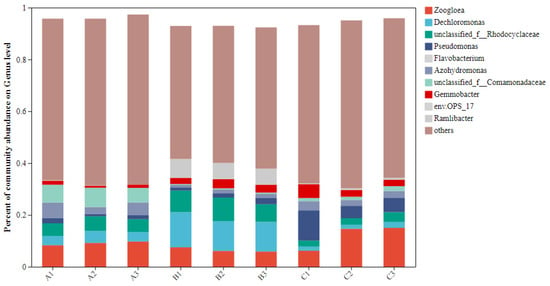
Figure 3.
The proportion of TOP10 bacteria in each group. Note: the horizontal/vertical coordinates indicate the sample name. The vertical/horizontal coordinates show the proportion of species in the sample. The different colors of the columns represent different species, and the lengths of the columns represent the size of the proportion of species.
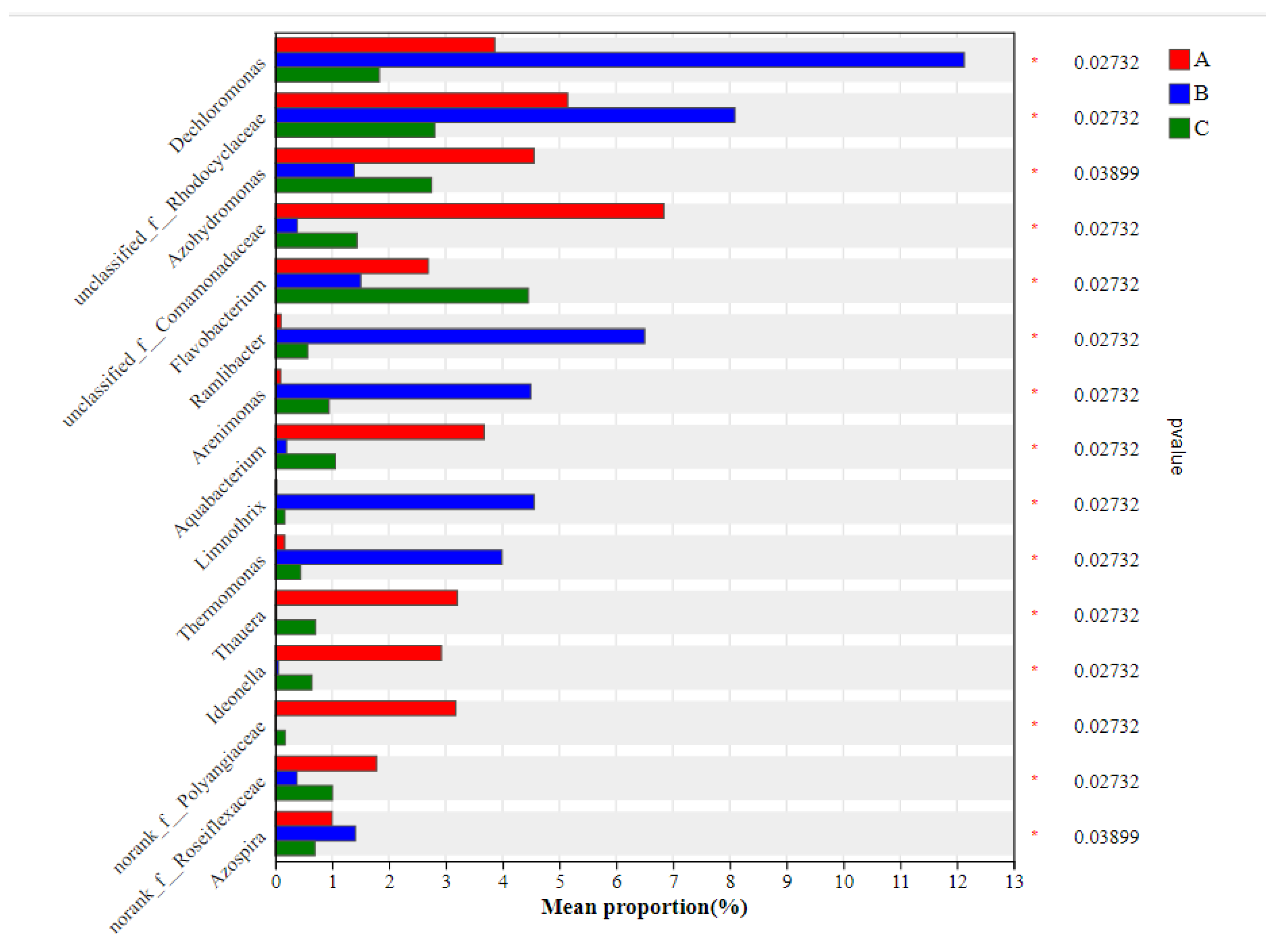
Group A was significantly enriched with genera such as Azohydromonas (4.557%), unclassified_f__Comamonadaceae (6.841%), Aquabacterium (3.667%), norank_f__Polyangiaceae (3.178%), Thauera (3.204%), Ideonella (2.925%), and norank_f__Roseiflexaceae (1.784%). Aquabacterium and unclassified_f__Comamonadaceae are known as heterotrophic anoxic denitrifiers [27,28], Ideonella as heterotrophic nitrifying-aerobic denitrifiers [29], and Thauera as facultative heterotrophic denitrifiers [30,31]. In group B, significantly enriched genera included Dechloromonas (12.13%), unclassified_f__Rhodocyclaceae (8.092%), Ramlibacter (6.503%), Arenimonas (4.499%), Limnothrix (4.559%), Thermomonas (3.992%), and Azospira (1.412%). Dechloromonas, Rhodocyclaceae, Azospira, and Arenimonas are heterotrophic nitrifying aerobic denitrifiers [32,33,34,35], and Thermomonas are autotrophic nitrifying aerobic denitrifiers [36]. In group C, Flavobacterium (4.454%) was significantly enriched as a heterotrophic nitrifying aerobic denitrifier (Figure 4).
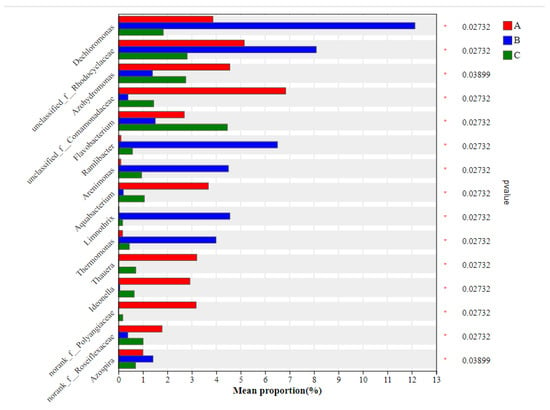
Figure 4.
Differences in the mean relative abundance of the same species among different groups. Note: the y-axis represents the species name at a certain taxonomic level; the x-axis represents the average relative abundance of the species in different groups; the colored bars represent different groups; and the column of numbers on the right-hand shows the p-values. The “*” on the right axis of the figure represents significant differences in the genus of bacteria among different groups.
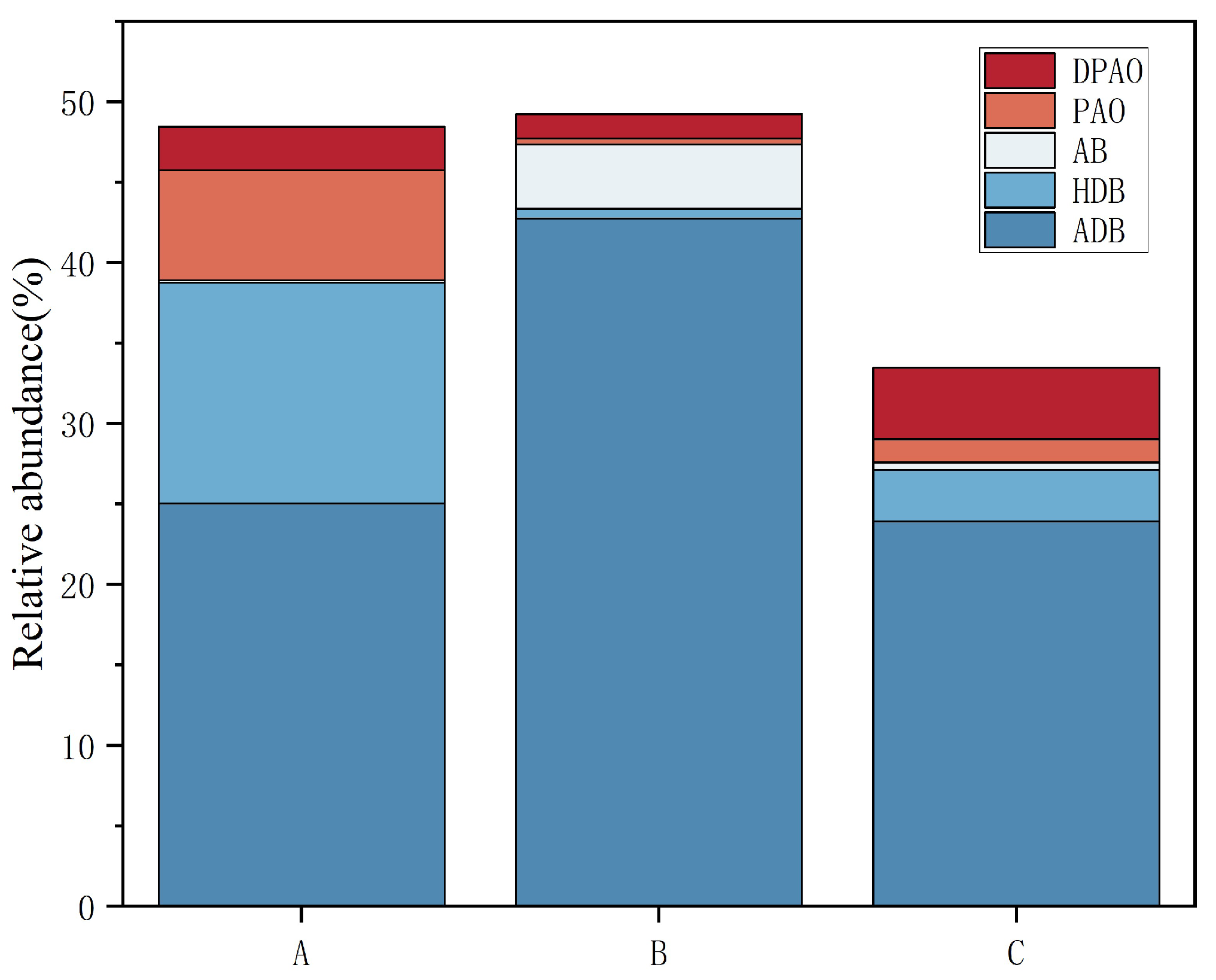
Further analysis identified that group A was predominantly enriched with heterotrophic denitrifying bacteria, with a total abundance of 13.71%, which was significantly higher than the 0.58% of group B and 3.21% of group C. Group B was mainly enriched with aerobic denitrifying bacteria, with a total abundance of 42.76%, which was significantly higher than the 25.03% and 23.92% of groups A and C, respectively (Figure 5). It is evident that with increasing gas-to-water ratios, the dominant denitrifying bacteria in the epiphytic biofilm changed from anoxic heterotrophic to aerobic denitrifiers. A further increase in the gas-to-water ratio will also inhibit the aerobic denitrifying bacteria, possibly as a result of over-oxidation of the carbon source from the inflow COD, which suppressed the growth of heterotrophic/aerobic denitrifiers, leading to a significantly lower abundance of total denitrifying bacteria in group C than in groups A and B. Coupled with wastewater TN removal analysis, group B significantly outperformed group A (Figure 1a), which is consistent with its higher denitrifier abundance. Aerobic denitrifiers such as Thermomonas in group B were more competitive in the denitrification of biodegradable organic matter than anoxic heterotrophic denitrifiers like Thauera [37]. No significant difference in TN removal rate was observed between groups A and C, possibly because of the low gas-to-water ratio in group A, thereby restricting the nitrification process and failing to supply sufficient nitrate electron acceptors for subsequent denitrification. The main phosphate-removing bacteria across the systems were Flavobacterium and unclassified_f__Comamonadaceae, with Flavobacterium identified as a denitrifying phosphate remover. The total proportion of polyphosphate-accumulating bacteria in the biofilm decreased (p < 0.05) as the gas-to-water ratio increased. However, no significant difference in TP removal rate was observed among the three groups, likely because systemic phosphorus removal requires residual sludge exclusion, and the experimental constructed wetland with Vallisneria had no sludge discharge.
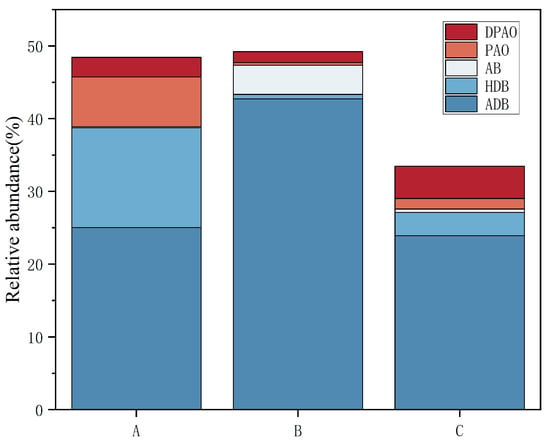
Figure 5.
Proportion of functional bacteria for nitrogen and phosphorus removal in each group under different gas-to-water ratios. Note: DPAO stands for denitrifying polyphosphate bacteria, PAO stands for polyphosphate bacteria, AB stands for autotrophic denitrifying bacteria, HDB stands for heterotrophic denitrifying bacteria, and ADB stands for aerobic denitrifying bacteria.
Additionally, within group A, the relative abundance of Zoogloea and Azohydromonas was relatively high. Zoogloea can promote the production of EPSs [38], and Azohydromonas mainly functions in alkalinization, with alkaline conditions being favorable for EPS growth. The pH value in group A was indeed significantly higher than in the other two groups, and this may be the main reason for the significant initial rise in EPS content under conditions of a low gas-to-water ratio (Figure 1c and Figure 2). In systems B and C, both Ramlibacter and Limnothrix were significantly higher than in group A (p < 0.05), and these bacteria have been reported to contribute to EPS accumulation [39,40,41]. However, the EPS content in systems B and C decreased from the initial state, possibly because these systems promoted the growth and metabolism of bacteria such as Pseudomonas and Flavobacterium that can decompose several polysaccharides. Flavobacterium also produces weak acids, which might be detrimental to EPS accumulation [42,43].
4. Conclusions
This study explored the impact of the gas-to-water ratio on the removal rate of pollutants and the epiphytic biofilm community structure in constructed wetland systems with Vallisneria. The findings clearly indicated that the gas-to-water ratio could significantly affect the TN removal rate, with the optimal conditions being a gas to water ratio of 15:1. Such a gas-to-water ratio had minimal effect on the influences on the removal efficiencies for COD, NH4+-N, and TP. An increase in the gas-to-water ratio led to a marked decline in biofilm EPSs. High-throughput sequencing analysis demonstrated that as the gas-to-water ratio increased, the content of denitrifying bacteria changed from anoxic heterotrophic denitrifiers to aerobic denitrifiers, with a significant rise in the abundance of denitrifier, although excessive ratios could suppress the growth of denitrifiers. Overall, a gas-to-water ratio of 15:1 presented the optimal condition for ecological restoration in Vallisneria wetland systems.
Author Contributions
Conceptualization, Z.J.; methodology, Z.J., H.M., C.B. and X.Y.; software, H.M., C.B. and X.J.; formal analysis, G.H.; investigation, S.L. and Z.J.; resources, X.Z. and M.Z.; writing—original draft preparation, H.M. and Z.J.; writing—review and editing, X.Z. and M.Z.; supervision, G.H. and X.J.; funding acquisition, Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52100197) and the Major Program of Institute for Eco-environmental Research of Sanyang Wetland, Wenzhou University (SY2022ZD-1002-06).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Alan K. Chang (Wenzhou University) for his kind help with revising the language of the manuscript.
Conflicts of Interest
Author Shiwen Lu is employed in Jiujiang Branch of Jiangsu Hongrun Biomass Energy Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Angeles-Núñez, J.G.; Cruz-Acosta, T. Aislamiento, caracterización molecular y evaluación de cepas fijadoras de nitrógeno en la promoción del crecimiento de frijol X1-Isolation, molecular characterization and evaluation of nitrogen-fixing strains in promoting the growth of beans. Rev. Mex. Cienc. Agrícolas 2015, 6, 929–942. [Google Scholar]
- Chen, H.; Zhang, S.; Lv, X.; Guo, S.; Ma, Y.; Han, B.; Hu, X. Interactions between suspended sediments and submerged macrophytes-epiphytic biofilms under water flow in shallow lakes. Water Res. 2022, 222, 118911. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, H.; Sun, S.; Li, Z. Cooperative denitrification in biocathodes under low carbon to nitrogen ratio conditions coupled with simultaneous degradation of ibuprofen in photoanodes. Bioresour. Technol. 2022, 351, 126988. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, S.; He, S. Iron plaque formation and its effect on key elements cycling in constructed wetlands: Functions and outlooks. Water Res. 2023, 235, 119837. [Google Scholar] [CrossRef]
- Fu, F.; Huang, S.; Hu, H.; Lu, Y.; Wang, Y.; Yuan, J.; Gong, Z.; Wu, J.; Zhang, Y. Transformation of N and S pollutants and characterization of microbial communities in constructed wetlands with Vallisneria natans. J. Water Process Eng. 2021, 42, 102186. [Google Scholar] [CrossRef]
- Gonzalez-Gil, G.; Sougrat, R.; Behzad, A.R.; Lens, P.N.; Saikaly, P.E. Microbial Community Composition and Ultrastructure of Granules from a Full-Scale Anammox Reactor. Microb. Ecol. 2015, 70, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Wu, H.; Zhang, Z.; Li, Q.; Zhang, W.; Zheng, Z.; Yang, K.; Miao, H.; Xu, J. Biological effects of harvesting harmful algal blooms on submerged macrophytes and leaf biofilms: A mesocosm experiment. J. Clean. Prod. 2022, 361, 132256. [Google Scholar] [CrossRef]
- Han, S.-F.; Jin, W.; Qu, F.; Hanelt, D.; Abomohra, A. Integrated municipal wastewater treatment and lipid accumulation by a self-flocculating/floating microalga Limnothrix sp. Bioresour. Technol. 2024, 394, 130165. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A. Textile Wastewater Treated by Constructed Wetlands–A Critical Review. J. Ecol. Eng. 2023, 24, 256–275. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, M.; He, D.; Zhu, J.; Yang, S.; Fang, F.; Yang, L. Submerged macrophyte promoted nitrogen removal function of biofilms in constructed wetland. Sci. Total Environ. 2024, 914, 169666. [Google Scholar] [CrossRef]
- Kumar, S.; Sangwan, V.; Kumar, M.; Deswal, S. A survey on constructed wetland publications in the past three decades. Environ. Monit. Assess. 2023, 195, 992. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, X.; Gu, P.; Yang, G.; Deng, H.; Zhang, J.; Zheng, Z. Transcriptomic analysis reveals phytohormone and photosynthetic molecular mechanisms of a submerged macrophyte in response to microcystin-LR stress. Aquat. Toxicol. 2022, 245, 106119. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, X.; Wu, Z.; Liu, J.; Hu, B.; Chen, H.; Li, B. Biotransformation of nitrogen and tetracycline by counter-diffusion biofilm system: Multiple metabolic pathways, mechanism, and slower resistance genes enrichment. Chem. Eng. J. 2023, 474, 145637. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, M.; Peng, X.; Ma, J.; Zhang, Y.; Yu, F.; Wu, Z.; Liu, B. Response of Vallisneria natans to aluminum phytotoxicity and their synergistic effect on nitrogen, phosphorus change in sediments. J. Hazard. Mater. 2020, 400, 123167. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Z.; Hu, X.B.; Yan, J.; Mei, L.Y.; Li, X.T. Effects of intermittent aeration under different operation conditions on the pollutant removal of a pilot-scale constructed wetland. Fresenius Environ. Bull. 2019, 28, 1312–1318. [Google Scholar]
- Liu, Y.; Feng, B.; Yao, Y. Research Trends and Future Prospects of Constructed Wetland Treatment Technology in China. Water 2024, 16, 738. [Google Scholar] [CrossRef]
- Meng, F.; Lyu, Y.; Zhao, H.; Lyu, F.; Bie, X.; Lu, Y.; Zhao, M.; Chen, Y.; Lu, Z. LsrR-like protein responds to stress tolerance by regulating polysaccharide biosynthesis in Lactiplantibacillus plantarum. Int. J. Biol. Macromol. 2023, 225, 1193–1203. [Google Scholar] [CrossRef]
- Nájera, A.F.; Serwecińska, L.; Szklarek, S.; Mankiewicz-Boczek, J. Seasonal and spatial changes of N-transforming microbial communities in sequential sedimentation-biofiltration systems—Influence of system design and environmental conditions. Int. Biodeterior. Biodegrad. 2021, 159, 105203. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Wan, J.; Yan, Z.; Ma, Y.; Zhang, G.; Zhu, B. The Nitrogen Removal Performance and Functional Bacteria in Heterotrophic Denitrification and Mixotrophic Denitrification Process. Water 2022, 14, 3603. [Google Scholar] [CrossRef]
- Seviour, R.J.; Mino, T.; Onuki, M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 2003, 27, 99–127. [Google Scholar] [CrossRef]
- Tian, J.; Han, Y.; Yin, P.; Zhang, J.; Guo, T.; Li, H.; Hou, Y.; Song, Y.; Guo, J. Response of dissimilatory perchlorate reducing granular sludge (DPR-GS) system to high-strength perchlorate and starvation stress in UASB reactor: Performance, kinetics and recovery mechanism. J. Environ. Chem. Eng. 2023, 11, 109414. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, J.; Huang, J.; Chen, G.; Zhao, Y. The metabolic process of aerobic granular sludge treating piggery wastewater: Microbial community, denitrification genes and mathematical model calculation. J. Environ. Chem. Eng. 2021, 9, 105392. [Google Scholar] [CrossRef]
- Tomás-Martínez, S.; Zwolsman, E.J.; Merlier, F.; Pabst, M.; Lin, Y.; van Loosdrecht, M.C.; Weissbrodt, D.G. Turnover of the extracellular polymeric matrix of granules performing biological phosphate removal. Appl. Microbiol. Biotechnol. 2023, 107, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. Agroindustrial Wastewater Treatment with Simultaneous Biodiesel Production in Attached Growth Systems Using a Mixed Microbial Culture. Water 2018, 10, 1693. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, S.; Zhou, Z.; Chen, S. Plant Compartments Shape the Assembly and Network of Vallisneria natans-Associated Microorganisms. Diversity 2023, 15, 676. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Q.; Wang, L.Y. Cadmium Toxicity and Phytochelatin Production in a Rooted-Submerged Macrophyte Vallisneria spiralis Exposed to Low Concentrations of Cadmium. Environ. Toxicol. 2009, 24, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Chen, N.; Piao, H.; Sun, D.; Ratnaweera, H.; Maletskyi, Z.; Bi, X. Characterization of Oxidation-Reduction Potential Variations in Biological Wastewater Treatment Processes: A Study from Mechanism to Application. Processes 2022, 10, 2607. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, X.; Li, J.; Zhao, Z.; Hou, X. Mixed culture of plants improved nutrient removal in constructed wetlands: Response of microbes and root exudates. Environ. Sci. Pollut. Res. 2022, 30, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, L.; Zhou, C.; Zhou, Y.; Xia, S.; Rittmann, B.E. Co-removal of 2,4-dichlorophenol and nitrate using a palladized biofilm: Denitrification-promoted microbial mineralization following catalytic dechlorination. J. Hazard. Mater. 2022, 422, 126916. [Google Scholar] [CrossRef]
- Wu, M.; Hao, H.; Ge, Y.; Pu, T.; He, Z.; Ge, D.; Rene, E.R.; Huang, Z. Treatment of Black-Odorous Water Using Submerged Plants: The Physiological Response of Vallisneria natans. Water 2023, 15, 653. [Google Scholar] [CrossRef]
- Wu, W.; Yang, L.; Wang, J. Denitrification performance and microbial diversity in a packed-bed bioreactor using PCL as carbon source and biofilm carrier. Appl. Microbiol. Biotechnol. 2013, 97, 2725–2733. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Xing, W.; Li, J.; Li, P.; Wang, C.; Cao, Y.; Li, D.; Yang, Y.; Zhou, J.; Zuo, J. Effects of residual organics in municipal wastewater on hydrogenotrophic denitrifying microbial communities. J. Environ. Sci. 2018, 65, 262–270. [Google Scholar] [CrossRef]
- Xu, P.; Xiao, E.; Wu, J.; He, F.; Zhang, Y.; Wu, Z. Enhanced nitrate reduction in water by a combined bio-electrochemical system of microbial fuel cells and submerged aquatic plant Ceratophyllum demersum. J. Environ. Sci. 2019, 78, 338–351. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Lin, D.; Guo, C.; Yan, L.; Wang, S.; He, Z. Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands. Sci. Total Environ. 2018, 622–623, 121–126. [Google Scholar] [CrossRef]
- Yu, G.; Chen, H.; Chen, J.; Chen, S.; Long, Y.; Huang, J.; Wang, Y.; He, S. Enhanced nitrogen removal through aerobic denitrifying bacteria in horizontal subsurface flow constructed wetlands: Influencing factors and microbial community structure. Chem. Eng. J. 2024, 481, 148654. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, F.; Qu, Q.; Wang, C.; Qin, Y.; Ma, L.; Jia, Y.; Ismael, M.; Jiang, Y.; Sun, T.; et al. Effects of ageing time on the properties of polysaccharide in tangerine peel and its bacterial community. Food Chem. 2023, 417, 135812. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, Z.; Li, Y.; Huang, S.; Zhang, J.; Zheng, Z. Response of submerged macrophytes and leaf biofilms to different concentrations of oxytetracycline and sulfadiazine. Chemosphere 2022, 308, 136098. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Xie, Y.; Ding, L.-Y.; Qiao, X.-J.; Tao, H.-C. Highly efficient ammonium removal through nitrogen assimilation by a hydrogen-oxidizing bacterium, Ideonella sp. TH17. Environ. Res. 2020, 191, 110059. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Zhou, J.; Ju, F. Active predation, phylogenetic diversity, and global prevalence of myxobacteria in wastewater treatment plants. ISME J. 2023, 17, 671–681. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Yang, J.; Zhou, S.; Du, J.; Zhang, M.; An, S. Mutual promotion of submerged macrophytes and biofilms on artificial macrophytes for nitrogen and COD removal improvement in eutrophic water. Environ. Pollut. 2021, 277, 116718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Hu, H.; Chow, A.T.; Chen, P.; Wang, Y.; Xu, X.; Gong, Z.; Huang, S. Evaluation of organic matter and nitrogen removals, electricity generation and bacterial community responses in sediment microbial fuel cell coupled with Vallisneria natans. J. Environ. Chem. Eng. 2023, 11, 110058. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, X.; Yang, X.; Zhou, H.; Fang, J.; Gong, S.; Yang, J.; Chen, J.; Lu, T.; Zeng, M.; et al. The nitrogen removal performance and microbial community on mixotrophic denitrification process. Bioresour. Technol. 2022, 363, 127901. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).